Abstract
Background
Digital single-operator cholangioscopes (digital SOCs), equipped with an improved image quality, have been recently introduced.
Objective
The aim of this study is to evaluate the safety and diagnostic and therapeutic efficacy of digital SOCs (Spyglass™ DS).
Methods
Sixty-seven digital SOC procedures performed between 2015 and 2017 were retrospectively analyzed.
Results
The most frequent indications for examination were indeterminate biliary strictures (61.2%) and biliary stone disease (23.9%). In 25 patients (37.3), visual findings predicted malignancy with a sensitivity of 88.9%, a specificity of 97.6%, a positive predictive value (PPV) of 96.0% and a negative predictive value (NPV) of 92.9%. For histological analysis, forceps biopsies were performed in 29 patients (43.2%). Compared with visual findings, forceps biopsies yield a lower diagnostic efficacy in diagnosing malignancy (sensitivity 62.5%, specificity 90.0%, PPV 90.9%, NPV 60.0%). Therapeutic interventions were performed in 19 patients with a technical success rate of 89.4%. Adverse events were observed in 17 patients (25.4%). Of these, 11 patients (16.4%) suffered from severe adverse events (pancreatitis, cholangitis or major bleeding), which led to a prolonged hospital stay.
Conclusion
Digital SOCs have excellent diagnostic and therapeutic efficacies, but are accompanied by high rates of adverse events; therefore, physicians should use digital SOCs in carefully selected cases.
Keywords: Cholangioscopy, SpyGlass DS, adverse events, digital single-operator cholangioscopy, indeterminate biliary strictures, complex biliary stone disease
Key summary
Established knowledge:
Digital single-operator cholangioscopes (digital SOCs) have been recently introduced.
New findings:
Digital SOCs have an excellent diagnostic accuracy for biliary tract diseases and a high efficacy for therapeutic interventions including treatment of complex biliary stone disease and biliary strictures.
Our results suggest a high incidence (16.4%) of severe adverse events associated with the examination, which is why digital SOCs should only be used in carefully selected cases.
Introduction
In 2015, a new digital single-operator cholangioscope (digital SOC; SpyGlass™ DS System, Boston Scientific, Marlborough, USA) was launched.1 Compared with the previous fibre-optic Spyglass System, this new cholangioscope provides digital imaging enabling an up to four-hold higher resolution.1–5 Moreover, it provides a 60% wider field of view and might lead to an easier scope-insertion into the biliary tract due to the tapered tip.1,5
To date, only a few studies have evaluated digital SOCs.6–10 Among these reports, three small studies examined the diagnostic and therapeutic efficacy of digital SOCs, but no study performed an in-depth investigation of adverse events (AEs) related to the examination.6,9,10 Therefore, the aim of this study was to evaluate the diagnostic efficacy, the success of therapeutic procedures and especially the safety of digital SOCs.
Methods
Study design
This retrospective study was performed at the Department of Medicine B for Gastroenterology and Hepatology of the University Hospital Muenster, Germany. The study was approved by the Ethics Board of the Westphalian Wilhelms-University of Muenster and the Medical Council of Westphalia-Lippe, Germany (date of approval: 1 September 2017) and conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in the prior approval by the institution's human research committee. As approved by the Ethics Board, informed patient consent was not required for this study because of its retrospective design. Data from all patients ≥18 years of age who underwent a digital SOC with the SpyGlass DS system between August 2015 and July 2017 were retrieved from the clinical data system.
Technical aspects of Spyglass cholangioscopy
All examinations were performed by two highly experienced endoscopists (F.L. and H.U.) according to the general accepted guidelines using an endoscopy retrograde cholangiography (ERC) case volume of greater than 200/year.11,12 Prior to cholangioscopy, all patients received a prophylactic, single shot antibiotic treatment with ceftriaxone. Indomethacin was not regularly administered before the procedure. CO2 insufflation was used during the examination. In all patients, endoscopic papillotomy was performed or had been previously performed. The SpyScope was inserted with guidewire assistance into the biliary duct; targeted biopsies were performed using SpyBite forceps (Boston Scientific, Marlborough, USA).
Diagnostics and safety using digital SOC
Visual findings were classified as suggestive of malignancy in the case of irregular vessels, easy bleeding, irregular surfaces and elevated masses protruding into the duct lumen.6,9,10 Tissue acquired by forceps biopsy was analyzed by an experienced pathologist and classified as suggestive of malignancy in case of detection of cancer or high-grade cell dysplasia.
The final diagnosis was based on a detailed evaluation of all available data. The median follow-up was 18 months (interquartile range (IQR) 13–23 months).
AEs were documented as follows: (a) new onset abdominal pain was diagnosed if pain occurred within 24 h of examination and required additional or new analgesia; (b) fever was diagnosed if the body temperature reached ≥38.0℃ within seven days of examination; (c) post-interventional pancreatitis was diagnosed if the onset of abdominal pain was accompanied by a three-fold increase in the serum lipase levels within 48 h of examination; (d) post-interventional cholangitis was defined as the onset of fever and newly or significantly higher inflammation markers requiring antibiotics within three days of examination; and (e) severe bleeding was diagnosed if blood transfusion was required. We defined the post-interventional onset of pancreatitis, cholangitis and severe bleeding as severe adverse events (SAEs).
Statistical analysis
The data analysis was performed using IBM SPSS Statistics 22.0 (IBM Corp., Armonk, USA). The contingency table-derived data were calculated using StatPages.13
Results
Study population
Digital SOCs were performed in 67 patients (Table 1). The median age was 66 years (IQR: 54–77 years). The mean total time of the procedure including ERC and digital SOC was 45 min (IQR 35–75 min) with a mean time for cholangioscopy of 11 min (IQR 7–15 min). The most frequent indication for digital SOC was indeterminate biliary stricture (61.2%) followed by cholelithiasis (23.9%) and selective guidewire insertion (7.5%; Table 1).
Table 1.
Characteristics of patients undergoing digital single-operator cholangioscope (SOC). Median and interquartile ranges are reported for continuous variables, and frequencies and percentages are reported for categorical variables.
| Variables | Patients (n = 67) |
|---|---|
| Age (years) | 66 (54–77) |
| Female (%) | 38 (56.7) |
| Male (%) | 29 (43.3) |
| Previous ERC (%) | 59 (88.1) |
| Cholangioscopy procedures (%) | 67 (100) |
| Time of examination in min (range) | |
| Total (ERC + cholangioscopy) | 45 (35–75) |
| Cholangioscopy time | 11 (7–15) |
| Indication for cholangioscopy | |
| Indeterminate biliary stricture | 41 (61.2) |
| Cholelithiasis | 10 (14.9) |
| Exclusion of residual cholelithiasis | 6 (9.0) |
| Selective guidewire insertion | 5 (7.5) |
| Mixed | 5 (7.5) |
| Final main diagnosis (%) | |
| Malignancy | 27 (40.3) |
| New diagnosis | 24 (35.8) |
| Already known diagnosis | 3 (4.5) |
| Entities of carcinoma | |
| - Cholangio-carcinoma | 18 (26.9) |
| - Pancreatic-carcinoma | 6 (9.0) |
| - Gallbladder-carcinoma | 1 (1.5) |
| - Other metastatic carcinoma | 2 (3.0) |
| Benignancy | 40 (59.7) |
| Cholelithiasis | 19 (28.4) |
| Strictures | 10 (14.9) |
| - Extrahepatic | 7 (10.4) |
| - Intrahepatic | 3 (4.5) |
| Follow-up after neoplasia removal | 3 (4.5) |
| Others | 8 (11.9) |
ERC: endoscopy retrograde cholangiography.
Forty patients (59.7%) had a final diagnosis of benign biliary disease with stone disease (28.4%), and benign biliary strictures (14.9%) as the most frequent. In 27 patients (40.3%), the diagnosis of malignancy was established as follows: cholangio-carcinoma (26.9%), pancreatic carcinoma (8.9%) and other malignancies (4.5%; Table 1).
In nearly all cases, the diagnostic procedures using digital SOCs were technically successful (98.5%); however, in one case, the SpyGlass DS System technically failed and could not be relaunched (1.5%; Table 2).
Table 2.
Analysis of cholangioscopy with digital single-operator cholangioscope (SOC). Percentages are reported for categorical variables. Median + interquartile ranges are reported for continuous variables. Severe adverse events are defined as complications such as pancreatitis, cholangitis and bleeding requiring a blood transfusion.
| Variables | Patients (n = 67) |
|---|---|
| Technical success of diagnostic procedures (%) | 66 (98.5) |
| Visual findings (%) | |
| Suggestive of malignancy | 25 (37.3) |
| Suggestive of benignancy | 42 (62.7) |
| Forceps biopsies, digital SOC guided (%) | |
| Patients undergoing SpyGlass biopsies | 29 (43.3) |
| Number of biopsies per patient | 3 (25) |
| Results of biopsies | |
| - Suggestive of malignancy | 9/29 (31.0) |
| - Suggestive of besnignancy | 17/29 (58.6) |
| - Inadequate material | 3/29 (10.3) |
| Adverse events (%) | |
| Adverse events | 17 (25.4) |
| Abdominal pain requiring analgesia | 16 (23.8) |
| Fever | 6 (8.9) |
| Pancreatitis | 6 (8.9) |
| Cholangitis | 5 (7.5) |
| Bleeding requiring blood transfusion | 1 (1.5) |
| Severe adverse events | 11 (16.4) |
| Pancreatitis | 6 (8.9) |
| Cholangitis | 5 (7.5) |
| Bleeding requiring blood transfusion | 1 (1.5) |
SpyBite forceps biopsies were performed in 29 patients (43.3%; mean number of biopsies per patient: 3). A SpyBite biopsy was not carried out in all patients in whom a digital SOC was performed due to the initial indication of an indeterminate stricture (e.g. cases where the stricture was unsuspicious during SOC, or biliary stone disease was identified as the true diagnosis). The biopsies were suggestive of benignancy in 17 patients (58.6%), and suggestive of malignancy in nine patients (31.0%); the acquired amount of tissue was not sufficient for histological diagnosis in three patients (10.3%; Table 2).
Therapeutic interventions
In 19 patients (28.4%), therapeutic procedures were performed with a high rate of technical success (89.4%; Table 3 and Figures 1 and 2). All nine electrohydraulic lithotripsies (EHL) were performed successfully: in eight patients EHL became necessary since prior attempts by conventional stone extraction were unsuccessful (Figure 1). Moreover, in one patient EHL was indispensable because cholelithiasis was not visible in standard ERC. The mean stone size was 22 mm (range 5–30 mm); on average 1.2 stones per patient were treated and apart from one stone, all were extrahepatic. In addition, we performed 10 SOC-assisted selective guidewire insertions in obstructed biliary segments that could not be cannulated using standard ERC (Figure 2). In eight of these cases therapeutic applications (cannulation, duct-dilatation and insertion of prosthesis) were technically successful (80%).
Table 3.
Subgroup analysis of therapeutic interventions during cholangioscopy with digital single-operator cholangioscope (SOC). Percentages are reported for categorical variables.
| Variables | Patients |
|---|---|
| Number of patients (of all digital SOCs; %) | 19/67 (28.4) |
| Technical success of therapeutic procedures | 17/19 (89.4) |
| Therapeutic procedure | |
| Electrohydraulic lithotripsy | 9/9 |
| - Stone size (mm; mean + range) | 22 (5–30) |
| - Treated stone number per patient | 1.2 |
| - Extrahepatic/intrahepatic stones | 10/1 |
| Selective guidewire insertion | 8/10 |
| Adverse events | |
| Adverse events in numbers of patients | 6/19 (31.6) |
| Severe adverse events in numbers of patients | 3/19 (15.8) |
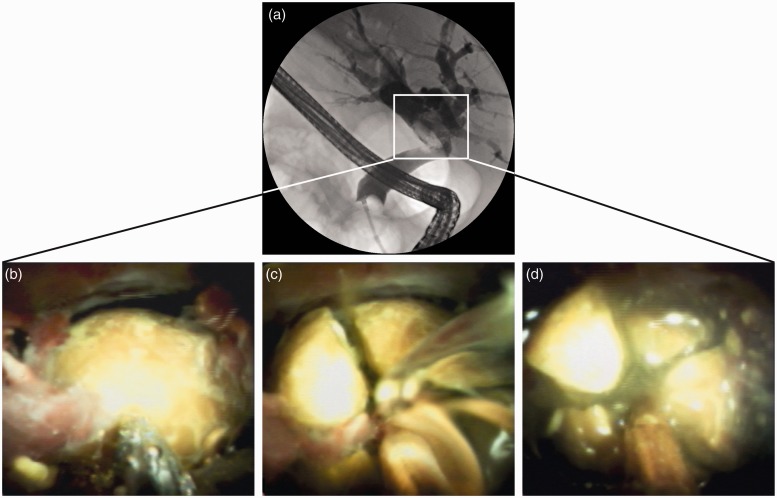
Figure 1.
Successful treatment of complex biliary stone disease using single-operator cholangioscope (SOC)-guided electrohydraulic lithotripsies (EHL). (a) Cholangiography showed an incarcerated biliary stone in the common bile duct. (b–d) Digital SOC enabled direct visualization of the incarcerated biliary stone and successful treatment using SOC-guided EHL.
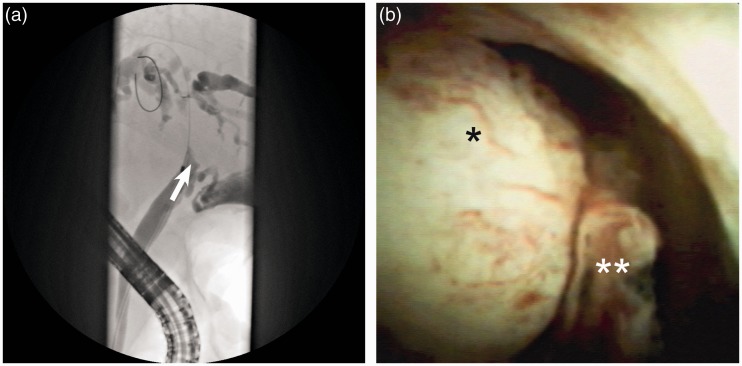
Figure 2.
Successful treatment of malignant stricture using single-operator cholangioscope (SOC)-guided selective guidewire insertion with subsequent endoprosthesis implantation. (a) Cholangiography indicated a suspicious obstruction of the hepatic ducts (arrow). (b) Cholangioscopy showed a protruding mass with irregular vessels suspicious for malignancy (*/**). Using SOC-guided selective guidewire insertion, an endoprosthesis was successfully implanted for biliary drainage.
Diagnostic efficacy of digital SOC for malignancy detection
We detected visual findings suggestive of malignancy in 25 patients (37.3%; Table 2). The visual findings consistent with malignancy predicted cancer with a sensitivity of 88.9% and a specificity of 97.5% (Table 4). The positive predictive value (PPV) was 96.0% and the negative predictive value (NPV) was 92.9% (Table 4).
Table 4.
Diagnostic efficacy of digital single-operator cholangioscope (SOC): comparison between visual findings and forceps biopsy in diagnosing malignancy. Percentages are reported for categorical variables; 95% confidence interval (CI).
| Variables | Sensitivity (%) | Specificity (%) | Pos. pred. value (%) | Neg. pred. value (%) |
|---|---|---|---|---|
| Visual findings (95% CI) | 88.9 (76.3–92.4) | 97.5 (89.0–99.9) | 96.0 (82.5–99.8) | 92.9 (84.8–95.1) |
| Forceps biopsy (95% CI) | 62.5 (44.5–68.4) | 90.0 (61.1–99.5) | 90.9 (64.7–99.5) | 60.0 (40.866.3) |
Forceps biopsy was used as a second tool to diagnose malignancy and had a sensitivity of 62.5% and a specificity of 90.0%. The forceps biopsies had a PPV of 90.9% and a NPV of 60.0% (Table 4).
AEs following digital SOC
No AEs were observed following digital SOC in 74.6% of the patients. However, AEs occurred in 17 patients (25.4%), and of these patients, 11 patients (16.4%) suffered from SAEs such as post-interventional pancreatitis (8.9%), cholangitis (7.5%) and major bleeding (1.5%). In general, the most frequent AE was post-interventional abdominal pain requiring analgesia (23.8%; Table 2).
According to a sub-analysis of digital SOCs performed along with therapeutic procedures, we observed 31.6% AEs and 15.8% SAEs. The rates of AE were similar to those in patients receiving diagnostic procedures only (p = 0.46; Table 3).
We analyzed the cases with SAEs in detail and found that all six patients suffering from post-interventional pancreatitis had a prolonged hospital stay. In five patients, pancreatitis symptoms resolved within 72 h and only in one case pancreatitis symptoms showed a prolonged disease course of seven days. None of these cases developed into a necrotizing pancreatitis. All patients (100%) suffering from post-interventional cholangitis required intravenous antibiotic treatment, which was accompanied by a prolonged hospital stay (median prolonged hospital stay: seven days (range 2–14 days)). Two patients had a challenging disease course requiring a change in antibiotic regimen; however, all patients were treated successfully. One patient developed a severe bleeding complication after endoprosthesis implantation into a contact-vulnerable malignant biliary stricture: however, this bleeding stopped spontaneously, and the patient required one pack of red blood cells. In summary, all cases of SOC-related complications were treated successfully by conservative therapeutic interventions.
Discussion
This study shows that peroral digital SOC is an efficient and easy-to-use tool for the diagnosis and treatment of indeterminate biliary strictures and complex biliary stone disease (Figures 1 and 2); however, our results suggest a high incidence (16.4%) of SAEs associated with ERC-assisted digital SOC.
The evaluation of indeterminate biliary strictures is challenging.14 To diagnose a malignant biliary stricture, conventional ERC combined with biopsies or brush cytology has a low sensitivity. A recent meta-analysis involving 730 patients found a sensitivity in diagnosing malignant biliary strictures of 48% for intraductal biopsies which could be increased to 59% if performed in combination with brush cytology.15 Beside a direct inspection of the stricture, cholangioscopy allows a targeted biopsy under visual control.16 In a meta-analysis involving 456 patients, the fibre-optic SOC system showed only a moderately higher sensitivity of 66.2% to diagnose malignant strictures using SOC-guided targeted biopsies compared with ERC-assisted biopsies.17 The first retrospective multicenter study using digital SOCs indicated a high sensitivity of 85% in 44 patients with indeterminate biliary strictures for targeted biopsies.9 Of note, onsite evaluation of the biopsies was performed by a cytopathologist, which may at least partially explain the high sensitivity rates.9,18 We performed no onsite evaluation of biopsies and observed a lower sensitivity of 62.5% in diagnosing malignancy, which is comparable to the sensitivity rates observed in fibre-optic SOC (66.2%).17 Therefore, we speculate that the sensitivity of targeted biopsies might not be improved significantly by digital image quality; however, the sensitivity could be increased by obtaining more biopsies or by the technique of sample processing.19,20
The above-mentioned digital SOC study by Navaneethan and colleagues reported a high sensitivity of 90% in the visual diagnosis of malignancy in 44 patients with indeterminate biliary strictures.9 In line with this finding, our study demonstrates a sensitivity of 88.9% in 25 patients for visual findings consistent with malignancy. In all studies evaluating the visual aspects during SOC, sensitivity and specificity rates might be biased by the investigator's awareness of the patient's history and prior tissue and imaging results.9,21 In summary, recent studies and our results support an improved imaging quality of digital SOCs that might increase the efficacy of optically diagnosing malignancy; however validated optical criteria for malignancy detection are missing.6,9,10
The second main indication for cholangioscopy was complex biliary stone disease. In our study, the digital SOC-guided EHL allowed complete biliary stone clearance in all patients (9/9). Our results are consistent with other digital SOC studies that report stone removal rates of 86–100%.7,9,10 According to a meta-analysis including 244 cholangioscopies in patients with difficult stone disease, the success rate for fibre-optic SOC was 87%.22 We conclude that compared with fibre-optic SOC, for complex stone disease, the improved digital imaging quality might not improve biliary stone clearance. Next to endoscopic digital SOC-guided EHL, the 10.5 Fr digital SOC might be useful for percutaneous-transhepatic-cholangioscopy-guided EHL in patients with complex biliary stone disease.23
The third indication for SOC was selective guidewire insertion into complex strictures, in which guidewire passage via conventional ERC had failed. In our study, 80% of the SOC-guided selective guidewire insertions and consecutive therapeutic procedures were successful (8/10 procedures). Only a few digital SOC studies reported selective guidewire insertion in a limited number of patients (4–7 patients) with success rates of 57–75%.7,10 Particularly in patients with complex strictures, the enhanced imaging quality of the digital SOC could help identify the ostium of the stricture and may prevent more invasive procedures.
In addition to the diagnostic and technical success rates, we focused on AEs related to digital SOC. In general, the incidence of AEs following ERC has been reported to be approximately 7%.24 In a well-documented prospective cohort of 84 fibre-optic SOC-procedures, AEs occurred in 21.4% of all patients.22 Only a few digital SOC studies involving more than 50 patients are available, and lower AE rates of 2.9–3.6% were reported in 55–105 patients.6,9 In contrast to previous studies, the rate of AEs was one of our key points; AEs, including post-interventional abdominal pain, occurred in a substantial proportion of patients (25%), and SAEs (such as SOC-associated pancreatitis, cholangitis and major bleeding) were documented in 16% of the patients. In contrast to previous digital SOC studies,6,9 we additionally defined abdominal pain and fever as AEs. Omitting these criteria, our overall AE rate dropped to 16%. Unlike in the previous digital SOC study published by Shah et al., we have provided a detailed methods section for defining AEs.6 Furthermore, in addition to a well-documented electronic health record, post-interventional lipase levels were determined in all patients, therefore even mild cases of pancreatitis were less likely to be missed. Taken together, this might help to explain why we observed higher rates of AEs than previous digital SOC studies. We conclude that complications following digital SOC might be comparable to those following fibre-optic SOC. Thus, due to the high complication rates, SOC should only be used in a carefully selected group of patients.
Pancreatitis rates following ERC were reported in 2–4% of unselected patients.24,25 Following fibre-optic SOC, pancreatitis rates lower than 2% have been described.22,26 In our study post-interventional pancreatitis occurred in 8.9% of the patients. A previous study identified intraductal ultrasound as a risk factor for post-interventional pancreatitis with an incidence of 8.3%.25 Given a digital SOC employing a rigid catheter might be comparable to an intraductal ultrasound probe, this may contribute to the risk of post-interventional pancreatitis. We did not routinely use rectal indomethacin for digital SOC presuming that cholangioscopy is not a high-risk factor for post-interventional pancreatitis. However, in light of our study results, rectal indomethacin administration in all patients undergoing digital SOC appears reasonable to reduce the risk of pancreatitis. Furthermore, pancreatitis rates can be increased due to a concomitant endoscopic sphincterotomy, a papillary balloon dilation or a pancreatic duct injection.27,28 Out of our six cases of post-interventional pancreatitis, two had a sphincterotomy and one had a papillary balloon dilation.
Cholangitis is another SAE occurring after SOC. After fibre-optic SOC procedures, cholangitis rates of 4–5% have been reported.22 In our study, we observed slightly higher cholangitis rates of 7.5%. Considering our results, appropriate steps to prevent SOC-associated cholangitis might be taken in future; this might include the performance of imaging like magnetic resonance cholangiopancreatography prior to examination to guide SOC, thereby avoiding an extended contrast injection and a prolonged procedure time. Furthermore, the risk of cholangitis depends on the completeness of post-interventional biliary drainage and on the opacified ducts.
Our study has several limitations. First, this was a retrospective study, and our final data set consisted of only 67 patients; however, we were able to collect a homogeneous and complete data set featuring detailed endoscopical reports. Second, we report results from a single-centre-study. However, we present the largest single-centre experience concerning digital SOCs, and we could ensure that examinations were performed by two highly experienced endoscopists, improving the reliability of our results. Third, the endoscopists were not blinded to previous imaging and laboratory results, and the visual impression of the endoscopists could have been biased; however, our patients were referred for digital SOC due to still indeterminate biliary strictures despite previously performed diagnostics.
Taken together, our data indicate that digital SOCs are highly effective for diagnosing and treating benign and malignant biliary disease; however, they may be associated with significant complication rates. Therefore, we recommend performing digital SOC with care and only in a selected group of patients. To strengthen these recommendations from our single-centre study, further studies are needed in the future.
Acknowledgements
FL, AB and HU designed the study, analyzed the data and wrote the manuscript; DG analyzed the data; TN and DB contributed to writing the manuscript. All authors edited the scientific contents of the manuscript and approved the final version prior to submission.
Declaration of conflicting interests
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not–for–profit sectors.
Ethics approval
The study was approved by the Ethics Board of the Westphalian Wilhelms-University of Muenster and the Medical Council of Westphalia-Lippe, Germany (date of approval: 1 September 2017) and conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in the prior approval by the institution's human research committee.
Informed consent
As approved by the Ethics Board, informed patient consent was not required for this study because of its retrospective design.
References
- 1.Boston Scientific. SpyglassTM DS Direct Visualization System, 2015. http://www.bostonscientific.com/content/dam/bostonscientific/endo/portfolio-group/SpyGlass%20DS/SpyGlass-DS-System-ebrochure.pdf (accessed 24 January 2018).
- 2.Tringali A, Lemmers A, Meves V, et al. Intraductal biliopancreatic imaging: European Society of Gastrointestinal Endoscopy (ESGE) technology review. Endoscopy 2015; 47: 739–753. [DOI] [PubMed] [Google Scholar]
- 3.Chen YK, Pleskow DK. SpyGlass single-operator peroral cholangiopancreatoscopy system for the diagnosis and therapy of bile-duct disorders: a clinical feasibility study (with video). Gastrointest Endosc 2007; 65: 832–841. [DOI] [PubMed] [Google Scholar]
- 4.Chen YK. Preclinical characterization of the Spyglass peroral cholangiopancreatoscopy system for direct access, visualization, and biopsy. Gastrointest Endosc 2007; 65: 303–311. [DOI] [PubMed] [Google Scholar]
- 5.Pereira P, Peixoto A, Andrade P, et al. Peroral cholangiopancreatoscopy with the SpyGlass(R) system: what do we know 10 years later. J Gastrointestin Liver Dis 2017; 26: 165–170. [DOI] [PubMed] [Google Scholar]
- 6.Shah RJ, Raijman I, Brauer B, et al. Performance of a fully disposable, digital, single-operator cholangiopancreatoscope. Endoscopy 2017; 49: 651–658. [DOI] [PubMed] [Google Scholar]
- 7.Imanishi M, Ogura T, Kurisu Y, et al. A feasibility study of digital single-operator cholangioscopy for diagnostic and therapeutic procedure (with videos). Medicine 2017; 96: e6619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong JC, Tang RS, Teoh AY, et al. Efficacy and safety of novel digital single-operator peroral cholangioscopy-guided laser lithotripsy for complicated biliary stones. Endosc Int Open 2017; 5: E54–E58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Navaneethan U, Hasan MK, Kommaraju K, et al. Digital, single-operator cholangiopancreatoscopy in the diagnosis and management of pancreatobiliary disorders: a multicenter clinical experience (with video). Gastrointest Endosc 2016; 84: 649–655. [DOI] [PubMed] [Google Scholar]
- 10.Ogura T, Imanishi M, Kurisu Y, et al. Prospective evaluation of digital single-operator cholangioscope for diagnostic and therapeutic procedures (with videos). Dig Endosc 2017; 29(7): 782–789. [DOI] [PubMed] [Google Scholar]
- 11.Loperfido S, Angelini G, Benedetti G, et al. Major early complications from diagnostic and therapeutic ERCP: a prospective multicenter study. Gastrointest Endosc 1998; 48: 1–10. [DOI] [PubMed] [Google Scholar]
- 12.Baron TH, Petersen BT, Mergener K, et al. Quality indicators for endoscopic retrograde cholangiopancreatography. Am J Gastroenterol 2006; 101: 892–897. [DOI] [PubMed] [Google Scholar]
- 13.John C. Pezzullo. 2-way Contingency Table Analysis. 2018. http://statpages.info/ctab2x2.html (accessed 24 January 2018).
- 14.Tabibian JH, Visrodia KH, Levy MJ, et al. Advanced endoscopic imaging of indeterminate biliary strictures. World J Gastrointest Endosc 2015; 7: 1268–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Navaneethan U, Njei B, Lourdusamy V, et al. Comparative effectiveness of biliary brush cytology and intraductal biopsy for detection of malignant biliary strictures: a systematic review and meta-analysis. Gastrointest Endosc 2015; 81: 168–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramchandani M, Reddy DN, Lakhtakia S, et al. Per oral cholangiopancreatoscopy in pancreatico biliary diseases: expert consensus statements. World J Gastroenterol 2015; 21: 4722–4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Navaneethan U, Hasan MK, Lourdusamy V, et al. Single-operator cholangioscopy and targeted biopsies in the diagnosis of indeterminate biliary strictures: a systematic review. Gastrointest Endosc 2015; 82: 608–614, e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Varadarajulu S, Bang JY, Hasan MK, et al. Improving the diagnostic yield of single-operator cholangioscopy-guided biopsy of indeterminate biliary strictures: ROSE to the rescue? (with video). Gastrointest Endosc 2016; 84: 681–687. [DOI] [PubMed] [Google Scholar]
- 19.Kawashima H, Itoh A, Ohno E, et al. Transpapillary biliary forceps biopsy to distinguish benign biliary stricture from malignancy: how many tissue samples should be obtained?. Dig Endosc 2012; 24(Suppl 1): 22–27. [DOI] [PubMed] [Google Scholar]
- 20.Feakins R, Allen D, Campbell F, et al. Tissue pathways for gastrointestinal and pancreatobiliary pathology, London: The Royal College of Pathologists, 2016. [Google Scholar]
- 21.El H, II, Shah RJ. Digital single-operator cholangioscopy: fully disposable yet valuable. Gastrointest Endosc 2016; 84: 656–658. [DOI] [PubMed] [Google Scholar]
- 22.Laleman W, Verraes K, Van Steenbergen W, et al. Usefulness of the single-operator cholangioscopy system SpyGlass in biliary disease: a single-center prospective cohort study and aggregated review. Surg Endosc 2017; 31: 2223–2232. [DOI] [PubMed] [Google Scholar]
- 23.Cannavale A, Bezzi M, Cereatti F, et al. Combined radiological-endoscopic management of difficult bile duct stones: 18-year single center experience. Ther Adv Gastroenterol 2015; 8: 340–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andriulli A, Loperfido S, Napolitano G, et al. Incidence rates of post-ERCP complications: a systematic survey of prospective studies. Am J Gastroenterol 2007; 102: 1781–1788. [DOI] [PubMed] [Google Scholar]
- 25.Meister T, Heinzow H, Heinecke A, et al. Post-ERCP pancreatitis in 2364 ERCP procedures: is intraductal ultrasonography another risk factor?. Endoscopy 2011; 43: 331–336. [DOI] [PubMed] [Google Scholar]
- 26.Kurihara T, Yasuda I, Isayama H, et al. Diagnostic and therapeutic single-operator cholangiopancreatoscopy in biliopancreatic diseases: Prospective multicenter study in Japan. World J Gastroenterol 2016; 22: 1891–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ding X, Zhang F, Wang Y. Risk factors for post-ERCP pancreatitis: A systematic review and meta-analysis. Surgeon 2015; 13: 218–229. [DOI] [PubMed] [Google Scholar]
- 28.Liu Y, Su P, Lin S, et al. Endoscopic papillary balloon dilatation versus endoscopic sphincterotomy in the treatment for choledocholithiasis: a meta-analysis. J Gastroenterol Hepatol 2012; 27: 464–471. [DOI] [PubMed] [Google Scholar]