Abstract
A copper-mediated synthesis of diaryl sulfides utilizing Cu(I)-thiophene-2-carboxylate (CuTC) is described. We demonstrate the use of CuTC as a soluble, non-basic catalyst in the coupling of aryl iodides and aryl thiols in the synthesis of synthetically advanced diaryl sulfides. This method allows for the successful coupling of challenging substrates including ortho-substituted and heteroaryl iodides and thiols. Additionally, most of the aryl iodide substrates used here contain the privileged piperazine scaffold bound to a pyrimidine, pyridine, or phenyl ring and thus this method allows for the elaboration of complex piperazine scaffolds into molecules of biological interest. The method described here enables the incorporation of late-stage structural diversity into diaryl sulfides containing the piperazine ring, thus enhancing the number and nature of derivatives available for SAR investigation.
Keywords: diarylsulfide, thioether, piperazine, CuTC
Graphical Abstract
The synthesis of aryl sulfides has been a major focus in organic chemistry in recent years because of their presence as intermediates in numerous biologically and pharmaceutically active molecules as well as in organic materials.1 A number of drugs in such diverse therapeutic areas as cancer, Alzheimer’s and Parkinson’s disease, diabetes, inflammation, immune and infectious diseases contain the aryl sulfide moiety.1d, e Since Migita and co-workers reported the first Pd-catalyzed cross-coupling reaction of aryl iodides and bromides with thiols,2 numerous reports have described transition-metal mediated synthesis of C(aryl)-S bonds through the coupling of thiols with aryl halides (iodides, bromides, chlorides) or pseudohalides (triflates). In addition to Pd,3 other metals including Cu,1c, 4 Ni,5 Co,6 and Fe7 have been used to effect this transformation. A number of useful protocols exist for each of these metals, typically in combination with a chelating ligand and a base. Of these, Cu-based methods offer some distinct advantages including functional simplicity, high substrate tolerance and low cost.8 Numerous copper-based procedures for the coupling of thiols with aryl halides are known including CuI/neocuproine,4b CuI/ethylene glycol,4c CuI/N-methylglycine or N,N-dimethylglycine,4e CuI/cis-1,2-cyclohexanediol.9 Additionally, ligand-free procedures are known utilizing CuO nanoparticles4a, 10 or CuI in NMP.11
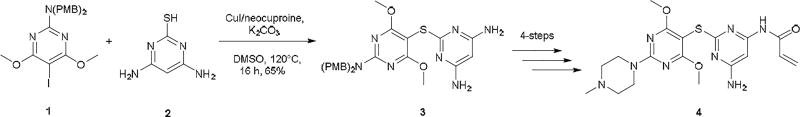
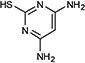
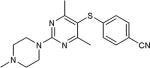
Our interest in this chemistry originates from efforts directed towards the synthesis of purine-scaffold heat shock protein 90 (Hsp90) inhibitors, whereby the key step was a C(aryl)-S(aryl) transformation.12 CuI/neocuproine catalyzed coupling of 8-mercaptoadenine to aryl iodides enabled the efficient creation of a library of 8-arylsulfanyl adenine derivatives as intermediates for Hsp90 structure activity relationship (SAR) studies.12b, c More recently, we have been engaged in the discovery of heat shock protein 70 (Hsp70) inhibitors and have reported a class of 2,5’-thiodipyrimidine and 5-(phenylthio)pyrimidine inhibitors.13 As before, the key step in the synthesis of these molecules involved the formation of a C(aryl)-S(aryl) bond. The coupling of p-methoxybenzyl (PMB)-protected 2-amino-5-iodopyrimidine 1 with thiol 2 was successfully accomplished with 20 mol% CuI/20 mol% neocuproine and K2CO3 after heating at 120 °C in DMSO for 16 h to yield disulfide 3 in 65% yield (Scheme 1). 3 was a key intermediate in SAR efforts which resulted in compound 4, a potent allosteric inhibitor of Hsp70 (Scheme 1). It also led to the identification of N-methylpiperazine as a favorable moiety in this series of compounds. However, attempts to couple 2-N-methylpiperazine-5-iodopyrimidines with a variety of thiols under the same conditions were not as successful. Generally, the reaction could be made to proceed but required excess CuI and neocuproine (≥ 30 mol%). Also, due to the basic nature of both desired product and neocuproine, as well as the increased amount of CuI, we found it difficult to purify the resulting product. Based on these results, we surmised that the reaction was hampered by chelation of copper by the N-methylpiperazine moiety. We also attempted this with little success using some established protocols including CuI/ethylene glycol,4c CuI/cis-1,2-cyclohexanediol,9 and Pd2(dba)3/Xantphos.3b As a result, we sought an alternative method for coupling 2-N-methylpiperazine-5-iodopyrimidines to aryl thiols.
Scheme 1.
CuI/neocuproine catalyzed coupling of PMB-protected 2-amino-5-iodopyrimidine 1 with thiol 2 to give 3 and conversion to the Hsp70 allosteric inhibitor 4.
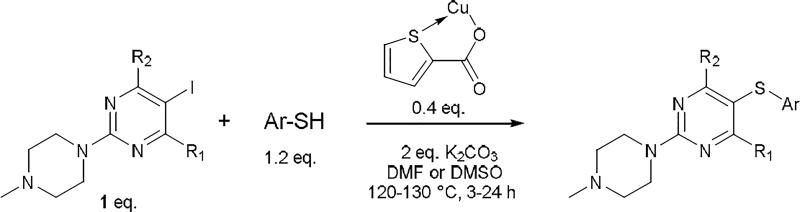
Since ligands function by chelating and solubilizing the metal, we hypothesized that more soluble copper sources such as copper(I) acetate (CuAc) or copper(I)-thiophene-2-carboxylate (CuTC) would yield better results. In an initial experiment evaluating these two as possible catalysts in the synthesis of 5 we found that CuTC was superior to CuAc in promoting the reaction. Whereas the reaction was incomplete with 1 eq. CuAc, the use of 0.3 eq. CuTC resulted in complete conversion to 5 in 74% yield (Table 1). CuTC represents an electron rich and soluble copper source that has previously been used in diverse organic transformations. For instance, it has been used in Stille cross-coupling of aryl-, heteroaryl- and alkenylstannanes with alkenyl and aryl iodides,14 to prepare thioethers from the reaction of boronic acids with N-thioimides,4g in Ullmann-like reductive coupling of substituted aromatic iodides and bromides, 2-iodoheteroaromatics, and alkenyl iodides,15 synthesis of ketones by coupling of thiol esters and boronic acids,16 and synthesis of 1-sulfonyl-1,2,3-triazoles from in situ generated copper(I) acetylides and sulfonyl azides.17 CuTC is a stable, dry, free flowing powder that is commercially available from a number of vendors.
Table 1.
CuTC promoted coupling of 4-substituted 2-N-methylpiperazine-5-iodopyrimidines with arylthiols.
| ||||
|---|---|---|---|---|
|
| ||||
| entry | R | HS-Ar | product | % yield |
| 5 | OMe |
|
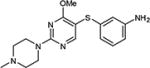
|
74a |
| 6 | OMe |
|
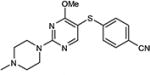
|
76 |
| 7 | OMe |
|

|
68 |
| 8 | OMe |
|
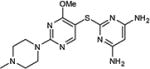
|
73 |
| 9 | OBn |
|
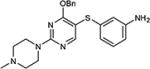
|
79 |
| 10 | OBn |
|
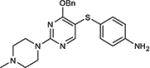
|
75 |
| 11 | OBn |
|
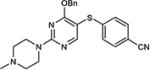
|
82 |
| 12 | OBn |
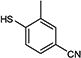
|
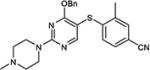
|
76 |
| 13 | OBn |
|
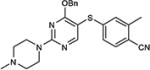
|
78 |
| 14 | OBn |
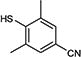
|
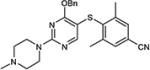
|
82 |
| 15 | OBn |
|
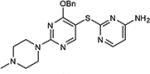
|
92 |
| 16 | OBn |
|
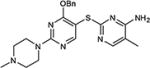
|
64 |
| 17 | OBn |
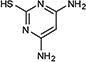
|
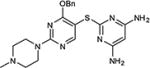
|
76 |
0.3 eq. CuTC used.
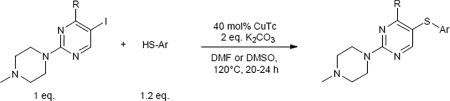
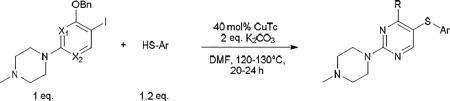
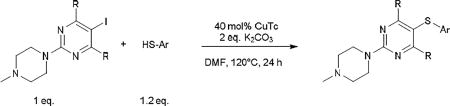
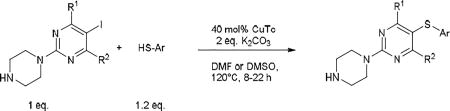
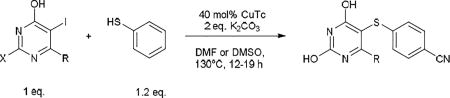
The standard protocol we developed to ensure complete reaction consisted of heating a mixture of aryl iodide (1 eq.), aryl thiol (1.2 eq.), CuTC (0.4 eq.) and K2CO3 (2 eq.) at 120–130 °C in DMF or DMSO for 3–24 h (Scheme 2).18 DMSO was used as solvent only in cases of thiopyrimidines which displayed limited solubility in DMF (see entry 8, 15–17, 24, 27). It should be noted that conditions were not optimized for reaction time. Using this general method, we were able to synthesize a variety of diaryl sulfides in good to moderate yields (Table 1–5). The scope of aryl iodide explored here include 2-N-methylpiperazine-5-iodopyrimidines (Table 1–2), 2-piperazine-5-iodopyrimidines (Table 3), 5-iodouracils (Table 4), 2-N-methylpiperazine-5-iodopyridines and 2-N-methylpiperazine-5-iodophenyls (Table 5). These were coupled to a variety of aryl thiols including substituted benzene, pyrimidine and pyridine.
Scheme 2.
Standard protocol for the CuTC-promoted coupling of aryl iodide with arylthiol.
Table 5.
CuTC promoted coupling of 4-benzyl 2-N-methylpiperazine-5-iodopyridine or -phenyl with arylthiols.
| ||||
|---|---|---|---|---|
|
| ||||
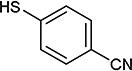
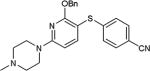
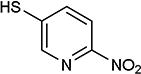
| entry | X1, X2 | HS-Ar | product | % yield |
| 28 | N, C |
|
|
85 |
| 29 | N, C |
|
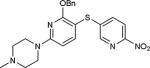
|
74 |
| 30 | N, C |
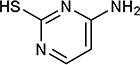
|
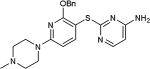
|
77 |
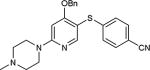
| 31 | C, N |
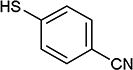
|
|
82 |
| 32 | C, C |
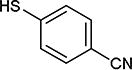
|
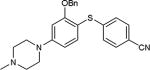
|
82 |
| 33 | C, C |
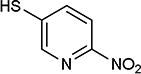
|
|
47 |
Table 2.
CuTC promoted coupling of 4,6-disubstitued 2-N-methylpiperazine-5-iodopyrimidines with arylthiols.
| ||||
|---|---|---|---|---|
|
| ||||
| entry | R | HS-Ar | product | % yield |
| 18 | OMe |
|
|
71 |
| 19 | OMe |
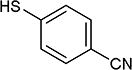
|
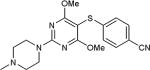
|
72 |
| 20 | Me |
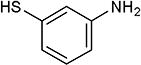
|
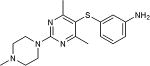
|
65 |
| 21 | Me |
|
|
83 |
Table 3.
CuTC promoted coupling of 2-piperazine-5-iodopyrimidines with arylthiols.
| ||||
|---|---|---|---|---|
|
| ||||
| entry | R1, R2 | HS-Ar | product | % yield |
| 22 | OBn, H |
|
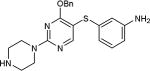
|
73 |
| 23 | OMe, OMe |
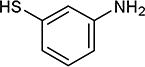
|
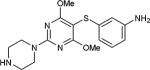
|
47 |
| 24 | OMe, OMe |
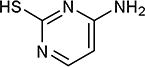
|
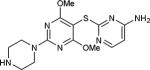
|
54 |
Table 4.
CuTC promoted coupling of 5-iodouracils with arylthiols.
| ||||
|---|---|---|---|---|
|
| ||||
| entry | X | R | product | % yield |
| 25 | OH | H |
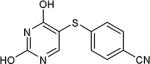
|
66 |
| 26 | OH | CF3 |
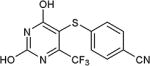
|
86 |
| 27 | NH2 | H |
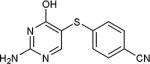
|
46 |
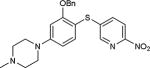
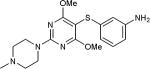
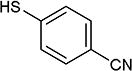
5-Iodopyrimidines containing methoxy (5–8) or benzyloxy (9–17) substituted at 4-position were efficiently coupled to a variety of mercaptobenzenes and mercaptopyrimidines in yields ranging from 64–92% (Table 1). A range of thiols were used and remarkably the reaction was not adversely affected by ortho-substitution. For example, comparison of entry 11 (82%), 12 (76%), and 14 (82%) shows no decrease in yield upon substitution of 4-mercaptobenzonitrile with one or two methyl groups in the ortho-position. This reaction was also tolerant of steric hindrance on the iodopyrimidine as the 4,6-dimethoxy (18–19) and 4,6-dimethyl (20–21) derivatives could also be prepared in yields ranging from 65–83% (Table 2). We were also able to successfully synthesize piperazine derivatives 22–24 (Table 3) in 47–73% yield.
As further example of the utility of this method we were able to couple 5-iodouracil and 5-iodo-4-trifluoromethyluracil to 4-mercaptobenzonitrile to give 25 and 26 in 66 and 86% yield, respectively (Table 4). We were also able to couple 2-amino-5-iodopyrimidin-4-ol to 4-mercaptobenzonitrile to give 27 in 46% yield. Finally, we wanted to investigate the effect of changing the pyrimidine group of the aryl iodide to pyridine or phenyl (Table 5). We found that the reaction was still successful with pyridine [either when X1=N, X2=C (entry 28–30) or X1=C, X2=N (entry 31)] or phenyl (entry 32–33) iodides. In the case of pyridine, the yields ranged from 74 to 85% (entry 28–31) while in the case for phenyl the yields were 47 to 82% (entry 32–33). Direct comparison of coupling with 4-mercaptobenzonitrile shows similar yields with pyrimidine 11 (82%), pyridine 28 (X1=N, X2=C; 85%), pyridine 31 (X1=C, X2=N; 82%) and phenyl 32 (82%), highlighting the broad substrate scope that can be successfully coupled in good yield using this method.
In summary, we report the synthesis of synthetically advanced diaryl sulfides through CuTC-mediated coupling of aryl iodides and aryl thiols. The couplings shown here include ortho-substituted and heteroaryl substrates, which are generally known to pose significant challenges.8 Additionally, most of the aryl iodides exemplified here contain a piperazine ring which is significant because it is considered a privileged scaffold and as such is a common motif in medicinal chemistry.19 The piperazine group can positively impact drug-like characteristics of molecules and enhance pharmacokinetic properties by improving water solubility and metabolic stability. The method described here allows for the ability to incorporate late-stage structural diversity into diaryl sulfides containing the piperazine ring and thus enhancing the diversity of derivatives available for SAR investigation. Therefore, we believe this method may be of great value and have broad appeal to medicinal chemists and other researchers engaged in SAR investigations.
Supplementary Material
Highlights.
-
-
A novel copper-mediated synthesis of synthetically advanced diaryl sulfides utilizing CuTC
-
-
CuTC is a soluble, non-basic catalyst used to couple aryl iodides and aryl thiols to diaryl sulfides
-
-
Coupling of challenging substrates including ortho-substituted and heteroaryl iodides and thiols
-
-
Incorporation of late-stage structural diversity into diaryl sulfides containing the piperazine ring
Acknowledgments
This work was supported in part by the NIH (R01 CA172546, R01 CA155226, P01 CA186866, P30 CA008748, P50 CA86438), and by Mr. William H. and Mrs. Alice Goodwin and the Commonwealth Foundation for Cancer Research and the Experimental Therapeutics Center of MSKCC. We also thank Dr. George Sukenick and Rong Wang of the NMR Analytical Core Facility at MSKCC for expert mass spectral analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.(a) Procter DJ. J. Chem. Soc., Perkin Trans. 2000;1:835–871. [Google Scholar]; (b) Procter DJ. J. Chem. Soc., Perkin Trans. 2001;1:335–354. [Google Scholar]; (c) Herradura PS, Pendola KA, Guy RK. Org. Lett. 2000;2:2019–2022. doi: 10.1021/ol005832g. [DOI] [PubMed] [Google Scholar]; (d) Liu G, Link JT, Pei Z, Reilly EB, Leitza S, Nguyen B, Marsh KC, Okasinski GF, von Geldern TW, Ormes M, Fowler K, Gallatin M. J. Med. Chem. 2000;43:4025–4040. doi: 10.1021/jm0002782. [DOI] [PubMed] [Google Scholar]; (e) Liu G, Huth JR, Olejniczak ET, Mendoza F, Fesik SW, Von Genldern TW. J. Med. Chem. 2001;44:1202–1210. doi: 10.1021/jm000503f. [DOI] [PubMed] [Google Scholar]; (f) Wang Y, Chackalamannil S, Hu Z, Clader JW, Greenlee W, Billard W, Binch H, Crosby G, Ruperto V, Duffy RA, McQuade R, Lachowicz JE. Bioorg. Med. Chem. Lett. 2000;10:2247–2250. doi: 10.1016/s0960-894x(00)00457-1. [DOI] [PubMed] [Google Scholar]; (g) Mori T, Nishimura T, Yamamoto T, Doi I, Miyazaki E, Osaka I, Takimiya K. J. Am. Chem. Soc. 2013;135:13900–13913. doi: 10.1021/ja406257u. [DOI] [PubMed] [Google Scholar]; (h) Takimiya K, Shinamura S, Osaka I, Miyazaki E. Adv. Mater. 2011;23:4347–4370. doi: 10.1002/adma.201102007. [DOI] [PubMed] [Google Scholar]; (i) Okamoto T, Mitsui C, Yamagishi M, Nakahara K, Soeda J, Hirose Y, Miwa K, Sato H, Yamano A, Matsushita T, Uemura T, Takeya J. Adv. Mater. 2013;25:6392–6397. doi: 10.1002/adma.201302086. [DOI] [PubMed] [Google Scholar]
- 2.(a) Kosugi M, Shimizu T, Migita T. Chem. Lett. 1978:13–14. [Google Scholar]; (b) Migita T, Shimizu T, Asami Y, Shiobara J, Kato Y, Kosugi M. Bull. Chem. Soc. Jpn. 1980;53:1385–1389. [Google Scholar]
- 3.(a) Mispelaere-Canivet C, Spindler J-F, Perrio S, Beslin P. Tetrahedron. 2005;61:5253–5259. [Google Scholar]; (b) Itoh T, Mase T. Org. Lett. 2004;6:4587–4590. doi: 10.1021/ol047996t. [DOI] [PubMed] [Google Scholar]; (c) Fernandez-Rodrıguez MA, Shen Q, Hartwig JF. J. Am. Chem. Soc. 2006;128:2180–2181. doi: 10.1021/ja0580340. [DOI] [PubMed] [Google Scholar]; (d) Murata M, Buchwald SL. Tetrahedron. 2004;60:7397–7403. [Google Scholar]; (e) Schopfer U, Schlapbach A. Tetrahedron. 2001;57:3069–3073. [Google Scholar]; (f) Li GY. Angew. Chem., Int. Ed. 2001;113:1561–1564. [Google Scholar]; (g) Barbieri RS, Bellato CR, Dias AKC, Massabni AC. Catal. Lett. 2006;109:171–174. [Google Scholar]; (h) Dickens MJ, Gilday JP, Mowlem TJ, Widdowson DA. Tetrahedron. 1991;47:8621–8634. [Google Scholar]; (i) Ishiyama T, Mori M, Suzuki A, Miyaura NJ. Organomet. Chem. 1996;525:225–231. [Google Scholar]; (j) Zheng N, McWilliams JC, Fleitz FJ, Armstrong JD, Volante RP. J. Org. Chem. 1998;63:9606–9607. [Google Scholar]; (k) Mann G, Baranano D, Hartwig JF, Rheingold AL, Guzei IA. J. Am. Chem. Soc. 1998;120:9205–9219. [Google Scholar]
- 4.(a) Rout L, Sen TK, Punniyamurthy T. Angew. Chem., Int. Ed. 2007;46:5583–5586. doi: 10.1002/anie.200701282. [DOI] [PubMed] [Google Scholar]; (b) Bates CG, Gujadhur RK, Venkataraman D. Org. Lett. 2002;4:2803–2806. doi: 10.1021/ol0264105. [DOI] [PubMed] [Google Scholar]; (c) Kwong FY, Buchwald SL. Org. Lett. 2002;4:3517–3520. doi: 10.1021/ol0266673. [DOI] [PubMed] [Google Scholar]; (d) Wu Y-J, He H. Synlett. 2003:1789–1790. [Google Scholar]; (e) Deng W, Zou Y, Wang Y-F, Liu L, Guo Q-X. Synlett. 2004:1254–1258. [Google Scholar]; (f) Palomo C, Oiarbide M, Lopez R, Gomez-Bengoa E. Tetrahedron Lett. 2000;41:1283–1286. [Google Scholar]; (g) Savarin C, Srogl J, Liebeskind LS. Org. Lett. 2002;4:4309–4312. doi: 10.1021/ol026948a. [DOI] [PubMed] [Google Scholar]; (h) Chen Y-J, Chen H-H. Org. Lett. 2006;8:5609–5612. doi: 10.1021/ol062339h. [DOI] [PubMed] [Google Scholar]; (i) Zhu D, Xu L, Wu F, Wan B. Tetrahedron Lett. 2006;47:5781–5784. [Google Scholar]
- 5.(a) Cristau HJ, Chabaud B, Chene A, Christol H. Synthesis. 1981:892–894. [Google Scholar]; (b) Millois C, Diaz P. Org. Lett. 2000;2:1705–1708. doi: 10.1021/ol0058184. [DOI] [PubMed] [Google Scholar]; (c) Percec V, Bae J-Y, Hill DH. J. Org. Chem. 1995;60:6895–6903. [Google Scholar]; (d) Takagi K. Chem. Lett. 1987:2221–2224. [Google Scholar]
- 6.Wong Y-C, Jayanth TT, Cheng C-H. Org. Lett. 2006;8:5613–5616. doi: 10.1021/ol062344l. [DOI] [PubMed] [Google Scholar]
- 7.(a) Correa A, Carril M, Bolm C. Angew. Chem. Int. Ed. 2008;47:2880–2883. doi: 10.1002/anie.200705668. [DOI] [PubMed] [Google Scholar]; (b) Wu J, Lin C, Lee C. Chem. Commun. 2009:4450–4452. doi: 10.1039/b907362k. [DOI] [PubMed] [Google Scholar]
- 8.Ley SV, Thomas AW. Angew. Chem. Int. Ed. 2003;42:5400–5449. doi: 10.1002/anie.200300594. [DOI] [PubMed] [Google Scholar]
- 9.Kabir MS, Lorenz M, van Linn ML, Namjoshi OA, Ara S, Cook JM. J. Org. Chem. 2010;75:3626–3643. doi: 10.1021/jo1004179. [DOI] [PubMed] [Google Scholar]
- 10.Jammi S, Sakthivel S, Rout L, Mukherjee T, Mandal S, Mitra R, Saha P, Punniyamurthy T. J. Org. Chem. 2009;74:1971–1976. doi: 10.1021/jo8024253. [DOI] [PubMed] [Google Scholar]
- 11.Sperotto E, van Klink GPM, de Vries JG, van Koten G. J. Org. Chem. 2008;73:5625–5628. doi: 10.1021/jo800491k. [DOI] [PubMed] [Google Scholar]
- 12.(a) He H, Llauger L, Rosen N, Chiosis G. J. Org. Chem. 2004;69:3230–3232. doi: 10.1021/jo049875c. [DOI] [PubMed] [Google Scholar]; (b) He H, Zatorska D, Kim J, Aguirre J, Llauger L, She Y, Wu N, Immormino RM, Gewirth DT, Chiosis G. J. Med. Chem. 2006;49:381–390. doi: 10.1021/jm0508078. [DOI] [PubMed] [Google Scholar]; (c) Llauger L, He H, Kim J, Aguirre J, Rosen N, Peters U, Davies P, Chiosis G. J. Med. Chem. 2005;48:2892–2905. doi: 10.1021/jm049012b. [DOI] [PubMed] [Google Scholar]
- 13.(a) Rodina A, Patel PD, Kang Y, Patel Y, Baaklini I, Wong MJ, Taldone T, Yan P, Yang C, Maharaj R, Gozman A, Patel MR, Patel HJ, Chirico W, Erdjument-Bromage H, Talele TT, Young JC, Chiosis G. Chem. Biol. 2013;20:1469–1480. doi: 10.1016/j.chembiol.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Kang Y, Taldone T, Patel HJ, Patel PD, Rodina A, Gozman A, Maharaj R, Clement CC, Patel MR, Brodsky JL, Young JC, Chiosis G. J. Med. Chem. 2014;57:1188–1207. doi: 10.1021/jm401551n. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Taldone T, Kang Y, Patel HJ, Patel MR, Patel PD, Rodina A, Patel Y, Gozman A, Maharaj R, Clement CC, Lu A, Young JC, Chiosis G. J. Med. Chem. 2014;57:1208–1224. doi: 10.1021/jm401552y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allred GD, Liebeskind LS. J. Am. Chem. Soc. 1996;118:2748–2749. [Google Scholar]
- 15.Zhang S, Zhang D, Liebeskind LS. J. Org. Chem. 1997;62:2312–2313. doi: 10.1021/jo9700078. [DOI] [PubMed] [Google Scholar]
- 16.Liebeskind LS, Srogl J. J. Am. Chem. Soc. 2000;122:11260–11261. [Google Scholar]
- 17.Raushel J, Fokin VV. Org. Lett. 2010;12:4952–4955. doi: 10.1021/ol102087r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.General procedure: A mixture of aryliodide (1 eq.), arylthiol (1.2 eq.) and K2CO3 (2 eq.) in DMF or DMSO was evacuated and backfilled with argon. CuTC (0.4 eq.) was added and evacuated and backfilled with argon. The reaction mixture was heated at 120–130 °C for 3–24 h under argon. Solvent was removed under reduced pressure and the residue was purified by column chromatography to afford the desired product.
- 19.(a) Dömling A, Huang Y. Synthesis. 2010;17:2859–2883. [Google Scholar]; (b) Shaquiquzzaman M, Verma G, Marella A, Akhter M, Akhtar W, Khan MF, Tasneem S, Alam MM. Eur. J. Med. Chem. 2015;102:487–529. doi: 10.1016/j.ejmech.2015.07.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.