Abstract
Background
Clinical computed tomography (CT) studies performed for other indications can be used to opportunistically assess vertebral bone without additional radiation or cost. Reference values for young women are needed to evaluate diagnostic accuracy and track changes in CT bone mineral density values across the lifespan. The purpose of this study was to determine reference values for lumbar trabecular CT attenuation (Hounsfield units [HU]) and determine the diagnostic accuracy of HU T-scores (T-scoreHU) for identifying individuals with osteoporosis.
Methods
We performed a retrospective single-center cohort study of patients undergoing CT of the lumbar spine. Reference values for lumbar spine Hounsfield units were determined from a reference sample of 190 young women aged 20-30 years undergoing CT scan of the lumbar spine. A separate sample of 252 older subjects undergoing CT and dual-energy X-ray absorptiometry (DXA) within a 6-month period that served as a validation cohort. Osteoporosis was defined by T-scoreDXA ≤ -2.5. Reference values were determined for lumbar HU from L1 to L4 from the reference cohort (24.0 ± 2.9 years). T-scoreHU was calculated in the validation cohort (58.9 ± 7.5 yrs). Receiver operating characteristic (ROC) curves were used to assess sensitivity and specificity of T-scoreHU for this task.
Results
Reference group HU ranged from 227 ± 42 at L3 to 236 ± 42 at L1 (P < 0.001). Validation group T-scoreDXA was -0.7 ± 1.5 and -0.9 ± 1.2 at lumbar and femoral sites respectively. Mean T-scoreHU was -2.3. T-scoreHU of -3.0, corresponding to 110 HU, was 48% sensitive and 91% specific for osteoporosis in the validation group. ROC area under the curve ranged from 0.825 to 0.853 depending on lumbar level assessed.
Conclusions
Although lumbar trabecular HU T-scores are lower than DXA T-scores, thresholds can be selected to achieve high sensitivity and specificity when screening for osteoporosis. Patients with a lumbar T-scoreHU ≤ -3.0 should be referred for additional evaluation. Further research into HU T-scores and clinical correlates may also provide a tool to assess changes in vertebral bone and the relationship to fracture risk across the lifespan.
Keywords: screening, computed tomography, dual-energy x-ray absorptiometry, osteoporosis
Introduction
In 2009, 10% of the U.S. population underwent computed tomography imaging (CT), accounting for 75 million scans.1 In addition to established clinical indications, CT imaging is actively being investigated as a screening tool for conditions such as colorectal polyps, lung cancer, coronary artery disease, and metabolic bone disease. Clinical CT exams, such as for evaluation of trauma patients, contain unused quantitative information that could provide an opportunity to screen large numbers of patients for metabolic bone disease at no additional radiation exposure and little cost when CT studies have been ordered for other indications.2-6
Osteoporosis is a condition of low bone mineral density and poor bone quality resulting in increased risk of fracture.7 Among women ≤ 65 years old with osteoporosis without prior vertebral fracture, 28% will experience vertebral fracture within 15 years.8 Still, osteoporosis is underdiagnosed and undertreated.9 The current standard of bone mineral density (BMD) assessment, dual-energy X-ray absorptiometry (DXA), is based upon a person’s BMD T-score at the hip and lumbar spine.10,11 These T-scores (T-scoreDXA) are calculated as the difference between the individual’s BMD and a reference population mean, divided by the standard deviation of the reference population. Osteoporosis is operationally defined by World Health Organization as a BMD 2.5 SD or more below the reference population mean, i.e. a T-score ≤ -2.5, whereas osteopenia is a state of low bone mass defined by a BMD T-score between -1 and -2.5.
Although World Health Organization diagnostic criteria apply only to DXA measures at the femoral neck and lumbar vertebrae, CT densitometry of the spine is equal to or superior to DXA for assessing vertebral fracture risk.12 One explanation for this is the ability to exclude cortical bone and vertebral posterior elements, which contribute less to vertebral compressive characteristics than trabecular bone but may change measured BMD due to degenerative changes or when deformity is present.13,14 CT attenuation numbers or values, measured in Hounsfield units (HU), can be attained prospectively or retrospectively from all clinical CT studies and can be used to estimate BMD without added costs or radiation.2 Traditionally, this required bone mineral phantoms and dedicated software to assess bone density. Recently, multiple studies have reported excellent reproducibility and strong accuracy identifying osteoporosis with simple CT attenuation measures of the spine, obviating the need for bone mineral reference phantoms or dedicated software.2-4,15,16 Appropriate HU reference values for 20 to 30 year old women have been reported from a relatively small sample.2 Expansion of this reference population could provide more robust CT reference intervals, thus improving CT-based screening for metabolic bone disease.
We hypothesized that vertebral CT attenuation, in HU, could identify individuals at risk for metabolic bone disease using T-scores based upon CT attenuation (T-scoreHU). To this end, we tested the reliability of vertebral HU measures and then measured a cohort of young women to create a reference standard in order to compare it to other HU measures. We gathered data from a second cohort that had undergone both DXA screening (the “gold standard” for osteoporosis screening and diagnosis) and abdominal CT to examine the diagnostic accuracy of T-scoreHU based upon T-scoreDXA.
Patient Sample
This retrospective cohort study was approved by a major U.S. academic medical center Institutional Review Board and exempted from informed consent. Records were identified by querying our picture archiving communication and storage (PACS) database. The reference cohort was identified by searching for lumbar CT scans performed on women aged 20 to 30 years, performed between January 2001 and July 2012. We identified 248 records in this cohort, with 58 subsequently excluded; exclusions included eleven repeat scans, 30 pre-existing vertebral fracture, five prior vertebral surgery, five radiologic contrast dye, two anatomical deformity, one severe degenerative disease, and four incompatible scan parameters (figure 1). This left 190 unique cases in the final reference cohort.
Figure 1.
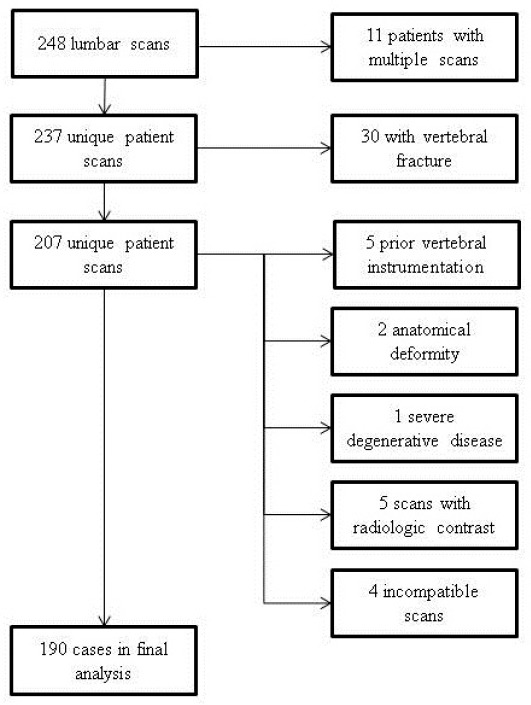
Flow diagram demonstrating frequencies of exclusions by criteria.
A separate validation cohort consisted of 252 older subjects undergoing CT colonography who also underwent DXA bone study within six months.
Sample size estimation was based on attaining enough precision for mean HU, measured as the width of the 95% confidence interval. Based on previously reported lumbar HU standard deviation of 38 HU, we predicted 95% confidence intervals of ± 5 HU for a sample of 225 subjects and ± 6 HU for 157 subjects, respectively (4). Therefore, our goal was 225 subjects for the reference cohort.
Methods
Imaging
Lumbar CT was performed using helical sixty four-channel CT scanner with automated exposure control, previously demonstrated to approximate trabecular bone mineral density (Light Speed Series, GE Healthcare).2 CT parameters included slice thickness of 1.25 mm with a 0.625 mm interval, tube voltage of 120 kVp, automated exposure control tube current of 300 mA (Smart mA/ Auto mA range, 150 to 750), and a bone reconstruction algorithm (window width/ window level, -3000/300).
CT images were retrospectively analyzed using a commercially available picture archiving and communication system (McKesson, San Francisco, California). Two-dimensional reconstructions were obtained in the axial and sagittal planes (figure 2). CT attenuation was measured in Hounsfield Units (HU) by placing a click-and-drag elliptical region of interest (ROI) within axial sections of vertebral trabecular bone. ROI were made as large as possible while avoiding vertebrobasilar complex, mild degenerative changes, and cortical surfaces. For the reference cohort three axial measures were included for each vertebra: inferior to cranial endplate, mid-body, and superior to caudal endplate. Dynamic oblique multiplanar reformatting (MPR) was used to align ROI parallel to the vertebral endplates in the sagittal plane. Cranial and caudal ROI were measured approximately 4 mm from the vertebral endplate. Lumbar vertebrae from L1 through L4 were assessed. L5 vertebrae were also measured, but were not reported due to potential difficulties that might arise when attempting to measure mid-body ROI without MPR reformatting due to lumbar lordosis and possible sacralization of the L5 vertebral body. HU measures in the older validation cohort were measured at a single mid-vertebral axial ROI without MPR reformatting, as these methods have been evaluated and shown to produce similar reliability.16
Figure 2.
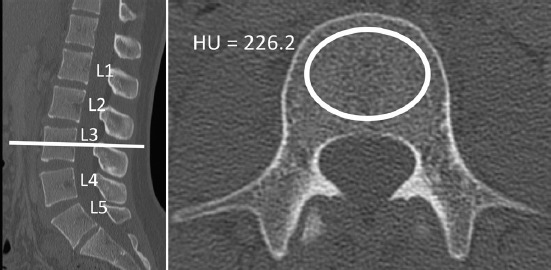
CT attenuation was measured by first locating the mid-vertebral body in the sagittal plane. Axial click-and-drag elliptical ROI were manually placed to be as large as possible while safely avoiding the cortical shell and vertebrobasilar complex. The picture archiving software reported the average CT attenuation of the ROI in Hounsfield units (HU)
Dual-energy x-ray absorptiometry was performed using standard techniques on Lunar Prodigy densitometers (GE Healthcare, Waukesha, WI). Central DXA BMD T-scores were recorded from the lumbar spine and hip. Subjects were categorized as having osteopenia (T-scoreDXA between -1.0 and -2.5 SD) or osteoporosis (T-scoreDXA ≤ -2.5 SD) based upon the lowest T-scoreDXA from either femoral or vertebral region.
Intra-rater and Inter-rater Reliability
Reliability of HU measures was assessed on a random sample of 20 subjects. Two separate readers performed HU measures from L1 to L3 at cranial, mid-body and caudal sections. Rater A, a research fellow, performed each measure on two separate occasions separated by more than one week and a rater B, an orthopaedic surgery resident, performed each measure once. Intra- and inter-rater reliability of HU measures was assessed using intraclass correlation coefficients (ICC) with an absolute agreement definition. ICC greater than 0.75 was considered excellent.17
Statistical analysis
Results are reported as mean ± standard deviation (SD), unless noted otherwise. A Wilcoxon rank-signed test was used to compare attenuations at different vertebral levels. Threshold for statistical significance was set at two-sided alpha = 0.05. Reference values including mean, SD and confidence intervals were calculated from the reference cohort.
Our goal was to assess whether T-score based on vertebral CT attenuation (T-scoreHU) is in close agreement with conventional DXA-derived T-scores (T-scoreDXA), considered here as the gold standard. To this end, we had access to both the CT and DXA data from Pickhardt et al., 2011.4 The trabecular CT attenuation at L1 through L4 were converted into T-scores by subtracting the reference mean and dividing the result by the reference standard deviation, (equation 1)
where HU represents CT attenuation in Hounsfield Units, and μref and σref are the mean and standard deviation of the reference values calculated from the reference cohort, respectively. T-score histograms were obtained at each level for both HU and DXA to assess whether these were approximately normally distributed.
T-scoreHU was plotted against central T-scoreDXA using the lower of two DXA T-scores measured at the lumbar spine and hip. The coefficients of determination (R2) and correlation (ρ) were obtained from regression analyses to assess the degree of linear association between T-scoreHU and T-scoreDXA. This same analysis was then repeated comparing T-scoreHU to T-scoreDXA from the lumbar spine to assess for changes in relationship when both T-scoreHU and T-scoreDXA are based on the same anatomical region. To assess the ability of T-scoreHU at different lumbar levels to identify osteoporosis, receiver operating characteristic (ROC) curves were obtained, and the area under the curve (AUC) was calculated.
Results
The reference cohort included 190 women in the third decade of life. The validation cohort included 239 women and 13 men. Mean age of the validation cohort was 58.9 ± 7.5 years.
Intra-rater and Inter-rater Reliability
Intra-rater reliability of lumbar HU measured at cranial, mid-body, and caudal ROI in 20 reference cohort subjects was excellent, with ICC exceeding 0.98 at each location measured within the L1 to L3 vertebral bodies (i.e. cranial, mid-body and caudal). Inter-rater reliability was also excellent, exceeding 0.98 at the mid-body locations. Cranial and caudal measures demonstrated more inter-rater variability than mid-body measures, as ICC ranged from 0.8 and 0.9.
Reference cohort
Mean lumbar CT attenuation from the reference cohort ranged from 229.7 at L3 to 236.3 HU at L1 (table 1). The L1 to L4 mean HU value was 232.4 ± 40.7 (95% CI for mean: 226.5, 238.2). HU were significantly higher at L1 and L2 compared to L3 and L4 (P<0.001).
Table I.
Reference CT attenuation reference values (Hounsfield units) from lumbar CT scans
| n | mean | std. dev | 95% CI | -1 SD | -2.5 SD | |
|---|---|---|---|---|---|---|
| L1 | 187 | 236.3 | 41.8 | 230.3, 242.3 | 194.5 | 131.8 |
| L2 | 188 | 234.3 | 42.1 | 228.3, 240.3 | 192.2 | 129.1 |
| L3 | 189 | 227.3 | 42.0 | 231.1, 243.9 | 185.3 | 122.3 |
| L4 | 188 | 229.7 | 42.6 | 223.6, 235.8 | 187.1 | 123.2 |
| L1 to L4Avg | 184 | 232.4 | 40.7 | 226.5, 238.2 | 192.0 | 129.6 |
Table 1. CT attenuation measures (Hounsfield units) are reported from single-slice mid-body trabecular ROI taken from lumbar CT scans performed on 190 20 to 30 year old women comprising our reference cohort. Column one lists the respective lumbar vertebral levels. Hounsfield unit values corresponding to T-scores of -1 and -2.5 are listed in the columns labeled -1 SD and -2.5 respectively.
Validation cohort
The mean T-scoreDXA in the validation cohort was -0.7 ± 1.5 for vertebral DXA (min -3.4, max 4.5) and -0.9 ± 1.2 for femoral DXA (min -3.6, max 3.7) (table 2). Mean lumbar T-scoreHU was -2.3 and ranged from -2.0 ± 0.9 at L1 to -2.4 ± 0.9 at L3. Similar to the reference cohort, lumbar CT attenuation was lowest at L3 and highest at L1 in the validation cohort.
Table II.
CT attenuation values (Hounsfield units) from lumbar CT scans of validation cohort
| mean | std. dev | |
|---|---|---|
| L1 | 152.9 | 38.3 |
| L2 | 143.1 | 39.3 |
| L3 | 130.5 | 38.6 |
| L4 | 133.2 | 38.3 |
Lumbar CT attenuation measures (Hounsfield units) from validation cohort of 239 women and 13 men aged 59 ± 7.5 years. Column one lists the respective lumbar vertebral levels.
All T-scores were approximately normally distributed, although large positive T-scores were slightly more likely than large negative T-scores. T-scoreHU was more negative than T-scoreDXA, with a difference in T-score of -1.1 to -1.6 depending on the anatomical region of interest compared (table 3). Regression analysis of T-scoreDXA compared to T-scoreHU resulted in coefficients of determination (R2) ranging from 0.32 to 0.36. Correlations between T-scoreDXA and T-scoreHU were similar for CT attenuation measured regardless of vertebral body measured from L1 to L4 (range 0.57-0.60). Comparing T-scoreHU to vertebral T-scoreDXA did not increase the correlation coefficient compared to using the lower of either vertebral or femoral T-scoreDXA, as is used in standard DXA osteoporosis screening.
Table III.
Validation cohort T-scores from DXA and CT measures
| CT T-score | DXA vertebral T-score | femoral T-score DXA | |
|---|---|---|---|
| L1 | -2.0 ± 0.9 | -0.7 ± 1.5 | -0.9 ± 1.2 |
| L2 | -2.2 ± 0.9 | ||
| L3 | -2.4 ± 0.9 | ||
| L4 | -2.3 ± 0.9 |
CT measures were based upon single-slice mid-body regions of interest. Regression analysis performed between externally validated lumbar trabecular CT T-score and the lowest DXA T-score from either the lumbar or femoral region, as well as between CT T-score and lumbar DXA T-score.
ROC analysis to assess the diagnostic accuracy of T-scoreHU for identifying individuals with osteoporosis resulted in area under the curve (AUC) ranging from 0.825 [0.709, 0.901] at L1 to 0.853 [0.741, 0.922] at L3 (reported as AUC [95% confidence interval]) (figure 3). L1, a T-scoreHU of -3.0 is 90% specific and 48% sensitive for osteoporosis, as defined by World Health Organization criteria of T-scoreDXA equal to or more negative than -2.5. For L1, a T-scoreHU of -2.0 is 50% specific and 93% sensitive for osteoporosis. These T-scoreHU values correspond to L1 HU thresholds of 110 and 153 HU, respectively.
Figure 3.
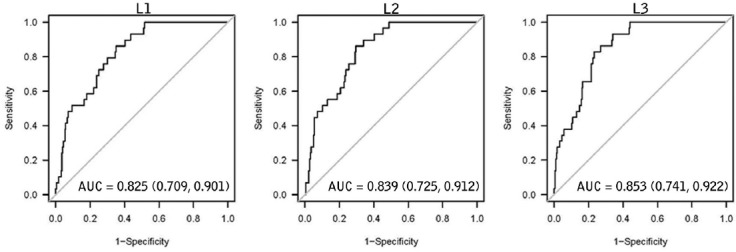
Receiver operating characteristic (ROC) curves assessing performance of lumbar spine Hounsfield Unit T-score (T-scoreHU) to identify subjects with osteoporosis as diagnosed by DXA BMD T-score (T-scoreDXA). Each point along the ROC curve represents the relationship between sensitivity (true positive rate) and the quantity 1-specificity (false positive rate). More negative T-scores occur closer to the origin. At the first lumbar vertebrae a T-scoreHU of -3.0 (110 HU) was 90% specific and 48% sensitive for osteoporosis, while a T-scoreHU 50% specific and 93% sensitive for osteoporosis as classified by DXA. of -2.0 was
Discussion
Our findings further validate previous publications applying CT modalities to opportunistic screening of metabolic bone disease. CT studies performed about the abdomen, pelvis, or lumbar spine can be used to assess bone density using Hounsfield Units to indicate patients for further assessment of metabolic bone disease.
Fundamental differences exist between DXA and CT vertebral densitometry, resulting in differences in bone density measures: DXA produces planar measures obtained in the anteroposterior plane and includes cortical bone, posterior elements, vascular calcification, and degenerative changes, potentially leading to spuriously elevated bone mineral density without correlating increase in vertebral body strength, and making DXA less sensitive to changes in fracture risk.18,19 Degenerative and arthritic changes in the posterior elements may reduce the sensitivity of DXA scans to decreases in trabecular bone density, at least partially explaining the greater absolute T-scores seen with DXA as compared to trabecular Hounsfield unit T-scores. These differences also help explain the low correlation between the two T-scores, with R2 only reaching 0.32. These issues may lead to significant false negative rates using DXA: Pickhardt et al. reported that 50% of subjects with moderate or severe radiographic vertebral fractures had non-osteoporotic T-scoreDXA and the majority of self-reported fractures in the National Osteoporosis Risk Assessment study were among women with non-osteoporotic T-scoreDXA.16,22 Helical CT technology produces volumetric measures and allows selective placement of regions of interest which greatly reduce the impact of these confounding factors. Thus, CT provides better accuracy and precision for measuring metabolically active trabecular BMD and monitoring changes over time.20,21
Our L1 to L4 average of 232 ± 41 HU is similar to previously reported lumbar HU values of 248 ± 52 and 222 ± 36 HU in similar aged women.2,23 Pickhardt et al. reported HU thresholds for L1 allowing clinicians to screen for osteoporosis with > 90% sensitivity and specificity using screening thresholds of 160 HU and 110 HU, respectively [16]. Our data uses subjects undergoing both DXA and CT measures to link these HU thresholds to T-scores based upon reference intervals from young women similar in age to those used in constructing the NHANES reference intervals for assessing DXA T-scores.10 T-scores have become a widely accepted parameter for diagnosis and treatment of osteoporosis. However, T-scoreDXA thresholds are not applicable to CT-based measures because DXA scans include cortical bone and posterior vertebral elements. Similar to previous reports, we found T-scoreHU was more negative than T-scoreDXA. In concordance with our results, previous comparisons of DXA and QCT T-scores found QCT T-score of -3.3 equal in sensitivity to spinal DXA for predicting vertebral fracture risk.12 Our data suggest that a T-scoreHU of -3.0 is highly specific for osteoporosis, meaning a high proportion of non-osteoporotics are correctly identified as such. Therefore, we recommend patients with T-scoreHU ≤ -3.0 be referred for further evaluation of metabolic bone disease. Likewise, a T-scoreHU of -2.0 was highly sensitive, meaning a high proportion of osteoporotics are correctly identified as such (figure 3). Based on these results, we recommend against referring patients with T-scoreHU ≥ -2.0 for additional workup, unless otherwise indicated by established screening guidelines or clinical presentation. ROC analysis, which is a comparison of sensitivity (true positive rate) and the quantity [1-specificity] (false positive rate), showed similar AUC for L1, L2, and L3 suggesting that T-scoreHU has good accuracy for discriminating between osteoporotic and non-osteoporotic bone regardless of which lumbar vertebrae is measured.
In addition to allowing calculation of T-scores from vertebral HU, our reference values provide insight into the distribution of vertebral CT attenuation in young women and allows for comparison with other cohorts at increased risk of fracture. These reference values are also a starting point for estimation of age-adjusted risk profiles that may allow future inclusion of vertebral HU as an input for predicting clinical fracture risk and informing treatment decisions.
The small geographic region sampled and a lack of demographic information for the women included in our analysis is a significant limitation to general applicability of these results. Our results are further limited by not screening subjects for conditions that can affect bone mineral density, including menopausal status. We selected reference cohort subjects undergoing lumbar CT independent of the clinical indication with the expectation that this would be a relatively heterogeneous cross-section of the regional population. These reference values should be expanded to include young women from other geographical regions and should be evaluated in prospective studies to determine age-adjusted fracture risk and response to treatment based upon trabecular spinal HU measures. Furthermore, we recognize the potential harm from radiation exposure and do not recommend CT analysis for the sole indication of osteoporosis screening, but rather recognize the clinically relevant information available in CT imaging acquired in appropriate clinical scenarios.
Our findings corroborate previous publications supporting the use of various CT modalities for screening subjects for metabolic bone disease. While it is not recommended for replacing central DXA as the standard of bone density assessment, T-scoreHU opportunistically measured from routine clinical CT studies is a time and cost efficient tool for identifying subjects who are likely to have metabolic bone disease and may benefit from additional studies or treatment.
References
- 1.Smith-Bindman R. Is computed tomography safe? N Engl J Med. 2010 Jul;363(1):1–4. doi: 10.1056/NEJMp1002530. [DOI] [PubMed] [Google Scholar]
- 2.Schreiber JJ, Anderson PA, Rosas HG, Buchholz AL, Au AG. Hounsfield units for assessing bone mineral density and strength: a tool for osteoporosis management. J Bone Joint Surg Am. 2011 Jun;93(11):1057–63. doi: 10.2106/JBJS.J.00160. [DOI] [PubMed] [Google Scholar]
- 3.Romme EA, Murchison JT, Phang KF, Jansen FH, Rutten EP, Wouters EF, et al. Bone attenuation on routine chest CT correlates with bone mineral density on DXA in patients with COPD. J Bone Miner Res. 2012 Nov;27(11):2338–43. doi: 10.1002/jbmr.1678. [DOI] [PubMed] [Google Scholar]
- 4.Pickhardt PJ, Lee LJ, del Rio AM, Lauder T, Bruce RJ, Summers RM, et al. Simultaneous screening for osteoporosis at CT colonography: bone mineral density assessment using MDCT attenuation techniques compared with the DXA reference standard. J Bone Miner Res. 2011 Sep;26(9):2194–203. doi: 10.1002/jbmr.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miyabara Y, Holmes D, Camp J, Miller VM, Kearns AE. Comparison of calibrated and uncalibrated bone mineral density by CT to DEXA in menopausal women. Climacteric. 2012 Aug;15(4):374–81. doi: 10.3109/13697137.2011.618566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Budoff MJ, Khairallah W, Li D, Gao YL, Ismaeel H, Flores F, et al. Trabecular bone mineral density measurement using thoracic and lumbar quantitative computed tomography. Acad Radiol. 2012 Feb;19(2):179–83. doi: 10.1016/j.acra.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 7. NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy, March 7- 29, 2000 highlights of the conference. South Med J. 2001 Jun;94(6):569–73. [PubMed] [Google Scholar]
- 8.Cauley JA, Hochberg MC, Lui LY, Palermo L, Ensrud KE, Hillier TA, et al. Long-term risk of incident vertebral fractures. JAMA. 2007 Dec;298(23):2761–7. doi: 10.1001/jama.298.23.2761. [DOI] [PubMed] [Google Scholar]
- 9.Curtis JR, Sharma P, Arora T, Bharat A, Barnes I, Morrisey MA, et al. Physicians’ explanations for apparent gaps in the quality of rheumatology care: results from the US Medicare Physician Quality Reporting System. Arthritis Care Res (Hoboken) 2013 Feb;65(2):235–43. doi: 10.1002/acr.21713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. World Health Organ Tech Rep Ser. 1994;843:1–129. [PubMed] [Google Scholar]
- 11.Kanis JA, Melton LJ, Christiansen C, Johnston CC, Khaltaev N. The diagnosis of osteoporosis. J Bone Miner Res. 1994 Aug;9(8):1137–41. doi: 10.1002/jbmr.5650090802. [DOI] [PubMed] [Google Scholar]
- 12.Engelke K, Adams JE, Armbrecht G, Augat P, Bogado CE, Bouxsein ML, et al. Clinical use of quantitative computed tomography and peripheral quantitative computed tomography in the management of osteoporosis in adults: the 2007 ISCD Official Positions. J Clin Densitom. 2008 Jan-Mar;11(1):123–62. doi: 10.1016/j.jocd.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 13.Perilli E, Briggs AM, Kantor S, Codrington J, Wark JD, Parkinson IH, et al. Failure strength of human vertebrae: prediction using bone mineral density measured by DXA and bone volume by micro-CT. Bone. 2012 Jun;50(6):1416–25. doi: 10.1016/j.bone.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 14.Bolotin HH. DXA in vivo BMD methodology: an erroneous and misleading research and clinical gauge of bone mineral status, bone fragility, and bone remodelling. Bone. 2007 Jul;41(1):138–54. doi: 10.1016/j.bone.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 15.Summers RM, Baecher N, Yao J, Liu J, Pickhardt PJ, Choi JR, et al. Feasibility of simultaneous computed tomographic colonography and fully automated bone mineral densitometry in a single examination. J Comput Assist Tomogr. 2011 Mar-Apr;35(2):212–6. doi: 10.1097/RCT.0b013e3182032537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pickhardt PJ, Pooler BD, Lauder T, del Rio AM, Bruce RJ, Binkley N. Opportunistic screening for osteoporosis using abdominal computed tomography scans obtained for other indications. Ann Intern Med. 2013 Apr;158(8):588–95. doi: 10.7326/0003-4819-158-8-201304160-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cicchetti DV, Sparrow SA. Developing criteria for establishing interrater reliability of specific items: applications to assessment of adaptive behavior. Am J Ment Defic. 1981 Sep;86(2):127–37. [PubMed] [Google Scholar]
- 18.Delmas PD, Seeman E. Changes in bone mineral density explain little of the reduction in vertebral or nonvertebral fracture risk with anti-resorptive therapy. Bone. 2004 Apr;34(4):599–604. doi: 10.1016/j.bone.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 19.Sambrook PN, Geusens P. The epidemiology of osteoporosis and fractures in ankylosing spondylitis. Ther Adv Musculoskelet Dis. 2012 Aug;4(4):287–92. doi: 10.1177/1759720X12441276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steiger P, Block JE, Steiger S, Heuck AF, Friedlander A, Ettinger B, et al. Spinal bone mineral density measured with quantitative CT: effect of region of interest, vertebral level, and technique. Radiology. 1990 May;175(2):537–43. doi: 10.1148/radiology.175.2.2326479. [DOI] [PubMed] [Google Scholar]
- 21.Guglielmi G, Floriani I, Torri V, Li J, van Kuijk C, Genant HK, et al. Effect of spinal degenerative changes on volumetric bone mineral density of the central skeleton as measured by quantitative computed tomography. Acta Radiol. 2005 May;46(3):269–75. doi: 10.1080/02841850510012661. [DOI] [PubMed] [Google Scholar]
- 22.Siris ES, Brenneman SK, Barrett-Connor E, Miller PD, Sajjan S, Berger ML, et al. The effect of age and bone mineral density on the absolute, excess, and relative risk of fracture in postmenopausal women aged 50-99 results from the National Osteoporosis Risk k (NORA) Osteoporos Int. 2006;17(4):565–74. doi: 10.1007/s00198-005-0027-4. [DOI] [PubMed] [Google Scholar]
- 23.Budoff MJ, Hamirani YS, Gao YL, Ismaeel H, Flores FR, Child J, et al. Measurement of thoracic bone mineral density with quantitative CT. Radiology. 2010 Nov;257(2):434–40. doi: 10.1148/radiol.10100132. [DOI] [PubMed] [Google Scholar]
- 24.Mueller DK, Kutscherenko A, Bartel H, Vlassenbroek A, Ourednicek P, Erckenbrecht J. Phantom-less QCT BMD system as screening tool for osteoporosis without additional radiation. Eur J Radiol. 2011 Sep;79(3):375–81. doi: 10.1016/j.ejrad.2010.02.008. [DOI] [PubMed] [Google Scholar]


