Abstract
Objective:
Previous studies have reported that a vegetarian diet may lower blood pressure (BP), but the effect of diet on BP in asymptomatic participants with proteinuria is unknown. We examined the association of diet and BP in individuals with or without proteinuria.
Materials and Methods:
This cross-sectional study analyzed data from participants who were more than 40 years old and received physical checkups at Taipei Tzu Chi Hospital from September 5, 2005, to December 31, 2016. Diets were assessed at baseline by a self-reported questionnaire and categorized as vegan, lacto-ovo vegetarian, or omnivore. There were 2818 (7.7%) vegans, 5616 (15.3%) lacto-ovo vegetarians, and 28,183 (77.0%) omnivores. The effect of different parameters on BP was determined using a multivariate multiple linear regression model with no intercept, with control for important characteristics and lifestyle confounders.
Results:
The vegan group had a lower mean systolic BP (−3.87 mmHg, P < 0.001) and diastolic BP (−2.48 mmHg, P < 0.001) than the omnivore group. Participants with proteinuria had a higher systolic BP (4.26 mmHg, P < 0.001) and diastolic BP (2.15 mmHg, P < 0.001) than those without proteinuria. Interaction analysis indicated that vegan participants with proteinuria had a lower systolic BP (−2.73 mmHg, P = 0.046) and diastolic BP (−2.54 mmHg, P = 0.013) than other participants with proteinuria. However, individuals in the lacto-ovo group with proteinuria had a BP similar to other participants with proteinuria.
Conclusions:
A vegan diet was associated with lower BP in asymptomatic participants with proteinuria. This diet could be a nonpharmacologic method to reduce BP.
KEYWORDS: Early chronic kidney disease, Hypertension, Proteinuria, Vegetarian diet
INTRODUCTION
High blood pressure (BP) accounts for approximately 50% of cardiovascular disease (CVD) morbidity worldwide [1] and is a major independent risk factor for global disease burden [2,3]. A nationwide survey in Taiwan indicated that the overall prevalence of hypertension (HTN) was 25% in men and 18% in women [4]. In addition, the prevalence of HTN in Taiwan has increased over time due to the increased prevalence rates of prehypertension, obesity, and metabolic syndrome [5]. HTN also increases the risk for deterioration of renal function [6]. Proteinuria is the earliest marker of kidney damage in clinical practice and also an important predictor of progression to end-stage renal disease [7]. Thus, screening for chronic kidney disease (CKD)-based proteinuria and aggressive control of CKD risk factors, such as HTN, are extremely important [8].
The 2015 guidelines of the Taiwan Society of Cardiology and the Taiwan Hypertension Society state that BP should be <130/80 mmHg for patients with CKD and proteinuria [9]. Similarly, a post hoc analysis of patients with proteinuria reported a lower BP target (<130/80 mmHg) and improved renal outcome [10].
Many recent studies have investigated the effects of different diets on BP [11,12,13]. Most of these studies had a relatively small number of participants and only examined groups of vegetarians. A recent meta-analysis reported lower BP in individuals with vegetarian than nonvegetarian diets [14]. However, no studies have yet examined the relationship between BP and a vegetarian diet in asymptomatic participants with proteinuria and Stage 1 or 2 CKD.
We hypothesized that, compared with an omnivore diet, a vegetarian diet could lower BP in participants with or without proteinuria. We examined this hypothesis by performing a large retrospective cross-sectional study.
MATERIALS AND METHODS
Study participants
This retrospective cross-sectional study examined the records of individuals admitted to the health checkup center of Taipei Tzu Chi Hospital (New Taipei City, Taiwan). We analyzed individuals over 40 years old who received self-paid health examinations from September 5, 2005, to December 31, 2016. We excluded individuals whose records had missing or incorrect identification and missing biochemical examination data. Participants who had more than one health checkup were recruited for analysis just once at the first checkup. This study was approved by our local Institutional Review Board (06-XD12-033) and followed the Strengthening the Reporting of Observational Studies in Epidemiology guidelines.
Clinical assessment
The presence of diabetic mellitus (DM), HTN, hyperlipidemia, and CVD was recorded. The participants were placed into four age groups for comparison: 40–49 years, 50–59 years, 60–69 years, and >70 years.
The dietary patterns and lifestyle variables of participants were collected using a self-reported questionnaire, which was completed when they first visited for the health examination. The questionnaire investigated alcohol consumption, betel nut chewing, cigarette smoking, amount of exercise, and type of diet (vegan, ovo-lacto vegetarian, or omnivore). An ovo-lacto vegetarian was defined as a person who consumes eggs or dairy products or both, but no other animal products; a vegan as one who only consumes plant-based foods; and an omnivore as one who consumes both plant- and animal-based foods. Regular exercise was defined exercise more than 3 times per week. Height and weight were measured by an automatic electronic meter (SECA GM-1000, Seoul, Korea), and body mass index (BMI, kg/m2) was calculated.
Proteinuria was determined using dipstick analysis with an automated urine analyzer (Arkray 4030, Tokyo, Japan). These results were graded as negative (<10 mg/dL), trace (±) (10–20 mg/dL), 1+ (30 mg/dL), 2+ (100 mg/dL), 3+ (300 mg/dL), or 4+ (1000 mg/dL). Patients with trace levels, 1+ levels, and above were defined as having proteinuria.
Statistical analysis
The characteristics of participants were analyzed using the Chi-square test for categorical variables and one-way analysis of variance for continuous variables. For categorical variables, if the observed values of each cell in the Chi-square test were <5, Fisher's exact test was used. A multivariate multiple linear regression model with no intercept was used to identify factors associated with the two dependent variables (systolic and diastolic BP), and the coefficient and standard error were calculated. The multivariate term refers to the two dependent variables (diastolic and systolic BPs) that are analyzed jointly. The factors considered in the multivariate analysis were age, sex, regular exercise, current smoking, alcohol drinking, betel nut chewing, DM, HTN, CVD, hyperlipidemia, BMI, and proteinuria. All statistical analyses were two-tailed, and P = 0.05 was considered statistically significant. SAS software version 9.4 (SAS, Cary, NC, USA) was used for the statistical analysis.
RESULTS
We examined the medical records of 36,617 individuals, 16,415 (44.8%) males and 20,202 (55.2%) females [Figure 1]. Table 1 shows the baseline characteristic of vegans (7.7%), lacto-ovo vegetarians (15.3%), and omnivores (77.0%). The vegan group was significantly older than the other groups (mean age of 64.1 years, 66.4% of participants older than 60 years). The prevalence of current smoking, alcohol drinking, and betel nut chewing was significantly lower in the vegan and lacto-ovo vegetarian groups than in the omnivore group (P < 0.001). The vegan group had a higher rate of regular exercise than the other two groups. The vegan and lacto-ovo vegetarian groups had higher percentages of DM and CVD than the omnivore group. The vegan group had a lower prevalence of HTN and proteinuria than the other groups (P < 0.001). The mean systolic and diastolic BP levels were 121 mmHg and 73.7 mmHg overall. Omnivores had a higher mean BMI (24 kg/m2) and a lower rate of regular exercise (P < 0.001). The proportion taking HTN medication showed no significant differences among the three dietary groups.
Figure 1.
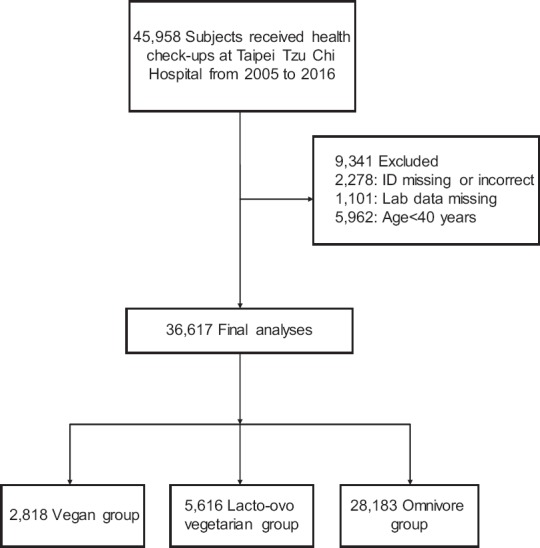
Participant selection process
Table 1.
Characteristics of individuals according to diet group
| Vegans | Lacto-ovo vegetarians | Omnivores | P | |
|---|---|---|---|---|
| N | 2818 | 5616 | 28183 | |
| Age, years (mean±SD) | 64.1±10.1 | 59.8±9.1 | 59.7±11.0 | <0.001 |
| Age, years (group), n (%) | ||||
| 40-49 | 196 (7.0) | 788 (14.0) | 5500 (19.5) | <0.001 |
| 50-59 | 751 (26.7) | 1981 (35.3) | 8800 (31.2) | |
| 60-69 | 1077 (38.2) | 2072 (36.9) | 8839 (31.4) | |
| ≥70 | 794 (28.2) | 775 (13.8) | 5044 (17.9) | |
| Sex, n (%) | ||||
| Male | 887 (31.5) | 1843 (32.8) | 13685 (48.6) | <0.001 |
| Female | 1931 (68.5) | 3773 (67.2) | 14498 (51.4) | |
| Current smoking n (%) | 3 (0.1) | 46 (0.8) | 1472 (5.2) | <0.001 |
| Alcohol drinking (%) | 125 (4.4) | 348 (6.2) | 7108 (25.2) | <0.001 |
| Betel chewing (%) | 0 (<0.1) | 0 (<0.1) | 77 (0.3) | <0.001 |
| Regular exercise (%) | 478 (17.0) | 667 (11.9) | 2702 (9.6) | <0.001 |
| Married (%) | 2540 (90.1) | 5238 (93.3) | 22037 (78.2) | <0.001 |
| Comorbidity, n (%) | ||||
| DM | 548 (19.4) | 1021 (18.2) | 4686 (16.6) | <0.001 |
| HTN | 622 (22.1) | 1340 (23.9) | 6482 (23.0) | <0.001 |
| HTN medication used | 513 (18.2) | 1027 (18.3) | 5177 (18.4) | 0.99 |
| Hyperlipidemia n (%) | 627 (22.2) | 1354 (24.1) | 6546 (23.2) | 0.16 |
| CVD n (%) | 580 (20.6) | 1081 (19.2) | 4777 (16.9) | 0.14 |
| Systolic BP (mmHg) (mean±SD) | 121 (17) | 120 (15) | 122 (16) | <0.001 |
| Diastolic BP (mmHg) (mean±SD) | 74 (12) | 72 (14) | 75 (12) | <0.001 |
| BMI (kg/m2), n (%) | ||||
| <25 | 2133 (75.7) | 4204 (74.9) | 18595 (66.0) | <0.001 |
| 25-29 | 603 (21.4) | 1227 (21.8) | 8122 (28.8) | |
| 30+ | 82 (2.9) | 185 (3.3) | 1466 (5.2) | |
| BMI (kg/m2) (mean±SD) | 23 (3.3) | 23.2 (3.3) | 24 (20.4) | <0.001 |
| Proteinuria, n (%) | ||||
| − | 2564 (91.0) | 4681 (83.4) | 25148 (89.2) | <0.001 |
| ±, 1+, and above n (%) | 254 (9.0) | 935 (16.6) | 3035 (10.8) |
Data are shown as n (%) or mean (SD). DM: Diabetes mellitus, HTN: Hypertension, CVD: Cardiovascular disease, BP: Blood pressure, BMI: Body mass index, SD: Standard deviation, - : negative (<10 mg/dL), trace (±) (10–20 mg/dL), 1+ (30 mg/dL), 2+ (100 mg/dL), 3+ (300 mg/dL), or 4+ (1000 mg/dL)
Analysis of these data by the multivariate linear regression model indicated that the vegan group had a significantly lower mean diastolic BP (−1.32 mmHg, P < 0.001) than the omnivore group [Table 2]. The lacto-ovo vegetarian group had both a lower mean systolic BP (−1.99 mmHg, P < 0.001) and diastolic BP (−3.18 mmHg, P < 0.001) than the omnivore group [Table 2]. In addition, participants with proteinuria had both a significantly higher mean systolic BP (2.85 mmHg, P < 0.001) and diastolic BP (1.28 mmHg, P < 0.001) [Table 2]. The multivariate multiple linear regression model with no intercept indicated that age had a strong effect on both systolic BP (1.66 mmHg, P < 0.001) and diastolic BP (0.99 mmHg, P < 0.001) [Table 3]. Systolic and diastolic BP was also significantly greater in males, current smokers, consumers of alcohol, and especially betel nut chewers (P < 0.001 for all comparisons) [Table 3].
Table 2.
Influence of different factors on systolic and diastolic blood pressure determined by a multivariate linear regression modela
| Systolic BP (mmHg) | Diastolic BP (mmHg) | |||||
|---|---|---|---|---|---|---|
| Coefficient | SE | P | Coefficient | SE | P | |
| Age | 0.47 | 0.008 | <0.001*** | 0.13 | 0.006 | <0.001*** |
| Male versus female | 4.78 | 0.17 | <0.001*** | 6.07 | 0.125 | <0.001*** |
| Current smoking | −0.65 | 0.427 | 0.13 | 1.74 | 0.321 | <0.001*** |
| Alcohol drinking | −0.55 | 0.21 | 0.008** | 1.21 | 0.158 | <0.001*** |
| Betel chewing | 8.80 | 1.86 | <0.001*** | 8.21 | 1.397 | <0.001*** |
| Regular exercise | 4.75 | 0.277 | <0.001*** | 0.75 | 0.209 | <0.001*** |
| Marriage | −3.26 | 0.219 | <0.001*** | −1.97 | 0.164 | <0.001*** |
| Comorbidity | ||||||
| DM | 4.58 | 0.225 | <0.001*** | 0.69 | 0.17 | <0.001*** |
| HTN | 12.40 | 0.192 | <0.001*** | 6.52 | 0.148 | <0.001*** |
| Hyperlipidemia | 12.31 | 0.191 | <0.001*** | 6.49 | 0.148 | <0.001*** |
| CVD | 4.22 | 0.223 | <0.001*** | 0.49 | 0.168 | 0.004** |
| BMI (kg/m2) | 0.05 | 0.005 | <0.001*** | 0.03 | 0.004 | <0.001*** |
| Proteinuria | 2.85 | 0.266 | <0.001*** | 1.28 | 0.2 | <0.001*** |
| Vegan versus omnivores | −0.14 | 0.322 | 0.67 | −1.32 | 0.241 | <0.001*** |
| Lacto-ovo vegetarian versus omnivores | −1.99 | 0.238 | <0.001*** | −3.18 | 0.178 | <0.001*** |
aAdjusted for age, gender, smoking, alcohol drinking, betel nut chewing, exercise, marital status, HTN, diabetes, CVD, hyperlipidemia, BMI, and proteinuria. DM: Diabetes mellitus, HTN: Hypertension, CVD: Cardiovascular disease, BP: Blood pressure, BMI: Body mass index, SE: Standard error, **P <0.01, ***P <0.001
Table 3.
Influence of different factors on systolic and diastolic blood pressure determined by a multivariate multiple linear regression model with no intercepta
| Systolic BP (mmHg) | Diastolic BP (mmHg) | |||||
|---|---|---|---|---|---|---|
| Coefficient | SE | P | Coefficient | SE | P | |
| Age | 1.66 | 0.004 | <0.001*** | 0.99 | 0.003 | <0.001*** |
| Male versus female | 6.08 | 0.223 | <0.001*** | 7.06 | 0.166 | <0.001*** |
| Current smoking | 7.05 | 0.542 | <0.001*** | 4.76 | 0.403 | <0.001*** |
| Alcohol drinking | 1.22 | 0.279 | <0.001*** | 0.90 | 0.208 | <0.001*** |
| Betel chewing | 18.07 | 2.306 | <0.001*** | 11.97 | 1.716 | <0.001*** |
| Regular exercise | −8.73 | 0.347 | <0.001*** | −7.07 | 0.258 | <0.001*** |
| Marriage | 16.72 | 0.255 | <0.001*** | 11.02 | 0.190 | <0.001*** |
| Comorbidity | ||||||
| DM | −3.70 | 0.684 | <0.001*** | −3.42 | 0.509 | <0.001*** |
| HTN | −2.94 | 1.781 | 0.10 | −2.30 | 1.326 | 0.08 |
| Hyperlipidemia | −1.90 | 0.675 | 0.005** | −1.73 | 0.502 | 0.001** |
| CVD | 6.11 | 1.774 | 0.001** | 3.81 | 1.320 | 0.004** |
| BMI (kg/m2) | 0.17 | 0.006 | <0.001*** | 0.11 | 0.004 | <0.001*** |
| Proteinuria | 4.26 | 0.386 | <0.001*** | 2.15 | 0.287 | <0.001*** |
| Vegan versus omnivores | −3.87 | 0.423 | <0.001*** | −2.48 | 0.315 | <0.001*** |
| Lacto-ovo vegetarian versus. omnivores | −0.34 | 0.327 | 0.30 | −1.58 | 0.243 | <0.001*** |
aAdjusted for age, gender, smoking, alcohol drinking, betel nut chewing, exercise, marital status, HTN, diabetes, CVD, hyperlipidemia, BMI, and proteinuria. DM: Diabetes mellitus, HTN: Hypertension, CVD: Cardiovascular disease, BP: Blood pressure, BMI: Body mass index, SE: Standard error, **P <0.01, ***P <0.001
The vegan group had a significantly lower mean systolic BP (−3.87 mmHg, P < 0.001) and diastolic BP (−2.48 mmHg, P < 0.001) than the omnivore group [Table 3]. The lacto-ovo vegetarian group had a lower mean diastolic BP (−1.58 mmHg, P < 0.001) than the omnivore group, but these groups had a similar systolic BP [Table 3]. Participants with proteinuria had a higher mean systolic BP (4.26 mmHg, P < 0.001) and diastolic BP (2.15 mmHg, P < 0.001) than those without proteinuria [Table 3]. Parameter estimates indicated that vegans with proteinuria had a lower mean systolic BP (−2.73 mmHg, P = 0.046) and diastolic BP (−2.54 mmHg, P = 0.013) than other groups with proteinuria [Table 4]. However, individuals in the lacto-ovo vegetarian group with proteinuria had systolic and diastolic BPs similar to other groups with proteinuria [Table 4].
Table 4.
Parameter estimates (β coefficient, 95% confidence interval) relating blood pressure and dietary pattern by proteinuria statusa
| Coefficient (95% CI) | ||||
|---|---|---|---|---|
| Systolic BP | Diastolic BP | |||
| No proteinuria | All | No proteinuria | All | |
| Vegan versus omnivores | −4.17 (−5.00-−3.35)*** | −3.87 (−4.70-−3.04)*** | −2.71 (−3.32-−2.11)*** | −2.48 (−3.09-−1.86)*** |
| Lacto-ovo vegetarian versus. omnivores | −0.61 (−1.25-0.03)*** | −0.34 (−0.98-0.30) | −1.78 (−2.24-−1.31)*** | −1.58 (−2.05-−1.10)*** |
| Proteinuria | No entries | 4.26 (3.50-5.02)*** | No entries | 2.15 (1.58-2.71)*** |
| Vegan with proteinuria | No entries | −2.73 (−5.41-−0.05)* | No entries | −2.54 (−4.53-−0.54)* |
| Lacto-ovo vegetarian with proteinuria | No entries | −0.90 (−2.50-0.69) | No entries | 0.65 (−0.53-1.84) |
*P <0.05, ***P <0.001. aAdjusted for age, gender, smoking, alcohol drinking, betel nut chewing, exercise, marital status, hypertension, diabetes, CVD, hyperlipidemia, and BMI. BP: Blood pressure, BMI: Body mass index, CVD: Cardiovascular disease, CI: Confidence interval
DISCUSSION
Previous studies investigated the relationship between diet and BP reported that vegetarians had lower BPs than omnivores [13,14,15,16]. However, these studies had relatively small number of patients. To the best of our knowledge, the present study is the largest cross-sectional study comparing systolic and diastolic BP in vegans, lacto-ovo vegetarians, and omnivores. Our hospital-based study is also the first study to examine the influence of diet on BP in asymptomatic CKD patients with proteinuria.
Beilin and Burke [17] reported that a diet with a low level of saturated fat, a high potassium/phosphorus ratio, and a high level of vegetable fiber, as in vegetarian diets, was associated with a clear reduction in BP. It is also possible that a vegetarian diet is associated with lower BP because this diet modulates baroreceptor sensitivity, leads to vasodilation, alters the catecholamine and renin–angiotensin system, and reduces blood viscosity [18]. Some studies also reported that BP declines following a change from a meat-based diet to a vegetarian diet [19,20]. Further studies are needed to identify the specific reasons why a vegetarian diet is associated with lower BP.
A 2014 meta-analysis of 39 studies (21,915 participants) concluded that a vegetarian diet was associated with a significantly lower mean systolic BP (−5.9 mmHg) and diastolic BP (−3.5 mmHg) than an omnivorous diet [14]. Similarly, our vegan group had a lower mean systolic BP (−3.87 mmHg, P < 0.001) and diastolic BP (−2.48 mmHg, P < 0.001) relative to the omnivore group [Table 3]. In our lacto-ovo vegetarian group, only the diastolic BP was significantly lower (−1.58 mmHg, P < 0.001) relative to the omnivore group [Table 3]. Some reviews reported a significantly lower prevalence of HTN in vegans relative to nonvegetarians and those with other vegetarian diet patterns [21,22,23]. However, few studies have examined the benefits of strict vegan diets.
We also found that diet had a significant effect on the prevalence of HTN. HTN was present in 22.1% of the vegans, 23.9% of the lacto-ovo vegetarians, and 23.0% of the ominovores [Table 1]. There is abundant evidence that HTN is a leading risk factor for CKD, and that poor BP control contributes to the deterioration of renal function [6,24,25]. Proteinuria is a marker of early-stage CKD [7,8,26], so screening of asymptomatic individuals for proteinuria and strict BP control is very important to prevent progression of CKD [27].
The participants in our study who had proteinuria also had significantly higher systolic and diastolic BP, but those in the vegan group with proteinuria had a lower systolic BP (−2.73 mmHg, P = 0.046) and diastolic BP (−2.54 mmHg, P = 0.013) than other groups with proteinuria [Table 4]. Some small studies reported that a plant-based diet can delay the progression of CKD, protect the renal endothelium, and decrease proteinuria [28,29]. Moreover, studies of CKD patients indicate that high intake of dietary fiber slows the decrease in the glomerular filtration rate and reduces inflammatory status [30,31].
The present study is the largest to date to investigate the association between diet and BP. Most participants were volunteers from the Buddhist Tzu Chi Foundation, suggesting they had stable dietary habits and accurately reported their diets. Moreover, we measured BP using an automatic electronic meter, which provides valid measurements of BP. Another novelty of our study is that it is the first to examine the effect of a vegan diet on BP in individuals with asymptomatic proteinuria. Some limitations of this study must also be acknowledged. First, selection bias was possible, in that participants motivated to have a health checkup may not be representative of the general population. Second, we had no information on portion size, a number of calories consumed, nutrient composition of food, or duration of dietary regimens in our participants. Third, this study had a cross-sectional design, so causality could not be established because of the temporal relationship between a vegan diet and BP is unclear. Nevertheless, this is the first study to show an association between BP and diet patterns in participants with proteinuria.
CONCLUSIONS
Our study demonstrated that a vegan diet was significantly associated with lower systolic and diastolic BP compared with a nonvegetarian diet. We observed the same benefit of a vegan diet in analysis of asymptomatic CKD patients who had proteinuria. Thus, a vegan diet could be an effective nonpharmacologic approach to reduce BP. If the association of a vegan diet with the outcomes observed here are indeed causal, this may be an important approach to slow the progression of CKD.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Lawes CM, Vander Hoorn S, Rodgers A. International Society of Hypertension. Global burden of blood-pressure-related disease, 2001. Lancet. 2008;371:1513–8. doi: 10.1016/S0140-6736(08)60655-8. [DOI] [PubMed] [Google Scholar]
- 2.Ezzati M, Riboli E. Behavioral and dietary risk factors for noncommunicable diseases. N Engl J Med. 2013;369:954–64. doi: 10.1056/NEJMra1203528. [DOI] [PubMed] [Google Scholar]
- 3.Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: A systematic analysis for the global burden of disease study 2010. Lancet. 2012;380:2224–60. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Su TC, Bai CH, Chang HY, You SL, Chien KL, Chen MF, et al. Evidence for improved control of hypertension in Taiwan: 1993-2002. J Hypertens. 2008;26:600–6. doi: 10.1097/HJH.0b013e3282f3b352. [DOI] [PubMed] [Google Scholar]
- 5.Chien KL, Hsu HC, Sung FC, Su TC, Chen MF, Lee YT, et al. Incidence of hypertension and risk of cardiovascular events among ethnic Chinese: Report from a community-based cohort study in Taiwan. J Hypertens. 2007;25:1355–61. doi: 10.1097/HJH.0b013e3280d94313. [DOI] [PubMed] [Google Scholar]
- 6.Klag MJ, Whelton PK, Randall BL, Neaton JD, Brancati FL, Ford CE, et al. Blood pressure and end-stage renal disease in men. N Engl J Med. 1996;334:13–8. doi: 10.1056/NEJM199601043340103. [DOI] [PubMed] [Google Scholar]
- 7.Iseki K, Ikemiya Y, Iseki C, Takishita S. Proteinuria and the risk of developing end-stage renal disease. Kidney Int. 2003;63:1468–74. doi: 10.1046/j.1523-1755.2003.00868.x. [DOI] [PubMed] [Google Scholar]
- 8.de Jong PE, Curhan GC. Screening, monitoring, and treatment of albuminuria: Public health perspectives. J Am Soc Nephrol. 2006;17:2120–6. doi: 10.1681/ASN.2006010097. [DOI] [PubMed] [Google Scholar]
- 9.Chiang CE, Wang TD, Ueng KC, Lin TH, Yeh HI, Chen CY, et al. 2015 guidelines of the Taiwan society of cardiology and the Taiwan hypertension society for the management of hypertension. J Chin Med Assoc. 2015;78:1–47. doi: 10.1016/j.jcma.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 10.Peterson JC, Adler S, Burkart JM, Greene T, Hebert LA, Hunsicker LG, et al. Blood pressure control, proteinuria, and the progression of renal disease. The modification of diet in renal disease study. Ann Intern Med. 1995;123:754–62. doi: 10.7326/0003-4819-123-10-199511150-00003. [DOI] [PubMed] [Google Scholar]
- 11.Huang CJ, Fan YC, Liu JF, Tsai PS. Characteristics and nutrient intake of Taiwanese elderly vegetarians: Evidence from a national survey. Br J Nutr. 2011;106:451–60. doi: 10.1017/S0007114511000195. [DOI] [PubMed] [Google Scholar]
- 12.Margetts BM, Beilin LJ, Vandongen R, Armstrong BK. Vegetarian diet in mild hypertension: A randomised controlled trial. Br Med J (Clin Res Ed) 1986;293:1468–71. doi: 10.1136/bmj.293.6560.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Appleby PN, Davey GK, Key TJ. Hypertension and blood pressure among meat eaters, fish eaters, vegetarians and vegans in EPIC-Oxford. Public Health Nutr. 2002;5:645–54. doi: 10.1079/PHN2002332. [DOI] [PubMed] [Google Scholar]
- 14.Yokoyama Y, Nishimura K, Barnard ND, Takegami M, Watanabe M, Sekikawa A, et al. Vegetarian diets and blood pressure: A meta-analysis. JAMA Intern Med. 2014;174:577–87. doi: 10.1001/jamainternmed.2013.14547. [DOI] [PubMed] [Google Scholar]
- 15.Garbett T, Garbett D, Wendorf A. Vegetarian diet: A prescription for high blood pressure?. A systematic review of the literature. J Nurs Pract. 2016;12:452–8.e6. [Google Scholar]
- 16.Agrawal R, Chaturvedi M, Singh S, Gupta SC. An epidemiological study of dietary and exercise habits as correlates of hypertension in persons aged 45 years and above in Agra District. Indian J Community Health. 2012;24:91–6. [Google Scholar]
- 17.Beilin LJ, Burke V. Vegetarian diet components, protein and blood pressure: Which nutrients are important? Clin Exp Pharmacol Physiol. 1995;22:195–8. doi: 10.1111/j.1440-1681.1995.tb01979.x. [DOI] [PubMed] [Google Scholar]
- 18.Ernst E, Pietsch L, Matrai A, Eisenberg J. Blood rheology in vegetarians. Br J Nutr. 1986;56:555–60. doi: 10.1079/bjn19860136. [DOI] [PubMed] [Google Scholar]
- 19.Scialla JJ, Anderson CA. Dietary acid load: A novel nutritional target in chronic kidney disease? Adv Chronic Kidney Dis. 2013;20:141–9. doi: 10.1053/j.ackd.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goraya N, Simoni J, Jo C, Wesson DE. Dietary acid reduction with fruits and vegetables or bicarbonate attenuates kidney injury in patients with a moderately reduced glomerular filtration rate due to hypertensive nephropathy. Kidney Int. 2012;81:86–93. doi: 10.1038/ki.2011.313. [DOI] [PubMed] [Google Scholar]
- 21.Woolf KJ, Bisognano JD. Nondrug interventions for treatment of hypertension. J Clin Hypertens (Greenwich) 2011;13:829–35. doi: 10.1111/j.1751-7176.2011.00524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berkow SE, Barnard ND. Blood pressure regulation and vegetarian diets. Nutr Rev. 2005;63:1–8. doi: 10.1111/j.1753-4887.2005.tb00104.x. [DOI] [PubMed] [Google Scholar]
- 23.Nguyen H, Odelola O, Rangaswami J, Amanullah A. A review of nutritional factors in hypertension management. Int J Hypertens. 2013;2013:698940. doi: 10.1155/2013/698940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Färbom P, Wahlstrand B, Almgren P, Skrtic S, Lanke J, Weiss L, et al. Interaction between renal function and microalbuminuria for cardiovascular risk in hypertension: The Nordic Diltiazem study. Hypertension. 2008;52:115–22. doi: 10.1161/HYPERTENSIONAHA.107.109264. [DOI] [PubMed] [Google Scholar]
- 25.Levey AS, Atkins R, Coresh J, Cohen EP, Collins AJ, Eckardt KU, et al. Chronic kidney disease as a global public health problem: Approaches and initiatives – A position statement from kidney disease improving global outcomes. Kidney Int. 2007;72:247–59. doi: 10.1038/sj.ki.5002343. [DOI] [PubMed] [Google Scholar]
- 26.Levin A, Hemmelgarn B, Culleton B, Tobe S, McFarlane P, Ruzicka M, et al. Guidelines for the management of chronic kidney disease. CMAJ. 2008;179:1154–62. doi: 10.1503/cmaj.080351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vassalotti JA, Fox CH, Becker BN. Risk factors and screening for chronic kidney disease. Adv Chronic Kidney Dis. 2010;17:237–45. doi: 10.1053/j.ackd.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 28.Cupisti A, Ghiadoni L, D’Alessandro C, Kardasz I, Morelli E, Panichi V, et al. Soy protein diet improves endothelial dysfunction in renal transplant patients. Nephrol Dial Transplant. 2007;22:229–34. doi: 10.1093/ndt/gfl553. [DOI] [PubMed] [Google Scholar]
- 29.D’Amico G, Gentile MG, Manna G, Fellin G, Ciceri R, Cofano F, et al. Effect of vegetarian soy diet on hyperlipidaemia in nephrotic syndrome. Lancet. 1992;339:1131–4. doi: 10.1016/0140-6736(92)90731-h. [DOI] [PubMed] [Google Scholar]
- 30.Xu H, Huang X, Risérus U, Krishnamurthy VM, Cederholm T, Arnlöv J, et al. Dietary fiber, kidney function, inflammation, and mortality risk. Clin J Am Soc Nephrol. 2014;9:2104–10. doi: 10.2215/CJN.02260314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu L, Huang YF, Wang MQ, Chen DX, Wan H, Wei LB, et al. Dietary fiber intake is associated with chronic kidney disease (CKD) progression and cardiovascular risk, but not protein nutritional status, in adults with CKD. Asia Pac J Clin Nutr. 2017;26:598–605. doi: 10.6133/apjcn.072016.08. [DOI] [PubMed] [Google Scholar]


