Abstract
Most gastrointestinal endoscopic procedures are now performed with sedation. Moderate sedation using benzodiazepines and opioids continues to be widely used, but propofol sedation is becoming more popular because its unique pharmacokinetic properties make endoscopy almost painless, with a very predictable and rapid recovery process. There is controversy as to whether propofol should be administered only by anesthesia professionals. According to published values, endoscopist-directed propofol has a lower mortality rate than endoscopist-delivered benzodiazepines and opioids, and a comparable rate to general anesthesia by anesthesiologists. Rapid recovery has a major impact on patient satisfaction, post-procedure education and the general flow of the endoscopy unit. According to estimates, the absolute economic benefit of endoscopist-directed propofol implementation in a screening setting is probably substantial, with 10-year savings of $3.2 billion in the USA. Guidelines concerning the use of propofol emphasize the need for adequate training and certification in sedation by non-anesthetists.
Keywords: Propofol, sedative agents, gastrointestinal endoscopy
Introduction
Although esophagogastroduodenoscopy (EGD) and colonoscopy can be performed without sedation, both procedures are better tolerated in terms of patient satisfaction and willingness to repeat the examination when sedation is administered.1 Endoscopic therapy is rarely performed without sedation.
Propofol (2,6-diisopropofol) is a phenolic derivative with satisfactory sedative and amnesic properties. It is highly lipophilic, and thus can rapidly cross the blood–brain barrier, resulting in an early onset of action.2 Propofol is a short-acting agent with rapid metabolism, which has a short recovery profile, regardless of the depth or length of the sedation period.3 Although propofol may lead to deep sedation or even dangerous adverse events that require cardiopulmonary support, it has been frequently used as a sedative agent for standard endoscopic procedures worldwide. In this article, the use of propofol in gastrointestinal endoscopy is reviewed.
Pharmacology of propofol
Propofol is a sedative-hypnotic drug with an amnestic effect, but only a minimal analgesic effect. Its hypnotic effect results from potentiating γ-aminobutyric acid (GABA) through the GABAA receptor in a manner similar to that of benzodiazepines.2 The depth of sedation increases in a dose-dependent manner. Serum levels of propofol should be greater than 1 µg/mL to produce sleep.4 The current formulation of propofol contains 1% propofol (10 mg/mL), 10% soybean oil, 2.25% glycerol and 1.2% purified egg phosphatide.5 Propofol is contraindicated in those with allergies to eggs or soybean.
Propofol is metabolized rapidly in the liver via hydroxylation and conjugation with glucuronide and sulfate, and its metabolites are excreted by the kidneys. The onset of effect for propofol is 0.5–1 minute, and the duration of effect is 4–8 minutes.5 The pharmacokinetic parameters of propofol vary with patient factors such as weight, sex and age. The dose should be reduced in elderly patients. Although impaired cardiac function potentiates the effects of propofol, chronic kidney disease and liver cirrhosis do not significantly alter propofol pharmacokinetics.
The major adverse effects are respiratory depression, hypotension and pain on injection. Hypotension results from the cardiovascular effects of propofol, which include decreased cardiac output and systemic vascular resistance. With overdosing, respiratory depression generally precedes clinically significant hypotension.6 Local pain during injection of propofol occurs in 30% of patients.5 There is no pharmacological antagonist to reverse its effect, although hypotension and respiratory depression typically respond rapidly to a dose reduction or interruption of drug infusion.
Propofol versus other agents
A 2005 meta-analysis of 12 randomized controlled trials (RCTs) summarized the potential benefits of propofol sedation during gastrointestinal endoscopy by comparing the cardiopulmonary complications (i.e. hypoxia, hypotension, arrhythmia and apnea) between propofol and traditional sedative agents, such as midazolam and meperidine.3
A 2013 meta-analysis of 22 RCTs found that propofol provides a shorter recovery time and better sedation than traditional sedative agents, without causing an increase in cardiopulmonary complications.7 Furthermore, two 2014 meta-analyses revealed the clear benefits of better sedation and shorter recovery in patients who underwent advanced endoscopic procedures such as endoscopic retrograde cholangiopancreatography and endoscopic submucosal dissection. These benefits were achieved without an increased risk of cardiopulmonary complications.8,9
A recent prospective study underlined the safety of sedation with propofol alone in comparison to other regimens. The multicenter electronic registry recorded a total of 36,206 endoscopies. Odds ratios for sedation-related complications are 1 (reference) for midazolam alone, 0.75 for propofol alone, 1.005 for propofol plus midazolam, 1.5 for midazolam plus opiate and 1.55 for other sedation regimens. The lowest risk was found with propofol alone.10
The short-acting selective alpha-2 agonist dexmedetomidine has been reported to facilitate the sedation of patients, while maintaining their consciousness and allowing maintenance of stable respiration and circulation.11 We previously conducted a meta-analysis of data from RCTs comparing dexmedetomidine with propofol.12 In gastrointestinal endoscopy, the patients’ satisfaction levels were higher with propofol administration than with dexmedetomidine administration, while the risk of complications was similar. In the future, propofol is expected to become an essential sedative agent for endoscopic examination.
Propofol in actual practice
An initial bolus of propofol (0.5–1 mg/kg) is administered intravenously, followed by a repeated bolus (10–20 mg) according to the patient’s condition, or a continuous propofol infusion (2–6 mg/kg/h, with an additional bolus administered as needed).9,12 The infusion rate is chosen according to the desired sedation depth and the patient risk profile.
Horiuchi et al. reported that low-dose nurse-administered propofol sedation was safe and practical for diagnostic EGD.13 The protocol for initial bolus injection was 40 mg for patients < 70 years old, 30 mg for patients aged 70–89 years, and 20 mg for those aged 90 years or older. When the target sedation level was not obtained, additional injections of 20 mg propofol were given. Furthermore, Horiuchi et al. reported that driving ability returned to baseline within 60 min following low-dose propofol sedation.14
Propofol is used in combination with an opioid analgesic such as pethidine or fentanyl, especially in advanced endoscopic procedures. However, the recovery time for a combination of propofol and opioid may be longer than that for propofol only.
Computer-assisted propofol sedation (CAPS) systems continuously monitor capnography, oxygen saturation, electrocardiogram and blood pressure, allowing non-anesthesiologists to administer propofol as a continuous infusion. In 2013, the SEDASYS® system (Ethicon Endo-Surgery, Inc., Cincinnati, OH, USA) was approved in the United States for the provision of moderate sedation to patients undergoing routine EGD and colonoscopy. However, the manufacturer of SEDASYS® closed down its CAPS division, as the company was unable to project profitability from this technology at the end of 2016.15
The safety of propofol by the non-anesthetist provider and legal issues
Poincloux et al. conducted an RCT comparing endoscopist-administered propofol sedation for colonoscopy with anesthetist-administered deep sedation.16 In comparison with anesthetist-administered deep sedation, it was found that endoscopist-administered propofol sedation for colonoscopy yielded fewer side effects, and a better level of satisfaction and patient willingness to undergo further colonoscopies under the same conditions.
Rex et al. reviewed a total of 646,080 cases of endoscopist-directed propofol sedation, although this study had a substantial weakness in the study design of meta-analysis plus retrospective data. Endotracheal intubations and deaths numbered 11 and 4, respectively.17 The overall death rate for endoscopist-directed propofol sedation was 0.6 per 100,000. Deaths occurred in two patients with pancreatic cancer, a severely handicapped patient with mental retardation and a patient with severe cardiomyopathy. By comparison, a recent retrospective evaluation of 324,737 cases of sedation by endoscopists using opioids and benzodiazepines reported 39 deaths (11 per 100,000).18 Recent studies have estimated anesthesia-related deaths during general anesthesia at 2–10 per 100,000.17,19–21 Thus, endoscopist-directed propofol has a far lower mortality rate than published values for endoscopist-delivered benzodiazepines and opioids, and a comparable rate to published values for general anesthesia by anesthesiologists.
However, the opinion of almost all anesthesiology societies concerning the use of propofol by non-anesthesiologists is definitely negative. They emphasize the fact that the manufacturers of propofol restrict its use solely to personnel trained in general anesthesia, and that the US Food and Drug Administration denied a petition by gastroenterologists seeking the removal of this particular restriction.22
Although the fears associated with the use of propofol by non-anesthetist providers are based on sound theoretical principles, they are not born out of scientific studies, but rather to aspects of professional politics. In the absence of any proven benefits such as a decrease in complication rates, anesthetist-administered deep sedation may be a waste of resources.
Training for propofol sedation
Guidelines concerning the use of propofol have been delivered by most major endoscopic associations worldwide.23 The guidelines of the Endoscopic Section of the German Society for Digestive and Metabolic Diseases suggest that
for simple endoscopic examinations and in low-risk patients, sedation with propofol should be induced by a properly qualified physician and can then be monitored by an experienced person with appropriate training. The person must not have any other tasks while monitoring the sedation.
Furthermore, they suggest that an anesthesiologist should be required only in patients with a high-risk profile.22
The European Society of Gastrointestinal Endoscopy and the European Society of Gastroenterology and Endoscopy Nurses and Associates have developed a non-anesthesiologist-administered propofol sedation curriculum and guidelines.24 The course structure includes two categories. First, a 3-day introductory course combines theory and practice with a focus on practical training. Next, clinical training consists of a learning phase of at least 2 weeks with a mentor and includes individual competency assessments.
In the USA, the multi-societies sedation curriculum for gastrointestinal endoscopy was developed by the American Association for the Study of Liver Diseases, the American College of Gastroenterology, the American Gastroenterological Association Institute, the American Society for Gastrointestinal Endoscopy, and the Society for Gastroenterology Nurses and Associates.25 However, the use of propofol is restricted to individuals trained in the administration of general anesthesia.
Novel propofol derivative
To overcome the disadvantages of propofol emulsions, such as pain on injection and infection risk, a water-soluble propofol pro-drug that can be converted to propofol in vivo has been developed. Fospropofol is a 2,6-diisopropyl phenol molecule with a methyl phosphate group substituted at the first carbon hydroxyl group on the propofol molecule (Figure 1). To be activated, alkaline phosphatases must cleave the molecule into the active propofol molecule, phosphate and formaldehyde.26 After bolus administration of fospropofol, the plasma concentration of liberated propofol has a slower upward slope, lower peak and prolonged plateau phase compared with an equipotent dose of propofol emulsion. Phase II and III clinical trials showed faster recovery and greater patient satisfaction after sedation with fospropofol in comparison with midazolam.27 Fospropofol causes less pain on injection than propofol, but is associated with a greater incidence of perineal paresthesia or pain. Fospropofol disodium has been approved by the US Food and Drug Administration for monitored anesthesia care in adults.
Figure 1.
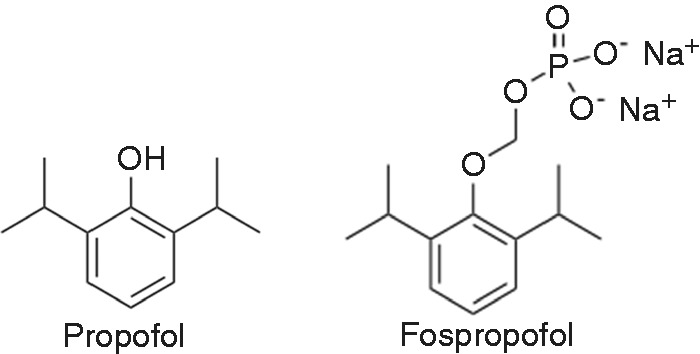
Chemical structural formulae of propofol and fospropofol.
Conclusion
The pharmacokinetic profile of propofol makes it a suitable sedative agent for endoscopic procedures. It has an excellent amnestic effect, rapid onset of action and a short half-life. Rapid recovery has a major impact on patient satisfaction, post-procedure education, the general flow of the endoscopy unit and the efficiency of recovery room staffing. According to estimates, the absolute economic benefit of endoscopist-directed propofol implementation in a screening setting is probably substantial, with 10-year savings of $3.2 billion in the USA.28
However, propofol has a narrow therapeutic window that can result in rapid depression of consciousness and cardiovascular functions. With respect to its potential side effects, the administrator should be aware of the risk of cardiopulmonary complications.
Acknowledgements
This work was supported by a Grant-in-Aid for Scientific Research (B) (16H05291 to H.S.) and Ministry of Education, Culture, Sports, Science and Technology (MEXT)-Supported Program for the Strategic Research Foundation at Private Universities (S1411003, to H.S.).
Declaration of conflicting interests
Over the last 2 years, H.S. received scholarship funds for research from Daiichisankyo Co Ltd, Otsuka Pharmaceutical Co Ltd and Tsumura Co Ltd, and received service honoraria from Astellas Pharm Inc, Astra-Zeneca K.K., EA Pharma Co Ltd, Mylan EPD Ltd, Otsuka Pharmaceutical Co Ltd, Takeda Pharmaceutical Co Ltd, Tsumura Co and Zeria Pharmaceutical Co Ltd. These sources of funding had no role in the design, practice or analysis of this study. There are no other conflicts of interest related to this article.
Funding
This work was supported by a Grant-in-Aid for Scientific Research (B) (16H05291 to H.S.) and MEXT-Supported Program for the Strategic Research Foundation at Private Universities (S1411003, to H.S.).
Ethics approval
Not applicable, as this paper is a review that does not involve subjects or patients.
Informed consent
Not applicable, as this paper is a review that does not involve subjects or patients.
References
- 1.Nishizawa T, Suzuki H, Arita M, et al. Pethidine dose and female sex as risk factors for nausea after esophagogastroduodenoscopy. J Clin Biochem Nutr. In press. [DOI] [PMC free article] [PubMed]
- 2.Shafer A, Doze VA, Shafer SL, et al. Pharmacokinetics and pharmacodynamics of propofol infusions during general anesthesia. Anesthesiology 1988; 69: 348–356. [DOI] [PubMed] [Google Scholar]
- 3.Qadeer MA, Vargo JJ, Khandwala F, et al. Propofol versus traditional sedative agents for gastrointestinal endoscopy: A meta-analysis. Clin Gastroenterol Hepatol 2005; 3: 1049–1056. [DOI] [PubMed] [Google Scholar]
- 4.Horn E, Nesbit SA. Pharmacology and pharmacokinetics of sedatives and analgesics. Gastrointest Endosc Clin N Am 2004; 14: 247–268. [DOI] [PubMed] [Google Scholar]
- 5.Moon SH. Sedation regimens for gastrointestinal endoscopy. Clin Endosc 2014; 47: 135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rex DK. Review article: moderate sedation for endoscopy: Sedation regimens for non-anaesthesiologists. Aliment Pharmacol Ther 2006; 24: 163–171. [DOI] [PubMed] [Google Scholar]
- 7.Wang D, Chen C, Chen J, et al. The use of propofol as a sedative agent in gastrointestinal endoscopy: A meta-analysis. PLoS One 2013; 8: e53311–e53311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sethi S, Wadhwa V, Thaker A, et al. Propofol versus traditional sedative agents for advanced endoscopic procedures: A meta-analysis. Dig Endosc 2014; 26: 515–524. [DOI] [PubMed] [Google Scholar]
- 9.Nishizawa T, Suzuki H, Matsuzaki J, et al. Propofol versus traditional sedative agents for endoscopic submucosal dissection. Dig Endosc 2014; 26: 701–706. [DOI] [PubMed] [Google Scholar]
- 10.Behrens A, Kreuzmayr A, Manner H, et al. Acute sedation-associated complications in GI endoscopy (ProSed 2 Study): results from the prospective multicentre electronic registry of sedation-associated complications. Gut. Epub ahead of print 9 March 2018. DOI: 10.1136/gutjnl-2018-316254. [DOI] [PubMed]
- 11.Nishizawa T, Suzuki H, Sagara S, et al. Dexmedetomidine versus midazolam for gastrointestinal endoscopy: A meta-analysis. Dig Endosc 2014; 27: 8–15. [DOI] [PubMed] [Google Scholar]
- 12.Nishizawa T, Suzuki H, Hosoe N, et al. Dexmedetomidine vs propofol for gastrointestinal endoscopy: A meta-analysis. United European Gastroenterol J 2017; 5: 1037–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horiuchi A, Nakayama Y, Hidaka N, et al. Low-dose propofol sedation for diagnostic esophagogastroduodenoscopy: Results in 10,662 adults. Am J Gastroenterol 2009; 104: 1650–1655. [DOI] [PubMed] [Google Scholar]
- 14.Horiuchi A, Nakayama Y, Katsuyama Y, et al. Safety and driving ability following low-dose propofol sedation. Digestion 2008; 78: 190–194. [DOI] [PubMed] [Google Scholar]
- 15.Lin OS. Sedation for routine gastrointestinal endoscopic procedures: A review on efficacy, safety, efficiency, cost and satisfaction. Intest Res 2017; 15: 456–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poincloux L, Laquiere A, Bazin JE, et al. A randomized controlled trial of endoscopist vs. anaesthetist-administered sedation for colonoscopy. Dig Liver Dis 2011; 43: 553–558. [DOI] [PubMed] [Google Scholar]
- 17.Rex DK, Deenadayalu VP, Eid E, et al. Endoscopist-directed administration of propofol: A worldwide safety experience. Gastroenterology 2009; 137: 1229–1237. quiz 518–519. [DOI] [PubMed] [Google Scholar]
- 18.Sharma VK, Nguyen CC, Crowell MD, et al. A national study of cardiopulmonary unplanned events after GI endoscopy. Gastrointest Endosc 2007; 66: 27–34. [DOI] [PubMed] [Google Scholar]
- 19.Kawashima Y, Takahashi S, Suzuki M, et al. Anesthesia-related mortality and morbidity over a 5-year period in 2,363,038 patients in Japan. Acta Anaesthesiol Scand 2003; 47: 809–817. [DOI] [PubMed] [Google Scholar]
- 20.Newland MC, Ellis SJ, Lydiatt CA, et al. Anesthetic-related cardiac arrest and its mortality: A report covering 72,959 anesthetics over 10 years from a US teaching hospital. Anesthesiology 2002; 97: 108–115. [DOI] [PubMed] [Google Scholar]
- 21.Arbous MS, Grobbee DE, van Kleef JW, et al. Mortality associated with anaesthesia: A qualitative analysis to identify risk factors. Anaesthesia 2001; 56: 1141–1153. [DOI] [PubMed] [Google Scholar]
- 22.Triantafillidis JK, Merikas E, Nikolakis D, et al. Sedation in gastrointestinal endoscopy: Current issues. World J Gastroenterol 2013; 19: 463–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee TH, Lee CK. Endoscopic sedation: From training to performance. Clin Endosc 2014; 47: 141–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dumonceau JM, Riphaus A, Beilenhoff U, et al. European curriculum for sedation training in gastrointestinal endoscopy: Position statement of the European Society of Gastrointestinal Endoscopy (ESGE) and European Society of Gastroenterology and Endoscopy Nurses and Associates (ESGENA). Endoscopy 2013; 45: 496–504. [DOI] [PubMed] [Google Scholar]
- 25.Vargo JJ, DeLegge MH, Feld AD, et al. Multisociety sedation curriculum for gastrointestinal endoscopy. Gastrointest Endosc 2012; 76: e1–e25. [DOI] [PubMed] [Google Scholar]
- 26.Feng AY, Kaye AD, Kaye RJ, et al. Novel propofol derivatives and implications for anesthesia practice. J Anaesthesiol Clin Pharmacol 2017; 33: 9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cohen LB, Cattau E, Goetsch A, et al. A randomized, double-blind, phase 3 study of fospropofol disodium for sedation during colonoscopy. J Clin Gastroenterol 2010; 44: 345–353. [DOI] [PubMed] [Google Scholar]
- 28.Hassan C, Rex DK, Cooper GS, et al. Endoscopist-directed propofol administration versus anesthesiologist assistance for colorectal cancer screening: a cost-effectiveness analysis. Endoscopy 2012; 44: 456–464. [DOI] [PubMed] [Google Scholar]


