Abstract
Objective:
Arthropod-borne viral diseases are a major burden on the health-care system worldwide. Only a few studies have reported on coinfection of dengue fever (DF) with the chikungunya virus in North India. We investigated the seroprevalence and significance of the clinicobiochemical profile of dengue and chikungunya coinfection. Besides this, the authors try to emphasize rationalize platelets transfusion.
Material and Methods:
The present study was conducted at the Heritage Institute of Medical Science, Varanasi, India, from July to December 2016. A total of 1800 suspected cases with acute viral febrile illness (age >18 years) were investigated to exclude other causes of acute febrile illnesses. Of these, 121 patients (6.72%) were diagnosed as seropositive for dengue and chikungunya mono or coinfection using IgM ELISA and were included in the study.
Results:
The male gender was predominant. The majority were in the 20–30-year age group with cases peaking in November. There were 102 (84.29%) cases of dengue, 6 (4.95%) cases of chikungunya, and 13 (10.74%) cases positive for coinfection. Fever was present in all cases. Headache followed by nausea/vomiting and generalized weakness were the most common symptoms in patients with DF while body aches and joint pain were most common in those with chikungunya fever. Deranged liver function and leukopenia were the most common complications in dengue.
Conclusion:
Joint-related symptoms (pain and restricted movements) were statistically significant in chikungunya monoinfection. Two patients with DF were died. There was no significant added severity of clinical features and blood investigations in patients with coinfection with dengue and chikungunya compared to those with monoinfections.
KEYWORDS: Chikungunya infection, Coinfection, Dengue hemorrhagic fever, Platelets transfusion
INTRODUCTION
Arthropod-borne viruses are a major burden on the health-care system worldwide. Dengue virus (DENV) and chikungunya virus (CHIKV) are the most rapidly spreading arboviruses (RNA virus). DENV is a Flavivirus in the family Flaviviridae and is prevalent in tropical and subtropical regions in Asia, the Pacific and Caribbean islands, and Central and South America [1]. It is estimated that there are 50–100 million cases of dengue fever (DF) per year worldwide, including more than 500,000 cases of severe dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS). Since 1996, dengue has been reported widely prevalent in different parts of India [2]. CHIKV is an alphavirus in the family Togaviridae, which is endemic in Africa and Asia [1]. It has been reported in nearly 40 countries, and it was listed as a category C priority pathogen by the US National Institute of Allergy and Infectious Diseases in 2008 [3,4]. Clinical management of dengue and chikungunya infection is entirely based on supportive therapy, and platelet transfusion is used for specific indications only, in dengue. We investigated the changing trends of the dengue clinical profile and prevalence of chikungunya coinfection in Uttar Pradesh, a state in North India.
MATERIALS AND METHODS
The present study was a prospective observational study, conducted in the Department of Medicine of Heritage Institute of Medical Sciences (HIMS) in collaboration with the Department of Microbiology, Institute of Medical Science, Banaras Hindu University (IMS, BHU), Varanasi, from July to December 2016. The study was approved by the Institutional Ethical Committee. Suspected cases (n = 1800) from outdoor and indoor of HIMS with acute febrile illness (age >18 years) were investigated to exclude other causes of acute febrile illnesses. All blood samples were investigated for DF using the Dengue Day 1 Test kit (Rapid test kit, J. Mitra and Co. Private Limited, New Delhi, India) at HIMS following the manufacturer's instructions. The study design is shown in Figure 1. There were 110 patients seropositive for dengue. Dengue Day 1 Test kit negative but suspected cases (n = 51) were sent at Microbiology Department, IMS, BHU, for the detection of dengue and chikungunya. Of those, 6 were positive for chikungunya and 5 were positive for dengue. On the other hand, suspected cases (rapid test positive dengue) were also sent at BHU for the detection of chikungunya. Of those, 13 cases were positive for chikungunya (coinfection). For detection of IgM antibodies against dengue and chikungunya, the DEN and CHIK MAC ELISA test kits (National Institute of Virology [NIV] Pune, India) were used in the Department of Microbiology, IMS, BHU. The sensitivity and specificity for the NIV Dengue IgM capture ELISA kit (version 2.4) were 98.53% and 98.84%, respectively, and for the NIV CHIK IgM capture ELISA kit (version 3.4) were 95% and 98%, respectively. Clinical signs and symptoms, severity, and outcomes with relevant laboratory parameters were compared in detail.
Figure 1.
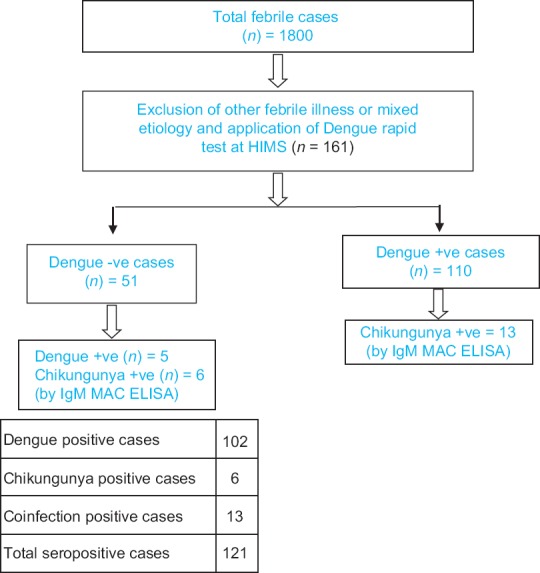
Design of study
Seropositive patients were treated with oral paracetamol and plenty of fluids with vitamin E supplementation. Tramadol, an opioid analgesic was used for arthralgia and symptomatic treatment was used for symptoms such as itching and vomiting. Intravenous drugs and platelet transfusions were given accordingly to guidelines.
Statistical analysis
Online data analysis was done using GraphPad software (Quick-Calcs version). The results of this study are presented as mean ± standard deviation (SD) and percentages. Mean and SD were computed for all continuous variables and comparisons were done using Student's t-test. A test of significance of the differences between two independent proportions was performed using Fischer's exact test. Values were judged extremely significant (P < 0.001), very significant (P = 0.001–0.01), significant (P = 0.01–0.05), or not significant (P ≥ 0.05).
RESULTS
A total of 121 (6.72%) of the 1800 cases of acute febrile illness were seropositive. Of these, 102 cases (84.3%) were DENV positive, 6 (5%) were CHIKV positive, and 13 (10.8%) cases were coinfections (DENV + CHIKV positive). There were 77 (63.6%) men and 44 (36.4%) women. The majority were in the 20–30-year age group. A seasonal peak with 42 (34.7%) cases was seen in November [Figure 2]. The patient characteristics and salient clinical features of acute dengue infection, acute chikungunya infection, and coinfection are compared in Tables 1 and 2. Rural residency was prominent in all groups.
Figure 2.
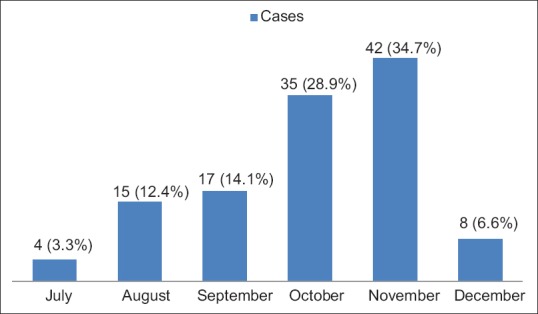
Seasonal variation in cases (n = 121)
Table 1.
Patient characteristics and clinical features (n=121)
| Characteristics | DENV (A=102) (%) | CHIKV (B=6) (%) | DENV + CHIKV (C=13) (%) | P | |
|---|---|---|---|---|---|
| Group A and B | Group A and C | ||||
| Age group (years) | |||||
| <20 | 9 (8.9) | 1 (16.7) | 1 (7.7) | - | - |
| 20-30 | 48 (47.1) | 2 (33.3) | 5 (38.5) | - | - |
| 31-40 | 19 (18.6) | 1 (16.6) | 5 (38.5) | - | - |
| 41-50 | 13 (12.85) | 1 (16.7) | 2 (15.4) | - | - |
| 51-60 | 6 (5.9) | 1 (16.7) | 0 | - | - |
| >60 | 7 (6.9) | 0 | 0 | - | - |
| Sex | |||||
| Male | 65 (63.7) | 3 (50) | 9 (69.2) | - | - |
| Female | 37 (36.3) | 3 (50) | 4 (30.8) | - | - |
| Residence | |||||
| Rural | 84 (82.4) | 4 (66.7) | 11 (84.6) | - | - |
| Urban | 18 (17.7) | 2 (33.3) | 2 (15.4) | - | - |
| Fever | 102 (100) | 6 (100) | 13 (100) | 1.0000 | 1.0000 |
| Headache | 60 (58.8) | 6 (100) | 13 (100) | 0.0796 | 0.0020 (S) |
| Low backache | 34 (33.3) | 1 (16.7) | 6 (46.6) | 0.6616 | 0.3702 |
| Nausea/vomiting | 68 (66.7) | 0 | 3 (23.1) | 0.0020 (VS) | 0.0045 (VS) |
| Rashes | 20 (19.6) | 0 | 2 (15.4) | 0.5905 | 1.0000 |
| Myalgia/body ache | 49 (48.1) | 4 (66.7) | 13 (100) | 0.4332 | 0.0002 (VS) |
| Joint pain | 23 (22.6) | 6 (100) | 13 (100) | 0.0002 (ES) | 0.0001 (ES) |
| Retroorbital pain | 7 (6.9) | 0 | 0 | 1.0000 | 1.0000 |
| Weakness | 80 (78.4) | 6 (100) | 13 (100) | 0.0796 | 0.0706 |
| Breathlessness | 11 (10.8) | 0 | 2 (15.4) | 1.0000 | 0.6405 |
| Diarrhea | 13 (12.8) | 0 | 0 | 1.0000 | 0.3561 |
| Bleeding diathesis | 17 (16.7) | 0 | 3 (23.1) | 0.5869 | 0.6963 |
| Restricted joint movement | 0 | 4 (66.7) | 6 (46.2) | 0.0001 (ES) | 0.0001 (ES) |
| Postinfectious hair loss | 3 (3) | 0 | 0 | 1.0000 | 1.0000 |
| Hypotension | 6 (5.9) | 0 | 1 (7.7) | 1.0000 | 0.5785 |
| Ascites | 5 (4.9) | 0 | 2 (15.4) | 1.0000 | 0.1790 |
| Pleural effusion | 4 (3.9) | 0 | 0 | 1.0000 | 1.0000 |
| Death | 2 (2) | 0 | 0 | 1.0000 | 1.0000 |
DENV: Dengue virus, CHIKV: Chikungunya virus, S: Significant, ES: Extremely significant, VS: Very significant
Table 2.
Distribution of biochemical parameters (n=121)
| Parameters | Normal range | DENV (A=102) (%) | CHIKV (B=6) (%) | DENV + CHIKV (C=13) (%) | P | |
|---|---|---|---|---|---|---|
| Group A and B | Group A and C | |||||
| Anemia | 11-16 g/dL | 34 (33.33) | 1 (16.67) | 1 (7.69) | 0.6616 | 0.1052 |
| Leukopenia | 4-11×103/mm3 | 53 (51.96) | 0 | 7 (53.84) | 0.0271 (S) | 1.0000 |
| Thrombocytopenia | 150-400×103/mm3 | 96 (94.11) | 2 (33.33) | 13 (100) | 0.0005 (ES) | 1.0000 |
| Hyponatremia | 136-146 mEq/L | 55 (53.92) | 0 | 8 (61.54) | 0.0120 (S) | 0.7695 |
| Hypokalemia | 3.5-5.1 mEq/L | 32 (31.37) | 2 (33.33) | 6 (46.15) | 1.0000 | 0.3507 |
| Elevated urea | 15-40 mg/dL | 13 (12.75) | 0 | 2 (15.38) | 1.0000 | 0.6775 |
| Elevated creatinine | 0.6-1.0 mg/dL | 23 (22.55) | 0 | 3 (23.07) | 0.3380 | 1.0000 |
| Elevated SGPT | 14-59 U/L | 68 (66.67) | 1 (16.67) | 10 (76.92) | 0.0225 (S) | 0.5443 |
| Elevated SGOT | 15-37 U/L | 88 (86.27) | 1 (16.67) | 10 (76.92) | 0.0006 (ES) | 0.4055 |
| Elevated ALP | 15-59 U/L | 100 (98.03) | 6 (100) | 13 (100) | 1.0000 | 1.0000 |
| Hypoalbuminemia | 3.4-5.0 g/dL | 44 (43.13) | 0 | 3 (23.07) | 0.0792 | 0.2341 |
S: Significant, DENV: Dengue virus, CHIKV: Chikungunya virus, ES: Extremely significant, SGPT: Serum glutamate pyruvate transaminase, SGOT: Serum glutamic-oxaloacetic transaminase, ALP: Serum alkaline phosphatase
Fever was seen in all cases (100%). In dengue infection, common symptoms included weakness, nausea/vomiting, headache, body pain, and bleeding diathesis. Deranged liver function was seen in 100 (98.1%) patients and deranged renal function in 23 (22.6%). Other complications included hypotension, ascites and pleural effusion. The symptomology of chikungunya included headaches, joint pain, weakness, and restricted joint movement. No complications were reported. All 13 patients with coinfection had headaches, body aches, joint pain, and weakness.
Biochemical investigations reported anemia in 34 (33.3%) dengue cases, leukopenia in 53 (52%) dengue cases, and 7 (53.8%) cases of coinfection but not in any cases of chikungunya. Thrombocytopenia was reported in 96 (94.1%) with dengue infection, 2 (33.3%) with chikungunya and 13 (100%) with coinfection. Elevated creatinine was seen in 23 (22.6%) cases of dengue and 3 (23.1%) cases of coinfection but no case of chikungunya. Elevated levels of serum glutamate-pyruvate transaminase alanine aminotransferase (ALT) and serum glutamic-oxaloacetic transaminase aspartate aminotransferase (AST) were seen in 68 (66.7%) and 88 (86.3%) dengue infections, respectively, and both were elevated in 10 (76.9%) patients with coinfection. Hypoalbuminemia was seen in 44 (43.1%) cases of dengue infection. The mean laboratory parameters are summarized in Table 3. In DF, lowering of platelets was a prominent parameter, and platelet transfusions were done in 13 (12.7%) dengue cases, as shown in Table 4. The degree of deranged liver enzymes in the groups is shown in Table 5.
Table 3.
Comparison of biochemical parameters (n=121)
| Parameters | Mean±SD | P | |||
|---|---|---|---|---|---|
| DENV (A=102) | CHIKV (B=6) | DENV + CHIKV (C=13) | Group A and B | Group A and C | |
| Hb | 11.76±2.25 | 12.25±1.75 | 12.43±1.64 | 0.6018 | 0.3018 |
| TLC | 4484.21±2351 | 7910±2371 | 4440±1476 | 0.0008 (ES) | 0.9475 |
| PC | 65,353±47,509 | 150,500±25,719.64 | 73,462±42,195 | 0.0001 (ES) | 0.5589 |
| HCT | 36.35±5.62 | 35.47±5.68 | 35.9±5.83 | 0.7102 | 0.7870 |
| RBS | 109.46±19.99 | 102.67±5.47 | 101.23±8.44 | 0.4102 | 0.1462 |
| Na+ | 134.54±5.29 | 138.67±2.34 | 136.08±5.20 | 0.0608 | 0.3241 |
| K+ | 3.69±0.52 | 4.02±0.96 | 3.89±0.60 | 0.1552 | 0.2019 |
| Urea | 29.53±15.22 | 18.83±2.56 | 25.76±12.09 | 0.0896 | 0.3927 |
| Creatinine | 1.05±0.67 | 0.83±0.16 | 1.04±0.54 | 0.4257 | 0.9589 |
| SGPT | 245.74±692.20 | 29±5.12 | 140.46±89.98 | 0.4468 | 0.5863 |
| SGOT | 338.11±1216.43 | 26.83±5.91 | 134.69±94.64 | 0.5339 | 0.5494 |
| ALP | 132.13±102.60 | 33.33±13.31 | 99.69±28.98 | 0.0208 (S) | 0.2608 |
| Protein | 6.92±0.91 | 7.6±0.65 | 7.41±0.67 | 0.0747 | 0.0634 |
| Albumin | 3.33±0.59 | 3.95±0.45 | 3.72±0.41 | 0.0130 (S) | 0.0228 (S) |
Hb: Hemoglobin, TLC: Total leukocyte count, PC: Platelets count, HCT: Hematocrit, RBS: Random blood sugar, Na+: Serum sodium, K+: Serum potassium, ALP: Serum alkaline phosphatase, DENV: Dengue virus, CHIKV: Chikungunya virus, ES: Extremely significant, SD: Standard deviation, SGPT: Serum glutamate pyruvate transaminase, SGOT: Serum glutamic-oxaloacetic transaminase, S: Significant
Table 4.
Thrombocytopenia in groups
| Group/platelets (/µL) | >150,000 (%) | 150,000-100,000 (%) | 100,000-50,000 (%) | 50,000-20,000 (%) | <20,000 (%) | <10,000 (%) | Platelets transfusion (%) |
|---|---|---|---|---|---|---|---|
| DENV | 6 (5.9) | 12 (11.8) | 32 (31.4) | 36 (35.3) | 13 (12.8) | 3 (3) | 13 (12.7) |
| CHIKV | 2 (33.3) | 3 (50) | 1 (16.7) | 0 | 0 | 0 | 0 |
| DENV+CHIKV | 0 | 5 (38.5) | 3 (23.1) | 4 (30.7) | 1 (7.7) | 0 | 1 (7.7) |
DENV: Dengue virus, CHIKV: Chikungunya virus
Table 5.
Deranged liver enzymes in groups
| Groups/parameters | SGPT (U/L) | SGOT (U/L) | ||||||
|---|---|---|---|---|---|---|---|---|
| 60-180 (%) | 181-500 (%) | 501-1000 (%) | >1000 (%) | 38-120 (%) | 121-500 (%) | 500-1000 (%) | >1000 (%) | |
| DENV | 39 (38.2) | 22 (21.6) | 4 (3.9) | 3 (2.9) | 45 (44.1) | 33 (32.4) | 6 (5.9) | 4 (3.9) |
| CHIKV | 1 (16.7) | 0 | 0 | 0 | 0 | 1 (16.7) | 0 | 0 |
| DENV + CHIKV | 6 (46.2) | 4 (30.7) | 0 | 0 | 4 (30.8) | 6 (46.2) | 0 | 0 |
DENV: Dengue virus, CHIKV: Chikungunya virus, SGPT: Serum glutamate pyruvate transaminase, SGOT: Serum glutamic-oxaloacetic transaminase
DISCUSSION
Dengue and chikungunya fever are globally important arboviral infections. The first outbreak of the CHIKV in India occurred in 1963 in Kolkata [3] followed by epidemics in Tamil Nadu, Andhra Pradesh, and Maharashtra [5]. By 2010, the disease had spread to more than 18 states/union territories within the country [6]. Over the past few years, many studies have reported chikungunya and dengue-coinfection in several parts of India [3,5]. The diseases share a common mode of transmission through different species of mosquitoes, that is, Aedes aegypti as a principle vector and Aedes albopictus as a secondary vector [1,7]. These infections have many common clinical features such as high-grade fever, rashes, nausea, headache, and body pain, so it is not always easy to differentiate the two infections clinically. Chikungunya fever is often misdiagnosed as dengue viral infection. In most cases of mild infection, symptoms subside spontaneously because the viral titer decreases in about 10 days [7]. Dengue infections are divided on the basis of clinical criteria into DF, DHF, DSS, and expanded dengue syndrome [8]. Expanded dengue syndrome includes atypical manifestations of dengue infection affecting the hepatic, gastrointestinal, neurological, pulmonary, and renal systems [9,10,11].
Chikungunya viral infection is not generally fatal but can cause neurological and optical manifestations. Severe joint pain is the prominent clinical manifestation and can persist for months to a year [7].
As DF has a high incidence and mortality rate, symptomatic patients are tested only for DENV and only in rare cases for chikungunya viral infection. This is an important reason why chikungunya cases go undiagnosed in dengue-endemic regions, and the true burden of the chikungunya viral infection has been missed. Thus, investigation for both viruses should be done, especially in endemic regions. Accurate and early diagnosis of coinfections would help inappropriate management [7].
The number of cases increases during and after the monsoon months because higher humidity lengthens the life span of mosquitoes and increased temperatures shorten the extrinsic incubation period [1]. Rising of the cases during the monsoon period with highest cases in different months had been reported in previous studies from India [1,3,12,13]. November was peak among the monsoon months in this study.
Previous studies using serological methods had the prevalence of coinfection ranging 0.9% to 19%, whereas in this study, it was 10.74%. A summary of studies from all over world is shown in Table 6 [14,15,16,17,18,19,20,21,22,23,24,25,26,27,28]. The present study showed that the 20–30-year age group males and patients from rural areas were more affected than their counterparts. Social bias may be a reason for higher rates of infections in males as India remains a male dominant country.
Table 6.
Summary of studies from different regions of the world
| Reference | Study (year) | Sample size | Diagnostic method | DENV (%) | CHIKV (%) | Coinfection (%) |
|---|---|---|---|---|---|---|
| Southeast Asia | ||||||
| Current study | Varanasi, Uttar Pradesh, India (2016) | 1800 | ELISA | 102 (84.29) | 6 (4.95) | 13 (10.7) |
| [14] | Vellore, India (1964) | 372 | ELISA | 11 (3) | 202 (54) | 7 (2) |
| [15] | Delhi, India (2006) | 69 | RT-PCR | 48 (70) | 17 (25) | 6 (9) |
| [16] | South India (2007) | 713 | ELISA and RT-PCR | 5.16 | - | 2.1 |
| [12] | West Bengal, India (2010) | 550 | ELISA | 104 (18.9) | 131 (23.8) | 68 (12.4) |
| [13] | Tirupati, India (2010) | 331 | ELISA | 40/331 (12.08) | 33/170 (19.4) | 2/72 (2.7) |
| [3] | Pune, India (2010) | 364 | ELISA | 96 | 54 | 25 |
| [17] | Delhi, India (2011) | 87 | ELISA and RT-PCR | 49 | 29 | 10 |
| [18] | Odisha, India (2011-2012) | 174 | ELISA and RT-PCR | 15 | 174 | 2 |
| [19] | Maharashtra and Odisha, India (2013) | 204 | ELISA | 96 (47) | 72 (32) | 43 (19) |
| [5] | Mumbai, India (2010-2015) | 300 | ELISA | 177 (59) | 6 (2) | 20 (6.7) |
| RT-PCR | 21 (7) | 105 (35) | 30 (10) | |||
| [20] | Sri Lanka (2006-2007) | 54 | ELISA | 20 | 21 | 3 |
| [21] | Myanmar (2010) | 116 | ELISA | 47 (40) | 6 (5) | 7 (6) |
| [22] | Thailand (2009) | 50 | ELISA | 10 | 32 | 1 |
| Eastern Mediterranean | ||||||
| [23] | Yemen (2012) | 440 | ELISA and RT-PCR | 116 (29) | 49 (12) | 13 (3.25) |
| The Americas | ||||||
| [24] | St. Martin (2013-2014) | 1502 | ELISA and RT-PCR | 570 (38) | 65 (4) | 16 |
| Africa | ||||||
| [25] | Gabon (2007) | 733 | RT-PCR | 257 | 54 | 28 |
| [26] | Gabon, Africa (2007-2010) | 4287 | RT-PCR | 376 (8.3) | 1567 (36.6) | 37 (0.9) |
| [27] | Madagascar (2006) | 38 | ELISA and RT-PCR | 10 | ||
| [28] | Tanzania (2013) | 93-364 | ELISA and RT-PCR | 29 (38.2) | 17 (4.7) | 4.34 (1.0) |
RT-PCR: Reverse transcription-polymerase chain reaction, DENV: Dengue virus, CHIKV: Chikungunya virus
The study showed a statistically significant presence of headache and myalgia/body ache in DF alone and coinfections, while joint involvement featured in patients with chikungunya infection alone and in coinfection with dengue. There were no significant differences in biochemical parameters, except for the mean serum albumin level in patients with DF and those with coinfections [Table 3]. In contrast to the study in Mumbai, this study results did not reveal any significant reduction of platelets in patients with coinfection [5]. Thus, symptoms such as headache, myalgia/body ache, and arthralgia may be helpful clinically in addressing the infections separately. Joint pain was persistent in chikungunya and coinfection patients for 1–2 months, while in those with dengue infection alone, joint pain subsided with improvement in the clinical condition. There were no reported cases of residual joint deformity. There were no statistically significant differences in the mean values of blood counts and chemistry in patients with dengue infection alone compared with those with coinfection. Many studies have shown changing trends in the clinical profile of dengue, so there is more concern about evaluating dengue properly. For management purposes, dengue cases are divided into mild, moderate, and severe dengue as follows [29]:
Mild dengue: Fever without any complications or evidence of capillary leakage
Moderate dengue: Fever with recurrent vomiting, abdominal pain/tenderness, generalized weakness/lethargy/restlessness/palpitations/breathlessness, decreased urine output, mild pleural effusion/ascites, hepatomegaly, increased hematocrit >20%, and DHF I and II with minor bleeding (scanty hemoptysis, hematemesis, hematuria, gum bleeding, increased menstrual flow, etc.)
Severe dengue: DF/DHF with significant hemorrhage, DHF with shock, severe organ involvement, and severe metabolic disorder. Patients with moderate and severe dengue are hospitalized and managed accordingly.
Anemia was observed in 33.33% and leukopenia in 51.96% of dengue cases. Thrombocytopenia occurred in all groups but was dominant in the dengue and coinfection groups. Decreased platelets in DF are due to (i) platelet destruction due to antibodies against platelets, (ii) disseminated intravascular coagulation, (iii) bone marrow suppression in the early stage, and (iv) peripheral sequestration of platelets [29]. A falling trend of platelets was seen in the dengue coinfection groups. This study divided cases into 4 categories based on the platelet count with close monitoring of vitals.
No risk category: Patients with a platelet count >100,000/μL
Low-risk category: Patients with a platelet count from 50,000 to 100,000/μL
Moderate-risk category: Patients with a platelet count from 10,000 to 50,000/μL
High-risk category: Patients with a platelet count <10,000/μL.
Platelet transfusion was done in high-risk category patients prophylactically and in patients with hemorrhage irrespective of the platelet count. Thirteen patients with DF alone and one with coinfection needed platelet transfusions.
No specific antiviral agent is indicated for dengue or chikungunya. Only paracetamol and/or tramadol are advised for fever and severe body aches, while nonsteroidal anti-inflammatory drugs, steroids, and antivirals are contraindicated. Judicious monitoring of intravascular volume replacement prevents severe dengue (DHF/DSS) and lowers the risk for hospitalization. Certainly, public awareness with incomplete information sometime creates nuisance in reference of decreasing trend of platelets count. In this situation, early platelets transfusion are forced irrespective of degree of thrombocytopenia. Therefore, correct information should be updated publically time to time. Furthermore, it is important to cross-check the platelet count manually before transfusion because automatic analyzers often underestimate the count if platelet clumping occurs. There are specific recommendations for platelet transfusion (i) prophylactic transfusion when the platelet count <10,000/μL and (ii) therapeutic transfusion when bleeding manifestations are present, irrespective of the platelet count, (iii) prolonged shock with coagulopathy and abnormal coagulogram and (iv) platelet transfusion may be needed with packed red cell transfusion in case of systemic bleeding [29]. Receiving multiple platelet transfusions may result in alloimmunization to many human leukocyte antigen-and platelet-specific antigens [29]. Hence, platelet transfusion should be done cautiously as per indications and single-donor apheresis platelets transfusion should be promoted over random donor platelets to decrease future transfusion-related risks.
Recent studies have reported increasing incidences of atypical hemorrhagic manifestations [30] and hepatic dysfunction [31]. Hepatic dysfunction was the most common complication in our dengue and coinfection groups. Elevated serum ALT occurred in 66.67% of dengue cases and elevated serum AST in 86.27%. Two patients in the DF group died, one (35 years old female) due to DHF and other (22 years old female) due to hepatic failure.
Many recent studies reported coinfection is associated with clinically severe disease leading to high mortality compared with monoinfection [3]. In contrast, our study supports findings from a study conducted in Africa that concluded coinfection had no added clinical manifestations [26]. Therefore, further studies involving larger sample sizes in endemic areas are needed to better understand the clinical and biochemical profile in dual viral infections.
CONCLUSION
Coinfection with chikungunya does not increase severity of dengue. However, clinically suspected cases should be tested for both viruses in endemic areas, especially in the monsoon season. This would help to calculate the true burden of dengue and chikungunya coinfection. Timely and appropriate management can assist in the prediction and control of outbreaks.
The limitations of study
First, reverse transcription polymerase chain reaction molecular diagnostic test was not performed in the present study which is more sensitive and specific than ELISA, and second, it was a cross-sectional design, conducted at a single center with limited cases.
Financial support and sponsorship
Nil.
Conflicts of interest
There is no conflict of interest.
Acknowledgment
We thank our junior residents, Dr Shishir, Dr Shivani, Dr Rajdeep, and Dr Nitesh, for patient care. We also thank to Mr. Vipin for technical help.
REFERENCES
- 1.Chang SF, Su CL, Shu PY, Yang CF, Liao TL, Cheng CH, et al. Concurrent isolation of chikungunya virus and dengue virus from a patient with coinfection resulting from a trip to Singapore. J Clin Microbiol. 2010;48:4586–9. doi: 10.1128/JCM.01228-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Annual Report 2007-08. New Delhi: Ministry of Health and Family Welfare; 2007-08. Government of India. [Google Scholar]
- 3.Dinkar A, Singh J, Prakash P, Das A, Nath G. Hidden burden of chikungunya in North India; A prospective study in a tertiary care centre. J Infect Public Health. 2017 doi: 10.1016/j.jiph.2017.09.008. pii: S1876-0341(17)30242-3. [DOI] [PubMed] [Google Scholar]
- 4.Gandhi BS, Kulkarni K, Godbole M, Dole SS, Kapur S, Satpathy P, et al. Dengue and chikungunya co-infection associated with more severe clinical disease than mono-infection. Int J Healthc Biomed Res. 2015;3:117–23. [Google Scholar]
- 5.Londhey V, Agrawal S, Vaidya N, Kini S, Shastri JS, Sunil S, et al. Dengue and chikungunya virus co-infections: The inside story. J Assoc Physicians India. 2016;64:36–40. [PubMed] [Google Scholar]
- 6.Ministry of Health and Family Welfare: Annual Report 2009-10. Government of India. Ministry of Health and Family Welfare. 2010 [Google Scholar]
- 7.Deeba F, Afreen N, Islam A, Naqvi IH, Broor S, Ahmed A, Parveen S. Co-infection with Dengue and Chikungunya Viruses. In: Rodriguez- Morales A, editor. Current Topics in Chikungunya. London-United Kingdom: InTech; 2016. [Last accessed on 2018 Jan 30]. DOI:10.5772/64308. Available from: http:// www.intechopen.com/books/current-topics-in-chikungunya/co-infectionwith- dengue-and-chikungunya-viruses . [Google Scholar]
- 8.Singh J, Dinkar A, Gupta KK, Singh AK, Kumar S, Himanshu D. Dengue encephalitis with acute intracerebral infarction and facial palsy; a rare presentation. Int J Sci Res. 2014;3:2251–3. [Google Scholar]
- 9.Singh J, Singh A, Dinkar A, Atam V. A rare presentation of dengue fever: Acute motor quadriparesis due to hypokalemia. Int J Res Med Sci. 2014;2:132–4. [Google Scholar]
- 10.Singh J, Dinkar A, Atam V, Misra R, Kumar S. A deadly combination of acute encephalitis and gastric hemorrhage in dengue fever: A rare case. J Med Sci Clin Res. 2014;2:3187–93. [Google Scholar]
- 11.Singh J, Dinkar A, Atam V, Misra R, Kumar S. An atypical complication of dengue haemorrhagic fever: Acute intracranial hemorrhage. Adv Bioresour. 2015;6:148–51. [Google Scholar]
- 12.Taraphdar D, Sarkar A, Mukhopadhyay BB, Chatterjee S. A comparative study of clinical features between monotypic and dual infection cases with chikungunya virus and dengue virus in West Bengal, India. Am J Trop Med Hyg. 2012;86:720–3. doi: 10.4269/ajtmh.2012.11-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalawat U, Sharma KK, Reddy SG. Prevalence of dengue and chickungunya fever and their co-infection. Indian J Pathol Microbiol. 2011;54:844–6. doi: 10.4103/0377-4929.91518. [DOI] [PubMed] [Google Scholar]
- 14.Carey DE, Myers RM, DeRanitz CM, Jadhav M, Reuben R. The 1964 chikungunya epidemic at Vellore, South India, including observations on concurrent dengue. Trans R Soc Trop Med Hyg. 1969;63:434–45. doi: 10.1016/0035-9203(69)90030-3. [DOI] [PubMed] [Google Scholar]
- 15.Chahar HS, Bharaj P, Dar L, Guleria R, Kabra SK, Broor S, et al. Co-infections with chikungunya virus and dengue virus in Delhi, India. Emerg Infect Dis. 2009;15:1077–80. doi: 10.3201/eid1507.080638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neeraja M, Lakshmi V, Dash PK, Parida MM, Rao PV. The clinical, serological and molecular diagnosis of emerging dengue infection at a tertiary care institute in Southern, India. J Clin Diagn Res. 2013;7:457–61. doi: 10.7860/JCDR/2013/4786.2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Afreen N, Deeba F, Khan WH, Haider SH, Kazim SN, Ishrat R, et al. Molecular characterization of dengue and chikungunya virus strains circulating in New Delhi, India. Microbiol Immunol. 2014;58:688–96. doi: 10.1111/1348-0421.12209. [DOI] [PubMed] [Google Scholar]
- 18.Mohanty I, Dash M, Sahu S, Narasimham MV, Panda P, Padhi S, et al. Seroprevalence of chikungunya in Southern Odisha. J Family Med Prim Care. 2013;2:33–6. doi: 10.4103/2249-4863.109939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saswat T, Kumar A, Kumar S, Mamidi P, Muduli S, Debata NK, et al. High rates of co-infection of dengue and chikungunya virus in Odisha and Maharashtra, India during 2013. Infect Genet Evol. 2015;35:134–41. doi: 10.1016/j.meegid.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 20.Kularatne SA, Gihan MC, Weerasinghe SC, Gunasena S. Concurrent outbreaks of chikungunya and dengue fever in Kandy, Sri Lanka, 2006-07: A comparative analysis of clinical and laboratory features. Postgrad Med J. 2009;85:342–6. doi: 10.1136/pgmj.2007.066746. [DOI] [PubMed] [Google Scholar]
- 21.Tun MM, Thant KZ, Inoue S, Nabeshima T, Aoki K, Kyaw AK, et al. Detection of East/central/South African genotype of chikungunya virus in Myanmar, 2010. Emerg Infect Dis. 2014;20:1378–81. doi: 10.3201/eid2008.131431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laoprasopwattana K, Kaewjungwad L, Jarumanokul R, Geater A. Differential diagnosis of chikungunya, dengue viral infection and other acute febrile illnesses in children. Pediatr Infect Dis J. 2012;31:459–63. doi: 10.1097/INF.0b013e31824bb06d. [DOI] [PubMed] [Google Scholar]
- 23.Rezza G, El-Sawaf G, Faggioni G, Vescio F, Al Ameri R, De Santis R, et al. Co-circulation of dengue and chikungunya viruses, al Hudaydah, Yemen, 2012. Emerg Infect Dis. 2014;20:1351–4. doi: 10.3201/eid2008.131615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Omarjee R, Prat C, Flusin O, Boucau S, Tenebray B, Merle O, et al. Importance of case definition to monitor ongoing outbreak of chikungunya virus on a background of actively circulating dengue virus, St. Martin, December 2013 to January 2014. Euro Surveill. 2014;19 doi: 10.2807/1560-7917.es2014.19.13.20753. pii: 20753. [DOI] [PubMed] [Google Scholar]
- 25.Leroy EM, Nkoghe D, Ollomo B, Nze-Nkogue C, Becquart P, Grard G, et al. Concurrent chikungunya and dengue virus infections during simultaneous outbreaks, Gabon, 2007. Emerg Infect Dis. 2009;15:591–3. doi: 10.3201/eid1504.080664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caron M, Paupy C, Grard G, Becquart P, Mombo I, Nso BB, et al. Recent introduction and rapid dissemination of chikungunya virus and dengue virus serotype 2 associated with human and mosquito coinfections in Gabon, central Africa. Clin Infect Dis. 2012;55:e45–53. doi: 10.1093/cid/cis530. [DOI] [PubMed] [Google Scholar]
- 27.Ratsitorahina M, Harisoa J, Ratovonjato J, Biacabe S, Reynes JM, Zeller H, et al. Outbreak of dengue and chikungunya fevers, Toamasina, Madagascar, 2006. Emerg Infect Dis. 2008;14:1135–7. doi: 10.3201/eid1407.071521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chipwaza B, Mugasa JP, Selemani M, Amuri M, Mosha F, Ngatunga SD, et al. Dengue and chikungunya fever among viral diseases in outpatient febrile children in Kilosa district hospital, Tanzania. PLoS Negl Trop Dis. 2014;8:e3335. doi: 10.1371/journal.pntd.0003335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.National Guidelines for Clinical Management of Dengue Fever. 2014. [Last accessed on 2017 Jan 03]. Available from: http://www.pbhealth.gov.in/Dengue-National- Guidelines-2014%20Compressed.pdf .
- 30.Singh J, Dinkar A, Atam V, Misra R, Kumar S, Gupta KK, et al. Intracranial hemorrhage in dengue fever: A case series. J Med Sci Clin Res. 2015;3:4447–52. [Google Scholar]
- 31.Singh J, Dinkar A, Atam V, Himanshu D, Gupta KK, Usman K, et al. Awareness and outcome of changing trends in clinical profile of dengue fever: A Retrospective analysis of dengue epidemic from January to December 2014 at a tertiary care hospital. J Assoc Physicians India. 2017;65:42–6. [PubMed] [Google Scholar]


