Abstract
Background:
The aims of this study were to assess the regulatory review process in South Africa from 2015 to 2017, identify the key milestones and timelines; evaluate the effectiveness of measures to ensure consistency, transparency, timeliness, and predictability in the review process; and to provide recommendations for enhanced regulatory practices.
Methods:
A questionnaire was completed by the Medicines Control Council (MCC) to describe the organization of the authority, record key milestones and timelines in the review process and to identify good review practices (GRevPs).
Results:
Currently, the MCC conducts a full assessment of quality, efficacy, and safety data in the review of all applications. The overall regulatory median approval time decreased by 14% in 2017 (1411 calendar days) compared with that of 2016, despite the 27% increase in the number of applications. However, the MCC has no target for overall approval time of new active substance applications and no targets for key review milestones. Guidelines, standard operating procedures, and review templates are in place, while the formal implementation of GRevPs and the application of an electronic document management system are planned for the near future.
Conclusions:
As the MCC transitions to the newly established South Africa Health Products Regulatory Authority, it would be crucial for the authority to recognize the opportunities for an enhanced regulatory review and should consider models such as abridged assessment, which encompass elements of risk stratification and reliance. It is hoped that resource constraints may then be alleviated and capacity developed to meet target timelines.
Keywords: Medicine Control Council (MCC), South Africa Health Products Regulatory Authority (SAHPRA), best practices, regulatory review, good review practices
Introduction
As part of a multicountry study on effective drug regulation, the World Health Organization (WHO) described 4 dimensions of medicine regulation, namely, administrative elements, regulatory functions, level of regulation, and technical elements.1 Further studies by Hill and Johnson recognized that regulators often operated in an environment with insufficient political support, resulting in inadequate legislative frameworks and financial resources, inconsistent application processes, and an inappropriate regulatory culture.2
During the past decade, regulatory authorities have acknowledged the need to develop efficient and effective regulatory review processes.3,4 Regulatory authorities are encouraged to facilitate the expedited approval of new medicines within mandated prerequisites of ensuring patients’ access to safe, effective, and quality medicines. Regulators face scientific, administrative, and legislative capacity constraints,2 yielding sometimes inoperable regulatory directives, limited solutions for timely evaluations, and a drive for maintaining sovereignty.
Many regulators have dedicated resources to improve the review processes and to develop indicators that go beyond the measurement of time and speed.3,4 The implementation of good review practices (GRevPs) plays a pivotal role in ensuring consistency, predictability, clarity, and efficiency in the product review process5,6 and contributes toward the evaluation of the performance of the regulatory authority. This review is the first to be carried out to evaluate the current South African regulatory review process, as it is has been applied by the Medicines Control Council (MCC), prior to the establishment of the South Africa Health Products Regulatory Authority (SAHPRA).
South Africa and the Medicines Control Council
The pharmaceutical market in South Africa was valued at approximately 45 billion Rand (US$3.2 billion) in 2015.7 The domestic manufacturing pharmaceutical industry almost exclusively produces generic products and the South African pharmaceutical sector is import dependent.7 In 2013, generic medicines accounted for 63% of the private pharmaceutical market and 80% market share in the South African government’s pharmaceutical use.7
Over the last 50 years, South Africa has developed a medicines regulatory authority with internationally recognized standing.8 The MCC is described as “the national medicines regulatory authority of South Africa, which is responsible, in terms of the Medicines and Related Substances Act, 1965 (Act 101 of 1965), to provide for the monitoring, evaluation, regulation, investigation, inspection, registration, and control of medicines, scheduled substances, clinical trials, and medical devices and related matters in the public interest.”9 Currently, the MCC operates through external experts who are members of Council Committee structures.8 The staff component of the Chief Directorate includes doctors, pharmacists, veterinarians, other scientists, project managers, and administrative staff.8
This study aimed to appraise the regulatory review process within the MCC, identify key milestones, and evaluate the review times for new active substances (NASs) and major line extensions (MLEs), from 2015 to 2017. It is hoped that the findings will provide a baseline for assessing the changes and improvements within the MCC, as the authority transitions into the newly established SAHPRA. This is the first study to evaluate the status quo of the regulatory review process of the MCC, since the promulgation of the Medicines and Related Substances Act, 1965, as amended on June 1, 2017.10
Study Objectives
The main objectives of this exploratory study were to:
assess the current regulatory review process in South Africa;
identify the key milestones, timelines and stages of the review process;
evaluate the effectiveness of the measures used to ensure consistency, transparency, timeliness, and predictability in the review process; and
review the challenges and opportunities for enhanced regulatory practices in South Africa with a view to improving patients’ access to innovative medicines.
Methods
This article does not contain any studies with human or animal subjects performed by any of the authors.
Data Collection Process
The Centre for Innovation in Regulatory Science (CIRS) developed a questionnaire that has been used to map the key milestones and activities associated with the review processes and practices within regulatory authorities. Through the use of the questionnaire, regulatory authorities are able to identify the models of review that are being used within the authority, identify target times and the main activities between milestones for registration, and identify the organization, structure, and the capacity of the authority.
The questionnaire information on the regulatory review process in South Africa was collected during an interview with the Registrar of Medicines for the MCC. The questionnaire was completed with a view to analyzing the quality measures that are currently in place, identify areas of capacity constraints, and to provide a baseline for the current review process, in light of the transition to the newly established SAHPRA.11 The questionnaire consisted of 3 parts, as listed below.
Part I: Organization of the Authority
This documents an introduction to the authority, its current structure and size, the resources available, and the review model(s) currently in place.
Part II: Key Milestones in the Registration of Medicines within the Review Process
This is based on a standard process map that was previously developed by CIRS, through the study of established and emerging regulatory authorities.12 This process map provided a detailed description of the pathway of a dossier, through administrative and technical screening steps, scientific evaluation, and Committee and Council processes. The completed process map enabled the collection of information in a standardized format, which could be used to simplify the comparison of the MCC and the current review process with the regulatory pathways used by other regulatory authorities.
Part III: Good Review Practice
Building quality into the assessment and registration processes provides an account of the activities and practices, currently in place at the MCC, which contribute toward improved consistency, transparency, timeliness, and predictability in the regulatory review and to the quality of the decision-making process. This questionnaire had been developed for use in the analysis of the regulatory environment in several emerging pharmaceutical markets.12
Results
Part I: Organization of the Authority
The current authority, MCC, was first established in 1965 and historically operated within the National Department of Health. Since then, the authority has undergone many changes, including its establishment as a 3A Public Entity, known as SAHPRA. Provision was made for the restructuring of the authority, through the amendment of the Medicines and Related Substances Act, 1965 (Act 101 of 1965), which was published on June 1, 2017.10
The scope of responsibility of the MCC includes medicinal products for human and veterinary use and medical devices. The MCC is mandated, through the Medicines and Related Substances Act, 1965 (Act 101 of 1965), to ensure the efficient, effective, and ethical evaluation or assessment and registration of medicines and medical devices that meet the defined standards of quality, safety, efficacy, and performance. The MCC also performs licensing activities, inspectorate and law enforcement functions, laboratory analysis of biological products, postmarket surveillance and vigilance activities, and controls the advertising of medicines and medical devices.
The MCC currently has a staff of approximately 200 full-time personnel, including management, technical and administrative personnel, and approximately 100 external consultants. At present, approximately 100 internal and external personnel are responsible for the technical evaluation of applications, which include NASs, generics, biologicals, veterinary, and complementary products. The majority of the staff responsible for the regulatory review process are qualified as pharmacists and many of the assessors have postgraduate qualifications.
Model of Assessment in South Africa
Three types of product review assessments are used by regulatory authorities: the verification review (type 1), an abridged review (type 2), and a full review (type 3).12 The MCC conducts a type 3 full assessment in the review of all applications, including NASs and generics for orthodox, biological, complementary, and veterinary medicinal products. A full independent assessment of quality, efficacy, and safety data is performed. The authority has access to assessors who have the relevant qualification and technical experience to perform a full assessment of the data provided. The majority of the assessors are external consultants who are not bound by contractual performance agreements. Over the last 2 years, the MCC has made major changes in building in-house capacity through assistance from external experts.
Data Requirements and Assessment
The Certificate of Pharmaceutical Product (CPP) is not essential for registration but a copy of the authorization letter should be provided if the product has been registered in a reference country (eg, for fast-track/priority products). Evidence of good manufacturing practice (GMP) status of the manufacturer and copies of labeling, for products authorized in reference countries, are also required. Full quality data (Module 3), full nonclinical data (Module 4), and full clinical data (Module 5) are required. A detailed assessment of the data is carried out by MCC and the relevant assessment reports prepared.
The MCC performs benefit-risk assessments, and the clinical opinion of the authority takes account of differences in medical culture/practice, ethnic factors, national disease patterns, and unmet medical needs. Where relevant, the authority will obtain internal assessment reports from other authorities and publicly available reports such as European Public Assessment Reports (EPARs). The MCC refers to pharmacovigilance reports and confirms GMP status and product compliance during the review process. Although registration elsewhere is not a prerequisite for making an application, information on existing registrations should be provided, where available.
Part II: South African Regulatory Review Process
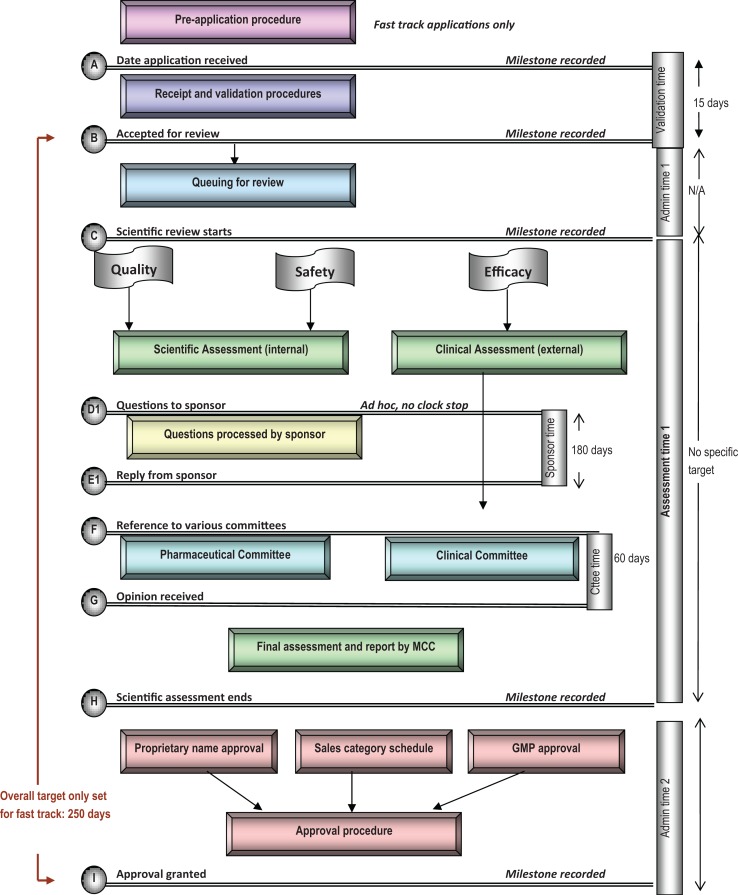
The South African regulatory review process is presented in Figure 1. The review process map illustrates the main steps in the review process and identifies the key milestone dates for monitoring and analyzing timelines for review. The map provides a simple representation of the review and authorization of applications for NASs and MLEs that are approved on the first cycle. The map does not describe the process, in the event that the application was refused. The appeal process that may be initiated, following refusal of an application, has also not been included in the review process map.
Figure 1.
Regulatory review process map for South Africa. Days reflected are calendar days. NAS, new active substance.
Queue Time
Applications for NASs are received by the Operations and Administration Unit, and administrative screening of applications is performed within 15 calendar days from the time of receipt. Applications are routed to the relevant unit, where they are allocated to an assessor to start the review process. There is no target set for the overall review time of an NAS application and there are no targets set for the key milestones identified in the review process. There is a mechanism in place whereby priority applications may be fast-tracked. Products that will be considered for expedited review are medicines on the Essential Drugs List (EDL) and new molecular entities that are considered essential for national health but that do not appear on the Essential Drugs List.13 Scientific data requirements do not differ between fast-track and other products, and the level of scientific assessment is the same. Once submitted, however, such products are always given priority in the queuing system, and an overall target of 250 calendar days is set for fast-track products. Currently, there is a substantial backlog because of the large number of applications received for the registration of generic medicines; however, applications for NASs are not placed in the same queue as generic medicine applications but are routed for allocation to assessors on completion of administrative screening.
Scientific Assessment
Scientific data presented in applications are assessed in parallel for quality, safety, and efficacy by different units within the MCC. The assessments are performed by internal as well as external assessors. While internal assessors are subject to annual performance appraisals, the external assessors are not contractually bound by service-level agreements, and this condition has an impact on review times. Detailed assessment reports and recommendations are prepared by the assessors, and these are peer reviewed and made part of the agenda at the relevant Scientific Committee meetings for discussion. The Scientific Committee then makes a recommendation to the MCC for ratification. Although there is no set timeline for the scientific assessment of applications, a request is sent to assessors to support completion of the assessment within 90 calendar days.
Questions to Sponsor (Applicant)
Recommendations pertaining to quality data are sent to sponsors, following ratification by the MCC. Sponsors who have submitted an application for an NAS are requested to provide a response to the recommendations within 180 calendar days. The response from the sponsor will be reviewed by an assessor and made part of the agenda at the next Scientific Committee meeting and subsequent Council meeting.
Questions pertaining to safety and efficacy data may be provided to the sponsor at any time during the assessment. Recommendations from the Scientific Committee are sent to the sponsor prior to ratification by the Council. Sponsors are required to respond to the recommendations within 180 calendar days. In the event that major deficiencies are identified in the data submitted, the response from the sponsor will be subjected to the full procedure of evaluation, discussion at the Scientific Committee meeting, and ratification at the Council meeting. The MCC has accepted responses that exceed the time limit.
Expert Committees
Applications for an NAS are referred to a number of Scientific Committees, which require at least 4 Committee reports to be available prior to the medicine’s consideration for registration by the MCC. These include the Pharmaceutical and Analytical Committee, the Clinical Committee, Good Compliance (eg, Good Manufacturing Practices, Good Distribution Practices, Good Clinical Practices, Good Laboratory Practices) Committee, and the Names and Scheduling Committee. There is no target time limit for the Committee procedure; however, routine committee meetings are held every 60 calendar days. Committee processes are conducted in parallel to support efficiencies in the review process. Council meeting dates are scheduled to accommodate the work of the Committees and prevent delays between the outcome of Committee meetings and Council ratification. The recommendations made by the Committees are made part of the agenda at the Council meeting, and the Council is responsible for the decision as to whether to grant authorization. This decision is based on the scientific assessment of the quality, safety, and efficacy data submitted by the sponsor. The Council will also base the decision for authorization or refusal on the approval of the proprietary name of the product, the allocation of a scheduling status to the active pharmaceutical ingredients, and the evaluation of the GMP status of the sponsor, the manufacturer, the assembler, the quality control laboratory, and the final product release responsibility. The decision for authorization or refusal is neither dependent on sample analysis nor on a pricing agreement. Based on the timing of the Council meetings, the authorization process can take up to 60 calendar days from receiving a positive recommendation from the Scientific Committees. Sponsors are informed of the decision of the Council within 7 calendar days after the Council meeting, and the target timelines for the MCC review process can be seen in Table 1.
Table 1.
Target Timelines for MCC Review Procedures.
| Process | Target |
|---|---|
| Validation | 15 calendar days |
| Scientific assessment | 90 calendar days |
| Sponsor response time (quality data) | 180 calendar days |
| Sponsor response time (safety and efficacy data) | 180 calendar days |
| Expert committee(s) | 60 calendar days |
| Authorization procedure | 60 calendar days |
| Notification of decision | 7 calendar days |
| Overall review time (fast track) | NASs: 250 calendar days |
| Overall review time | NASs: No target |
Abbreviations: MCC, Medicines Control Council; NASs, new active substances.
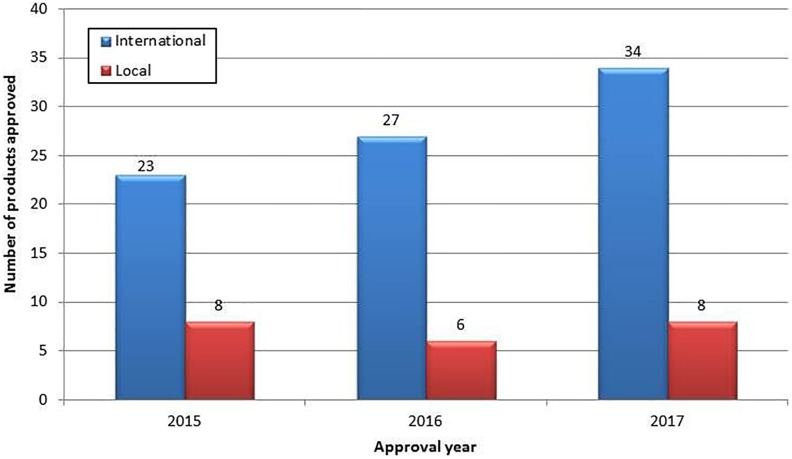
The majority of NASs approved over the period 2015-2017 were submitted by international companies, while local companies were responsible for 21% of these approvals. The number of approved NASs from international and local companies during the period 2015-2017 is shown in Figure 2.
Figure 2.
Number of approved new active substances from local and international companies (2015-2017).
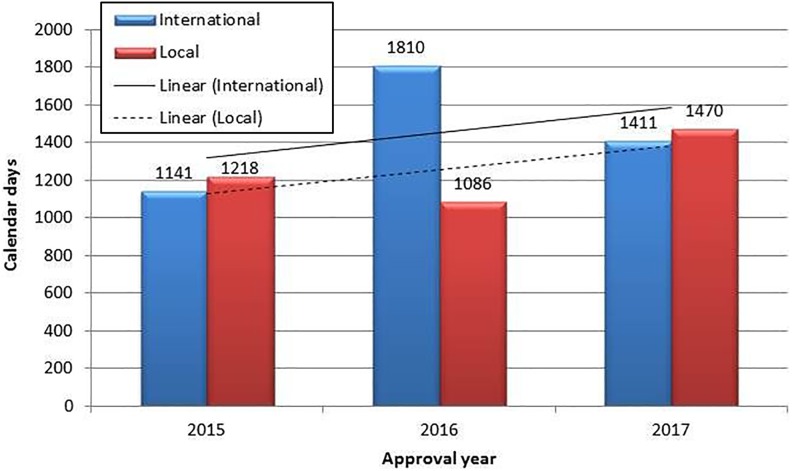
During the studied time period, the highest number of approved NASs for international companies was 34 in 2017, while the highest number of approved NASs for local companies was 8, in both 2015 and 2017. The highest number of NASs was approved in 2017 (n = 42), with a median approval time of 1411 calendar days for applications made by international companies and 1470 calendar days for applications made by local companies (Figure 3). In 2016, 33 NASs were approved, and in 2015, 31 NASs were approved. During the period 2015-2017, the highest median approval time (1810 calendar days) was observed in 2016, for applications made by international companies. The lowest median approval time (1086 calendar days) was also observed in 2016, for applications made by local companies (Figure 3).
Figure 3.
Median approval timelines for new active substances for local and international companies (2015-2017).
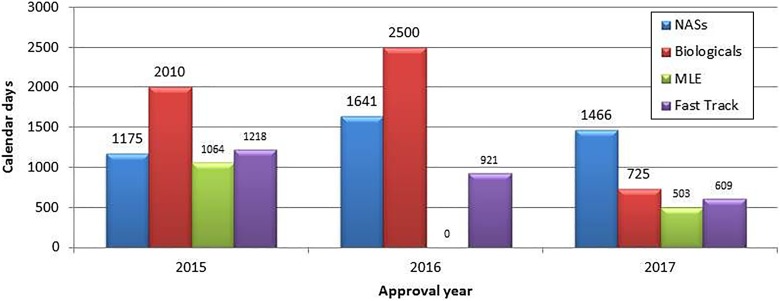
In 2015 and 2016, the approval times for biological products were longer than for NASs (Figure 4). However in 2017, the median approval time for biological products (n = 5) was less than for NASs (n = 31). In 2016 and 2017, fast-track products had shorter approval times in comparison to NASs. Fast-track products also had shorter approval times in 2015-2017, when compared with biologicals. In 2015 and 2017, MLEs had the shortest approval times, when compared with NASs, biologicals, and fast-track products. The most commonly approved NASs, by therapeutic class, during the period 2015-2017 included cytostatic agents (14 products); analgesics (8 products); anticonvulsants, including antiepileptics (6 products); and nonsteroidal anti-inflammatory drugs (6 products). During this time period, only 1 NAS per class was approved for local anesthetics, vasoconstrictors, ophthalmic preparations, medicines against protozoa, and macrolides and lincosamides.
Figure 4.
Median approval time for new active substances compared with biologicals, major line extensions, and fast track products (2015-2017).
Part III: Good Review Practices: Building Quality into the Registration and Review Processes
General Measures Used to Achieve Quality
The MCC has developed an internal quality policy that describes the overall intentions and direction of the authority, related to the quality of the review process. Within the next 2 years, the authority intends to formally implement a quality policy and prescribe the measures that will be used to achieve and continuously improve quality. GRevPs are defined as a framework, applied to the process and documentation related to regulatory review procedures. GRevP measures aim to standardize and improve overall documentation and to ensure timeliness, predictability, consistency, and high quality in reviews and assessment reports. The MCC has initiated the development and implementation of a GRevP framework; however, it is acknowledged that the system is still evolving. Table 2 provides an overview of the status of the implementation of GRevPs by the MCC and demonstrates that there are a number of elements of the framework that need to be formalized and improved.
Table 2.
Status of Implementation of Good Review Practices by the MCC.
| Indicator | Status | Comments | |
|---|---|---|---|
| Quality measures | |||
| Internal quality policy | ✓ | Planned to formally implement | |
| Good review practice system | ✓ | Planned to formally implement | |
| Standard operating procedures for guidance of assessors | ✓ | Planned to formally implement | |
| Assessment templates | ✓ | Planned to formalize the use of a single, common template | |
| Dedicated quality department | ✗ | Establishment of a dedicated quality department is planned | |
| Scientific committee | ✓ | ||
| Shared and joint reviews | ✓ | ||
| Transparency and communication parameters | |||
| Feedback to industry on submitted dossiers | ✓ | ||
| Details of technical staff to contact | ✓ | Contact details are made available on an ad hoc basis | |
| Presubmission scientific advice to industry | ✓ | Meetings are held with industry on an ad hoc basis | |
| Official guidelines to assist industry | ✓ | ||
| Industry can track progress of applications | ✗ | Implementation of electronic document management system is planned | |
| Publicly available Summary Basis of Approval (SBA) | ✗ | Summary is available but is currently not published | |
| Approval times | ✓ | Approval times are not made available to the public | |
| Advisory committee meeting dates | ✓ | ||
| Approval of products | ✓ | ||
| Continuous improvement initiatives | |||
| External quality audits | ✓ | External quality audits are not performed routinely | |
| Internal quality audits | ✗ | Planned | |
| Internal tracking systems | ✓ | Implementation of electronic document management system is planned | |
| Review of assessors’ feedback | ✓ | ||
| Reviews of stakeholders’ feedback | ✓ | Planned to be formally and routinely reviewed | |
| Training and education | |||
| International workshops/conferences | ✓ | ||
| External courses | ✓ | ||
| In-house courses | ✓ | Training program to be formalized | |
| On-the-job training | ✓ | Training program to be formalized | |
| External speakers invited to the authority | ✓ | ||
| Induction training | ✓ | Training program to be formalized | |
| Sponsorship of postgraduate degrees | ✓ | ||
| Placements and secondments in other regulatory authorities | ✓ | ||
▪ Formally implemented
▪ Informally implemented
▪ Not implemented
The MCC has also recognized that the currently implemented elements of the GRevP framework have been underused by staff. Additional training to learn and understand GRevPs would be valuable so that the benefits of formally implementing a comprehensive GRevP framework within the authority may be fully realized. Furthermore, the MCC intends to formally codify the critical elements of GRevPs so that they may be written into the internal organizational policy. The authority also aims to develop a quality management system to support the successful application of GRevPs. Standard operating procedures (SOPs) are available to describe the routine procedure for the regulatory review process, and these provide guidance for the scientific assessors and the advisory committee who are consulted during the review process. These need to be revitalized to provide a detailed description of processes that have been enhanced, and there are plans to update these SOPs within the next 2 years.
Assessment templates, which set out the content and format of written reports on scientific reviews, are available and both external and internal peer reviews are carried out when an NAS is assessed. Elements included in this assessment template are drug substance, drug product, comments on the product label, nonclinical data, clinical pharmacology, safety and efficacy, good clinical practice aspects, and a list of recommendations to the sponsor.
The scientific committees involved in the regulatory review process meet approximately every 60 calendar days to review NAS applications. The assessment reports discussed at these meetings are prepared by both internal and external assessors, but these are not published on the MCC website. The recommendations made by the scientific committees are made part of the agenda at the MCC meeting, where the decision for acceptance or refusal of the application is made.
Quality Management
The MCC recognizes the importance of introducing quality measures throughout the authority in order to ensure consistency, increase transparency, improve efficiencies, and enhance allocation of regulatory resources. The MCC holds regular meetings with external stakeholders, in the form of Industry Task Group (ITG) meetings, which provide a forum for candid discussion between the industry and the regulator. The MCC maintains an open-door policy, whereby meetings with the regulator are routinely facilitated. Furthermore, the industry and interested parties are invited to participate in workshops hosted by the regulator, through which opinions, feedback, and complaints may be received and channeled into corrective and preventive actions.
Currently, the MCC does not have a dedicated unit for assessing the quality of the review process for new medicines; however, contingencies have been put in place to establish such a unit. This unit will be responsible for developing a quality system for the authority, for performing internal quality audits, and for implementing strategies geared for continuous improvement, through retrospective evaluation of the assessment and authorization process. Provision has also been made to employ the use of an electronic document management system (EDMS). The tracking functionality of the EDMS will allow for internal monitoring of the process, thus contributing to efficiency and accuracy in the review process. The quality unit will also be responsible for ensuring that the requirements of the quality management system of the authority are fulfilled, in order to be certified to the quality standards of the International Standardization Organization (ISO) and to ensure that the South African regulatory system is recognized by the WHO.
Quality in the Review and Assessment Process
The MCC has implemented a number of mechanisms in an effort to improve the quality of applications received from sponsors and the scientific review of such applications. Guidelines for industry have been developed and have been published on the MCC website and in official publications. These guidelines are also available on request from the regulator and through industry associations. There is no policy for providing preapplication scientific advice to a sponsor, and such advice is not routinely monitored. Preapplication scientific advice may be provided following a request from the sponsor, who is also given the contact details of technical staff who may be contacted to discuss an application during the review. Formal contact, such as scheduled meetings with the regulator, is possible during product development and assessment and in this time there is also an extensive amount of informal contact between the sponsor and the regulator, via telephone or email.
Shared and Joint Reviews
The MCC takes part in joint reviews through the ZaZiBoNa collaborative process that aims to harmonize regulatory efforts across Africa. The process started as a partnership between the regulatory authorities in Zambia, Zimbabwe, Botswana, and Namibia, and participation by interested South African Development Community (SADC) Member States is encouraged.14 In order to be eligible to participate in the ZaZiBoNa collaborative process, the sponsor is required to submit the application for registration to 2 of the participating authorities.13 Products that have been registered by recognized regulatory authorities are eligible for an abridged review process provided that the assessment report from the authorizing authority is available. The collaborative process aims to complete product authorization or refusal within 11 months. Products may be considered for 2 review cycles, and sponsors are required to respond to the consolidated list of regulatory assessment questions within a period of 60 days. The overall review target for the collaborative process is 210 days. Participating regulatory authorities maintain the right to make a final determination on any application, and the final national regulatory decisions are the responsibility of individual participating authorities.14
Training and Education
Training and professional development of internal and external assessors continues to contribute to the element of quality within the MCC review process (Table 2). Although the training program has not been formalized, assessors are required to take part in induction and on-the-job training. Mentorship programs between experienced assessors and less experienced assessors have been developed to provide support. The National Department of Health provides financial support to assessors enrolled in postgraduate studies and external courses. Assessors have the opportunity to be seconded to other regulatory authorities for further training and regularly attend international workshops and conferences to enrich their learning. Participation in training provided by the WHO, on topics such as the prequalification process and quality managements systems, as well as training provided by the European Directorate for the Quality of Medicine has formed an integral part in the training of assessors.
Transparency of the Review Process
The MCC assigns a high priority to being open and transparent in relationships with the public, health professionals, and industry. The MCC has recognized the need to increase confidence in the regulatory system and to provide assurances on safety safeguards as the main drivers for assigning resources to activities that enhance the transparency of the regulatory system. Table 2 provides an overview of the measures that have been put into place by the MCC in an effort to promote transparency and improve communication with stakeholders.
The MCC has a manual system in place that is used to trace applications that are under review and identify the stage at which the application is in the process. Currently, sponsors are able to track the status of their applications, via telephone and email contact, but the MCC is progressing toward the use of an EDMS that is capable of signaling any target review dates that may have been exceeded, recording the terms of the authorization once granted, and providing searchable archiving of information on applications. The MCC publishes the list of licensed manufacturers, wholesalers, and quality control laboratories; committee meeting dates; and a list of registered products, on the MCC website, where such relevant information is published in the Government Gazette.
Discussion
The regulatory authority in South Africa strives to be an authority of international standing and is one of the most developed authorities in the African region. The authority has taken into account international best practices in the development of its legislation, guidelines, and SOPs. Regulatory authorities in low- and middle-income countries continuously face a number of challenges and are often not sufficiently resourced to provide an efficient and effective service. The MCC has historically faced similar difficulties, resulting in a track record of slow decision making and unnecessary delays in effecting regulatory mandates. Currently review times for NASs are in excess of 4 years, whereas for mature agencies this is of the order of 10 to 16 months.15 This subsequent delay with respect to patients’ access to new medicines is the rationale for the establishment of the new regulatory authority, SAHPRA, and the re-engineering of the current regulatory processes in South Africa. The success of the new system is imperative as the South African authority strives to be considered alongside other comparable agencies.
This study evaluated the overall regulatory approval times for NASs, biologicals, MLEs, and fast-track applications in South Africa from 2015-2017. The number of products approved by the MCC has been increasing each year, and during 2015-2017, 79% were sponsored by international companies. While local companies do submit applications for NASs, these companies often do not have the resources and dedicated research facilities to develop such products in-house, but rather enter into contractual agreements with international companies to develop the products abroad or to sell the product under license.
Significant pro-access policies, which were implemented historically by the Department of Health to support medicine access by the state sector, have resulted in an inundation of generic medicine applications, notwithstanding the number of registered products of the same molecule already available on the market. Efforts to address the increasing volume of applications that have been received have to date failed and resources have been stretched to capacity, resulting in the development of a significant backlog and extended timelines for product registration. Furthermore, inappropriate operational processes and a structure that is heavily reliant on overcommitted external assessors and devoid of effective performance contracts do not provide a sustainable solution for timely evaluations.
The MCC has recognized the importance of building confidence into the system and the support from expert review committees as factors that may contribute to the effectiveness and efficiency of the review and decision-making processes for NAS applications. While outdated mechanisms for review could be improved through the re-engineering of the operational process and decision model, consideration of an appropriate benefit-risk model is recommended. With the amendment of the Medicines and Related Substances Act, 1965 (Act 101 of 1965) in support of liaising with other regulatory authorities, in the spirit of harmonization, the MCC could consider the use of an alternative risk stratification model incorporating reliance strategies on other regulatory authorities. It is also evident that firm target times for the review process must be written into the organizational policy and should be tracked through the use of an electronic management system in order to realize effective regulatory mandates.
Conclusions
This study has evaluated the current MCC regulatory review process, as it has been applied prior to the establishment of SAHPRA. Key milestones and timelines within the regulatory review process have been identified and the measures used for GRevP have been considered. Currently the MCC continues to perform a full review assessment for applications for registration, including NASs and generics for orthodox, biological, complementary, and veterinary medicinal products. The value added in codifying the guidelines for GRevP and formalizing the quality policy and quality management system have been recognized. The findings from this study suggest that the MCC has identified the opportunities for an enhanced regulatory review and may consider an abridged assessment model, which encompasses elements of risk stratification and reliance. As the MCC transitions to the newly established SAHPRA it is hoped that the resource constraints may be alleviated and capacity developed to meet target timelines. The intersection of regulatory frameworks, regulatory performance, and improved access, availability, and affordability of quality, safe, and effective health care products in South Africa has become an essential area for advancement. In the light of the findings of this study, the evaluation of the regulatory environment in South Africa with a view to improving the review process and patients’ access to medicines will be prioritized.
Acknowledgments
The authors acknowledge the assistance of the Deputy Director: Operations and Administration of the Medicines Control Council, for the collection of the data pertaining to metrics on the approval process for new active substances.
Footnotes
Declaration of Conflicting Interests: Andrea Keyter was employed by the South African National Department of Health in the Cluster: Food Control, Pharmaceutical Trade and Product Regulation during the time of this study. Dr Joey Gouws was the Registrar for the Medicines Control Council and was employed by the South African National Department of Health in her capacity as Acting Cluster Manager: Food Control, Pharmaceutical Trade and Product Regulation during the time of this study.
Funding: The authors did not receive any financial support for carrying out this work.
ORCID iD: Sam Salek, RPh, PhD, FFPM, FRPS, MCMS, FESCP  http://orcid.org/0000-0002-4612-5699
http://orcid.org/0000-0002-4612-5699
References
- 1. Ratanawijitrasin S, Wondemagegnehu E. Effective Drug Regulation: A Multi-country Study. Geneva: World Health Organization; 2002. [Google Scholar]
- 2. Hill S, Johnson K. Emerging challenges and opportunities in drug registration and regulation in developing countries, DFID Issue Paper, 2004. http://heart-resources.org/wp-content/uploads/2012/10/Emerging-challenges-and-opportunities-in-Drug-registration-and-regulation.pdf. Accessed November 5, 2017.
- 3. Cone M, Walker S. Workshop Report: Building Quality Into Regulatory Dossiers and the Review Process: Knowing and Meeting Customer Expectations. Surrey, UK: CMR International Institute; 2005. [Google Scholar]
- 4. Cone M, McAuslane N. R&D Briefing 46: Building Quality Into Regulatory Activities: What Does It Mean? Epsom, UK: CMR International Institute, 2006. [Google Scholar]
- 5. Al-Essa R, Salek S, Walker S. An appraisal of good regulatory review practices in the Gulf Cooperation Council States. Drug Info J. 2012;46:57. [Google Scholar]
- 6. World Health Organization. Good review practice guidelines for regulatory authorities (draft for comment). 2014. http://www.who.int/biologicals/GRevPGuidelines-RHSC-endorsed-for-WHO_QAS14-576_27022014.pdf. Accessed November 5, 2017.
- 7. Soomaroo S. The Department of Trade and Industry’s involvement in the State’s procurement of ARVs. Presented to the Portfolio Committee on Economic Development, Pretoria, South Africa, 2017. https://www.thedti.gov.za/parliament/2017/Pharmaceuticals.pdf. Accessed November 3, 2017. [Google Scholar]
- 8. Medicines Control Council. Publications. http://www.mccza.com/About. Accessed October 29, 2017.
- 9. Medicines Control Council. Business Plan, 2006.
- 10. Republic of South Africa. Medicines and Related Substances Act, 1965 (Act 101 of 1965). Government Gazette 40869, May 26, 2017.
- 11. National Treasury. Guide for creating, merging, rescheduling and disestablishment of entities on the national or provincial sphere of government, 1 December 2015.
- 12. McAuslane N, Cone M, Collins J, Walker S. Emerging markets and emerging authorities: a comparative study of how key regulatory authorities in Asia, Latin America, the Middle East and Africa are developing regulatory processes and review models for new medicinal products. Drug Info J. 2009;43:349–359. [Google Scholar]
- 13. Medicines Control Council. General Guideline: Registration of Medicines, version 8, August 2012 http://www.mccza.com/documents/1d9c57df2.01_General_information_Jul12_v8_showing_changes.pdf. Accessed November 2, 2017.
- 14. Regulatory Resources for Africa. ZAZIBONA Registration Pathway, version 1, 9 June 2015 http://www.rrfa.co.za/harmonisation/. Accessed November 3, 2017.
- 15. Centre for Innovation in Regulatory Science. R&D Briefing 65: New drug approvals in six major authorities 2007-2016: Focus on the internationalisation of medicines 2017, London. [Google Scholar]