Abstract
Background
Omentin-1 a new anti-inflammatory adipokine has been identified as a major visceral (omental) secretory adipokine which plays important roles in glucose homeostasis, lipid metabolism, insulin resistance and diabetes. The aim of our study was to evaluate serum omentin-1 levels in type 2 diabetic obese females and assess its relation with glycemic control, insulin resistance and metabolic parameters.
Methods
The study included 60 obese type 2 diabetic females and 30 healthy female subjects formed the control group. They subjected to full clinical examination, weight, height, waist and hip circumference. Fasting (blood glucose, insulin, lipid profile, omentin-1) and HbA1c were measured. BMI and HOMA-IR were calculated. Our data analyzed and expressed in terms of mean ± SD. Pearson correlation performed to study the correlation of serum omentin-1 in relation to glycemic control, insulin resistance and metabolic parameters in the studied groups.
Results
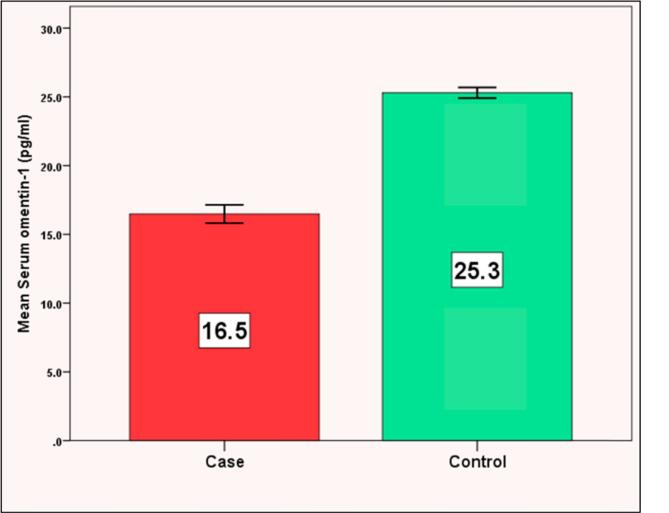
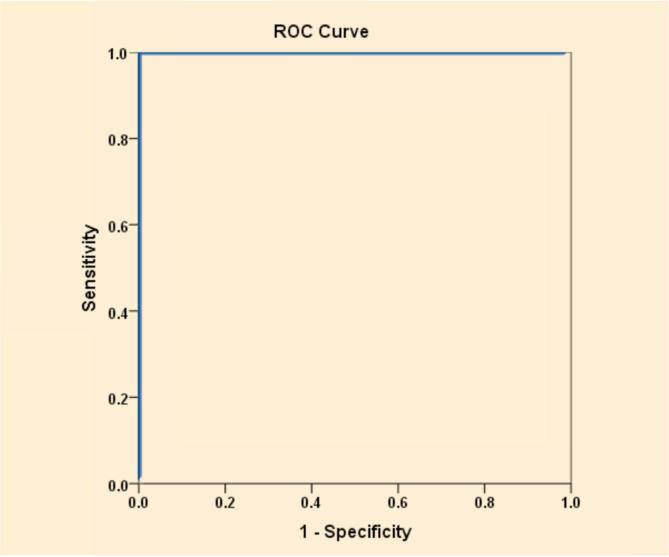
We found significant decrease in serum omentin-1 levels in cases with mean ± SD (16.5 ± 2.6 pg/ml) compared to controls (25.3 ± 1.0 pg/ml) (P < 0.001). We also found strong significant negative correlations between serum omentin-1 and (BMI, fasting insulin, HOMA-IR) (r = −0.909, −0.853, −0.511) respectively (P < 0.001) and systolic blood pressure (r = −0.274, p = 0.031). The best cut off point of serum omentin-1 was 22.2 pg/ml to differentiate cases from controls using ROC curve analysis.
Conclusion
Our study has shown significant low levels of serum omentin-1 in obese type 2 diabetic females in comparison to healthy subjects. Omentin-1 inversely related to obesity, insulin resistance and SBP. No significant associations with glycemic control and fasting lipids. Serum omentin-1 can be used as a biomarker for obesity related metabolic disorders.
Keywords: Omentin-1, Type 2 diabetes, Insulin resistance, Obesity
Introduction
Type 2 diabetes is a metabolic disorder presented by decrease insulin secretion from pancreatic B cells, insulin resistance and hyperglycaemia [1]. It was estimated by International diabetes federation (IDF) that diabetes affected 387 million people worldwide in 2014 and 592 million people by the year 2035 are expected to suffer from diabetes with increase by 55% [2]. Mostly the increase prevalence is present in Asia and Africa by 2030 [3]. It is estimated that Egypt will have 8.6 million adults with diabetes to be one of the 10th largest country affected by diabetes by the year 2030 [4] and the sixth most important cause of disability burden in Egypt [5]. The rise in diabetes prevalence is mostly linked to obesity and bad lifestyle habits [6].
Obesity is a major health problem mostly linked to type 2 diabetes and leads to increased morbidity and mortality [7]. Increased abdominal fat leads to secretion of inflammatory bioactive peptides (adipokines) from the adipose tissue [8], [9]. These adipokines have crucial effects on glucose and lipid metabolism [10], insulin resistance, diabetes, atherosclerosis, vascular endothelium, inflammation, and cardiovascular function [11]. Therefore novel adipokines linked to obesity related disorder are important subjects for research. Adiponectin, resistin, leptin, TNFα, IL-6 are some examples of many secreted adipokines [12]. Yang et al. had discovered omentin gene which extracted from the human omental fat also named as intelectin, endothelial lectin and intestinal lactoferrin receptor [13].
Omentin gene is mainly expressed in cDNA library from the human omental adipose tissue. Omentin consists of 313 amino acid protein of full length 1269 bp (base pair) which contain a secretory protein sequence and a fibrinogen related domain [13]. Omentin has been identified in two homologous isoforms, omentin-1 and omentin-2, where omentin-1 is the main omentin present in the human blood [14]. The two omentin genes were found to be located in 1q22-q23 region adjacent to each other which have linked to type 2 diabetes in various populations [15]. Omentin-1 is mainly expressed in the human omental adipose tissue specifically in the visceral not the subcutaneous tissue and to less extent in the intestine, lung, heart and rarely found in muscle and kidney and not found in other tissues [15]. Omentin-1 is detected in the human blood and its concentration varies from one human to another [13].
Omentin-1 is an anti-inflammatory adipokine [16] and plays a significant role in modulating insulin sensitivity by paracrine and endocrine factor where it enhances the insulin sensitivity and glucose metabolism on the local level of omental adipose tissue, as it increases the insulin signal transduction through activation the protein kinase (Akt/protein kinase B) so it modulates the body fat distribution between the visceral and subcutaneous fat depot [13]. Omentin-1 accelerates only insulin mediated glucose transport and has no effect on the basal glucose transport which indicates that it hasn’t intrinsic insulin like activity [13]. Removal of visceral rather than subcutaneous adipose tissue has been shown to improve insulin sensitivity [17]. On the other hand since it is secreted in the human blood it accelerates insulin sensitivity and glucose metabolism at distant sites as muscles, liver, subcutaneous fat so it may take part in the process of food storage and breakdown [18], [19].
Due to the main expression of omentin-1 in the human visceral omental tissue, omentin-1 levels are decreased in obese subjects [14]. Also Previous studies have shown decreased omentin-1 levels in type 2 diabetes [18], [19] glucose intolerance, newly diagnosed and untreated diabetes and negative correlations with parameters of insulin resistance [18] and obesity, so omentin-1 may represent a biomarker of different metabolic diseases [14]. Our study aimed to evaluate serum omentin-1 levels in type 2 diabetes mellitus obese women and compare it with omentin-1 levels in healthy female subjects and detect its relation with insulin resistance, glycemic control and metabolic parameters.
Material and methods
Subjects
The study was conducted on 90 female subjects were divided into 2 groups, group 1 included 60 obese female patients with type 2 diabetes mellitus who had attended the diabetes and endocrine clinic at Kasr Al Ainy Hospital, Cairo University and group 2 included 30 female healthy age matched control subjects. The study was performed from May 2016 to April 2017. Type 2 diabetes was defined according to American Diabetes Association 2012 [20]. All patients were diagnosed to have diabetes mellitus for at least 5 years.
We excluded male gender, type 1 diabetes mellitus, lean patients and patients with intrinsic renal or hepatic disease. Ethical aspects: Research protocols were approved by the medical ethics committee of Kasr Al Ainy Medical School, Cairo University. All participants provided an informed consent after the research protocols were carefully explained to them.
All subjects were subjected to full history taking and clinical examination. Fasting blood glucose (FBG), fasting lipids (total cholesterol (TC), triglycerides (TG), low density lipoprotein (LDL-C), high density lipoprotein (HDL-C), glycosylated hemoglobin (Hb A1c), fasting insulin and serum omentin-1 levels were measured. Weight, height, waist and hip circumference and blood pressure were measured. Body mass index (BMI) and homeostasis model assessment insulin resistance (HOMA-IR) were calculated. Weight and height were measured while the subjects wearing light clothes and no shoes, BMI was calculated as (kg/m2).
Our study included female patients with BMI equal or more than 30 kg/m2. The waist and hip circumference was measured using a measuring tape. Waist circumference was measured at the level midway between the lower rib margin and the iliac crest in the standing position while breathing out gently. Hip circumference was recorded as the maximum circumference over the buttocks.
Three ml of fasting (12–16 h) venous blood samples were taken from each participant and divided into 2 parts: The 1st part was put in a tube containing EDTA for HbA1c. The 2nd part was left to clot and the serum was separated by centrifugation at 3000×g for 10 min. The separated serum was stored at −20 °C for the determination of fasting blood glucose (immediate determination), lipid profile, insulin and omentin-1. The determination of fasting blood glucose, serum cholesterol and serum triglyceride were carried out on Hitachi 912 (Roche Diagnostics GmbH, D-68298 Mannheim, USA) by colorimetric techniques. For determination of HDL-cholesterol, phosphotungestic acid and magnesium ions are used for precipitating all lipoproteins except HDL fraction that was present in the supernatant and measured by Hitachi 912 auto analyzer. LDL cholesterol was measured by Friedwald formula [21]. Haemoglobin A1C measurement was performed using turbidimetric inhibition immunoassay method on Dimension xpand plus supplied from Siemens (Siemens Healthcare Diagnostics Inc., Newark, DE 19714, USA) [22]. Fasting serum insulin was determined using radio immuno assay [23]. Insulin resistance was calculated according to the homeostasis model assessment (HOMA-IR) using the following equation: HOMA-IR = fasting blood glucose (mg/dl) x fasting serum insulin (μIU/ml)/405 [24]. HOMA-IR cut-off value used was 2.7 (>2.7 was considered insulin resistant and <2.7 was considered insulin sensitive) [24].
Serum omentin-1 was measured using quantitative sandwich enzyme immunoassay technique supplied from Cusabio (Wuhan Hi-tech Medical Devices Park, Building B11, 818 Gaoxin Road, Donghu Hi-Tech development area, Wuhan, Hubei Province 430206, P.R. China) [25].
Statistical methods
Pre-coded data was entered on the computer using “Microsoft Office Excel Software” program (2010) for windows. Data was then transferred to the Statistical Package of Social Science Software program, version 21 (SPSS) to be statistically analyzed. Data was summarized using mean, and standard deviation for quantitative variables and frequency and percentage for qualitative ones. Comparison between groups was performed using independent sample t test for quantitative variables and Chi square or Fisher’s Exact test for qualitative ones.
Pearson correlation coefficients were calculated to signify the association between different quantitative variables. Receiver Operating Characteristics (ROC) analysis was conducted to explore the discriminated ability of serum omentin-1 in differentiating cases from controls. P values less than 0.05 were considered statistically significant, and less than 0.01 were considered highly significant.
Results
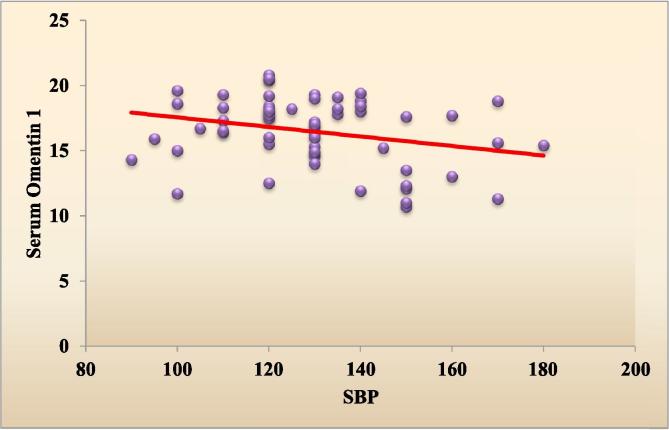
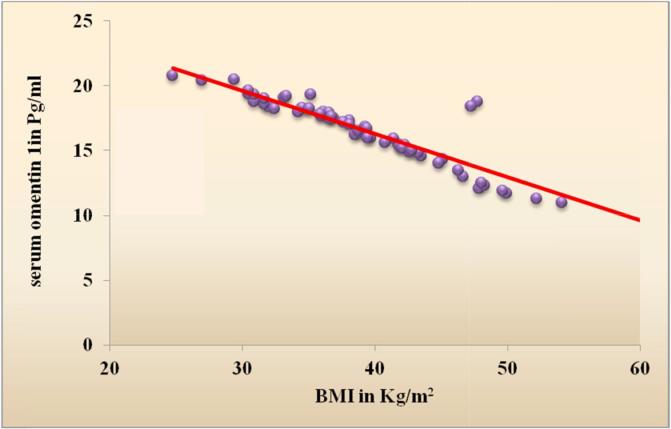
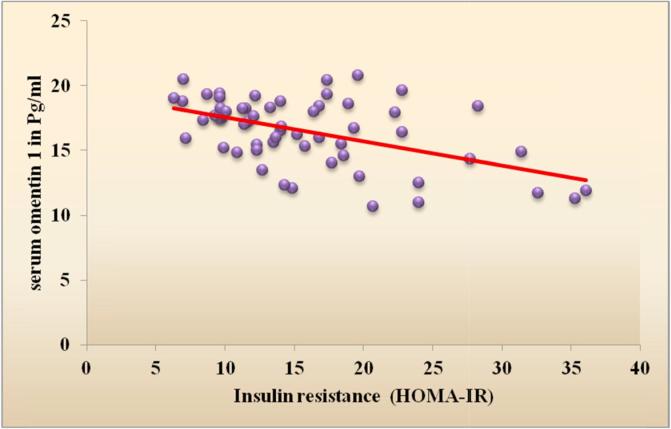
Table 1 shows the demographic data of our studied groups. Group 1 included 60 diabetic obese females with their age ranging from 40 to 60 years with mean (49.5 ± 6.0) years and group 2 included 30 healthy females with mean age (43.7 ± 7.4) years. There were significant difference regarding weight, height, BMI, waist and hip circumference (P < 0.001). Our cases included 31 (51.7%) hypertensive patients and 29 (48.3%) non-hypertensive patients. There was significant difference between the 2 groups regarding SBP (P < 0.01), DBP showed non-significant difference between the 2 groups (Table 1). The laboratory findings of the 2 groups were compared and showed significant difference between the 2 groups according to their FBG, HbA1c, fasting insulin, HOMA-IR and lipid profile (P < 0.001) (Table 1). Comparison between serum omentin-1 among the 2 groups revealed statistically significant decrease in serum omentin-1 levels in cases compared to controls (P < 0.001) (Table 1, Fig. 1). By generating a receiver operating characteristic (ROC) curve we found the cut-off value of serum omentin-1 levels was 22.2 pg/ml (yielding sensitivity and specificity values of 100.0%) these result emphasize the usefulness of the discriminated ability of serum omentin-1 to differentiate between diabetic cases and non diabetic controls (Table 2, Fig. 2). According to the correlation between omentin-1 and different parameters in our cases we found significant positive correlation between serum omentin-1 and age in our diabetic patients (Table 3). Strong negative correlation was found between omentin-1 and SBP (r = −0.274, p = 0.031) (Table 3, Fig. 3) in contrast no significant correlation was found between omentin-1 and DBP (r = 0.223, p = 0.087) (Table 3). We found strong negative correlations between serum omentin-1 levels and weight (r = −0.849, P < 0.001), waist circumference (r = −0.697, P < 0.001), hip circumference (r = −0.778, P < 0.001) and BMI (r = −0.909, P < 0.001) (Table 3, Fig. 4) also strong negative correlations were detected between fasting insulin (r = −0.853, P < 0.001), HOMA IR (r = −0.511, P < 0.001) and omentin-1 (Table 3, Fig. 5) while no significant correlations were detected between fasting glucose, HbA1c, lipid profile and omentin-1 (Table 3). Within controls Strong negative correlations were also detected between weight (r = −0.814, P < 0.001), BMI (r = −0.984, P < 0.001), fasting insulin (r = −0.965, P < 0.001), insulin resistance (r = −0.932, P < 0.001) and serum omentin-1 levels (Table 4).
Table 1.
Demographic and laboratory data of the studied groups.
| Group I diabetic cases | Group II controls | P value | |
|---|---|---|---|
| Age (years) | 49.5 ± 6.0 | 43.7 ± 7.4 | <0.001* |
| Height (cm) | 157.3 ± 5.4 | 163.3 ± 4.4 | <0.001* |
| Weight (kg) | 97.6 ± 19.3 | 57.3 ± 4.6 | <0.001* |
| BMI (kg/m2) | 39.4 ± 7.0 | 21.5 ± 2.1 | <0.001* |
| WC (cm) | 124.1 ± 11.7 | 66.1 ± 6.5 | <0.001* |
| Hip circumference (cm) | 120.7 ± 11.7 | 163.3 ± 4.4 | <0.001* |
| SBP (mmHg) | 129.4 ± 19.6 | 120.7 ± 11.7 | <0.01* |
| DBP (mmHg) | 72.4 ± 18.1 | 73.0 ± 10.2 | 0.9 |
| FBG (mg/dl) | 230.0 ± 82.1 | 91.7 ± 5.8 | <0.001* |
| HbA1c% | 9.0 ± 2.0 | 5.3 ± 0.5 | <0.001* |
| Fasting insulin (μIU/ml) | 28.0 ± 7.2 | 10.2 ± 2.7 | <0.001* |
| (HOMA-IR) | 15.8 ± 7.1 | 2.3 ± 0.6 | <0.001* |
| TC (mg/dl) | 197.8 ± 39.8 | 153.8 ± 19.8 | <0.001* |
| HDL-C (mg/dl) | 33.5 ± 7.2 | 44.4 ± 5.6 | <0.001* |
| LDL-C (mg/dl) | 134.2 ± 41.8 | 87.0 ± 24.4 | <0.001* |
| TG (mg/dl) | 144.0 ± 38.7 | 72.4 ± 7.0 | <0.001* |
| Omentin-1 (pg/ml) | 16.5 ± 2.6 | 25.3 ± 1.0 | <0.001* |
Values are expressed as means ± SD, *P < 0.05 is significant, SBP, systolic blood pressure, DBP, diastolic blood pressure, BMI, body mass index, WC, waist circumference, FBG, fasting blood glucose, HbA1c, glycated haemoglobin, TG, triglycerides, TC, total cholesterol, HDLC, high-density lipoprotein cholesterol, LDLC, low-density lipoprotein cholesterol, HOMA-IR, homeostasis model assessment insulin resistance.
Fig. 1.
It shows the comparison between serum omentin-1 among our studied groups.
Table 2.
ROC curve analysis.
| Tested variable | AUC | 95% CI | P value | Cut-off point | Sensitivity | Specificity |
|---|---|---|---|---|---|---|
| Omentin-1 | 1.0 | 1.0–1.0 | <0.001 | ≤22.2 | 100.0% | 100.0% |
AUC; area under the curve, CI; confidence interval.
Fig. 2.
ROC curve to explore the discriminated ability of serum omentin-1 to differentiate diabetic cases from controls.
Table 3.
Correlation of serum Omentin-1 with different parameters in our type 2 diabetic patients.
| r | P value | |
|---|---|---|
| Age | 0.274 | 0.034* |
| Height | −0.027 | 0.840 |
| Weight | −0.849 | <0.001* |
| BMI | −0.909 | <0.001* |
| Waist circumference | −0.697 | <0.001* |
| Hip circumference | −0.778 | <0.001* |
| SBP | −0.274 | 0.031* |
| DBP | 0.223 | 0.087 |
| FBG | 0.011 | 0.931 |
| HbA1c | 0.065 | 0.621 |
| Fasting insulin | −0.853 | <0.001* |
| HOMA-IR | −0.511 | <0.001* |
| Total cholesterol | 0.027 | 0.839 |
| HDL-C | 0.074 | 0.576 |
| LDL-C | −0.020 | 0.882 |
| TG | 0.142 | 0.278 |
r = Pearson correlation coefficient. * P < 0.05 is significant.
Fig. 3.
Scatter plot graph showing the negative correlation between SBP and serum omentin-1 among type 2 diabetic patients.
Fig. 4.
Scatter plot graph showing the strong negative correlation between BMI & serum Omentin-1 among type 2 diabetic patients.
Fig. 5.
Scatter plot graph showing the negative correlation between Insulin resistance & serum omentin-1 among type 2 diabetic patients.
Table 4.
Correlation of serum Omentin-1 with different parameters in our control subjects.
| r | P value | |
|---|---|---|
| Age | 0.105 | 0.582 |
| Height | 0.547 | 0.002* |
| Weight | −0.814 | <0.001* |
| BMI | −0.984 | <0.001* |
| Waist circumference | 0.144 | 0.448 |
| Hip circumference | −0.030 | 0.877 |
| SBP | −0.220 | 0.242 |
| DBP | 0.051 | 0.790 |
| FBS | 0.209 | 0.268 |
| HbA1c | −0.070 | 0.714 |
| Fasting insulin | −0.965 | <0.001* |
| HOMA-IR | −0.932 | <0.001* |
| Total cholesterol | 0.008 | 0.967 |
| HDL-C | −0.182 | 0.335 |
| LDL-C | −0.079 | 0.679 |
| TG | 0.172 | 0.364 |
r = Pearson correlation coefficient. * P < 0.05 is significant.
Discussion
Omentin-1 is an anti-inflammatory adipokine produced mainly in visceral adiposity has insulin sensitivity effects and has linked to obesity and obesity related disorders as insulin resistance and diabetes. The exact physiological role of omentin-1 in glucose homeostasis is still understood. Circulating omentin-1 levels were documented to be negatively correlated to waist circumference, BMI, HOMA-IR, fasting glucose and insulin and positively correlated to HDL [14], [26]. Therefore we aimed to compare serum omentin-1 levels between type 2 diabetic obese females and healthy female subjects and to assess the association of serum omentin-1 to glycemic control, insulin resistance and metabolic parameters.
Our study included only female subjects in order to obtain a homogenous group where circulating omentin-1 levels had found to be higher in women in comparison to men [14]. Researchers supposed sex difference to be an independent factor for circulating omentin-1 abnormalities [26] due to difference in the pattern of body fat distribution regarding android versus gynoid or due to difference in sex hormones gene expression in adipose tissue [27]. The mean age distribution within our study groups was (49.5 ± 6.0 years) within cases and (43.7 ± 7.4 years) within controls which has supported by the IDF update where the greatest number of people suffered from diabetes are between 40 and 59 years of age [2].
Various studies have reported decreased omentin-1 levels in type 2 diabetes, impaired glucose tolerance and obesity [14], [18], [19]. In our study we found statistically significant decrease in the mean serum omentin-1 levels in diabetic obese patients compared to control group (P value <0.001) and we found the value 22.2 pg/ml of serum omentin-1 the cut-off value to differentiate diabetic cases from controls. Our result supported by Egyptian study had done by El-Mesallamy et al. [28] who documented significant lower omentin-1 levels in diabetic patients in comparison to controls with or without ischemic heart disease before and after adjustment the BMI. Similarly Abd-Elbaky et al. [29] reported significant decreased levels of omentin-1 in diabetic obese Egyptian patients with or without cardiovascular disease in comparison to controls.
The exact mechanisms leading to decreased omentin-1 levels in obesity and type 2 diabetes are still unknown. It has reported that insulin and glucose significantly decrease the omentin mRNA expression and omentin protein production in vitro omental adipose tissue therefore hyperinsulinemia leads to decrease the circulating omentin-1 level significantly in normal subjects and this lead insulin and glucose play a role in the regulation of omentin-1 synthesis either directly or indirectly [30]. Urbanova et al. [31] hypothesized the abnormalities found in the circulating omentin-1 might only reflect the metabolic disturbances that occur in the adipose tissue. Also Catoi et al. [32] supposed the decreased omentin-1 levels clarify the decreased insulin mediated glucose uptake in the insulin responsive tissues which might play a role in the development of type 2 diabetes in obese patients.
Regarding the relation of omentin-1to glycemic control we didn’t find any significant correlations between fasting glucose, HbA1c and serum omentin-1 within cases and controls. Similarly Urbanova et al. [31] failed to find any significant association between serum omentin-1 and fasting glucose levels in both obese and type 2 diabetic patients relative to control subjects. Catoi et al. [32] also failed to find significant association between serum omentin-1 and fasting glucose in morbidly obese patients but after using multiple regression analysis they found fasting glucose might be an independent factor for the changes that might occur in the circulating omentin-1levels. In contrast others [14], [18], [19], [33] reported negative correlation between omentin-1 and fasting glucose.
Insulin resistance is a compensatory mechanism that occurs initially to maintain glucose levels in normal ranges where there be decreased response to insulin effects at the level of muscles and adiposity which leads to glucose intolerance. Therefore insulin resistance plays a crucial role in type 2 diabetes development [33]. In our study we found statistical significant difference between our diabetic obese women and control group in mean fasting insulin and HOMA-IR (P value <0.001). We found serum omentin-1 inversely correlated with both HOMA-IR and fasting insulin in patients and controls (P value <0.001). Nanda et al. [33] showed closely similar results to ours however they recruited 50 males newly diagnosed with type 2 diabetes. Our result is also consistent with other studies [14], [18], [30]. These results support the role of omentin-1 on insulin sensitivity. Omentin-1 has found to enhance the insulin sensitivity and glucose metabolism as it increases the insulin transduction by activating the Akt protein kinase B in both visceral and subcutaneous adiposity [13]. On the contrary Hossein-Nezhad et al. [34] failed to find significant correlation between fasting insulin, insulin resistance and omentin-1.
Visceral adiposity has found to be more pathogenic than subcutaneous adiposity through accelerating insulin resistance, type 2 diabetes and cardiovascular disorders [26]. Body fat distribution, waist and hip circumference were known to reflect the visceral adiposity. It has suggested that differences in adipose tissue distribution may influence the secretion of adipokines [35]. In our study we recruited obese female with BMI (mean ± SD 39.4 ± 7.0 kg/m2) and we documented statistically significant difference in the anthropometric measurements between diabetic patients and controls (P < 0.001). This was supported by previous studies [14], [34].
Regarding the relation of serum omentin-1 to obesity we found strong statistically significant inverse correlations between weight, BMI, waist, hip circumference and serum omentin-1. Various studies [14], [18], [19], [30] also documented inverse correlation between BMI and omentin-1. On the contrary Auguet et al. [36] and Catoi et al. [32] didn’t find significant correlation between omentin-1 and BMI in morbidly obese women.
Due to the strong association between omentin-1 and obesity researchers had studied the effect of caloric restriction and exercise for treatment of obesity on circulating omentin-1 levels and they had found significant increase in omentin-1 levels associated with significant improvement on WC, body fat percent, glycemic control and insulin resistance [26], [37]. Regarding these observations changes in circulating omentin-1may be used as a marker for leanness and a useful marker to counteract the obesity related metabolic disorders [8], [14].
Our patients were obese and had insulin resistance and these 2 factors were found to be associated with increased internal cholesterol synthesis with decreased cholesterol absorption when compared to healthy subjects [38]. In our study we found statistically significant increased levels in fasting lipid profile (P < 0.001) in patients compared to controls. Similarly De Souza Batista et al. had shown that visceral obesity strongly associated with dyslipidemia [14]. On the contrary the study by Hossein-nezhad et al. [34] didn’t show any significant difference in lipid profile between the studied groups (obese and non-obese).
Regarding the relation between omentin-1 and fasting lipids we didn’t find any significant correlations between serum omentin-1 and lipid profile as markers of lipid metabolism. Similarly Hossein-Nezhad et al. [34] didn’t find any significant correlations between omentin-1 and lipid profile in patients with metabolic syndrome. On the contrary the study done by Moreno-Navarrete et al. [26] had reported negative correlations between omentin-1 and (TG, TC and LDL-C). Also Abd-ELbaky et al. [29] reported significant inverse relation between omentin-1 and TC in their obese diabetic groups. This may signify the role of omentin-1 in lipid metabolism where it has found that omentin-1can stimulates 5-AMP-activated protein kinase which acts as cholesterol synthesis inhibitor [39], [40]. In addition other studies [14], [31] showed positive correlation between omentin-1 and HDL. It had supposed that the relation between HDL-C and omentin-1 might be due to impairment in insulin signalling that might occur as a result of changes in circulating omentin-1levels [41].
Hypertension has linked to type 2 diabetes and obesity. It had hypothesized that insulin has anti-natriuretic effect to be one of the leading factors for hypertension in these groups of patients [42]. In our study we found significant increase in mean systolic blood pressure in our patients compared to controls (P < 0.01). Also De Souza–Batista et al. [14] had found significant increase in mean SBP and DBP in over weight and obese patients compared to healthy subjects. In addition we found strong negative correlation between SBP and omentin-1. This result also documented by Auguet et al. [36] and Moreno-Navarrete et al. [43]. On the contrary De Souza–Batista et al. [14] didn’t document this association. Omentin-1 has known to have anti-atherogenic effects through its role in endothelial dysfunction [43], preventing arterial calcification [45], vasodilator effect on isolated blood vessels through inducing the secretion of endothelial nitric oxide [44], inhibiting TNF-α which induce vascular endothelial inflammation [46] and as inhibitor to the inflammatory cascade [47]. Accordingly these data document the possible role of omentin-1 in preventing obesity related metabolic vascular disorders.
Conclusion
Our study showed significant decrease in serum omentin-1 levels in type 2 diabetic obese insulin resistant females. Serum omentin-1 levels inversely related to obesity, insulin resistance and systolic blood pressure. No significant associations have found between fasting glucose, HbA1c, fasting lipids and serum omentin-1 levels. Regarding our results the abnormalities in circulating omentin-1 may be used as a biomarker for obesity and associated metabolic and vascular disorders. Further large scale studies are needed to detect the exact role of omentin-1 on glucose homeostasis and related genes and receptors which may be used as a targeted therapy for future prevention of obesity induced metabolic vascular disorders.
Declaration of interest
None. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Harris M.I. Impaired glucose tolerance in the U.S. population. Diabetes Care. 1989;12:464–474. doi: 10.2337/diacare.12.7.464. [DOI] [PubMed] [Google Scholar]
- 2.International Diabetes Federation. IDF Diabetes Atlas. Sixth Edition Poster update 2014 (17/12/2014).
- 3.Lawrence J.M., Contreras R., Chen W., Sacks D.A. Trends in the prevalence of preexisting diabetes and gestational diabetes mellitus among a racially/ethnically diverse population of pregnant women, 1999–2005. Diabetes Care. 2008;31(5):899–904. doi: 10.2337/dc07-2345. [DOI] [PubMed] [Google Scholar]
- 4.Shaw J.E., Sicree R.A., Zimmet P.Z. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87(1):4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 5.National Information Center of Health and Population (NICHP) Egypt. The Burden of Disease and Injury in Egypt (Mortality and Morbidity); 2004.
- 6.Sullivan P.W., Morrato E.H., Ghushchyan V., Wyatt H.R., Hill J.O. Obesity, inactivity, and the prevalence of diabetes and diabetes-related cardiovascular comorbidities in the U.S., 2000–2002. Diabetes Care. 2005;28:1599–1603. doi: 10.2337/diacare.28.7.1599. [DOI] [PubMed] [Google Scholar]
- 7.Conway B., Miller R.G., Costacou T., Fried L., Kelsey S., Evans R.W. Temporal patterns in overweight and obesity in Type 1 diabetes. Diabet Med. 2010;27:398–404. doi: 10.1111/j.1464-5491.2010.02956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tan B.K., Adya R., Randeva H.S. Omentin: a novel link between inflammation, diabesity and cardiovascular disease. Trends Cardiovasc Med. 2010;20:143–148. doi: 10.1016/j.tcm.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 9.Rabe K., Lehrke M., Parhofer K.G., Broedl U.C. Adipokines and insulin resistance. Mol Med. 2008;14:741–751. doi: 10.2119/2008-00058.Rabe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Després J.P., Lemieux I., Bergeron J., Pibarot P., Mathieu P., Larose E. Abdominal obesity and metabolic syndrome. Arterioscler Thromb Vasc Biol. 2008;28(7):1039–1049. doi: 10.1161/ATVBAHA.107.159228. [DOI] [PubMed] [Google Scholar]
- 11.Zhong X., Zhang H.Y., Tan H., Zhou Y., Liu F.L., Chen F.Q. Association of serum omentin-1 levels with coronary artery disease. Acta Pharmacol Sin. 2011;32:873–878. doi: 10.1038/aps.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shah A., Mehta N., Reilly M.P. Adipose inflammation, insulin resistance and cardiovascular disease. J Parenteral Enteral Nutr. 2008;32(6):638–644. doi: 10.1177/0148607108325251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang R.Z., Lee M.J., Hu H., Pray J., Wu H.B., Hansen B.C., Shuldiner A.R., Fried S.K., McLenithan J.C., Gong D.W. Identification of omentin-1 as a novel depot-specific adipokine in human adipose tissue: possible role in modulating insulin action. Am J Physiol Endocrinol Metab. 2006;290(6):1253–1261. doi: 10.1152/ajpendo.00572.2004. E1253-61. [DOI] [PubMed] [Google Scholar]
- 14.de Souza Batista C.M., Yang R.Z., Lee M.J., Glynn N.M., Yu D.Z., Pray J. Omentin plasma levels and gene expression are decreased in obesity. Diabetes. 2007;56:1655–1661. doi: 10.2337/db06-1506. [DOI] [PubMed] [Google Scholar]
- 15.Fu M., Gong D.W., Damcott C., Sabra M., Yang R., Pollin T. Systematic analysis of omentin 1 and omentin 2 on 1q23 as candidate genes for type 2 diabetes in the Old Order Amish. Diabetes. 2004;53:A59. [Google Scholar]
- 16.Senthilkumar G.P., Anithalekshmi M.S., Yasir M., Parameswaran S., Packirisamy R.M., Bobby Z. Role of omentin 1 and IL-6 in type 2 diabetes mellitus patients with diabetic nephropathy. Diab Metab Syndrome: Clin Res Rev. 2018;12:23–26. doi: 10.1016/j.dsx.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 17.Thörne A., Lönnqvist F., Apelman J., Hellers G., Arner P. A pilot study of long-term effects of a novel obesity treatment: omentectomy in connection with adjustable gastric banding. Int J Obes Relat Metab Disord. 2002;26:193–199. doi: 10.1038/sj.ijo.0801871. [DOI] [PubMed] [Google Scholar]
- 18.Pan H.Y., Guo L., Li Q. Changes of serum omentin-1 levels in normal subjects and in patients with impaired glucose regulation and with newly diagnosed and untreated type 2 diabetes. Diabetes Res Clin Pract. 2010;88(1):29–33. doi: 10.1016/j.diabres.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 19.Cai R.C., Wei L., Di J.Z., Yu H.Y., Bao Y.Q., Jia W.P. Expression of omentin-1 in adipose tissues in obese and type 2 diabetic patients. Zhonghua Yi Xue Za Zhi. 2009;89(6):381–384. [PubMed] [Google Scholar]
- 20.Diabetes Association Diabetes Care. 2012;35:S11–S65. [Google Scholar]
- 21.Friedwald W.T., Levy R.I., Fredrickson D.S. Estimation of the concentration of low density lipoprotein cholesterol in plasma without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 22.Lakshmy R., Gupta R. Measurement of glycated hemoglobin A1c from dried blood by turbidimetric immunoassay. J Diabetes Sci Technol. 2009;3(5):1203–1206. doi: 10.1177/193229680900300527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perez-Fontan M., Cordido F., Rodriguez-Carmona A., Peteiro J., Garcia-Naveiro R., Garcia-Buela J. Plasma ghrelin levels in patients undergoing hemodialysis and peritoneal dialysis. Nephrol Dial Transplant. 2004;19:2095–2100. doi: 10.1093/ndt/gfh313. [DOI] [PubMed] [Google Scholar]
- 24.Wallace T.M., Levy J.C., Matthews D.R. Use and abuse of HOMA modeling. Diabetes Care. 2004;27(6):1487–1495. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- 25.Eisinger K., Krautbauer S., Wiest R., Karrasch T., Hader Y., Scherer M.N. Portal vein omentin is increased in patients with liver cirrhosis but is not associated with complications of portal hypertension. Eur J Clin Invest. 2013;43:926–932. doi: 10.1111/eci.12122. [DOI] [PubMed] [Google Scholar]
- 26.Moreno-Navarrete J.M., Catalan V., Ortega F., Gómez-Ambrosi J., Ricart W., Frühbeck G. Circulating omentin concentration increases after weight loss. Nutr Metab (Lond) 2010;7:27. doi: 10.1186/1743-7075-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luque-Ramirez M., Martinez-Garcia M.A., Montes-Nieto R., Fernandez-Duran E., Insenser M., Alpanes M. Sexual dimorphism in adipose tissue function as evidenced by circulating adipokine concentrations in the fasting state and after an oral glucose challenge. Hum Reprod. 2013;28:1908–1918. doi: 10.1093/humrep/det097. [DOI] [PubMed] [Google Scholar]
- 28.El-Mesallamy H.O., El-Derany M.O., Hamdy N.M. Serum omentin-1 and chemerin levels are interrelated in patients with type 2 diabetes mellitus with or without ischaemic heart disease. Diabet Med. 2011;28:1194–1200. doi: 10.1111/j.1464-5491.2011.03353.x. [DOI] [PubMed] [Google Scholar]
- 29.Abd-Elbaky A., Abo-ElMatty D.M., Mesbah N.M., Ibrahim S.M. Omentin and apelin concentrations in relation to obesity diabetes mellitus type two and cardiovascular diseases in egyptian population. Endocrinol Metab Int J. 2015;2(2):18. [Google Scholar]
- 30.Tan Bee K., Adya Raghu, Farhatullah S., Lewandowski Kris C., O’Hare Paul, Lehnert Hendrik. Omentin-1, a novel adipokine, is decreased in overweight insulin-resistant women with polycystic ovary syndrome: ex vivo and in vivo regulation of omentin-1 by insulin and glucose. Diabetes. 2008;57:801–808. doi: 10.2337/db07-0990. [DOI] [PubMed] [Google Scholar]
- 31.Urbanová M., Dostálová I., Trachta P., Drápalová J., Kaválková P., Haluzíková D. Serum concentration and subcutaneous adipose tissue mRNA expression of omentin in morbid obesity and type II diabetes mellitus: the effect of very-low – calorie diet, physical activity and laparoscopic sleeve gastrectomy. Physiol Res. 2014;63:207–218. doi: 10.33549/physiolres.932530. [DOI] [PubMed] [Google Scholar]
- 32.Cătoi A.F., Suciu Ş., Pârvu A.E., Copăescu C., Galea R.F., Buzoianu A.D. Increased chemerin and decreased omentin-1 levels in morbidly obese patients are correlated with insulin resistance, oxidative stress and chronic inflammation. Clujul Med. 2014;87:19–26. doi: 10.15386/cjm.2014.8872.871.afc1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nanda B., Mahapatra S., Devi N., Swain S., Padhy R.K., Rattanet R. Study of serum omentin-1 in relation to insulin resistance in type II diabetes mellitus. IOSR J Dental Med Sci. 2015;14(12):12–21. [Google Scholar]
- 34.Hossein-nezhad A., Mirzaei K., Alatab S., Ahmadivand Z., Najmafshar A. Circulating omentin-1 in obesity and metabolic syndrome status compared to control subjects. Endocrinol Metabol Syndrome. 2012;S1:008. [Google Scholar]
- 35.Wajchenberg B.L. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev. 2000;21(6):697–738. doi: 10.1210/edrv.21.6.0415. [DOI] [PubMed] [Google Scholar]
- 36.Auguet T., Quintero Y., Riesco D., Morancho B., Terra X., Crescenti A. New adipokines vaspin and omentin. Circulating levels and gene expression in adipose tissue from morbidly obese women. BMC. Med Genet. 2011;12:60. doi: 10.1186/1471-2350-12-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saremi A., Asghari M., Ghorbani A. Effects of aerobic training on serum omentin-1 and cardiometabolic risk factors in overweight and obese men. J Sports Sci. 2010;28:993–998. doi: 10.1080/02640414.2010.484070. [DOI] [PubMed] [Google Scholar]
- 38.Simonen P., Gylling H., Howard A., Miettinen T.A. Introducing a new component of the metabolic syndrome: low cholesterol absorption. Am J Clin Nutr. 2000;72:82–88. doi: 10.1093/ajcn/72.1.82. [DOI] [PubMed] [Google Scholar]
- 39.Kataoka Y., Shibata R., Ohashi K., Kambara T., Enomoto T., Uemura Y. Omentin prevents myocardial ischaemic injury through AMP- activated protein kinase – and Akt-dependent mechanisms. J Am Coll Cardiol. 2014;63:2722–2733. doi: 10.1016/j.jacc.2014.03.032. [DOI] [PubMed] [Google Scholar]
- 40.Yu D. Omentin activates AMP – activated protein kinase and plays a role in energy metabolism and immune response [PhD dissertation]. Molecular Medicine. University of Maryland: Baltimore, MD, USA; 2011.
- 41.Yan P., Liu D., Long M., Ren Y., Pang J., Li R. Changes of serum omentin levels and relationship between omentin and adiponectin concentrations in type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes. 2011;119:257–263. doi: 10.1055/s-0030-1269912. [DOI] [PubMed] [Google Scholar]
- 42.Brands M.W., Manhiani M.M. Sodium-retaining effect of insulin in diabetes. Am J Physiol. 2012;303(11):R1101–R1109. doi: 10.1152/ajpregu.00390.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moreno-Navarrete J.M., Ortega F., Castro A., Sabater M., Ricart W., Ferna’ndez-Real J.M. Circulating omentin as a novel biomarker of endothelial dysfunction. Obesity. 2011;19:1552–1559. doi: 10.1038/oby.2010.351. [DOI] [PubMed] [Google Scholar]
- 44.Yamawaki H., Tsubaki N., Mukohda M., Okada M., Hara Y. Omentin, a novel adipokine, induces vasodilation in rat isolated blood vessels. Biochem Biophys Res Commun. 2010;393:668–672. doi: 10.1016/j.bbrc.2010.02.053. [DOI] [PubMed] [Google Scholar]
- 45.Duan X.Y., Xie P.L., Ma Y.L., Tang S.Y. Omentin inhibits osteoblastic differentiation of calcifying vascular smooth muscle cells through the PI3K/Akt pathway. Amino Acids. 2011;41:1223. doi: 10.1007/s00726-010-0800-3. [DOI] [PubMed] [Google Scholar]
- 46.Yamawaki H., Kuramoto J., Kameshima S., Usui T., Okada M., Hara Y. Omentin, a novel adipocytokine inhibits TNF-induced vascular inflammation in human endothelial cells. Biochem Biophys Res Commun. 2011;408:339–343. doi: 10.1016/j.bbrc.2011.04.039. [DOI] [PubMed] [Google Scholar]
- 47.Watanabe Kaho, Watanabe Rena, Konii Hanae, Shirai Remina, Sato Kengo, Matsuyama Taka-aki. Counteractive effects of omentin-1 against atherogenesis. Cardiovasc Res. 2016;110:118–128. doi: 10.1093/cvr/cvw016. [DOI] [PubMed] [Google Scholar]