Abstract
Background
Over the past decades, multiple approaches to aspiration sclerotherapy of large symptomatic hepatic cysts have been investigated. However, comparative data are scarce.
Objective
The objective of this article is to compare cyst reduction, symptomatic relief, and adverse events between ethanol sclerotherapy and polidocanol sclerotherapy.
Methods
This retrospective study included adults having a symptomatic hepatic cyst treated at a European tertiary referral center with ethanol sclerotherapy (Center 1) or polidocanol-sclerotherapy (Center 2). We compared cyst diameter reduction (%) and symptom improvement (yes/no) within 12 months’ post-treatment between centers using multivariate regression analyses adjusted for confounding factors. Finally, we compared adverse events using Fisher’s exact test.
Results
We included 71 patients from Center 1 and 66 patients from Center 2 (median age 57 years; 126/137 (92%) female). Cyst reduction was comparable between Centers 1 and 2: 37.5% (IQR 15.7–61.0%) versus 44.2% (IQR 24.6–60.5%), respectively (p = 0.35). Correspondingly, symptomatic relief was comparable: 30/53 (56.6%) versus 43/66 (65.2%), respectively (p = 0.88). Center 1 reported significantly more (11 versus 3; p = 0.047) adverse events than Center 2.
Conclusion
We found comparable cyst reduction and symptomatic relief rates between ethanol- and polidocanol sclerotherapy, while adverse events occurred more often in the ethanol group. Prospective studies focused on clinical response are needed to further explore differences between approaches.
Keywords: Aspiration sclerotherapy, liver, cyst, polycystic liver disease, efficacy, safety
Key summary
Established knowledge
Aspiration sclerotherapy is an effective and safe method to reduce hepatic cysts.
Although multiple different treatment approaches have been published in the last decades, formal comparisons are scarce.
Significant and/or new findings
In this non-randomized study, hepatic cyst reduction and symptomatic relief rates after ethanol- or polidocanol-sclerotherapy approaches seemed highly comparable.
Although numbers were small, we found more adverse events after ethanol-sclerotherapy compared to polidocanol-sclerotherapy.
Introduction
Hepatic cysts are fluid-filled cavities located in the liver that arise from congenital malformations of the biliary tract. Prevalence is estimated around 2.5% to 18.0% of the general population.1–3 Hepatic cysts can occur as solitary or multiple lesions in the context of polycystic liver disease (PLD).4 Although typically asymptomatic, a proportion of patients with large hepatic cysts may develop symptomatic disease. Treatment should be considered in these cases and encompasses surgical or radiological interventions.5
Aspiration sclerotherapy consists of ultrasonography (US) guided puncture and drainage of the hepatic cyst with subsequent instillation of a sclerosing agent aiming to eradicate the lining cystic epithelium. Lysis of these fluid-producing cells results in regression of the cyst.
Multiple protocols for aspiration sclerotherapy are available with a number of variables that differ between approaches. Examples of these variables are the type of sclerosing agent (e.g. ethanol, tetracycline, and polidocanol), the injected volume of the sclerosing agent, and instillation time.6–12 The literature mainly contains case series that highlight one specific protocol. However, few have compared the results between different approaches.
For this study, we compared two institutes that were subjected to a distinct aspiration sclerotherapy protocol including the use of different sclerosing agents: ethanol and polidocanol. Historically, ethanol has been generally applied as a sclerosing agent in most centers.13 However, because of side effects of pain and intoxications, other sclerosing agents has been explored in recent decades. One of these alternative agents is polidocanol. Inspired by the success rates in varices and renal cysts, polidocanol was injected into hepatic cysts with both toxic and anesthetic effects on the epithelium.14,15 Our aim was to compare efficacy in terms of cyst reduction, clinical response, and safety between these different standardized aspiration sclerotherapy approaches.
Materials and methods
Study design and population
In this study we evaluated treatment outcome after aspiration sclerotherapy of hepatic cysts. This retrospective cohort consists of patients who were treated in the following two tertiary referral centers: (a) Radboud University Medical Center, Nijmegen, the Netherlands, and (b) the Medical School of Hannover, Hannover, Germany. Ethics approval was obtained by the local medical ethics committee of both centers. As this was an observational, retrospective cohort, informed consent was not deemed necessary by the ethics boards.
We included adult (≥18 years) patients who underwent aspiration sclerotherapy of a symptomatic, non-parasitic, non-neoplastic hepatic cyst between 2004 and 2012 (Center 1) and 2007 and 2013 (Center 2). Indication for aspiration sclerotherapy was based on the presence of symptoms (e.g. abdominal distension, pain, dyspnea, and early satiety) that were likely to result from compression by a large hepatic cyst. Both centers treated solitary cysts and dominant cysts within the context of PLD. A dominant cyst was identified as the largest hepatic cyst surrounded by smaller cysts. The treated cyst had to be measured by US or computed tomography (CT) within six months before and within 12 months after aspiration sclerotherapy. We excluded a patient if the treated cyst could not be identified from other (nontreated) cysts on postinterventional imaging. Measurements that were recorded after a second intervention of the cyst (i.e. second aspiration sclerotherapy or surgical fenestration) were not included.
Aspiration sclerotherapy procedures and follow-up
Both centers worked with standardized, single-session procedures. Center 1 performed US-guided drainage of the cyst using a 5-French pigtail catheter (Cook Medical, Bloomington, IN, USA).16 Subsequently, 100% ethanol was injected into the cyst with a volume of 10% of the aspirated cyst fluid volume. For safety reasons a maximal amount of 50 ml was instilled. Patients did not change position during the procedure. After 10 minutes, ethanol was re-aspirated from the cyst cavity. To prevent ethanol leakage, the catheter was withdrawn while applying suction. The procedure was performed using conscious sedation and local anesthesia. In Center 2, cysts were aspirated under US guidance (20 G Chiba needle), followed by instillation of polidocanol 1% (Aetoxisclerol 1%, Kreussler Pharma, Wiesbaden, Germany) in a volume of 10% of aspirated volume with a safety margin of 120 ml. After five minutes polidocanol was partially re-aspirated and a maximum of 60 ml polidocanol remained in the cyst.17 In Center 2, only local anesthesia was applied.
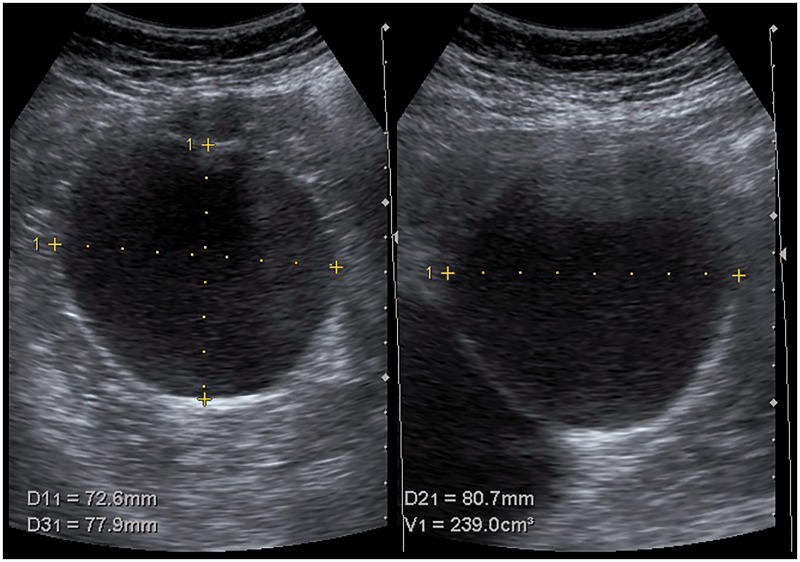
In both centers, patients visited the outpatient clinic before (<6 months) and after (<12 months) aspiration sclerotherapy. During these visits, US was performed to assess the three orthogonal cyst diameters from which one mean diameter was calculated (Figure 1). In addition, during both visits, patient symptoms of were monitored. Treatment protocols remained unchanged during the study period.
Figure 1.
Orthogonal measurement of a treated hepatic cyst.
Study endpoints
The primary endpoint of this study was proportional (%) cyst diameter reduction compared between treatment strategies of Centers 1 and 2. To calculate proportional diameter reduction, we used the baseline and last available measurement within 12 months after aspiration sclerotherapy. Secondary endpoints were symptomatic change and safety. Symptomatic change was extracted from the medical charts and dichotomized into two categories: reduction or no reduction of symptoms. To evaluate safety, we documented type, rate, and date of any complication following treatment within 12 months.
Data collection
We performed an Electronic Patients Database search and included all patients who underwent aspiration sclerotherapy between January 2004 to December 2012 (Center 1) and January 2007 to December 2013 (Center 2). Data were subtracted from medical charts. Patient history and US or CT images were used to assess patient eligibility. We assembled a dataset with all endpoints. In addition, we included the following variables: age, gender, PLD (> 20 hepatic cysts, yes/no), relevant medical history, and dates of aspiration sclerotherapy and follow-up. Data were entered into a pooled database by one of the two primary researchers and cross-checked for errors.
Statistical analysis
Frequency tables were provided for all demographic information and compared between centers. Continuous and categorical data were compared between centers using the Mann–Whitney U test and Pearson’s Chi-square test, respectively. By linear regression analysis, we compared proportional cyst diameter reduction between centers. We included proportional diameter reduction as a dependent variable and center as an independent variable. Moreover, we included the following variables to correct for confounding: age, gender, PLD, baseline cyst diameter, amount of sclerosing agent, and follow-up time. Similarly, we compared symptomatic change (dependent variable) between centers (independent variable) using logistic regression analysis including the aforementioned confounding variables.
Within each treatment regimen, we compared cyst reduction between patients with or without symptomatic relief using the Mann–Whitney U test. In addition, we compared cyst reduction and symptomatic relief between patients with or without PLD, by the Mann–Whitney U and Chi-square test, respectively. Finally, adverse event rates were compared using Fisher’s exact test. We performed statistical analysis using IBM SPSS statistics (SPSS Inc, Chicago, IL, USA). All reported p values were two tailed, and values < 0.05 were considered statistically significant.
Results
Baseline characteristics
We identified 111 patients in Center 1 and 121 patients in Center 2 (Figure 2). From these 232 patients, 137 were eligible for inclusion: 71 from Center 1 and 66 patients from Center 2. Absence of baseline (n = 12) or follow-up (n = 32) imaging or insufficient documentation (n = 19) were main reasons for exclusion. All treated cysts could be identified after treatment. Re-intervention rates were comparable between centers (Figure 2). Age, gender, underlying diagnosis and location of the cyst were comparable between centers (Table 1). The occurrence of renal cysts (21.1% versus 51.5%; p < 0.01) and baseline cyst diameter (11.2 cm (interquartile range (IQR) 8.4–16.0 cm) versus 8.4 cm (IQR 7.0–10.7 cm); p < 0.01) differed between centers.
Figure 2.
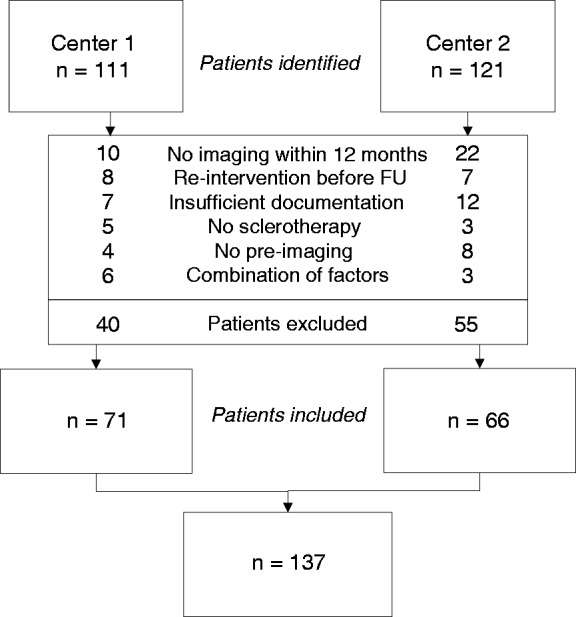
Flowchart: identification and selection of patients in Centers 1 and 2. FU: follow-up.
Table 1.
Demographics.
| Center 1 (n = 71) | Center 2 (n = 66) | p valuea | |
|---|---|---|---|
| Age at treatment, years | 55 (47–65) | 58 (52–67) | 0.16 |
| Gender, female (%) | 68 (95.8) | 58 (87.9) | 0.09 |
| Diagnosis, PLD (%) | 56 (78.9) | 50 (75.8) | 0.67 |
| Renal cysts, yes (%) | 15 (21.1) | 34 (51.5) | <0.01 |
| Baseline cyst diameter, cm | 11.2 (8.4–16.0) | 8.4 (7.0–10.7) | <0.01 |
| Liver lobe, rightb | 39 (54.9) | 38 (57.6) | 0.76 |
Continuous values by Mann–Whitney U test; categorical data by Pearson Chi-Square test.
In both centers, five patients had a centrally located cyst.
PLD: polycystic liver disease (>20 hepatic cysts).
Cyst reduction
In Center 1, cysts reduced to a mean diameter of 6.7 cm (IQR 3.7–10.6 cm) after a follow-up of two months (IQR 1–5 months), corresponding with a reduction of 37.5% (IQR 15.7–61.0%). Patients treated in Center 2 had a median diameter of 4.9 cm (3.5–6.8 cm) after seven months (IQR 3–9 months), which was a proportional reduction of 44.2% (IQR 24.6–60.5 months) (Figure 3). By multivariable linear regression analysis we found no statistically significant difference between centers (p = 0.35). Although median follow-up time differed between centers, this was not associated with a difference in cyst reduction between centers (p = 0.87).
Figure 3.
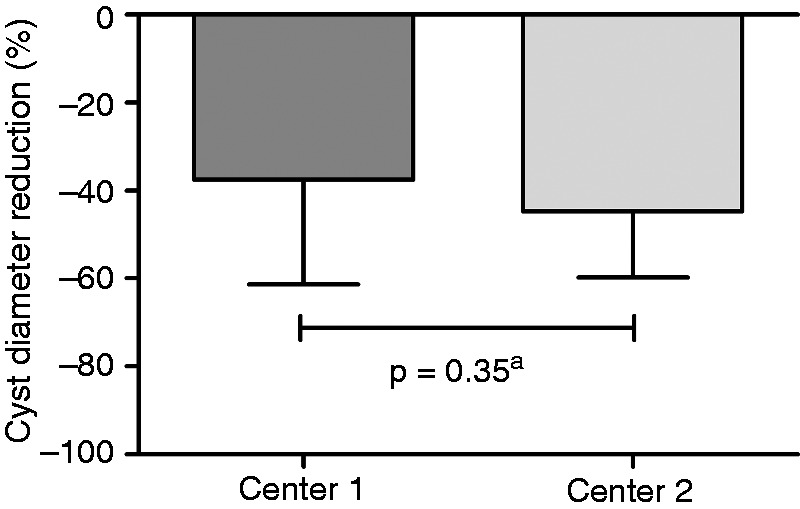
Cyst diameter reduction compared between Centers 1 and 2.
aMultivariate regression analysis.
Symptomatic relief
In Center 1, 30 out of 53 (56.6%) patients reported symptomatic relief, compared to 43 out of 66 (65.2%) in Center 2. In Center 1, clinical response could not be retrieved from the medical charts for 18 patients. Logistic regression showed no differences between centers (odds ratio (OR) 0.90; 95% confidence interval (CI) 0.23–3.52; p = 0.88). Again, median follow-up time was not associated with differences in symptom response (OR 1.02; 95% CI 0.85–1.20; p = 0.90).
Cyst reduction and symptomatic relief
In both centers, higher efficacy of cyst reduction was associated with improved clinical response. In Center 1, we found significantly larger median cyst reduction in patients who reported a decrease in symptoms compared to patients without symptom improvement: 48.8% (32.0–65.3%) versus 23.4% (10.3–61.0%), respectively (p = 0.02). In Center 2, we found similar results: 53.2% (32.5–68.7%) versus 28.7% (7.9–47.0%), respectively (p = 0.03).
Cyst reduction and symptomatic relief compared between diagnosis
In general, we observed higher cyst and symptom reduction rates in patients without PLD.
In Center 1, non-PLD and PLD patients had comparable cyst reduction rates: 42.7% (15.2–86.7%) versus 34.8% (16.1–55.1%); p = 0.38. In contrast, in Center 2, cysts of patients without PLD had stronger regression rates: 70.3% (55.0–84.6%) versus 36.3% (20.9–53.4%); p < 0.01.
Correspondingly, symptom reduction was higher in patients without PLD compared to PLD patients in both centers: 7/10 (70.0%) versus 23/43 (53.5%; p = 0.34) in Center 1, and 16/16 (100%) versus 27/54 (54.0%; p < 0.01) in Center 2.
Safety
In total, Center 1 reported 11 adverse events: Five patients had pain following treatment, five patients had a suspected cyst hemorrhage, and one patient developed a hepatic cyst infection (Table 2). No ethanol-intoxications were reported. In Center 2, three adverse events were reported: one hepatic cyst hemorrhage and two cyst infections; no patients reported pain. Center 1 reported significantly more adverse events (p = 0.047). All adverse events were treated conservatively (analgesics or antimicrobial treatment) and resolved without sequelae.
Table 2.
Adverse events.
| Center 1 (n = 71) | Center 2 (n = 66) | p valuea | |
|---|---|---|---|
| Post-procedural pain, n/N | 5/71 (7.0%) | 0/66 | 0.06 |
| Cyst hemorrhage, n/N | 5/71 (7.0%) | 1/66 (1.5%) | 0.21 |
| Cyst infection, n/N | 1/71 (1.4%) | 2/66 (3.0%) | 0.61 |
| Total, n/N | 11/71 (15.4%) | 3/66 (4.5%) | 0.047 |
Fisher’s exact test.
Discussion
The primary finding of this study is that we found comparable efficacy rates of cyst reduction and clinical relief between two different aspiration sclerotherapy approaches. In contrast, ethanol sclerotherapy resulted in more adverse events compared to polidocanol sclerotherapy.
In the past decades, multiple centers have described their experience with sclerotherapy protocols.13 More recently, studies compared single variables within these protocols.18,19 Nonetheless, head-to-head comparisons between these strategies are scarce. In this study, we compared two distinct treatment protocols using different sclerosing agents, volumes and time intervals. Despite these two different approaches, we found highly comparable cyst reduction, re-intervention, and symptomatic relief rates.
Compared to previous studies, we found lower cyst diameter reduction and clinical response rates.20,21 These smaller reduction rates may be explained by our short follow-up interval. Previous studies reported continuous cyst reduction in the first year after aspiration sclerotherapy.22 As cyst reduction continues over time, our follow-up may have been too short to reach comparable efficacy rates.23 In addition, we assessed cyst response in terms of proportional diameter reduction as cyst volume measurements could not be retrieved (or calculated accurately) from our medical reports. Compared to cyst diameter reduction, volume reduction automatically results in higher proportional reduction rates because of mathematical differences.
In line with cyst reduction, our clinical response rates were relatively small. One cohort of 57 patients24 reported symptom reduction in 95% of patients; another study of 25 patients20 found reduction of symptoms in 72%. Both studies described high cyst reduction rates. Possibly, these high levels of symptom relief could be explained by the stronger cyst reduction rates. Indeed, in this study the amount of cyst reduction was associated with clinical response. Second, our study included a relatively high number of patients with PLD (77%). This may have reduced our overall clinical response rate as PLD was associated with restricted symptomatic relief (88% in solitary cysts versus 52% in PLD patients). Indeed, in the aforementioned series the number of PLD patients was lower (33% and 56%, respectively).20,24
Ethanol is the most common sclerosing agent reported in the literature as it is cheap, safe and widely available. On the downside, ethanol sclerotherapy has side effects; mainly pain and intoxications are described.13 Polidocanol was introduced as sclerosing agent for hepatic cysts aiming to reduce peri- and post-procedural pain. Indeed, in our ethanol sample, pain was reported more frequently. Interestingly, ethanol intoxications were not encountered, which may be explained by the restricted maximal volume of 50 ml and relatively short sclerotherapy duration of 10 minutes.
How do our findings affect clinical practice? First, although our treatment protocols included different variables, we found comparable cyst reduction and clinical response rates. This implies that the currently available approaches probably have similar efficacy that results from cyst wall destruction leading to regression of the cyst and thereby reduction of symptoms. As efficacy rates between ethanol and polidocanol are highly comparable, we could argue that polidocanol should be preferred as a sclerosing agent as adverse event rates were smaller. To draw definite conclusions, a prospective comparison between these agents is needed. In addition, we found that PLD patients had less chance to gain symptomatic relief. Several studies reported comparable findings.20,24 This confined clinical response may be explained by the remaining large liver volume. In contrast, patients with solitary cysts proved to be excellent candidates for this intervention.
The main strength of this study is the large number of patients included from two tertiary centers. This large sample size allowed us to correct for known confounders. Our study was limited by several factors. First, median follow-up time in Center 1 was short. Transitory reaccumulation of the treated cyst in this period may have blurred our results and reduced efficacy of ethanol sclerotherapy. We have adjusted for follow-up time in our regression analysis. Nevertheless, longer follow-up periods are necessary to provide possible long-term efficacy differences. Second, although we included confounding variables in the regression analysis, some factors (e.g. needle size, amount of sclerosing agent, sclerosing time, positional change) were collinear with each center and could therefore not be included. Although these factors may have affected our outcome, the individual effect of each variable could not be evaluated. Third, exclusion of patients for lack of imaging or sufficient documentation may have led to selection bias. Possibly, patients with a strong or minimal clinical response may have been excluded (loss to follow-up or early re-intervention, respectively), which may have affected our efficacy rates. Finally, our clinical response and safety measurements were not assessed by standardized instruments and therefore susceptible to bias. Recently, a disease-specific questionnaire has been validated to assess severity and frequency of symptoms in patients with hepatic cysts.25 This instrument opens the door for a prospective observational study to observe long-term clinical efficacy rates. Hepatic cysts are benign lesions; therefore, we strongly recommend using such patient-reported outcome measures as primary endpoints to evaluate efficacy of aspiration sclerotherapy.
To conclude, despite two distinct sclerotherapy protocols, we found comparable efficacy rates of cyst reduction and clinical relief. Polidocanol sclerotherapy had fewer adverse events than ethanol sclerotherapy. Future prospective studies are needed that primarily focus on clinical relief and safety using validated instruments.
Declaration of conflicting interests
None declared.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
Ethics approval was obtained by the local medical ethics committees of Radboud University Medical Center, Nijmegen, the Netherlands, and the Medical School of Hannover, Hannover, Germany.
Informed consent
As this was an observational, retrospective cohort, informed consent was not deemed necessary by the ethics boards of Radboud University Medical Center, Nijmegen, the Netherlands, and the Medical School of Hannover, Hannover, Germany.
References
- 1.Larssen TB, Rorvik J, Hoff SR, et al. The occurrence of asymptomatic and symptomatic simple hepatic cysts. A prospective, hospital-based study. Clin Radiol 2005; 60: 1026–1029. [DOI] [PubMed] [Google Scholar]
- 2.Gaines PA, Sampson MA. The prevalence and characterization of simple hepatic cysts by ultrasound examination. Br J Radiol 1989; 62: 335–337. [DOI] [PubMed] [Google Scholar]
- 3.Carrim ZI, Murchison JT. The prevalence of simple renal and hepatic cysts detected by spiral computed tomography. Clin Radiol 2003; 58: 626–629. [DOI] [PubMed] [Google Scholar]
- 4.Gevers TJ, Drenth JP. Diagnosis and management of polycystic liver disease. Nat Rev Gastroenterol Hepatol 2013; 10: 101–108. [DOI] [PubMed] [Google Scholar]
- 5.Drenth JP, Chrispijn M, Nagorney DM, et al. Medical and surgical treatment options for polycystic liver disease. Hepatology 2010; 52: 2223–2230. [DOI] [PubMed] [Google Scholar]
- 6.Bean WJ, Rodan BA. Hepatic cysts: Treatment with alcohol. AJR Am J Roentgenol 1985; 144: 237–241. [DOI] [PubMed] [Google Scholar]
- 7.Goldstein HM, Carlyle DR, Nelson RS. Treatment of symptomatic hepatic cyst by percutaneous instillation of Pantopaque. AJR Am J Roentgenol 1976; 127: 850–853. [DOI] [PubMed] [Google Scholar]
- 8.Hagiwara H, Kasahara A, Hayashi N, et al. Successful treatment of a hepatic cyst by one-shot instillation of minocycline chloride. Gastroenterology 1992; 103: 675–677. [DOI] [PubMed] [Google Scholar]
- 9.vanSonnenberg E, Wroblicka JT, D’Agostino HB, et al. Symptomatic hepatic cysts: Percutaneous drainage and sclerosis. Radiology 1994; 190: 387–392. [DOI] [PubMed] [Google Scholar]
- 10.Potthoff A, Sandkühler F, Boozari B, et al. Long-term follow up of 100 patients with polycystic liver disease—Efficacy of percutaneous US-guided polidocanol sclerotherapy. J Hepatol 2011; 54: S359–S359. [Google Scholar]
- 11.Nakaoka R, Das K, Kudo M, et al. Percutaneous aspiration and ethanolamine oleate sclerotherapy for sustained resolution of symptomatic polycystic liver disease: An initial experience. AJR Am J Roentgenol 2009; 193: 1540–1545. [DOI] [PubMed] [Google Scholar]
- 12.Jusufović R, Zerem E. Percutaneous treatment of symptomatic non-parasitic benign liver cysts with 20% NaCl solution. Med Arh 2011; 65: 35–37. [PubMed] [Google Scholar]
- 13.Wijnands TF, Gortjes AP, Gevers TJ, et al. Efficacy and safety of aspiration sclerotherapy of simple hepatic cysts: A systematic review. AJR Am J Roentgenol 2017; 208: 201–207. [DOI] [PubMed] [Google Scholar]
- 14.Zellweger U, Meyenberger C, Bühler H, et al. Ultrasonically-guided sclerosing of kidney and liver cysts using polidocanol [article in German]. Schweiz Rundsch Med Prax 1990; 79: 1412–1415. [PubMed] [Google Scholar]
- 15.Spârchez Z, Radu P, Zaharie F, et al. Percutaneous treatment of symptomatic non-parasitic hepatic cysts. Initial experience with single-session sclerotherapy with polidocanol. Med Ultrason 2014; 16: 222–228. [DOI] [PubMed] [Google Scholar]
- 16.Wijnands TF, Gevers TJ, Kool LJ, et al. Aspiration sclerotherapy combined with pasireotide to improve reduction of large symptomatic hepatic cysts (SCLEROCYST): Study protocol for a randomized controlled trial. Trials 2015; 16: 82–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dietrich CF, Chiorean L, Potthoff A, et al. Percutaneous sclerotherapy of liver and renal cysts, comments on the EFSUMB guidelines. Z Gastroenterol 2016; 54: 155–166. [DOI] [PubMed] [Google Scholar]
- 18.Yan-Hong F, Lin-Xue Q, Hai-Ma G, et al. Sclerotherapy of simple hepatic cysts by repeated aspiration and alcohol instillation. Turk J Gastroenterol 2012; 23: 359–365. [DOI] [PubMed] [Google Scholar]
- 19.Zerem E, Imamović G, Omerović S. Percutaneous treatment of symptomatic non-parasitic benign liver cysts: Single-session alcohol sclerotherapy versus prolonged catheter drainage with negative pressure. Eur Radiol 2008; 18: 400–406. [DOI] [PubMed] [Google Scholar]
- 20.Tikkakoski T, Mäkelä JT, Leinonen S, et al. Treatment of symptomatic congenital hepatic cysts with single-session percutaneous drainage and ethanol sclerosis: Technique and outcome. J Vasc Interv Radiol 1996; 7: 235–239. [DOI] [PubMed] [Google Scholar]
- 21.Yang CF, Liang HL, Pan HB, et al. Single-session prolonged alcohol-retention sclerotherapy for large hepatic cysts. AJR Am J Roentgenol 2006; 187: 940–943. [DOI] [PubMed] [Google Scholar]
- 22.Larssen TB, Rosendahl K, Horn A, et al. Single-session alcohol sclerotherapy in symptomatic benign hepatic cysts performed with a time of exposure to alcohol of 10 min: Initial results. Eur Radiol 2003; 13: 2627–2632. [DOI] [PubMed] [Google Scholar]
- 23.Hahn ST, Han SY, Yun EH, et al. Recurrence after percutaneous ethanol ablation of simple hepatic, renal, and splenic cysts: Is it true recurrence requiring an additional treatment? Acta Radiol 2008; 49: 982–986. [DOI] [PubMed] [Google Scholar]
- 24.Benzimra J, Ronot M, Fuks D, et al. Hepatic cysts treated with percutaneous ethanol sclerotherapy: Time to extend the indications to haemorrhagic cysts and polycystic liver disease. Eur Radiol 2014; 24: 1030–1038. [DOI] [PubMed] [Google Scholar]
- 25.Neijenhuis MK, Gevers TJ, Hogan MC, et al. Development and validation of a disease-specific questionnaire to assess patient-reported symptoms in polycystic liver disease. Hepatology 2016; 64: 151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]