Role of combination therapy in treating raised LDL cholesterol in high-risk patients
Cholesterol carried in low-density lipoprotein cholesterol (LDL-C) and other apolipoprotein B-containing lipoproteins plays a causal role in atherogenesis,1–3 and, accordingly, is a key target in the prevention of atherosclerotic cardiovascular disease (ASCVD).2,3 Clinical trial evidence indicates that the magnitude of the benefit from LDL-C lowering is independent of the means by which it is achieved and is proportionate to the absolute decrease in lipoprotein level.1–4 Further, the relative risk reduction (RRR) appears the same, regardless of patient demographics and background medical history.1–4 Most guidelines (e.g. Piepoli et al 2016)2 recommend goals for LDL-C lowering that, while somewhat artificial and idealized constructs because the association between LDL-C and risk is continuous,1 have been useful clinically as a metric of therapeutic success. In clinical practice, however, not all patients achieve their LDL-C goal with statins alone, and increasing recognition of this treatment gap has led to the need to consider the routine use of combination lipid-lowering therapies.
The cholesterol absorption inhibitor ezetimibe added to statin achieves an incremental reduction in LDL-C of typically 20–25% and has been shown to provide a further decrease in ASCVD risk.5 Hence, this agent is now recommended for patients not achieving their lipid goal on maximum tolerated statin dose.2,3 Recently, the introduction of proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors has made possible more profound LDL-C lowering of the order of 50–60%,6,7 and both currently marketed agents—evolocumab and alirocumab—have been approved for use in selected patient groups [those with severe ASCVD and those with primary hypercholesterolaemia, especially familial hypercholesterolaemia (FH)] with an inadequate response to maximally tolerated statin therapy or statin intolerance. With the publication of the FOURIER trial, we have evidence that the addition of PCSK9 inhibitors to statin delivers significant reduction in the risk of cardiovascular events, in line with the predicted benefit from meta-regression analysis.8
Cost-effectiveness analyses of PCSK9 inhibitor therapy
Although current European and US guidelines indicate that the use of PCSK9 inhibitors is appropriate in specific patients,9,10 uptake has been low, in most part due to their perceived expense.11 Cost-effectiveness analyses are essential in determining whether, how, and when PCSK9 inhibitor treatment meets accepted metrics of value for money. However, the different models published to date have produced widely varying and potentially confusing results. Eight reports on the health economics of PCSK9 inhibitor use have appeared: four academic-led12–15 and four supported by industry.16–19 Of these, three analyses were based on the FOURIER data.14,15,19 In addition, health technology appraisals were undertaken by the UK National Institute for Health and Care Excellence (NICE) for alirocumab and evolocumab.20
According to the US Institute for Clinical and Economic Review (ICER) analysis, the use of PCSK9 inhibitors did not meet a threshold of cost-effectiveness, defined in the study as $100 000 per quality-adjusted life year (QALY) gained, in patients with heterozygous FH (primary prevention) or ASCVD.12 The analysis was based on the Cardiovascular Disease Policy Model that adopted a US health system perspective and a price for PCSK9 inhibitors of $14 350 per annum. Estimates of LDL-C reduction were based on the published literature, and statins, ezetimibe, and PCSK9 inhibitors were all assumed to achieve similar reductions in the risk of cardiovascular events per mg/dL decrease in LDL-C. Addition of a PCSK9 inhibitor to statin in ASCVD patients was estimated to save 4.3 million major cardiovascular events with an increment of 7.9 million QALYs at a cost of $414 000 per QALY gained. This analysis was updated recently using the findings of the FOURIER trial.14 Cost-effectiveness was recalculated at $450 000 per QALY in ASCVD patients, compared with eztimibe, a less optimistic value than the initial estimate; the authors stated that price reductions of the order of >71% would be required to meet the $100 000 per QALY threshold.
In a further US-based analysis, Arrieta et al.13 assumed annual costs of $14 000–$15 000, and a hypothetical patient population based on those enrolled in the OSLER-1 and -2 extension studies,6 with starting LDL-C levels of 120 mg/dL. Again, the authors concluded that PCSK9 inhibitors are not cost-effective, with an estimated incremental cost of approximately $350 000 per QALY compared with background therapy. In contrast, the US-based model developed by Gandra et al.16 estimated an incremental cost-effectiveness ratio of only $75 800 per QALY for the lifetime use of evolocumab (based on an annual price of $14 139) in patients with heterozygous FH. Corresponding estimates for patients with ASCVD and statin-intolerant ASCVD were $141 700 and $100 300 per QALY. In this model comparing evolocumab with background therapy, baseline LDL-C levels were high at 156.5 mg/dL in heterozygous FH, 141.3 mg/dL in ASCVD, and 189.4 mg/dL in statin-intolerant ASCVD. The magnitude of LDL-C lowering was based on data from Phase 3 studies and estimated effects on cardiovascular event rates on the meta-analysis of statin trials (22% reduction in events per 1.0 mmol/L reduction in LDL-C).4
The model by Toth et al.17 also reported cost-effectiveness of evolocumab, based on ‘real-world’ cardiovascular disease (CVD) burden data (which in general reveal patients to be at higher risk than is seen in clinical trial cohorts) from the UK Clinical Practice Research Datalink, and assuming a payer discount of >20% for the price of evolocumab (list price $14 100). The target patient population was derived from eligibility criteria in the FOURIER study8 and predicted reductions in cardiovascular event rates were based on meta-regression.4 For patients with ASCVD and baseline LDL-C levels ≥70 mg/dL and ≥100 mg/dL, the justifiable value-based price range under a willingness-to-pay threshold of $150 000 per QALY gained for evolocumab was $11 990–$16 856.
In a study from the Spanish National Health System perspective, Villa et al.18 concluded that evolocumab may be cost-effective, assuming a target population with FH or prior cardiovascular event history, LDL-C >100 mg/dL, LDL-C lowering as in Phase 3 trials, cardiovascular event reduction again based on meta-regression,4 and lifetime treatment at a cost of €4969 per annum. Incremental cost-effectiveness ratios were found to be €30 893 for FH and €45 340 for the secondary prevention population.
Finally, based on the FOURIER data applied to a real-life US population and assuming a 29% price discount, Fonarow et al.19 found an incremental cost-effectiveness ratio of $166 000 per QALY. These authors did not compare with ezetimibe but with standard background therapy. The baseline risk level corresponded to a total (initial plus recurrent) cardiovascular event rate of 6.4 per 100 patient-years.
Hernandez did not perform a cost-effectiveness analysis but calculated the societal impact of a health outcomes-based agreement whereby industry would not charge for PCSK9 inhibitors for those patients with events despite treatment.15 The author concluded that such a scheme would not lead to sufficiently low prices.
Assessments of alirocumab and evolocumab conducted by NICE indicated that these drugs could be cost-effective in the UK National Health Service setting.20 The approach assumed annual alirocumab costs of £4383 and starting LDL-C levels of 3.5 mmol/L for heterozygous FH patients with CVD and non-FH patients at very high risk, 4.0 mmol/L for non-heterozygous FH patients at high risk for CVD, and 5.0 mmol/L for heterozygous FH patients without CVD. LDL-C lowering was informed by the ODYSSEY trials,7 and the hazard ratio per 1.0 mmol/L reduction in LDL-C was estimated at 0.79.4 Example incremental costs per QALY gained for alirocumab were estimated as £37 000 (LDL-C ≥4 mmol/L) for heterozygous FH primary prevention, £24 000 (LDL-C ≥2.5 mmol/L) for heterozygous FH secondary prevention, £44 300 (LDL-C ≥2.5 mmol/L) for patients with ‘high-risk’ CVD, and £34 000 (LDL-C ≥2.5 mmol/L) for patients at ‘very high risk’.
There are noteworthy issues in the models described above. For example, Villa et al.18 assumed that the risk reduction of heart failure was the same as that of myocardial infarction. If that assumption is not made, the cost-effectiveness ratio increases to €62 000 per QALY. A further consideration is the treatment of stroke. In the report by Kazi et al.,12 the disutility applied to this outcome was surprisingly low, and because FOURIER found a significant stroke benefit from only 2 years of treatment,8 this would lead to an underestimate of cost-effectiveness.
However, as indicated by Toth et al.,17 the variation in outcome from these cost-effectiveness studies is driven (next to the assumed acquisition cost of the medicines) primarily by the estimation of absolute risk (of both first and recurrent events) rather than fundamental differences in modelling approach. Ongoing risk of future cardiovascular events is hard to define precisely for the populations of interest, given the dearth of long-term follow-up data in FH and the wide range of risk in subjects with established ASCVD.2,3,21 With the above economic results in mind, it seems more appropriate at the moment to base the use of these agents on a stratification of risk and benefit than to promulgate general deployment in secondary prevention.
‘Highest risk–highest benefit’ approach to PCSK9 inhibitor use
The first element of a potentially useful ‘stratified-medicine’ strategy is to define ‘highest risk’, i.e. those with the highest baseline event rate. The level of risk required to justify the use of PCSK9 inhibitors will preclude their use in asymptomatic individuals without prevalent vascular disease (primary prevention), with the exception of FH with high LDL-C despite statin therapy, and so secondary prevention is the main focus. ‘Highest risk’ categories are polyvascular disease, ASCVD with co-morbidities such as chronic kidney disease or diabetes with end-organ damage or FH patients with a CVD event (Table 1).20–22 In this context, it would be worthwhile applying the risk stratification tools as has been done for acute coronary syndrome patients with ezetimibe/statin combination therapy.21 The second element is to identify patients receiving the ‘highest benefit’. Because the RRR is proportionate to the absolute decrease in LDL-C level in mmol/L,1–4 and the magnitude of percent LDL-C reduction with PCSK9 inhibitor therapy appears similar across baseline LDL-C subgroups,6,7 then patients with the highest starting LDL-C will achieve the greatest absolute reduction in LDL-C and hence the greatest RRR on the drugs.
Table 1.
Categories of ‘highest risk’ for ASCVD (around or above a benchmark of 30% 10-year risk) on statin therapy, based on published trial data
| Category | Projected 10-year risk on moderate- or high-intensity statin therapy (%) |
|---|---|
| Clinical ASCVD + diabetes | 28–38 |
| No CKD | 26–29 |
| With CKD | 28–43 |
| Clinical ASCVD + CKD | 34–35 |
| Recent acute coronary syndrome (<3 months) | 32 |
| CHD and poorly controlled risk factors | 28–41 |
| CHD and peripheral vascular disease | 43–55 |
| CHD and age ≥65 years | 21–54 |
| Stroke/transient ischaemic attack and male | 31 |
| CHD and familial hypercholesterolaemia (baseline LDL-C ≥190 mg/dL) | 41 |
ASCVD, atherosclerotic cardiovascular disease; CHD, coronary heart disease; CKD, chronic kidney disease.
Adapted from Robinson et al.22
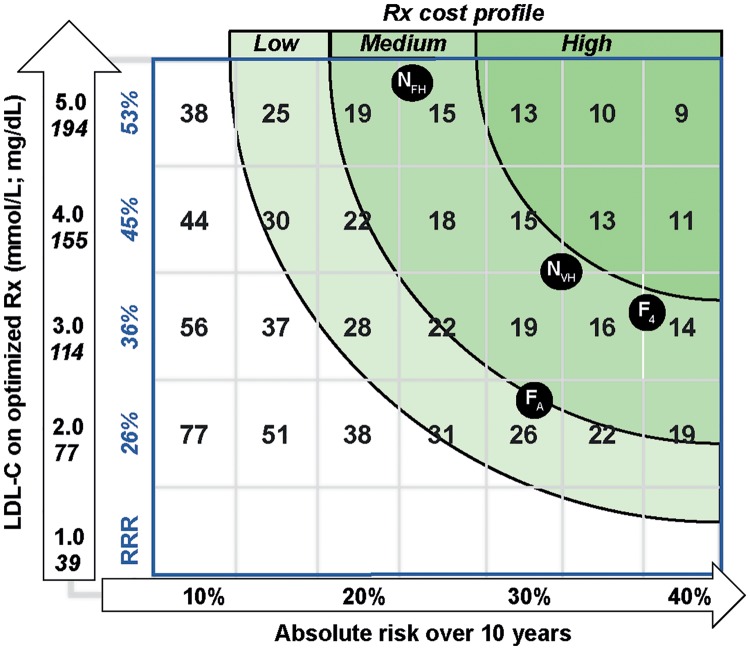
Given the above, it is possible to construct a relatively simple matrix that provides a framework for optimizing the use of these agents. Figure 1 depicts the gradient of ‘number needed to treat’ (NNT, over 5 years) based on the estimated risk of a future ASCVD event, the starting LDL-C, and the average RRR associated with a drug-induced LDL-C drop of 60%.6–8 Estimated NNT is lower (incremental cost-effectiveness ratio lower) in individuals with the greatest CVD risk and largest absolute LDL-C reduction. This is a conceptual expansion of the tables generated by NICE (in TA393 and TA394)20 and the approach advocated by Robinson et al.22
Figure 1.
‘Highest risk–highest benefit’ strategy for proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor use. The schematic shows LDL-C on optimum statin/ezetimibe therapy on the vertical axis and risk of a cardiovascular disease event on the horizontal axis. Predicted relative risk reduction (RRR) associated with a PCSK9 inhibitor-induced 60% decrease in LDL-C is in the first column. This is based on a 22% risk reduction per 1.0 mmol/L drop in LDL-C as derived by meta-regression4 and confirmed by the FOURIER investigators.8 Number-needed-to-treat (NNT) (reciprocal of the absolute risk reduction) is provided per 5% increment in risk and 1.0 mmol/L increment in LDL-C in the other columns. These are given for a 5-year timescale. Varying cost profiles are represented by the shades of green. A low-cost jurisdiction may permit NNT <30 as an acceptable threshold; medium-cost NNT <20; and high-cost NNT <15.22 For illustration purposes, markers are provided for the approximate NNT for the average FOURIER subject (FA; LDL-C 92 mg/dL, estimated annual risk 3.3%), a FOURIER subject in the top LDL-C quartile (F4; LDL-C 126 mg/dL; annual risk 3.8%),8 and the National Institute for Health and Care Excellence (NICE)-approved categories (NVH—very high-risk, i.e. polyvascular disease or multiple events, LDL-C >3.5 mmol/L; NFH—FH no event with LDL-C >5.0 mmol/L). Rx, prescription.
Depending on the prevailing price of PCSK9 inhibitors, bands of NNT that are payer-acceptable could be defined as an aid to prescribers—this is shown in the shaded green regions in Figure 1. For example, in the NICE alirocumab appraisal, it was calculated that, to achieve the cost-effectiveness threshold of £30 000 per QALY gained, the baseline LDL-C level would need to be ∼5.0 mmol/L for primary prevention in FH and ∼3.5 mmol/L in very high-risk patients. Similarly, Robinson et al.22 showed that addition of a PCSK9 inhibitor provides a 5-year NNT ≤50 to prevent one cardiovascular event in very high-risk and high-risk patients with LDL-C ≥70 mg/dL and a 5-year NNT ≤30 for very high-risk and high-risk patients with LDL-C ≥130 mg/dL. It should be noted that in most health care systems, it is likely that the ‘prevailing price’ will need to be substantially below the list price for these drugs to be cost-effective in a broad range of target patient types (and greater transparency on drug discounts would aid decision-making). The strategy depicted in Figure 1 captures not only the potential clinical benefit for patient types at a given LDL-C level but also could be used to formulate the cost-effectiveness to society based on the range of NNT considered appropriate to an overall budget for these agents.
Conclusion
A simple strategy is needed to help physicians in the selection of patients suitable for PCSK9 inhibitor therapy, and we commend the ‘highest risk–highest benefit’ concept and advocate the development of simple aids (such as Figure 1) for clinicians to implement this framework. The aids should be adapted to the prevalent cost of these drugs in various pricing jurisdictions.
Authors’ contributions
All authors contributed to the conceptual background and content of the publication.
Acknowledgements
Medical writing support was provided by Liz Anfield, Prime, Knutsford, Cheshire, UK, funded by Regeneron Pharmaceuticals, Inc. and Sanofi. Responsibility for opinions, conclusions, and interpretation of the data lies with the authors.
Funding
This work was supported by Regeneron Pharmaceuticals, Inc. and Sanofi.
Conflict of interest: L.A. reports grants received from Sanofi and Amgen for participating at advisory boards and providing lectures. C.J.P. reports grants received from Roche and MSD, honoraria from Amgen, MSD, Pfizer, Regeneron Pharmaceuticals, Inc., and Sanofi for lectures and advisory boards. A.B. reports receiving consultancy payments from Sanofi and Amgen. K.K.R. reports grants from Sanofi, Regeneron Pharmaceuticals, Inc., Pfizer, Amgen, and MSD and personal fees from Sanofi, Amgen, Regeneron Pharmaceuticals, Inc., Lilly, Medicines Company, Astra Zeneca, Pfizer, Kowa, Algorithm, Ionis, Esperion, Novo Nordisk, Takeda, Boehringer Ingelheim, Resverlogix, and AbbVie.
Footnotes
The opinions expressed in this article are not necessarily those of the Editors of the European Heart Journal or of the European Society of Cardiology.
References
- 1. Ference BA, Ginsberg HN, Graham I, Ray KK, Packard CJ, Bruckert E, Hegele R, Krauss RM, Raal FJ, Schunkert H, Watts GC, Borén J, Fazio S, Horton JD, Masana L, Nicholls SJ, Nordestgaard BG, van de Sluis B, Taskinen MR, Tokgözoglu L, Landmesser U, Laufs U, Wiklund O, Stock JK, Chapman MJ, Catapano AL.. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J 2017;38:2459–2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, Cooney M-T, Corrà U, Cosyns B, Deaton C, Graham I, Hall MS, Hobbs FDR, Løchen M-L, Löllgen H, Marques-Vidal P, Perk J, Prescott E, Redon J, Richter DJ, Sattar N, Smulders Y, Tiberi M, van der Worp HB, van Dis I, Verschuren WMM, De Backer G, Roffi M, Aboyans V, Bachl N, Bueno H, Carerj S, Cho L, Cox J, De Sutter J, Egidi G, Fisher M, Fitzsimons D, Franco OH, Guenoun M, Jennings C, Jug B, Kirchhof P, Kotseva K, Lip GYH, Mach F, Mancia G, Bermudo FM, Mezzani A, Niessner A, Ponikowski P, Rauch B, Rydén L, Stauder A, Turc G, Wiklund O, Windecker S, Zamorano JL.. 2016 European guidelines on cardiovascular disease prevention in clinical practice. Eur J Prev Cardiol 2016;23:NP1–NP96. [DOI] [PubMed] [Google Scholar]
- 3. Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd-Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC Jr, Watson K, Wilson PW, Eddleman KM, Jarrett NM, LaBresh K, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC Jr, Tomaselli GF.. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. Circulation 2014;129(25 Suppl 2):S1–S45. [DOI] [PubMed] [Google Scholar]
- 4. Cholesterol Treatment Trialists’ (CTT) Collaboration; Fulcher J, O’Connell R, Voysey M, Emberson J, Blackwell L, Mihaylova B, Simes J, Collins R, Kirby A, Colhoun H, Braunwald E, La Rosa J, Pedersen TR, Tonkin A, Davis B, Sleight P, Franzosi MG, Baigent C, Keech A.. Efficacy and safety of LDL-lowering therapy among men and women: meta-analysis of individual data from 174, 000 participants in 27 randomised trials. Lancet 2015;385:1397–1405. [DOI] [PubMed] [Google Scholar]
- 5. Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, Darius H, Lewis BS, Ophuis TO, Jukema JW, De Ferrari GM, Ruzyllo W, De Lucca P, Im K, Bohula EA, Reist C, Wiviott SD, Tershakovec AM, Musliner TA, Braunwald E, Califf RM.. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med 2015;372:2387–2397. [DOI] [PubMed] [Google Scholar]
- 6. Sabatine MS, Giugliano RP, Wiviott SD, Raal FJ, Blom DJ, Robinson J, Ballantyne CM, Somaratne R, Legg J, Wasserman SM, Scott R, Koren MJ, Stein EA.. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med 2015;372:1500–1509. [DOI] [PubMed] [Google Scholar]
- 7. Robinson JG, Farnier M, Krempf M, Bergeron J, Luc G, Averna M, Stroes ES, Langslet G, Raal FJ, El Shahawy M, Koren MJ, Lepor NE, Lorenzato C, Pordy R, Chaudhari U, Kastelein JJ.. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med 2015;372:1489–1499. [DOI] [PubMed] [Google Scholar]
- 8. Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, Kuder JF, Wang H, Liu T, Wasserman SM, Sever PS, Pedersen TR; FOURIER Steering Committee Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med 2017;376:1713–1722. [DOI] [PubMed] [Google Scholar]
- 9. Lloyd-Jones DM, Morris PB, Ballantyne CM, Birtcher KK, Daly DD Jr, DePalma SM, Minissian MB, Orringer CE, Smith SC.. Jr. 2016 ACC expert consensus decision pathway on the role of non-statin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: a report of the American College of Cardiology task force on clinical expert consensus documents. J Am Coll Cardiol 2016;68:92–125. [DOI] [PubMed] [Google Scholar]
- 10. Landmesser U, John Chapman M, Farnier M, Gencer B, Gielen S, Hovingh GK, Luscher TF, Sinning D, Tokgozoglu L, Wiklund O, Zamorano JL, Pinto FJ, Catapano AL.. European Society of Cardiology/European Atherosclerosis Society task force consensus statement on proprotein convertase subtilisin/kexin type 9 inhibitors: practical guidance for use in patients at very high cardiovascular risk. Eur Heart J 2017;38:2245–2255. [DOI] [PubMed] [Google Scholar]
- 11. Sabatine MS. Proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors: comparing and contrasting guidance across the Atlantic. Eur Heart J 2017;38:2256–2258. [DOI] [PubMed] [Google Scholar]
- 12. Kazi DS, Moran AE, Coxson PG, Penko J, Ollendorf DA, Pearson SD, Tice JA, Guzman D, Bibbins-Domingo K.. Cost-effectiveness of PCSK9 inhibitor therapy in patients with heterozygous familial hypercholesterolemia or atherosclerotic cardiovascular disease. JAMA 2016;316:743–753. [DOI] [PubMed] [Google Scholar]
- 13. Arrieta A, Page TF, Veledar E, Nasir K, Cox D.. Economic evaluation of PCSK9 inhibitors in reducing cardiovascular risk from health system and private payer perspectives. PLoS One 2017;12:e0169761.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kazi DS, Penko J, Coxson PG, Moran AE, Ollendorf DA, Tice JA, Bibbins-Domingo K.. Updated cost-effectiveness analysis of PCSK9 inhibitors based on the results of the FOURIER trial (letter). JAMA 2017;318:748–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hernandez I. Revisiting outcomes–based pricing propositions for the PCSK9 inhibitor evolocumab. JAMA Int Med 2017;177:1388–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gandra SR, Villa G, Fonarow GC, Lothgren M, Lindgren P, Somaratne R, van Hout B.. Cost-effectiveness of LDL-C lowering with evolocumab in patients with high cardiovascular risk in the United States. Clin Cardiol 2016;39:313–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Toth PP, Danese M, Villa G, Qian Y, Beaubrun A, Lira A, Jansen JP.. Estimated burden of cardiovascular disease and value-based price range for evolocumab in a high-risk, secondary-prevention population in the US payer context. J Med Econ 2017;20:555–564. [DOI] [PubMed] [Google Scholar]
- 18. Villa G, Lothgren M, Kutikova L, Lindgren P, Gandra SR, Fonarow GC, Sorio F, Masana L, Bayes-Genis A, Hout BV.. Cost-effectiveness of evolocumab in patients with high cardiovascular risk in Spain. Clin Ther 2017;39:771–786. [DOI] [PubMed] [Google Scholar]
- 19. Fonarow GC, Keech AC, Pedersen TR, Giugliano RP, Sever PS, Lindgren P, van Hout B, Villa G, Qian Y, Somaratne R, Sabatine MS.. Cost-effectiveness of evolocumab therapy for reducing cardiovascular events in patients with atherosclerotic cardiovascular disease. JAMA Cardiol 2017;2:1069–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. National Institute for Health and Care Excellence. Alirocumab for treating primary hypercholesterolaemia and mixed dyslipidaemia. Technology appraisal guidance [TA393]. 22 June 2016, and Evolocumab for treating primary hypercholesterolaemia; and mixed dyslipidaemia. Technology appraisal guidance [TA394]. 22 June 2016. https://www.nice.org.uk/guidance/ta393 and https://www.nice.org.uk/guidance/TA394 (23 February 2017).
- 21. Bohula EA, Morrow DA, Giugliano RP, Blazing MA, He P, Park JG, Murphy SA, White JA, Kesaniemi YA, Pedersen TR, Brady AJ, Mitchel Y, Cannon CP, Braunwald E.. Atherothrombotic risk stratification and ezetimibe for secondary prevention. J Am Coll Cardiol 2017;69:911–921. [DOI] [PubMed] [Google Scholar]
- 22. Robinson JG, Huijgen R, Ray K, Persons J, Kastelein JJ, Pencina MJ.. Determining when to add nonstatin therapy: a quantitative approach. J Am Coll Cardiol 2016;68:2412–2421. [DOI] [PubMed] [Google Scholar]