Abstract
Background
This study was conducted to evaluate the effects of commonly used injection medication combinations on supraspinatus tenocyte cell viability and tissue metabolism.
Methods
Twenty adult dogs underwent ultrasound guided injection of the canine equivalent of the subacromial space, based on random assignment to one of four treatment groups (n=5/group): normal saline, 1.0% lidocaine/methylprednisolone, 1.0% lidocaine/triamcinolone or 0.0625% bupivacaine/triamcinolone. Full-thickness sections of supraspinatus tendon were harvested under aseptic conditions and evaluated on days 1 and 7 post-harvest for cell viability and tissue metabolism. Data were analyzed for significant differences among groups.
Results
Tendons exposed to 1% lidocaine/ methylprednisolone had significantly lower cell viability at day 1 as compared to all other groups and control. All local anesthetic/ corticosteroid combination groups had decreased cell viability at day 7 when compared to the control group.
Conclusions
This study demonstrated significant in vivo supraspinatus tenotoxicity following a single injection of combination local anesthetic/ corticosteroid when compared to saline controls.
Level of Evidence
Level II
Keywords: local anesthetic, injections, corticosteroid, tendon, tenotoxicity
Introduction
Peri-articular injections are commonly performed for diagnostic and therapeutic purposes when managing acute and chronic soft tissue conditions about the shoulder and other joints.1 Many different combinations and dosages have been used, based on the condition being treated and individual surgeon preference.2 Injection of local anesthetic, corticosteroid, or combination agents may improve diagnostic accuracy and provide short-term pain relief for a variety of acute inflammatory and chronic degenerative soft tissue conditions.3-5 Despite widespread clinical use for rotator cuff pathology, the long-term consequences and potential toxicity of subacromial injection on supraspinatus tenocytes and other peri-articular structures has not been fully elucidated. Numerous in vitro studies have demonstrated the damaging effects of local anesthetics, corticosteroids, or combination agents on intra-articular chondrocytes.3,5-15 Several in vitro studies have confirmed similar toxicity to tenocytes, demonstrating decreased cell proliferation and viability following even single exposure to these agents.16-21 Previous in vitro supraspinatus tendon explant studies have demonstrated significant toxicity of various concentrations of lidocaine, bupivacaine, and several cortisone derivatives on tenocyte viability and metabolism.17,18,22
Despite growing concern, there has been a paucity of in vivo studies investigating these effects on supraspinatus tendons. There exists a disparity between the apparent clinical safety of routine combination subacromial injections and the detrimental results reported from in vitro models. It has been postulated that the extracellular matrix of the intact tendon may provide protection for tenocytes, thus mitigating the damaging effects observed using in vitro monolayer cell culture models.16,18,23 Therefore, the purpose of the present study was to use a translational in vivo model to investigate the effects of a single subacromial injection of local anesthetic/ corticosteroid on supraspinatus tenocyte viability and cell metabolism. Our hypothesis was that there would be toxic effects of local anesthetic/ corticosteroid injectates on supraspinatus tenocytes following single peri-tendon injection in this in vivo model.
Methods
With Institutional Animal Care and Use Committee (IACUC) approval, adult purpose-bred dogs (n=20; mean weight = 28.6 kg) were sedated (medetomidine 0.04 mg/kg) and prepared for aseptic injection of the left supraspinatus tendon. Each dog (shoulder) was randomly assigned to one of four groups (N=5 per group):
Control = 5 ml of 0.9% saline
L/ M = 4 ml of 1% lidocaine (pH 6.5) + 1 ml of 40 mg/ ml methylprednisolone (pH 6.5)
L/T = 4 ml of 1% lidocaine + 1 ml of 40 mg/ ml triamcinolone (pH 7.0)
B/T = 4 ml of 0.0625% bupivacaine (pH 6.5) + 1 ml of 40 mg/ ml triamcinolone
The dosage of 40 mg corticosteroid was selected as it is the recommended dose for the human wrist, which would be similar in size/volume to the canine shoulder. Additionally, the recommended clinical veterinary dose for the canine shoulder is 30 mg to 40 mg. For each treatment, a 1.5-inch 22 gauge needle was inserted to be immediately superficial to the supraspinatus tendon 1 cm from its insertion on the greater tubercle and just distal to the acromion, verified by ultrasonography (GE Logiq i portable ultrasound machine with 10-14 MHz linear transducer; GE Healthcare, Milwaukee, WI, USA) (Fig. 1), and then used to deliver the entire volume of the respective injectate into the area. The dogs were recovered from sedation and allowed full activity in their runs. The dogs were euthanatized 24 hours after injection as part of another IACUC-approved study. The injected tissues were aseptically harvested and full-thickness sections from the supraspinatus tendon were placed in sterile closed containers filled with tissue culture media, transported to the laboratory, and assessed immediately (day 1) or processed for tissue culture.
Figure 1 –
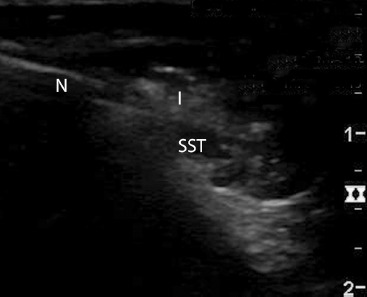
Image from a canine in this study showing ultrasound guided injection. A 1.5-inch 22 gauge needle (N) is used to inject the respective injectate (I) immediately superficial to the supraspinatus tendon (SST) 1 cm from its insertion on the greater tuberosity.
Tissue Culture
Two 4 mm diameter explants per canine were aseptically prepared from the harvested tendon tissue using a dermal biopsy punch (Fray Products, Buffalo, NY). One explant was used for day 1 assessment of tissue viability, and the other explant was placed in a well of 24-well tissue culture plates (Becton Dickinson Labware, Franklin Lakes, NJ) containing 1 ml Dulbecco’s modified Eagle’s medium with high glucose (Gibco, Invitrogen, Carlsbad, CA) supplemented with 1% ITS, penicillin, streptomycin, amphotericin B, L-ascorbic acid, L-glutamine, and nonessential amino acids. Explants were cultured in dedicated incubators at 37 °C with 5% CO2 at 95% humidity, and the media was changed on day 1 and 3 of culture.
Toxicity Assessments
Tendon explants were assessed immediately after harvest (day 1) and on day 7 of culture. Cell viability was determined using a live-dead cell assay (Invitrogen, Carlsbad, CA). Tissues were incubated with Calcein AM (live cell stain) and Sytox Blue (dead cell stain) using manufacturer’s instructions and then assessed by fluorescent microscopy to determine the number of live and dead cells in each section. Images of each section were captured using commercially available software (Microsuite, Olympus, Tokyo, Japan), and a subjective assessment of viability was performed by 6 investigators blinded to treatment. Each tendon tissue explant was given a score from 0 (0% viability) to 5 (100% viability). The scores from all observers were averaged to give a mean tenocyte subjective viability score (VS) for each explant.
Tissue metabolism was assessed on day 1 and 7 of explants cultured for 7 days using the resazurin (Sigma-Aldrich, St. Louis, MO) fluorescent metabolic assay. Resazurin is converted to a fluorescent compound (resorufin) by metabolically active cells. The degree of fluorescence detected in the media provides a quantitative measure of the number of viable cells in tissue.24 For day 1 testing, 100 uL of resazurin reagent was added to 900 uL of media of each tissue section and incubated overnight at 37 °C. Fluorescence was measured (Ex: 530, Em: 590) on a 200 uL sample of the media the following day using a Synergy HT plate reader (BioTek, Winooksi, VT). After testing on day 1, the media was removed from the tissue, the tissue explant was washed twice with clean supplemented media, and the tissue explant then placed in a fresh 1 ml of media for culture as described above. On day 6 the media was removed and a fresh 900 uL of media and 100 uL of resazurin stain was added to the tissue explants and tested as described above.
Statistical Analysis
Tendon VS and metabolic assay fluorescence data were assessed for statistically significant differences among groups at each assessment time point using a one-way ANOVA with Holm-Sidak post-hoc analyses. Significance was set at p < 0.05.
Results
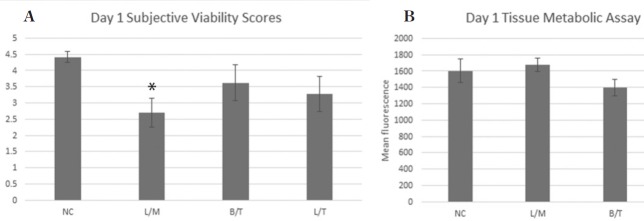
At day 1 (24 hours after injection) the viability score for tendon tissue exposed to L/M was significantly (p=0.006) lower than the saline injected control (Fig. 2a). However, the resazurin assay did not identify a statistically significant (p=0.28-0.69) difference in tissue metabolic activity among groups at the day 1 time point (Fig. 2b). After 7 days of culture, tendon tissue exposed to L/ M (p=0.001), L/T (p=0.041), and B/T (p=0.004) all had significantly lower viability scores compared to the saline injected control (Fig. 3a). No statistically significant differences in tissue metabolism among groups were identified by the resazurin assay on day 7 (Fig. 3b).
Figure 2 –
Day 1 (24 hours after intra-articular injection) Subjective viability score (SVS) (a) and tissue metabolic activity (b) for tendon explants in each treatment group. Tendon tissue exposed to L/M (*) had significantly (p=0.006) lower SVS compared to the saline injected control. No statistically significant differences in tissue metabolism levels were noted between groups.
Figure 3 –
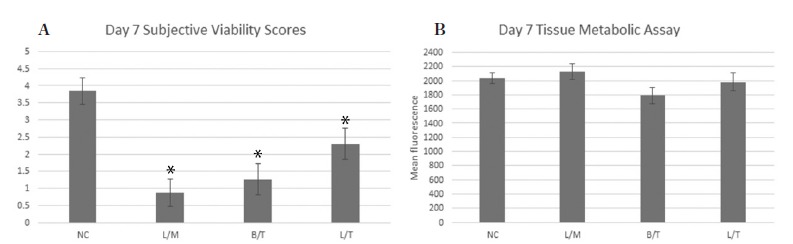
Day 7 Subjective viability score (SVS) (a) and tissue metabolic activity (b) for tendon explants in each treatment group. Tendon tissue exposed to L/M (p=0.001), L/T (p=0.041), and to B/T (p=0.004) had significantly (*) lower SVS compared to the saline injected control. No statistically significant differences in tissue metabolism levels were noted between groups.
Discussion
The results of this study using an in vivo model demonstrate that a single injection of local anesthetic/ corticosteroid into the subacromial space had deleterious effects on supraspinatus tenocyte viability when compared to a saline control. Compared to saline, all treatment groups showed decreased cell viability at days 1 and 7, with the 1% lidocaine/methylprednisolone group reaching statistically significant lower levels at post-injection day 1. This confirmed our hypothesis that there would be significant toxic effects of these injectates on supraspinatus tenocytes after a single peri-tendon injection.
In a systematic review, Dean et al. found significant negative effects of corticosteroids in both in vitro and in vivo tendon studies.25 In vitro findings included reduced cell viability, cell proliferation, and collagen synthesis; and in vivo studies showed increased collagen disorganization and necrosis in the limited series.25 Many laboratory studies demonstrate tenotoxicity at the cellular level with use of these agents, and clinical studies suggest increased risk of delayed tendon healing or tendon rupture.2,18-21,24-28 Specific to local anesthetics, Scherb et al. showed reduced tenocyte proliferation after bupivacaine exposure and Piper et al. demonstrated incrementally damaging effects of lidocaine on bovine tenocytes.16,17 Similarly, our study showed decreased cell viability at day 1 for lidocaine, and at day 7 for both lidocaine and bupivacaine. Regarding independent corticosteroid use, Wong et al. and Scutt et al. both showed that dexamethasone reduced tenocyte collagen synthesis and cell proliferation and viability.18,20 In an in vivo study, Dean et al. compared histological and immunohistochemical effects of glucocorticoid injection versus surgical rotator cuff repair for rotator cuff tendinopathy.29 They noted increases in cell proliferation, vascularity and hypoxia inducible factor 1α after the surgical repairs but not after the injections, and concluded that further tendon damage may result after glucocorticoid injection. Similar to these studies, our studies showed decreased cell viability with both dexamethasone and triamcinolone at day 7. Our study did not show significant effects of the triamcinolone group at day 1, however. Another difference from previous studies was that our study did not show significant differences in cellular metabolism in any of the study groups. A potential reason for this could be that the specimens were tendon explants, thus limiting the release of matrix metalloproteinases that could influence metabolism. We attempted to mitigate this factor by preserving the extracellular matrix and cell heterogeneity of the tendon itself.
Previous in vitro studies have shown tenotoxicity to a single exposure with the individual injectates 1% lidocaine, methylprednisolone, bupivacaine, and triamcinolone.17-22 Most injections administered clinically are given in combination, however. Data from previous in vitro results, along with common clinical combinations, provided the rationale for the combination injectate groups used in this in vivo translational study. Based on data from previous screening studies, number of dogs available, and a pre-study power analysis, three local anesthetic-corticosteroid combinations were chosen: 1%L/M as the “worst case scenario”, 0.0625%B/T as the “best case scenario”, and 1%L/T as the “mismatched” group to help determine whether local anesthetic or corticosteroid might be most influential in terms of in vivo effects. Dosage and volume of subacromial injectate was chosen to directly correspond to those used in human patients, as well as current standard of care in veterinary medicine.
In general, physicians often utilize combination injections in clinical practice. The local anesthetic provides initial pain relief and allows the practitioner to perform a Neer’s Impingement Test to differentiate the injected subacromial space as a major pain generator or not, while the corticosteroid is included to decrease inflammation so that the patient may rehabilitate effectively. Piper et al. investigated the effects of independent usage of local anesthetic and cortisone versus combined use. They found that longer acting ropivacaine alone was not found to have significantly negative toxic effects, but short acting lidocaine was noted to have dose dependent toxic effects. More importantly, they demonstrated that when both anesthetics were combined with dexamethasone, there was noted to have significantly increased toxicity to tenocytes.13 Previous in vitro data demonstrates similar independent toxicity with lidocaine and less toxicity with longer acting dilute anesthetic (i.e., 0.0625% bupivacaine).22 Similar to results from Piper et al., however, this study exhibited that the combination of agents that may be safe in isolation (i.e., bupivacaine), remain a significant concern when used in combination. The synergistic and deleterious mechanism of action for tenotoxicity is unknown and should be the subject of future research, but our results are nonetheless potentially valuable to the clinician. Unfortunately, limitations in number of animals did not allow for independent testing of local anesthetic or corticosteroid injection alone in this study. Future study is warranted, particularly to answer whether agents such as triamcinolone and dilute bupivacaine are safe when used in isolation, as they have been shown to be in some in vitro studies.18-22
Based on the current study and the available data in the literature, the authors have now discontinued the use of lidocaine in our practices for both intra-articular and peri-tendinous injections. We now use low dose (0.0625%) bupivacaine sparingly for diagnostic purposes only. Our data and others also support potential toxicity with the use of methylprednisolone or dexamethasone, which we also avoid for subacromial injection. Data regarding triamcinolone is mixed, with some studies demonstrating toxic effects only when used as a combination agent and others demonstrating no long-term negative effects of independent injection.18-20,23 In all of the intra-articular and subacromial in vitro and in vivo studies conducted at our institution, we have demonstrated no deleterious effects of independent usage of triamcinolone versus negative control. Based on the available data, the authors now clinically use isolated triamcinolone with a normal saline carrier for intra-articular and subacromial injections.
Financial and ethical limitations dictated the number of specimens for this study and prohibited the use of more canines and potentially more treatment groups. Treatment groups of local anesthetic-corticosteroid pairs were chosen based on previous in vitro studies evaluating each substance alone, prior peer reviewed literature, pre-study power analysis, and the common clinical practice pattern of combination injections. Future in vivo study should investigate these and other substances (i.e., ropivacaine, dexamethasone) individually to control for variables that may have influenced the results. In this study, saline injection was used as negative control, as the contralateral shoulder was unavailable and being utilized for a different study. While it can be argued that saline injection is not equivalent to an untouched normal shoulder, our results in the saline group consistently demonstrated high viability and metabolic function for all samples at all time points. This profile compares favorably with results from historical controls using normal canine tenocytes. When financial and/ or ethical considerations limit use of research animals, placebo or sham controls are preferred over unaltered or normal controls and are considered adequate for hypotheses testing. In fact, placebo or sham controls (e.g., saline injection as in the present study) are required by most regulatory bodies, whereas unaltered, normal controls are not. Regarding the potential issue involving the use of the canines for multiple simultaneous studies, it is noted that this was IACUC approved and addresses the NIH mandate of “Reduce, Refine, and/ or Replace.” Canines did not experience lameness or dysfunction in the 24 hours of study duration and the other study involving the contralateral shoulder joint did not involve any systemic treatments. As such, we do not think this in any way effected the study results.
Other limitations include the use of normal non-pathologic canine supraspinatus tendons. While the canine shoulder is very similar to its human counterpart in terms of pathophysiology and clinical treatment—including injections—differences do exist and should be taken into account when applying the results to a human patient population. Moreover, the use of healthy tendons may not replicate the exact biologic responses a pathologic subacromial space might have to an injection, but we believe the use of normal tendons shows even a stronger impact of the potential damaging effects of the medications tested. Nonetheless, these results should be replicated in a model of tendon pathology before definitive treatment recommendations can be made. Another limitation could be the use of only two early time points. While significant effects on cell viability were noted at these time points, there were not significant differences noted in cell metabolism. It is possible that further time points could exhibit differences between groups. It is also possible that the methodology employed for the metabolism assay influenced these results. Overnight incubation of the explants in the indicator dye resulted in high-metabolism samples reaching the maximum level of fluorescence, such that relative differences among these samples could not be distinguished. Therefore, shorter incubation times may be used for ongoing studies. However, cell viability and cell metabolism can, and often are, uncoupled, especially with respect to anabolism versus catabolism. As such, cell viability was considered the most important factor for clinical applicability in the present study and therefore was the primary outcome measure.
The negative effects on viability at even these early time points, however, raises concern that long-term clinically significant toxicity is possible and should be investigated prior to recommending routine use of the combinations shown to be toxic in this study.
This study demonstrated significant in vivo supraspinatus tenotoxicity following a single injection of combination local anesthetic/ corticosteroid when compared to saline controls. This data raises significant concern regarding the clinical use of combination peri-tendinous injections near supraspinatus tendons.
REFERENCES
- 1.Hill JJ Jr, Trapp RG, Colliver JA. Survey on the use of corticosteroid injections by orthopaedists. Contemp Orthop. 1989;18::39–45. [Google Scholar]
- 2.Cole BJ, Schumacher HR. Injectable corticosteroids in modern practice. J Am Acad Orthop Surg. 2005;13(1):37–46. doi: 10.5435/00124635-200501000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Farkas B, Kvell K, ly T, s T, Bárdos T. Increased chondrocyte death after steroid and local anesthetic combination. Clin Orthop Relat Res. 2010;468(11):3112–3120. doi: 10.1007/s11999-010-1443-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Busch CA, Shore BJ, Bhandari R, et al. Efficacy of periarticular multimodal drug injection in total knee arthroplasty: a randomized trial. J Bone Joint Surg Am. 2006;88(5):959–963. doi: 10.2106/JBJS.E.00344. [DOI] [PubMed] [Google Scholar]
- 5.Cassuto J, Sinclair R, Conderovic M. Antiinflammatory properties of local anesthetics and their present and potential clinical implications. Acta Anaesthesiol Scand. 2006;50(3):265–282. doi: 10.1111/j.1399-6576.2006.00936.x. [DOI] [PubMed] [Google Scholar]
- 6.Chu CR, Coyle CH, Chu CT, et al. In vivo effects of single intra-articular injection of 0.5% bupivacaine on articular cartilage. J Bone Joint Surg Am. 2010;92(3):599–608. doi: 10.2106/JBJS.I.00425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dragoo JL, Braun HJ, Kim HJ, Phan HD, Golish SR. The in vitro chondrotoxicity of single-dose local anesthetics. Am J Sports Med. 2012;40(4):794–799. doi: 10.1177/0363546511434571. [DOI] [PubMed] [Google Scholar]
- 8.Dragoo JL, Korotkova T, Kim HJ, Jagadish A. Chondrotoxicity of low pH, epinephrine, and preservatives found in local anesthetics containing epinephrine. Am J Sports Med. 2010;38(6):1154–1159. doi: 10.1177/0363546509359680. [DOI] [PubMed] [Google Scholar]
- 9.Anz A, Smith MJ, Stoker A, et al. The effect of bupivacaine and morphine in a coculture model of diarthrodial joints. Arthroscopy. 2009;25(3):225–231. doi: 10.1016/j.arthro.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 10.Grishko V, Xu M, Wilson G, Pearsall IV AW. Apoptosis and mitochondrial dysfunction in human chondrocytes following exposure to lidocaine, bupivacaine, and ropivacaine. J Bone Joint Surg Am. 2010;92(3):609–618. doi: 10.2106/JBJS.H.01847. [DOI] [PubMed] [Google Scholar]
- 11.Gomoll AH, Yanke AB, Kang RW, et al. Long-term effects of bupivacaine on cartilage in a rabbit shoulder model. Am J Sports Med. 2009;37(1):72–77. doi: 10.1177/0363546508323748. [DOI] [PubMed] [Google Scholar]
- 12.Piper SL, Kim HT. Comparison of ropivacaine and bupivacaine toxicity in human articular chondrocytes. J Bone Joint Surg Am. 2008;90(5):986–991. doi: 10.2106/JBJS.G.01033. [DOI] [PubMed] [Google Scholar]
- 13.Piper SL, Kramer JD, Kim HT, Feeley BT. Effects of local anesthetics on articular cartilage. Am J Sports Med. 2011;39(10):2245–2253. doi: 10.1177/0363546511402780. [DOI] [PubMed] [Google Scholar]
- 14.Syed HM, Green L, Bianski B, Jobe CM, Wongworawat MD. Bupivacaine and triamcinolone may be toxic to human chondrocytes: A pilot study. Clin Orthop Relat Res. 2011;469::2941–2947. doi: 10.1007/s11999-011-1834-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dvorak LD, Cook JL, Kreeger JM, Kuroki K, Tomlinson JL. Effects of carprofen and dexamethasone on canine chondrocytes in a three-dimensional culture model of osteoarthritis. Am J Vet Res. 2002;63(10):1363–1369. doi: 10.2460/ajvr.2002.63.1363. [DOI] [PubMed] [Google Scholar]
- 16.Sherb MB, Han SH, Courneya JP, Guyton GP, Schon LC. Effect of bupivacaine on cultured tenocytes. Orthopedics. 2009;;32(1):26.) doi: 10.3928/01477447-20090101-19. [DOI] [PubMed] [Google Scholar]
- 17.Piper SL, Laron D, Manzano G, et al. A comparison of lidocaine, ropivacaine and dexamethasone toxicity on bovine tenocytes in culture. J Bone Joint Surg Br. 2012;94(6):856–862. doi: 10.1302/0301-620X.94B6.29063. [DOI] [PubMed] [Google Scholar]
- 18.Wong MW, Lui WT, Fu SC, Lee KM. The effect of glucocorticoids on tendon cell viability in human tendon explants. Acta Orthop. 2009;80(3):363–367. doi: 10.3109/17453670902988386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wong MW, Tang YN, Fu SC, Lee KM, Chan KM. Triamcinolone suppresses human tenocyte cellular activity and collagen synthesis. Clin Orthop Relat Res. 2004;421::277–281. doi: 10.1097/01.blo.0000118184.83983.65. [DOI] [PubMed] [Google Scholar]
- 20.Scutt N, Rolf CG, Scutt A. Glucocorticoids inhibit tenocyte proliferation and tendon progenitor cell recruitment. J Orthop Res. 2006;24(2):173–182. doi: 10.1002/jor.20030. [DOI] [PubMed] [Google Scholar]
- 21.Zhang J, Keenan C, Wang JH. The effects of dexamethasone on human patellar tendon stem cells: implications of dexamethasone treatment of injury. J Orthop Res. 2013;31(1):105–110. doi: 10.1002/jor.22193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nuelle CW, Cook CR, Stoker AM, Cook JL, Sherman SL. In vitro toxicity of local anesthetics and corticosteroids on tenocytes. J Ortho Translat. 2017;8::20–24. doi: 10.1016/j.jot.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raynauld J-P, Buckland-Wright C, Ward R, et al. Safety and efficacy of long-term intraarticular steroid injections in osteoarthritis of the knee: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2003;48(2):370–377. doi: 10.1002/art.10777. [DOI] [PubMed] [Google Scholar]
- 24.Perrot S, Dutertre-Catella H, Martin C, Rat P, Warnet JM. Resazurin metabolism assay is a new sensitive alternative test in isolated pig cornea. Toxicol Sci. 2003;72(1):122–129. doi: 10.1093/toxsci/kfg014. [DOI] [PubMed] [Google Scholar]
- 25.Dean BJ, Lostis E, Oakley T, Rombach I, Morrery ME, Carr AJ : The risks and benefits of glucocorticoid treatment for tendinopathy: A systematic review of the effects of local glucocorticoid on tendon. Sem Arthritis and Rheum. 2014;43(4):570–576. doi: 10.1016/j.semarthrit.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 26.Coombes BK, Bisset L, Vicenzino B. Efficacy and safety of corticosteroid injections and other injections for management of tendinopathy: a systematic review of randomised controlled trials. Lancet. 2010;376(9754):1751–1767. doi: 10.1016/S0140-6736(10)61160-9. [DOI] [PubMed] [Google Scholar]
- 27.Turmo-Garuz A, Rodas G, Balius R, et al. Can local corticosteroid injection in the retrocalcaneal bursa lead to rupture of the Achilles tendon and the medial head of the gastrocnemius muscle? Musculoskelet Surg. 2014;98(2):121–126. doi: 10.1007/s12306-013-0305-9. [DOI] [PubMed] [Google Scholar]
- 28.Nepple JJ, Matava MJ. Soft tissue injections in the athlete. Sports Health. 2009;1(5):396–404. doi: 10.1177/1941738109343159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dean BJ, Franklin SL, Murphy RJ, Javaid MK, Carr AJ : Glucocorticoids induce specific ion-channelmediated toxicity in human rotator cuff tendon: a mechanism underpinning the ultimately deleterious effect of steroid injection in tendinopathy. Br J Sports Med. 2014;48(22):1620–1626. doi: 10.1136/bjsports-2013-093178. [DOI] [PubMed] [Google Scholar]