Abstract
Aims
To objectively appraise evidence for possible adverse effects of long-term statin therapy on glucose homeostasis, cognitive, renal and hepatic function, and risk for haemorrhagic stroke or cataract.
Methods and results
A literature search covering 2000–2017 was performed. The Panel critically appraised the data and agreed by consensus on the categorization of reported adverse effects. Randomized controlled trials (RCTs) and genetic studies show that statin therapy is associated with a modest increase in the risk of new-onset diabetes mellitus (about one per thousand patient-years), generally defined by laboratory findings (glycated haemoglobin ≥6.5); this risk is significantly higher in the metabolic syndrome or prediabetes. Statin treatment does not adversely affect cognitive function, even at very low levels of low-density lipoprotein cholesterol and is not associated with clinically significant deterioration of renal function, or development of cataract. Transient increases in liver enzymes occur in 0.5–2% of patients taking statins but are not clinically relevant; idiosyncratic liver injury due to statins is very rare and causality difficult to prove. The evidence base does not support an increased risk of haemorrhagic stroke in individuals without cerebrovascular disease; a small increase in risk was suggested by the Stroke Prevention by Aggressive Reduction of Cholesterol Levels study in subjects with prior stroke but has not been confirmed in the substantive evidence base of RCTs, cohort studies and case–control studies.
Conclusion
Long-term statin treatment is remarkably safe with a low risk of clinically relevant adverse effects as defined above; statin-associated muscle symptoms were discussed in a previous Consensus Statement. Importantly, the established cardiovascular benefits of statin therapy far outweigh the risk of adverse effects.
Keywords: Statin, Adverse effects, Glucose homeostasis, Metabolic syndrome, Cognitive function, Renal function, Liver function, Haemorrhagic stroke, Cataract
Introduction
Statins [3-hydroxy-3-methylglutaryl coenzyme A reductase (HMG-CoA) inhibitors] are recommended as the treatment of first choice for management of hypercholesterolaemia and combined hyperlipidaemia by European guidelines for cardiovascular disease (CVD) prevention and lipid management.1,2 The efficacy of these agents in decreasing low-density lipoprotein cholesterol (LDL-C), a causal factor in the pathophysiology of atherosclerotic cardiovascular disease, and in preventing both first and recurrent cardiovascular events (with or without type 2 diabetes), is indisputable.2–4
Large randomized controlled trials (RCTs) have clearly established the benefit/risk ratio of this treatment.4,5 Since several trials are evaluating the effects of a statin-containing polypill on modifiable risk factors,6 the use of statins is likely to expand into a wider cross-section of the population. Consequently, critical appraisal of evidence relating to possible unintended effects of long-term statin therapy is needed, on the one hand to accurately assess their incidence, and on the other, to place often exaggerated perceptions of side effects among patients, the general public and some healthcare providers, in their correct perspective.
Data from RCTs provide reliable information on the safety of statin therapy, but this information relates to the specific patient populations which fulfilled the inclusion criteria and were treated for a relatively short duration, typically less than 5 years. Less frequent adverse effects of treatment may only emerge after long-term exposure in very large numbers of patients. For example, while single studies were contradictory with respect to the risk of new-onset diabetes mellitus (DM),7,8 meta-analyses and large data bases provided clear evidence, especially in susceptible individuals with the risk factor cluster of the metabolic syndrome who may already be in a pre-diabetic state.9
It remains to be seen if the pharmacology of different statins (Table 1) is relevant to the issue of statin side effects.10 Indeed, the metabolism of statins is distinct. For example, genetic differences in the activity of the cytochrome P450 (CYP) system can affect statin interactions with other drugs, whereas genetic differences in membrane transporters can alter first pass hepatic uptake, a major determinant of residual circulating concentrations and ultimately of peripheral tissue exposure.11 The issues described above highlight the critical need for an objective appraisal of adverse effects attributed to statins in order to differentiate the perception from the reality of the potential risks associated with statin therapy, specifically on glucose homeostasis, and cognitive, renal and hepatic function, as well as the risk for haemorrhagic stroke and cataract. This appraisal will provide important evidence-based information not only for patients, clinicians and the wider spectrum of healthcare professionals, but also for public health policy makers.
Table 1.
Comparative pharmacology of statins
| Increasing lipophilicity |
|||||||
|---|---|---|---|---|---|---|---|
| Lovastatin | Simvastatin | Atorvastatin | Pitavastatin | Fluvastatin | Rosuvastatin | Pravastatin | |
| IC50 HMG-CoA reductase (nM) | 2–4 | 1–2 (active metabolite) | 1.16 | 0.1 | 3–10 | 0.16 | 4 |
| Oral absorption (%) | 30 | 60–85 | 30 | 80 | 98 | 50 | 35 |
| Bioavailability (%) | 5 | <5 | 12 | 60 | 30 | 20 | 18 |
| Protein binding (%) | >98 | >95 | >98 | 96 | >98 | 90 | 50 |
| Half life (h) | 2–5 | 2–5 | 7–20 | 10–13 | 1–3 | 20 | 1–3 |
| Metabolism by CYP450 | 3A4 (?2C8) | 3A4 (2C8, 2D6) | 3A4 (2C8) | (2C9) | 2C9 | 2C9 (2C19) | (3A4) |
| Cellular transporter | OATP1B1 | (MRP2) | OATP1B1 | OATP1B1 (MRP2) | OATP1B1 | OATP1B1 | OATP1B1 (MRP2) |
| Daily dose (mg) | 10–40 | 10–40 | 10–80 | 1–4 | 80 (retard formulation) | 5–40 | 10–40 |
Adapted from Sirtori.10
Figures in parentheses indicate a minor metabolic pathway or transporter.
CYP450, cytochrome P450; IC50, 50% inhibitory concentration; HMG-CoA, 3-hydroxy-3-methylglutaryl coenzyme A; MRP2, multidrug resistance-associated protein 2; OATP1B1, Organic Anion Transporting Polypeptide 1B1.
Statin-associated muscle symptoms
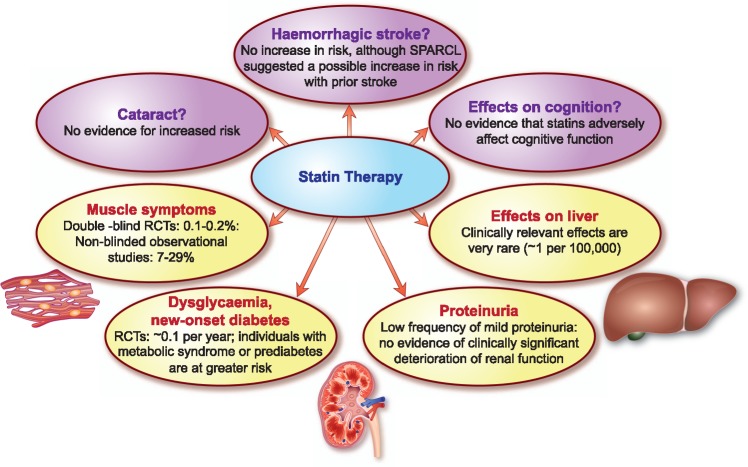
Statin-associated muscle symptoms (SAMS, the focus of a separate Consensus Statement)12 are the predominant adverse effect encountered in clinical practice (Figure 1), and impact adherence and ultimately clinical outcomes (Box 1).13,14 A much-debated issue is whether SAMS represent real or nocebo effects. A nocebo effect is caused by negative expectations about the effects of treatment, arising from information provided by clinicians and/or the media about possible side effects, which lead to higher reporting rates for adverse effects of the treatment than would otherwise be expected.12,15,16 The Anglo-Scandinavian Cardiac Outcomes Trial—Lipid-Lowering Arm (ASCOT-LLA) Study Group addressed this issue by comparing the incidence of four different types of adverse events with statin therapy, including muscle-related symptoms, during both the blinded, placebo-controlled trial and its open-label extension study. They concluded that a nocebo effect may explain the higher incidence of SAMS in observational studies vs. RCTs,17 although others have noted that the overall rate of muscle-related events decreased from 2.03% in the blinded phase to 1.26% when subjects were aware that they were on a statin. Perhaps the take home message for clinicians is that they should be cautious about prematurely attributing muscle symptoms to statin therapy, without further investigation of their cause.
Figure 1.
Overview of the relative prevalence of the main types of adverse effects reported with statin therapy. RCT, randomized controlled trial; SPARCL, Stroke Prevention by Aggressive Reduction in Cholesterol Levels.
Box 1.
Key points about SAMS for clinicians
|
Search strategy
The literature was searched using Medline, Current Contents, PubMed, and relevant references with the terms ‘statin safety’, ‘statin adverse effects’, ‘statin AND cognitive function’, ‘statin AND plasma glucose’, ‘statin AND diabetes’, ‘statin AND renal function’, ‘statin AND hepatic function’, ‘statin AND stroke’, ‘statin AND peripheral neuropathy’, ‘statin AND cardiovascular disease’, ‘statin AND atherosclerosis’, ‘statin AND atherothrombosis’. Main articles published in English between 2000 and 2017 were included, as well as European guidelines on CVD prevention and lipid management.1,2 This Review was based on discussions at meetings of the EAS Consensus Panel organized and chaired by M.J.C. and H.N.G., where the search results and drafts of the Review were critically and comprehensively appraised. The content of this Review resulted from a consensus of considered opinions and insights of the expert members of the Panel.
Effects on glucose homeostasis
Statin therapy is known to be associated with a small increment in fasting blood glucose levels.2 In a meta-analysis of 13 RCTs involving 91 140 subjects without diabetes at baseline, statin treatment increased incident DM by ∼9%, representing one additional case of diabetes (12.23 cases with statin vs. 11.25 cases with control) per 1000 patients per year of exposure, but also prevented five first CVD events. This is, however, an underestimate as multiple recurrent events were not considered.9 Another meta-analysis including ∼40 000 patients with stable coronary heart disease or recent acute coronary syndrome in five RCTs showed that high intensity statin therapy increased the risk of incident DM by 12%, but also reduced the risk of CVD events by 16%, or in absolute terms, prevented 3.5 CVD events for each additional case of diabetes.18 In this analysis, a ‘case of diabetes’ was defined by serum glycated haemoglobin (HbA1c) >6.5, a laboratory finding that has no immediate impact on the quality of life, and therefore should not be compared with outcomes such as stroke or death from myocardial infarction.
The risk of incident DM with statin treatment increases with an increasing number of components of the metabolic syndrome, as shown by post hoc analyses of the Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER), Treating to New Targets (TNT), Incremental Decrease in End Points Through Aggressive Lipid Lowering (IDEAL), and Stroke Prevention by Aggressive Reduction of Cholesterol Levels (SPARCL) trials, especially in individuals with the highest fasting blood glucose levels at initiation of statin therapy; this effect may be substantially higher in women than men.8,19–21 In the Metabolic Syndrome in Men (METSIM) cohort in 8749 men (2142 on a statin) aged 45–73 years with features of the metabolic syndrome but without a diabetes diagnosis, intense statin treatment was associated with a 46% increase in incident DM (11.2% vs. 5.8% in those not on a statin, P < 0.001) over 5.9 years follow-up, representing 10 new cases per 1000 patients per year of exposure.22 These individuals were older, more obese, less physically active, and exhibited lower levels of high-density lipoprotein cholesterol (HDL-C) and higher triglycerides, fasting blood glucose and HbA1c.22 To put these findings in context, the rate of conversion to DM in subjects with confirmed impaired glucose tolerance not on a statin was 110 per 1000 subjects per year of exposure in the Diabetes Prevention Program,23 and 200 per 1000 Japanese participants per year of exposure in the J-PREDICT trial (Odawara M, Late Breaking Studies, American Diabetes Association Congress, 2013).
Among such high risk patients who developed new- onset DM, the risk of CVD events was lower on statin therapy supporting the notion that, at least within the time scale of these trials, potential adverse effects of hyperglycaemia do not negate the benefits of LDL-C reduction.8,24 Furthermore, observational data show that patients who developed DM while receiving a statin not only had a lower rate of macrovascular disease but also microvascular disease complications normally linked to diabetes.25 Thus, the net benefit among high risk patients in need of statins favours their use, consistent with the Joint Task Force guidelines recommendations.1,2,4,5 These data are consistent with findings among patients with DM treated with statins who derive a similar relative risk (RR) reduction per unit reduction in LDL-C but a greater absolute benefit.4,26
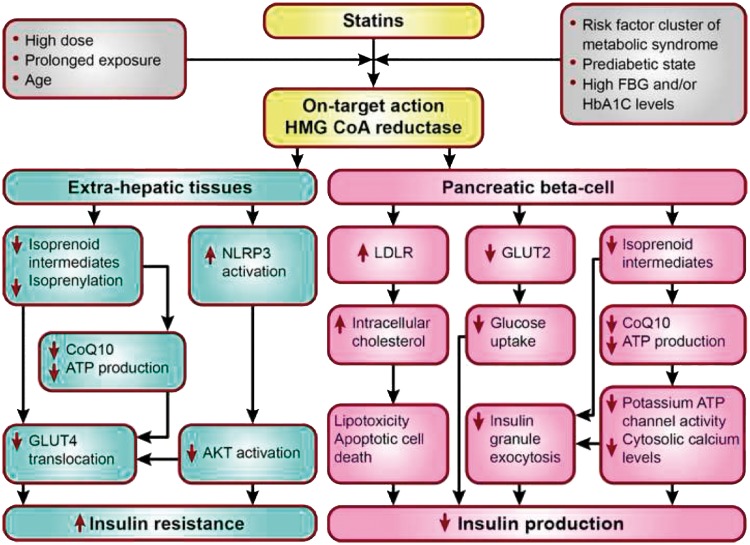
Determining whether the effect of statins on DM risk is an on-target (i.e. inhibition of HMG-CoA reductase) or off-target action will help in understanding whether the effect of a statin on glucose metabolism is a drug or drug class effect. Mechanistically, statins could increase blood glucose by increasing insulin resistance, possibly mediated by changes in circulating free fatty acids,27 impairing beta- cell function, or alternative mechanisms, or a combination of these (Figure 2).28 Indeed, a meta-analysis of new-onset DM and weight change data from up to 20 major RCTs (n = 129 170) also showed that patients who received a statin gained on average 0.24 kg compared with control at study close.29 This overall question was clarified by a Mendelian randomization study in ∼200 000 individuals, in which the associations between common genetic variants (rs17238484 and rs12916) of the HMGCR gene, the target of statins, and body weight, body mass index (BMI), waist circumference, plasma insulin and glucose, and DM risk were evaluated.29 These two variants were not only associated with lower LDL-C at a genome wide level of significance, but also a small increase in the risk of DM, and higher blood glucose, insulin levels, body weight, waist circumference and BMI (Table 2).29–34 Other meta-analyses of genome-wide association studies of BMI30 and plasma insulin31 revealed directionally concordant associations of the same variants (or suitable proxies) with both these traits, although associations of both variants with fasting insulin were not statistically significant after adjustment for BMI. Long-term follow-up from the METSIM cohort showed that the increased DM risk with statin therapy was attributable to decreases in insulin sensitivity and insulin secretion,21 although recent reports associated the gut microbiota and the metabolomic profile with these metabolic traits, as well as the effects of statin treatment on such traits.32,33
Figure 2.
Factors favouring diabetogenic effects of statins and candidate mechanisms in extrahepatic tissues and pancreatic beta-cells. AKT, alpha serine-threonine-protein kinase; ATP, adenosine triphosphate; CoQ10, Coenzyme Q10, also known as ubiquinone; FBG, fasting blood glucose; GLUT, glucose transporter; HbA1c, glycated haemoglobin; HMG CoA reductase, 3-hydroxy-3-methylglutaryl coenzyme A reductase; LDLR, low-density lipoprotein receptor; NLRP3, NOD-like receptor family, pyrin domain containing 3.
Table 2.
Summary of the evidence that the effect of statins on diabetes risk is an on-target action
| Year of citations | Description of studies | Results | Conclusion |
|---|---|---|---|
| 201030 | Genome wide association study (GWAS) of genetic variants for BMI (n = 249 796) |
|
The effect of statins on diabetes risk is at least partly explained by an on-target effect on body weight/BMI |
| 201231 | GWAS of genetic variants for insulin (n = 133 010) |
|
|
| 201529 | Mendelian randomization study (n ∼200 000 subjects) of common HMGCR gene variants | Each allele of the HMGCR gene variant rs17238484G was associated with significant increases in
|
|
| 201529 | Meta-analysis of 20 RCTs (n = 129 170) |
|
|
| 201632 | Mendelian randomization study using genetic risk scores for variants in HMCGR and PCSK9 genes associated with lower LDL-C levels (n = 112 722) |
|
The effect of statins on diabetes risk may be mediated by an effect of LDL on beta- cell function |
| 201633 | Meta-analyses of genetic association studies for LDL-lowering alleles in or near NPC1L1, HMGCR, PCSK9, ABCG5/G8, LDLR involving 50 775 individuals with T2DM and 270 269 controls |
|
|
| 201734 | Mendelian randomization study of PCSK9 variants associated with lower LDL-C levels (n = >550 000) |
|
BMI, body mass index; CI, confidence interval; LDL-C, low-density lipoprotein cholesterol; RCT, randomized controlled trial; T2DM, type 2 diabetes mellitus.
Alternatively, this effect on glucose homeostasis may be a class effect of statins mediated via LDL. Three large genetic studies which assessed life-long exposure to lower LDL-C levels due to carriage of genetic variants of other LDL-lowering drug targets, namely PCSK934,35 and NPC1L1,36 showed an increased risk of DM but only in those individuals with impaired glucose tolerance. Whilst this predicted increased risk has not been observed so far at very low LDL-C levels attained with add-on treatment with a PCSK9 (proprotein convertase subtilisin/kexin type 9) inhibitor,37,38 or ezetimibe,39 prolonged drug exposure particularly among those more at risk of developing diabetes may be required to observe an effect. It is also noteworthy that a reduced incidence of diabetes has been observed in individuals with causative LDLR mutations for familial hypercholesterolaemia.40 On the other hand, causative APOB mutations for familial hypercholesterolaemia were not associated with diabetes.41 Clearly, the relationship of circulating LDL to predisposition to diabetes is unresolved, as highlighted by the Randomized EValuation of the Effects of Anacetrapib Through Lipid-modification (REVEAL) trial with the cholesteryl ester transfer protein inhibitor, anacetrapib, in which a lower risk of diabetes was observed despite an additional 17% reduction in LDL-C on top of background statin treatment with ∼100 000 person years of exposure.42
Thus, evidence suggests that statins affect glucose homeostasis and are associated with a small risk of incident DM. Caution is needed, however, as studies have generally not included glucose tolerance testing, the gold standard for the diagnosis of diabetes, before and after statin treatment. Moreover, while this effect has been thought to be a drug class effect, recent insights suggest that this may not be the case.43–45 Both pravastatin and pitavastatin have been recognized as neutral for effects on glycaemic parameters in patients with and without DM, as reflected by regulatory labelling.46,47 In the absence of head-to-head studies, definitive statements as to whether any of the statins differ in their effect on glycaemia are not possible.
Take home messages
Concordant evidence from RCTs and genetic studies indicate that statin treatment is associated with a modest increase in the risk of new-onset DM of approximately one case per 1000 patients per year of exposure but also prevents five new CVD events.
People with features of the metabolic syndrome or prediabetes are at significantly greater risk of this adverse effect, although conversion to DM without statin is also higher.
In most studies diagnosis of ‘DM’ was based on a laboratory finding of an HbA1c >6.5 without symptoms; the relevance of this HbA1c based conversion to diabetes for long-term morbidity and mortality will require long-term follow-up.
Patients should be reassured that the benefits of statins in preventing CVD events far outweigh the potential risk from elevation in plasma glucose, especially in individuals with increased HbA1c.
Cognitive function
Whether statin treatment has a possible effect on cognitive function is an important issue, especially with the pandemic of dyslipidaemia associated with diabetes and insulin resistance on the one hand, and changing demographic patterns affecting the prevalence of dementia on the other. Epidemiological studies have documented an association between high cholesterol levels and increased risk of Alzheimer’s disease,48,49 leading some to suggest that improved vascular function with statin treatment could be beneficial in the context of several pathologies that cause dementia.50 On the other hand, it has been suggested that reduction in cholesterol levels with statin therapy may be potentially detrimental for cognitive function.51 Yet the view that statins directly affect the brain is simplistic, given the brain-blood barrier and the fact that the brain is largely self-sufficient with respect to endogenous cholesterol synthesis.52
The variable quality of data pertaining to this question is also problematic. Most clinical trials rely on patient self-report of neurological symptoms such as memory impairment, but have not incorporated rigorous objective testing for cognitive function. Furthermore, the study populations were at low risk for cognitive decline and the study duration may not have been sufficient to observe a cognitive effect. In the post-marketing setting, case reports and observational studies predominate (Table 3).39,53–60 Additionally, whether factors present in midlife that are known to be associated with impaired physical function in the longer-term, equally impact cognitive function is often overlooked.61–64
Table 3.
Summary of evidence evaluating possible effects of statins on cognitive function
| Year of citations | Description of studies | Results | Conclusion |
|---|---|---|---|
| 201353 | Meta-analysis of eight prospective cohort studies (n = 57 020 and 2851 cases of dementia) |
|
Statin use was associated with reduction in the risk of dementia |
| 201354,55 | Systematic review of RCTs and cohort, case–control, and cross-sectional studies and FDA post surveillance marketing database | Among statin users, there was:
|
Published data do not suggest an adverse effect of statins on cognition |
| 201456 | Cochrane review of 4 RCTs (n = 1154 with probable or possible dementia) |
|
Statin therapy does not delay deterioration of cognitive function in patients with dementia |
| 201557 | Meta-analysis of 25 RCTs (n = 46 836); 23 RCTs included cognitive testing (n = 29 012) |
|
Statin therapy is not associated with cognitive impairment |
| 201739,58 | IMPROVE-IT (n = 15 281)39 FOURIER (n = 25 982)58 |
|
Very low LDL-C levels do not adversely affect cognitive function |
| 201759 | EBBINGHAUS; prospective nested cohort study of the FOURIER study (n = 1204). Cognitive function was assessed prospectively using the Cambridge Neuropsychological Test Automated Battery |
|
Low LDL-C levels were not associated with adverse effects on cognitive function as assessed prospectively over 19 months |
| 201760 | Mendelian randomization studies:
|
|
Low LDL-C levels due to PCSK9 and HMGCR variants mimicking PCSK9 inhibitor and statin treatment had no causal effect on the risk of Alzheimer’s disease, vascular dementia, any dementia, or Parkinson’s disease |
CI, confidence interval; EBBINGHAUS, Evaluating PCSK9 Binding antiBody Influence oN coGnitive HeAlth in high cardiovascUlar risk Subjects; FDA, Food and Drug Administration; FOURIER, Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk; IMPROVE-IT Examining Outcomes in Subjects With Acute Coronary Syndrome: Vytorin (Ezetimibe/Simvastatin) vs Simvastatin; LDL-C, low-density lipoprotein cholesterol; RCT, randomized controlled trial.
In a review of published literature, the Food and Drug Administration (FDA) concluded that there was no evidence that statins increase the incidence of dementia, mild cognitive impairment, or decline in cognitive performance.54 Despite this, the labelling for statins was amended to include cognitive side effects such as memory loss and confusion, although the FDA emphasized that the cardiovascular benefits of statins outweighed these possible effects.54 Similar conclusions were reported in an updated review.55 These findings are supported by data from prospective studies. The Heart Protection Study used the Telephone Interview for Cognitive Status at final follow-up to assess cognitive performance, and showed no differences between simvastatin and placebo groups for the proportion of patients classified as cognitively impaired, either overall or by baseline age subgroups.65 Additionally, in the Pravastatin in elderly individuals at risk of vascular disease (PROSPER) study, which assessed cognitive function at six different time points during the study using four neuropsychological performance tests, there was no difference in cognitive decline between pravastatin and placebo groups over a mean follow-up of 42 months.66
Subsequent analyses have also addressed this question. Prospective observational data analysis (>57 000 subjects followed for a median of 4 years) showed that statin use was associated with a lower risk of dementia [RR 0.62, 95% confidence interval (CI) 0.43–0.81; P = 0.001].53 A meta-analysis of more than 46 000 patients in 25 RCTs (23 with cognitive testing), did not identify any significant negative effect of statins on cognitive function, both for cognitively normal subjects or those with Alzheimer’s disease.57 Added to this, a Cochrane review of four trials including 1154 patients with probable or possible Alzheimer’s disease found no significant differences in the Alzheimer’s Disease Assessment Scale—cognitive subscale and the Minimal Mental State Examination between patients treated with statin or placebo,56 implying that statins do not delay cognitive deterioration in patients with known dementia. While transient global amnesia has been linked with statin use in case reports,67 there is no evidence to support causality from the totality of evidence to date.
Another question is whether there is any risk of adverse effects on cognitive function with the very low LDL-C levels attained with the combination of a statin and ezetimibe or a PCSK9 inhibitor. A prespecified analysis of the [Examining Outcomes in Subjects With Acute Coronary Syndrome: Vytorin (Ezetimibe/Simvastatin) vs. Simvastatin] IMPROVE-IT trial showed no increase in neurocognitive adverse events with ezetimibe compared with placebo when associated with exposure to LDL-C levels <0.78 mmol/L (<30 mg/dL) for up to 6 years.39 Data from the Open-Label Study of Long-term Evaluation Against LDL-C (OSLER) trial involving treatment with evolocumab for up to 4 years, and a pooled analysis of studies of alirocumab treatment for up to 2 years, add further support.68,69 Even at the very low LDL-C levels (<0.5 mmol/L or <20 mg/dL) attained with evolocumab plus moderate or high intensity statin therapy in the Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk (FOURIER) trial, there was no increase in neurocognitive adverse events compared with placebo (statin alone).58
The Evaluating PCSK9 Binding antiBody Influence oN coGnitive HeAlth in high cardiovascUlar risk Subjects (EBBINGHAUS) study59 assessed the effect of very low LDL-C levels on cognitive function in a subset of 1204 patients who were enrolled in the FOURIER trial over a mean follow-up of 1.8 years. This study used the Cambridge Neuropsychological Test Automated Battery (CANTAB, http://www.cambridgecognition.com), a computerized assessment tool that is specifically designed to assess cognitive function across a range of domains, including episodic and working memory, executive function, psychomotor speed, and attention. Assessment is independent of nuances in language and culture, and therefore suitable for application in large multinational clinical studies. Even at very low LDL-C levels [interquartile range 0.28–0.44 mmol/L (11–17 mg/dL) for the lowest LDL-C subgroup] attained with the addition of evolocumab to moderate to high intensity statin therapy in some patients in the FOURIER trial, there was no change in cognitive function over the trial. Indeed, as reported by the authors, the changes seen over time in each group were an order of magnitude less than the changes found in patients with mild cognitive impairment preceding dementia.70
Finally, in a Mendelian randomization study involving 111 194 individuals from the Danish general population, the Copenhagen General Population Study and the Copenhagen City Heart Study, low LDL-C levels associated with PCSK9 and HMGCR variants had no causal effect on the risk of Alzheimer’s disease, vascular dementia, any dementia, or Parkinson’s disease (Table 3).60 Summary level data from the International Genomics of Alzheimer’s Project on risk of Alzheimer’s disease for variants of PCSK9, HMGCR, or other variants associated with LDL-C lowering supported the same conclusion.60
Take home messages
Statin treatment does not adversely affect cognitive function.
At very low LDL-C levels attained with the combination of statin plus ezetimibe or a PCSK9 inhibitor, there was no signal for any adverse effect on cognitive function.
Mendelian randomization analyses support the finding that low LDL-C levels, due to PCSK9 and HMGCR variants mimicking PCSK9 inhibitors and statins, had no causal effect on the risk of Alzheimer’s disease, vascular dementia, any dementia, or Parkinson’s disease.
Effects on renal function
With the exception of the hydrophilic statins pravastatin and rosuvastatin, statins are metabolized by the liver and cleared minimally by the kidney. The Kidney Disease: Improving Global Outcomes (KDIGO) guideline has provided recommendations for lipid management in chronic kidney disease (CKD).71 Dose reduction based on estimated glomerular filtration rate may be prudent in patients with severe kidney dysfunction who are receiving intensive statin regimens.71
While few studies have been performed in CKD patients, recent meta-analyses indicate that statin treatment reduces CVD risk in patients with CKD, especially those with mild kidney disease.72–75 There was, however, no clear benefit in patients on dialysis.72,76–78 Given that statins reduce CVD events by 20% in CKD,79 this has prompted guidelines to recommend statin therapy in CKD patients except those on dialysis.71,75
Mild proteinuria, often transient, is seen at low frequency with high dose statin treatment but is not associated with impaired renal function (as reviewed previously80,81). This may be caused by reduced tubular reabsorption of albumin, related to inhibition of HMG-CoA reductase and reduced prenylation of proteins involved in endocytosis.82,83 A potential concern, however, is whether high dose statin therapy increases the risk of acute kidney disease.84–86 One retrospective analysis involving more than two million statin users (59 636 with CKD) newly treated with a statin between 1997 and 2008, reported a 34% higher RR of acute renal injury within 120 days of initiation of high vs. moderate intensity statin treatment, although this was attenuated with prolonged statin exposure. This was not seen in patients with CKD.84 While this retrospective analysis may raise concerns, data from RCTs have not shown any increase in risk. A meta-analysis of 24 RCTs involving 15 000 patient years exposure reported no change in the risk of acute renal impairment, and no increase in serious adverse renal events during statin treatment.87 Furthermore, in a number of meta-analyses that have focused on CKD patients, there was no increase in progression of CKD or acute renal events on statin therapy.75,88,89 Indeed, it has been suggested that statins may have potential renoprotective effects, or even slow progression of CKD,88–94 although no such benefit on renal function was evident in other studies.75,79,95
Take home messages
Statin treatment is not associated with clinically significant deterioration of renal function.
Dose reduction based on estimated glomerular filtration rate may be prudent in patients with severe kidney dysfunction who are receiving intensive statin regimens.
A protective effect of statins on the kidney cannot be excluded but further study is merited.
Effects on hepatic function
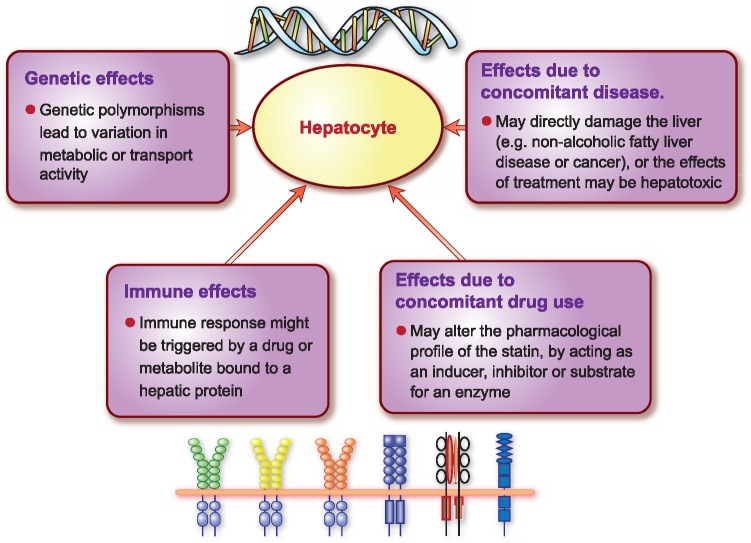
It is difficult to determine the role of statins in the extremely rare cases of severe liver injury associated with statins. Drug-induced liver injury (DILI) is the most frequent cause of acute liver failure and the need for liver transplantation in Western countries.96 The most common biomarkers for DILI are alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma glutamyl transferase (GGT), serum total bilirubin and alkaline phosphatase (ALP).97,98 Hepatocellular injury is generally detected by elevations in serum ALT or AST, elevated ALP marks injury to cells in the bile excretory ducts, and elevated serum total or conjugated bilirubin is indicative of reduced excretory function of the liver.96 In most cases, DILI is rare, idiosyncratic and unpredictable. Moreover, estimating the frequency of DILI is challenging due to potential genetic, epigenetic, environmental and clinical factors that may confound accurate diagnosis. Liver-mediated drug metabolism and transport have also been implicated in mechanisms underlying DILI (Figure 3).99,100 These interacting factors plus the rarity of severe liver toxicity associated with statins, contribute to the difficulty in assessing the role of statins in DILI.
Figure 3.
Factors that may affect susceptibility to drug induced liver injury, either by influencing drug metabolism or transport mechanisms.
Elevation in liver enzymes
Mild elevation in liver transaminases occurs in 0.5–2.0% of patients on any statin, usually within 3 months of initiation of therapy. This may not differ significantly from placebo, and in isolation, is unlikely to be clinically relevant.1,2,101 A systematic meta-analysis of 135 RCTs involving more than 246 000 patients reported that statins as a class produced ∼50% higher risk of transaminase elevation compared with control or placebo. There was a clear dose–response relationship for atorvastatin, lovastatin, and simvastatin. These elevations were transient, and usually normalized with continuing therapy.102 Clinically relevant ALT elevations are rare. An analysis of 49 trials involving more than 14 000 patients, reported persistent elevations in hepatic transaminases [>3× upper limit of normal (ULN)] in 0.1%, 0.6%, and 0.2% of patients on atorvastatin 10 mg, atorvastatin 80 mg, and placebo (Table 4).103
Table 4.
Summary of evidence for possible adverse effects of statin treatment on hepatic function
| Year of citations | Description of studies | Results | Conclusion |
|---|---|---|---|
| 2006103 | Retrospective pooled analysis of 49 trials (n = 14 236); patients were treated with atorvastatin (10 mg or 80 mg) or placebo |
|
Clinically relevant transaminase elevation with statin therapy is rare; higher doses are associated with a higher risk of transaminase elevation |
| 2013102 | Network meta-analysis of 135 RCTs (n = 246 955) |
|
|
| 2009104 | Swedish Adverse Drug Reactions Advisory Committee (1998–2010) | Only cases with transaminase elevation >5× ULN and/or ALP elevation >2× ULN were included
|
Statin-induced liver injury is very rare |
| 2016105 | UK General Practice Database (1997-2006) | Evaluated data for patients with a first prescription for simvastatin or atorvastatin with no prior liver disease, alcohol-related diagnosis, or liver dysfunction. Moderate to severe liver toxicity was defined as bilirubin >60 μmol/L, transaminase >200 U/L or ALP >1200 U/L
|
|
| 201197 | FDA Adverse Drug Event Reporting System database |
|
ALP, alkaline phosphatase; LT, alanine aminotransferase; CI, confidence interval; FDA, Food and Drugs Administration; RCT, randomized controlled trial; ULN, upper limit of the normal range.
In patients with mild ALT elevation due to steatosis or non-alcoholic fatty liver disease, statin therapy does not result in worsening of liver disease,106 although caution may be needed in patients with pre-existing primary biliary cirrhosis.107 Moreover, the cardiovascular benefits of statin therapy are likely to outweigh any potential safety issues, as highlighted by the Joint Task Force guidelines.1,2,108 Indeed, an updated meta-analysis in more than 120 000 patients with chronic liver disease showed that statin use was associated with a lower risk of hepatic decompensation and mortality, and possibly reduced portal hypertension.109 Statins should not be prescribed, however, in patients with active hepatitis B virus infection until serum levels of AST, ALT, GGT, total bilirubin, and ALP have normalized.110
Drug-induced liver injury
Idiosyncratic liver injury associated with statins is rare but can be severe. Previous studies of drug-related adverse events have suggested that statins may be implicated in 1–3% of all DILI.104,105,111,112 In a real-world setting using the United Kingdom General Practice Research Database (1997–2006),105 moderate to severe hepatotoxicity (bilirubin >60 μmol/L, AST or ALT >200 U/L, or ALP >1200 U/L) was reported in 0.09% (71/76 411) patients on atorvastatin vs. 0.06% (101/164 407) on simvastatin (hazard ratio for atorvastatin 1.9, 95% CI 1.4–2.6; P < 0.001). Reporting rates were higher at higher doses (40–80 mg/day) (0.44% on atorvastatin and 0.09% on simvastatin).105 Data from the Swedish Adverse Drug Reactions Advisory Committee (1998–2010),104 reported that 1.2 per 100 000 patients had DILI (defined as transaminase elevation >5× ULN and/or ALP >2× ULN) on statin therapy. A similar pattern of liver injury was produced on re-exposure after recovery. Despite increasing statin prescription since the late 1990s, however, the FDA Adverse Event Reporting System database did not identify any increase in the rates of fatal or severe liver injury cases caused by statin use.97 Reports of statin-associated serious liver injury were extremely low (≤2 per one million patient-years). There were 75 reports of severe liver injury, including requirement for liver transplant (n = 11) or death (n = 37), of which 30 (14 deaths, 7 liver transplantations, and 9 cases of severe liver injury) were assessed as possibly or probably associated with statin therapy. No cases were assessed as highly likely or definitely associated with statin therapy (Table 4).97 A recent update from the US National Lipid Association’s Statin Liver Safety Task Force concluded that recorded hepatotoxicity due to statins remains a very rare event.113
Clinically apparent liver injury is likely to be a class effect of statins occurring any time after initiation of statin treatment.114,115 Autoimmune hepatitis is perhaps the most common phenotype for DILI of statin-induced hepatotoxicity. Statins may trigger idiopathic inflammatory myositis or immune-mediated necrotizing myopathy,12 with antibodies against HMG-CoA reductase. Similar mechanisms could contribute to a statin-associated autoimmune hepatitis.
Monitoring liver enzyme elevation
Routine periodic monitoring of liver enzymes during statin therapy is not supported by current evidence, and is thus not recommended in asymptomatic patients.1,2,116 Indeed, routine periodic monitoring could identify patients with isolated increased ALT, AST, or GGT levels, and prompt physicians to reduce or discontinue statin therapy, thereby placing patients at increased risk for CVD events. It is, however, reasonable to measure hepatic function if symptoms suggestive of hepatotoxicity arise (e.g. unusual fatigue or weakness, loss of appetite, abdominal pain, dark-coloured urine, or yellowing of the skin or sclera). If the patient develops ALT levels >3× ULN (or lower when combined with a new increase in bilirubin levels), the statin should be discontinued. Other potential aetiologies should be considered before assuming that the elevated liver enzymes are due to the statin.
Take home messages
Mild ALT elevation in isolation in asymptomatic statin users is not clinically relevant. In patients with mild ALT elevation due to steatosis or non-alcoholic fatty liver disease, statin therapy does not worsen liver disease.
Clinically apparent liver injury with statin therapy is very rare and likely to be a class effect of statins.
Routine periodic monitoring of liver enzymes is not justified.
Liver enzymes should be measured in the rare patient who develops symptoms suggestive of hepatotoxicity.
Haemorrhagic stroke
There is substantive evidence from RCTs that statin treatment reduces the risk of ischaemic stroke by 26% (99% CI 15–35%) per mmol/L reduction in LDL-C.117 While this benefit on ischaemic stroke is established, lower LDL-C levels have been associated with an increase in haemorrhagic stroke in the general population.118 The possibility that statins increase the risk of haemorrhagic stroke was suggested by a meta-analysis of over 8000 patients with a history of cerebrovascular events, which showed a higher risk of haemorrhagic stroke events (RR 1.73, 95% CI 1.19–2.50).119 These results were mainly driven by the SPARCL trial, which evaluated atorvastatin 80 mg/day in patients with a prior stroke or transient ischaemic attack and with LDL-C levels of 2.6–4.9 mmol/L (100–190 mg/dL).120 Atorvastatin reduced ischaemic stroke in SPARCL (218 events with atorvastatin vs. 274 with placebo), but produced a numerically higher number of haemorrhagic strokes (55 vs. 33). This event was more frequent in older individuals, men, or those with prior haemorrhagic stroke.121A meta-analysis of eight RCTs (38 153 patients on statin therapy), showed a trend between attained LDL-C level and risk for haemorrhagic stroke, although the absolute number of haemorrhagic strokes was low.122
A subsequent meta-analysis including 248 391 patients, however, found no significant increased risk of intracerebral haemorrhage based on data from RCTs (RR 1.10, 95% CI 0.86–1.41), cohort studies (RR 0.94, 95% CI 0.81–1.10), and case–control studies (RR 0.60, 95% CI 0.41–0.88).123 A further meta-analysis of these patients found no association between the risk of intracerebral haemorrhage and the magnitude of LDL-C reduction.124 Moreover, even at very low attained LDL-C levels in FOURIER, there was no increase in the risk of haemorrhagic stroke.58
Take home messages
Statin treatment reduces the risk of first or subsequent ischaemic strokes by 15–35% per mmol/L reduction in LDL-C.
While SPARCL suggested a small increase in haemorrhagic stroke in subjects with prior stoke, this possible increased risk associated with LDL-C reduction has not been confirmed by analysis of a substantive evidence base of RCTs, cohort studies, and case–control studies.
No alteration in the statin regimen in patients with a history of cerebrovascular disease is indicated.
Cataract
Age-related lens opacity (cataract) is the main cause of vision loss in the older population. Whether statin use exacerbates this risk has been a potential concern. Investigation of this question, however, has been hampered by methodological issues such as the lack of standardized definition of cataract as an outcome,125 as well as failure to account for the impact of statin adherence and the frequency of ophthalmological check-ups.
Observational data and limited preclinical studies suggested a possible link between cataract and statin use.126,127 A propensity score-matched analysis of a US administrative dataset of 46 249 subjects, including 13 262 statin users, showed that the risk of cataract was slightly higher (by 9%) with statin treatment.128 In addition, both the Heart Outcomes Prevention Evaluation (HOPE)-3 study and a retrospective nested case–control study showed an increase in risk for cataract surgery with statin use.129,130
On the other hand, evidence from RCTs provides reassurance on this question. In the Expanded Clinical Evaluation of Lovastatin (EXCEL) study in 8032 patients randomized to lovastatin (40 mg or 20 mg once or twice daily) or placebo, there were no significant differences in ocular opacities, visual acuity, or cataract extraction over a follow-up of 48 weeks.131 The Oxford Cholesterol Study Group trial in 539 patients randomized to simvastatin (40 mg or 20 mg daily) or placebo also showed no differences in visual outcomes or cataract grading after 18 months of treatment.132 Similarly, the Simvastatin and Ezetimibe in Aortic Stenosis (SEAS) study in 1873 patients with asymptomatic aortic stenosis and no history of diabetes, coronary heart disease, or other serious co-morbidities (average follow-up of 4.3 years) found that the risk of cataract was significantly lower with the use of simvastatin and ezetimibe compared to placebo (hazard ratio 0.56, 95% CI 0.33–0.96).133 A subsequent meta-analysis of 313 200 patients in cohort trials (n = 6, follow-up duration of up to 5 years), case–control studies (n = 6, follow-up duration of up to 5 years), and RCTs (n = 5, follow-up duration 0.9–5.4 years) did not show any association between statin use and the development of cataracts.134 Mechanistically, it has been suggested that the antioxidant and anti-inflammatory effects of statins could slow the development of cataracts,135,136 although further study is needed.
Take home messages
Statin treatment is not associated with cataract development.
No change in cardiovascular prevention strategies are indicated, even in patients with cataracts.
Conclusion
Public perception of the adverse effects of statins is often exaggerated, in part as a consequence of media reports.13,15 While statins generally have an acceptable safety profile,2 questions have been raised regarding possible unintended effects on glucose homeostasis, and cognitive, renal, and hepatic function, as well as the risk for haemorrhagic stroke or cataract. This Consensus Panel Statement therefore addressed these persistent uncertainties.
We conclude that statin treatment is remarkably safe. While there is a modest risk (about one new case per 1000 patients per year of exposure) of new onset DM with long-term statin treatment, this comes with the benefit of five new CVD events avoided. Patients with the metabolic syndrome or prediabetes are at higher risk of DM. In the absence of head-to-head studies, however, definitive statements as to whether any of the statins differ in their effect on glucose homeostasis are not possible. Statin use is not associated with adverse effects on cognitive function or clinically significant deterioration of renal function and does not increase the risk of cataract or haemorrhagic stroke in individuals without prior stroke, although the SPARCL data suggested statins may possibly increase the risk of haemorrhagic stroke in those with prior stroke. Clinical liver injury with statin therapy is very rare.
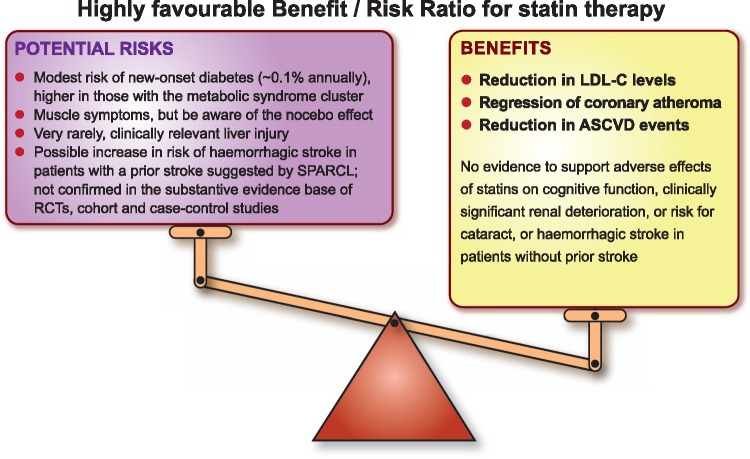
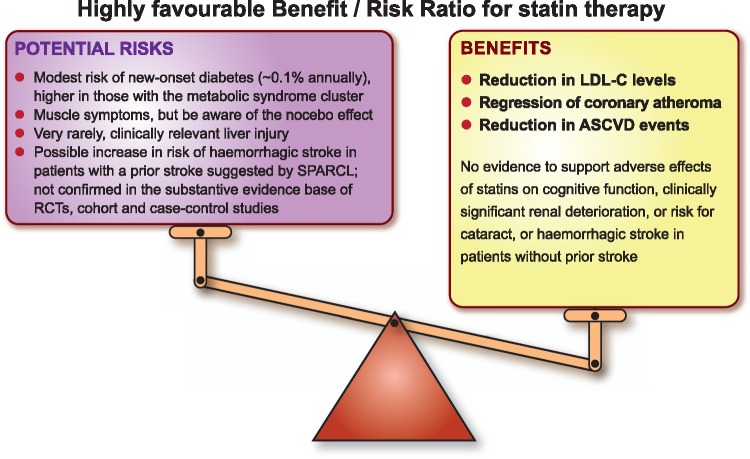
Finally, clinicians should be reassured by the long-term safety of statins, and the low risk of clinically relevant adverse effects, as discussed above. Importantly, and reinforcing recommendations from the recent European guidelines on CVD prevention and lipid management,1,2 the Panel emphasizes that the established cardiovascular benefits of statin therapy far outweigh the risk of any such adverse effects (Take home figure).
Take home figure.
The cardiovascular benefit of long-term statin therapy far outweighs potential risks. ASCVD, atherosclerotic cardiovascular disease; LDL-C, low-density lipoprotein cholesterol; RCT, randomized controlled trial; SPARCL, Stroke Prevention by Aggressive Reduction in Cholesterol Levels.
Acknowledgements
We acknowledge literature research support (Cognitive function subsection) from Ms Aliki Buhayer (Prism Scientific Sarl).
Funding
The Panel met in London and Barcelona at meetings chaired by M.J.C. and H.N.G. to comprehensively and critically appraise and discuss the literature for this review. Funding for attendance of the Panel members at these meetings was provided by unrestricted educational grants to the European Atherosclerosis Society from Amgen, AstraZeneca, Eli Lilly, Esperion, Merck, Pfizer, and Sanofi-Regeneron. These companies were not present at the Consensus Panel meetings, had no role in the design or content of the manuscript, and had no right to approve or disapprove the final document. The Writing Group comprised F.M., K.K.R., O.W., A.C., A.L.C. and the Co-Chairs.
Conflict of interest: The following authors report disclosures outside the submitted work. F.M. has received research grants from Amgen, AstraZeneca and MSD, and honoraria for consultancy from Amgen, AstraZeneca, MSD and Pfizer. K.K.R. has received research grants from Sanofi, Regeneron, Pfizer, Amgen and MSD, and honoraria for lectures, advisory boards and/or as a steering committee member from Sanofi, Amgen, Regeneron, Lilly, The Medicines Company, AstraZeneca, Pfizer, Kowa, IONIS, Esperion, Takeda, Boehringer Ingelheim. O.W. has received honoraria for lectures from Sanofi, Amgen, MSD, and AstraZeneca. A.C. has received fees for consulting and research grants from Amgen, Sanofi, Pfizer, Mediolanum Farmaceutici, MSD, Mylan, Recordati and AstraZeneca. A.L.C. has received research grants to his institution from Amgen, AstraZeneca, Merck, Regeneron/Sanofi, and Sigma Tau, and honoraria for advisory boards, consultancy and/or speaker bureau from Abbott, Aegerion, Amgen, AstraZeneca, Eli Lilly, Genzyme, Merck/MSD, Mylan, Pfizer, Rottapharm and Sanofi-Regeneron. E.B. has received research grants from Aegerion and Amgen, and honoraria for advisory boards, consultancy and/or speaker bureau from Aegerion, MSD, Sanofi, Amgen, Unilever, Chiesa, Lilly, Genfit, AstraZeneca, Rottapharm-MEDA, IONIS, Akcae and Institut Benjamin Delessert. R.A.H. has received research grants from Amgen. Pfizer and Sanofi, and honoraria for advisory boards, consultancy and/or speakers bureau from Aegerion, Akcea/IONIS, Boston Heart Diagnostics, Eli Lilly, Sanofi and Valeant. K.G.H. has received honoraria for advisory boards, consultancy and/or speakers bureau from Amgen, Genzyme, Merck, Pfizer, Roche and Sanofi-Regeneron. T.A.J. has received research grants from AstraZeneca, Merck and Sanofi-Aventis/Regeneron. R.K. has received research grants from ISIS, Ligand Pharmaceuticals, Madrigal Pharmaceuticals, MedChefs, Merck, Metabolex, Quest Diagnostics and Sanofi-Aventis/Regeneron. U.L. has received honoraria for advisory boards, consultancy and/or speakers bureau from Amgen, MSD, Sanofi, Lilly and Pfizer. L.A.L. has received research grants to his institution from Amgen, Eli Lilly, Merck, Pfizer, Regeneron/Sanofi and The Medicines Company, and honoraria for advisory boards, consultancy and/or speakers bureau from Amgen, Eli Lilly, Esperion, Kowa, Merck, Regeneron/Sanofi, The Medicines Company and Aegerion. W.M. has received grants and personal fees from Siemens Diagnostics, Aegerion, Amgen, AstraZeneca, BASF, Berlin Chemie, Danone Research, Pfizer, Numares AG, personal fees from Hoffmann LaRoche, MSD, Sanofi, Synageva, grants from Abbott Diagnostics, and other fees from Synlab Holding Deutschland GmbH. B.G.N. has received lecture and/or consultancy honoraria from AstraZeneca, Merck, Sanofi, Regeneron, IONIS, Dezima, Amgen, and Kowa. F.J.R. has received a research grant from the University of Witwatersrand, Johannesburg, South Africa, fees for conducting clinical trials with evolocumab and alirocumab in subjects with heterozygous and homozygous familial hypercholesterolaemia, and honoraria for advisory boards, consultancy and/or speakers bureau and nonfinancial support from Pfizer, Amgen and Sanofi/Regeneron. M.R. has received research grants from Boehringer Ingelheim, Novartis, AstraZeneca and Nutricia Danone, and honoraria for advisory boards, consultancy and/or speakers bureau from Novo, Sanofi, Merck, Poxel and Lilly. R.D.S. has received honoraria for advisory boards, consultancy and/or speakers bureau from AstraZeneca, Biolab, BristolMyersSquibb, Amgen, Aegerion, Genzyme, Boehringer-Ingelheim, ISIS, Nestle, Novo-Nordisk, Sanofi/Regeneron, Pfizer, Merck, Unilever and Novartis. E.A.S. has received modest consultancy honoraria from Amgen, Regeneron, Sanofi, Roche/Genentech related to PCSK9 inhibitor development and AstraZeneca related to statins. E.S.S. has received research grants to his institution from Amgen, Merck, IONIS, Chiesa, Sanofi/Regeneron and Athera. L.T. has received research funding, and/or honoraria for advisory boards, consultancy or speaker bureau from Abbott, Actelion, Amgen, AstraZeneca, Bayer, Merck, Mylan, Novartis, Pfizer, Recordati, Sanofi-Regeneron and Servier. J.K.S. has received an honorarium for consultancy from Aegerion. H.N.G. has received grants and honoraria for advisory boards, consultancy or speaker bureau from Sanofi Regeneron, Amgen, and Merck, and honoraria for advisory boards, consultancy or speaker bureau from Pfizer, AstraZeneca and BristolMyersSquibb. M.J.C. has received research grants from MSD, Kowa, Pfizer, and Randox, and honoraria for consultancy/lectures from Amgen, Kowa, Merck, Sanofi, Servier, Regeneron and Unilever. G. D.B., B.G., P.D.T. and G.D.V. report no conflict of interest.
References
- 1. Catapano AL, Graham I, De Backer G, Wiklund O, Chapman MJ, Drexel H, Hoes AW, Jennings CS, Landmesser U, Pedersen TR, Reiner Ž, Riccardi G, Taskinen MR, Tokgozoglu L, Verschuren WM, Vlachopoulos C, Wood DA, Zamorano JL.. 2016 ESC/EAS Guidelines for the management of dyslipidaemias. Eur Heart J 2016;37:2999–3058. [DOI] [PubMed] [Google Scholar]
- 2. Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, Cooney MT, Corrà U, Cosyns B, Deaton C, Graham I, Hall MS, Hobbs FD, Løchen ML, Löllgen H, Marques-Vidal P, Perk J, Prescott E, Redon J, Richter DJ, Sattar N, Smulders Y, Tiberi M, van der Worp HB, van Dis I, Verschuren WM; Authors/Task Force Members. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: the Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J 2016;37:2315–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ference BA, Ginsberg HN, Graham I, Ray KK, Packard CJ, Bruckert E, Hegele RA, Krauss RM, Raal FJ, Schunkert H, Watts GF, Borén J, Fazio S, Horton JD, Masana L, Nicholls SJ, Nordestgaard BG, van de Sluis B, Taskinen MR, Tokgözoglu L, Landmesser U, Laufs U, Wiklund O, Stock JK, Chapman MJ, Catapano AL.. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J 2017; 38:2459–2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Collins R, Reith C, Emberson J, Armitage J, Baigent C, Blackwell L, Blumenthal R, Danesh J, Smith GD, DeMets D, Evans S, Law M, MacMahon S, Martin S, Neal B, Poulter N, Preiss D, Ridker P, Roberts I, Rodgers A, Sandercock P, Schulz K, Sever P, Simes J, Smeeth L, Wald N, Yusuf S, Peto R.. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet 2016;388:2532–2561. [DOI] [PubMed] [Google Scholar]
- 5. Cholesterol Treatment Trialists' (CTT) Collaboration, Fulcher J, O’Connell R, Voysey M, Emberson J, Blackwell L, Mihaylova B, Simes J, Collins R, Kirby A, Colhoun H, Braunwald E, La Rosa J, Pedersen TR, Tonkin A, Davis B, Sleight P, Franzosi MG, Baigent C, Keech A.. Efficacy and safety of LDL-lowering therapy among men and women: meta-analysis of individual data from 174,000 participants in 27 randomised trials. Lancet 2015;385:1397–1405. [DOI] [PubMed] [Google Scholar]
- 6. Lafeber M, Webster R, Visseren FL, Bots ML, Grobbee DE, Spiering W, Rodgers A; Programme to Improve Life and Longevity (PILL) Collaborative Group. Estimated cardiovascular relative risk reduction from fixed-dose combination pill (polypill) treatment in a wide range of patients with a moderate risk of cardiovascular disease. Eur J Prev Cardiol 2016;23:1289–1297. [DOI] [PubMed] [Google Scholar]
- 7. Freeman DJ, Norrie J, Sattar N, Neely RD, Cobbe SM, Ford I, Isles C, Lorimer AR, Macfarlane PW, McKillop JH, Packard CJ, Shepherd J, Gaw A.. Pravastatin and the development of diabetes mellitus: evidence for a protective treatment effect in the West of Scotland Coronary Prevention Study. Circulation 2001;103:357–362. [DOI] [PubMed] [Google Scholar]
- 8. Ridker PM, Pradhan A, MacFadyen JG, Libby P, Glynn RJ.. Cardiovascular benefits and diabetes risks of statin therapy in primary prevention: an analysis from the JUPITER trial. Lancet 2012;380:565–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sattar N, Preiss D, Murray HM, Welsh P, Buckley BM, de Craen AJ, Seshasai SR, McMurray JJ, Freeman DJ, Jukema JW, Macfarlane PW, Packard CJ, Stott DJ, Westendorp RG, Shepherd J, Davis BR, Pressel SL, Marchioli R, Marfisi RM, Maggioni AP, Tavazzi L, Tognoni G, Kjekshus J, Pedersen TR, Cook TJ, Gotto AM, Clearfield MB, Downs JR, Nakamura H, Ohashi Y, Mizuno K, Ray KK, Ford I.. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet 2010;375:735–742. [DOI] [PubMed] [Google Scholar]
- 10. Sirtori CR. The pharmacology of statins. Pharmacol Res 2014;88:3–11. [DOI] [PubMed] [Google Scholar]
- 11. DeGorter MK, Tirona RG, Schwarz UI, Choi YH, Dresser GK, Suskin N, Myers K, Zou G, Iwuchukwu O, Wei WQ, Wilke RA, Hegele RA, Kim RB.. Clinical and pharmacogenetic predictors of circulating atorvastatin and rosuvastatin concentrations in routine clinical care. Circ Cardiovasc Genet 2013;6:400–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stroes ES, Thompson PD, Corsini A, Vladutiu GD, Raal FJ, Ray KK, Roden M, Stein E, Tokgözoğlu L, Nordestgaard BG, Bruckert E, De Backer G, Krauss RM, Laufs U, Santos RD, Hegele RA, Hovingh GK, Leiter LA, Mach F, März W, Newman CB, Wiklund O, Jacobson TA, Catapano AL, Chapman MJ, Ginsberg HN; European Atherosclerosis Society Consensus Panel. Statin-associated muscle symptoms: impact on statin therapy-European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur Heart J 2015;36:1012–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nielsen SF, Nordestgaard BG.. Negative statin-related news stories decrease statin persistence and increase myocardial infarction and cardiovascular mortality: a nationwide prospective cohort study. Eur Heart J 2016;37:908–916. [DOI] [PubMed] [Google Scholar]
- 14. Serban MC, Colantonio LD, Manthripragada AD, Monda KL, Bittner VA, Banach M, Chen L, Huang L, Dent R, Kent ST, Muntner P, Rosenson RS.. Statin intolerance and risk of coronary heart events and all-cause mortality following myocardial infarction. J Am Coll Cardiol 2017;69:1386–1395. [DOI] [PubMed] [Google Scholar]
- 15. Matthews A, Herrett E, Gasparrini A, Van Staa T, Goldacre B, Smeeth L, Bhaskaran K.. Impact of statin related media coverage on use of statins: interrupted time series analysis with UK primary care data. BMJ 2016;353:i3283.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tobert JA, Newman CB.. The nocebo effect in the context of statin intolerance. J Clin Lipidol 2016;10:739–747. [DOI] [PubMed] [Google Scholar]
- 17. Gupta A, Thompson D, Whitehouse A, Collier T, Dahlof B, Poulter N, Collins R, Sever P; ASCOT Investigators. Adverse events associated with unblinded, but not with blinded, statin therapy in the Anglo-Scandinavian Cardiac Outcomes Trial-Lipid-Lowering Arm (ASCOT-LLA): a randomised double-blind placebo-controlled trial and its non-randomised non-blind extension phase. Lancet 2017; 389:2473–2481. [DOI] [PubMed] [Google Scholar]
- 18. Preiss D, Seshasai SR, Welsh P, Murphy SA, Ho JE, Waters DD, DeMicco DA, Barter P, Cannon CP, Sabatine MS, Braunwald E, Kastelein JJ, de Lemos JA, Blazing MA, Pedersen TR, Tikkanen MJ, Sattar N, Ray KK.. Risk of incident diabetes with intensive-dose compared with moderate-dose statin therapy: a meta-analysis. JAMA 2011;305:2556–2564. [DOI] [PubMed] [Google Scholar]
- 19. Waters DD, Ho JE, Boekholdt SM, DeMicco DA, Kastelein JJ, Messig M, Breazna A, Pedersen TR.. Cardiovascular event reduction versus new-onset diabetes during atorvastatin therapy: effect of baseline risk factors for diabetes. J Am Coll Cardiol 2013;61:148–152. [DOI] [PubMed] [Google Scholar]
- 20. Mora S, Glynn RJ, Hsia J, MacFadyen JG, Genest J, Ridker PM.. Statins for the primary prevention of cardiovascular events in women with elevated high-sensitivity C-reactive protein or dyslipidemia: results from the Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) and meta-analysis of women from primary prevention trials. Circulation 2010;121:1069–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Goodarzi MO, Li X, Krauss RM, Rotter JI, Chen YD.. Relationship of sex to diabetes risk in statin trials. Diabetes Care 2013;36:e100–e101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cederberg H, Stančáková A, Yaluri N, Modi S, Kuusisto J, Laakso M.. Increased risk of diabetes with statin treatment is associated with impaired insulin sensitivity and insulin secretion: a 6 year follow-up study of the METSIM cohort. Diabetologia 2015;58:1109–1117. [DOI] [PubMed] [Google Scholar]
- 23. Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kohli P, Waters DD, Nemr R, Arsenault BJ, Messig M, DeMicco DA, Laskey R, Kastelein JJP.. Risk of new-onset diabetes and cardiovascular risk reduction from high-dose statin therapy in pre-diabetics and non-pre-diabetics: an analysis from TNT and IDEAL. J Am Coll Cardiol 2015;65:402–404. [DOI] [PubMed] [Google Scholar]
- 25. Nielsen SF, Nordestgaard BG.. Statin use before diabetes diagnosis and risk of microvascular disease: a nationwide nested matched study. Lancet Diabetes Endocrinol 2014;2:894–900. [DOI] [PubMed] [Google Scholar]
- 26. Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Neil HA, Livingstone SJ, Thomason MJ, Mackness MI, Charlton-Menys V, Fuller JH; CARDS Investigators. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet 2004;364:685–696. [DOI] [PubMed] [Google Scholar]
- 27. Szendroedi J, Anderwald C, Krssak M, Bayerle-Eder M, Esterbauer H, Pfeiler G, Brehm A, Nowotny P, Hofer A, Waldhausl W, Roden M.. Effects of high-dose simvastatin therapy on glucose metabolism and ectopic lipid deposition in nonobese type 2 diabetic patients. Diabetes Care 2009;32:209–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Betteridge DJ, Carmena R.. The diabetogenic action of statins—mechanisms and clinical implications. Nat Rev Endocrinol 2016;12:90–110. [DOI] [PubMed] [Google Scholar]
- 29. Swerdlow DI, Preiss D, Kuchenbaecker KB, Holmes MV, Engmann JE, Shah T, Sofat R, Stender S, Johnson PC, Scott RA, Leusink M, Verweij N, Sharp SJ, Guo Y, Giambartolomei C, Chung C, Peasey A, Amuzu A, Li K, Palmen J, Howard P, Cooper JA, Drenos F, Li YR, Lowe G, Gallacher J, Stewart MC, Tzoulaki I, Buxbaum SG, van der ADL, Forouhi NG, Onland-Moret NC, van der Schouw YT, Schnabel RB, Hubacek JA, Kubinova R, Baceviciene M, Tamosiunas A, Pajak A, Topor-Madry R, Stepaniak U, Malyutina S, Baldassarre D, Sennblad B, Tremoli E, de Faire U, Veglia F, Ford I, Jukema JW, Westendorp RG, de Borst GJ, de Jong PA, Algra A, Spiering W, Maitland-van der Zee AH, Klungel OH, de Boer A, Doevendans PA, Eaton CB, Robinson JG, Duggan D. DIAGRAM Consortium; MAGIC Consortium; InterAct Consortium Kjekshus J, Downs JR, Gotto AM, Keech AC, Marchioli R, Tognoni G, Sever PS, Poulter NR, Waters DD, Pedersen TR, Amarenco P, Nakamura H, McMurray JJ, Lewsey JD, Chasman DI, Ridker PM, Maggioni AP, Tavazzi L, Ray KK, Seshasai SR, Manson JE, Price JF, Whincup PH, Morris RW, Lawlor DA, Smith GD, Ben-Shlomo Y, Schreiner PJ, Fornage M, Siscovick DS, Cushman M, Kumari M, Wareham NJ, Verschuren WM, Redline S, Patel SR, Whittaker JC, Hamsten A, Delaney JA, Dale C, Gaunt TR, Wong A, Kuh D, Hardy R, Kathiresan S, Castillo BA, van der Harst P, Brunner EJ, Tybjaerg-Hansen A, Marmot MG, Krauss RM, Tsai M, Coresh J, Hoogeveen RC, Psaty BM, Lange LA, Hakonarson H, Dudbridge F, Humphries SE, Talmud PJ, Kivimäki M, Timpson NJ, Langenberg C, Asselbergs FW, Voevoda M, Bobak M, Pikhart H, Wilson JG, Reiner AP, Keating BJ, Hingorani AD, Sattar N.. HMG-coenzyme A reductase inhibition, type 2 diabetes, and bodyweight: evidence from genetic analysis and randomised trials. Lancet 2015;385:351–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Speliotes EK, Willer CJ, Berndt SI, Monda KL, Thorleifsson G, Jackson AU, Lango Allen H, Lindgren CM, Luan J, Mägi R, Randall JC, Vedantam S, Winkler TW, Qi L, Workalemahu T, Heid IM, Steinthorsdottir V, Stringham HM, Weedon MN, Wheeler E, Wood AR, Ferreira T, Weyant RJ, Segrè AV, Estrada K, Liang L, Nemesh J, Park JH, Gustafsson S, Kilpeläinen TO, Yang J, Bouatia-Naji N, Esko T, Feitosa MF, Kutalik Z, Mangino M, Raychaudhuri S, Scherag A, Smith AV, Welch R, Zhao JH, Aben KK, Absher DM, Amin N, Dixon AL, Fisher E, Glazer NL, Goddard ME, Heard-Costa NL, Hoesel V, Hottenga JJ, Johansson A, Johnson T, Ketkar S, Lamina C, Li S, Moffatt MF, Myers RH, Narisu N, Perry JR, Peters MJ, Preuss M, Ripatti S, Rivadeneira F, Sandholt C, Scott LJ, Timpson NJ, Tyrer JP, van Wingerden S, Watanabe RM, White CC, Wiklund F, Barlassina C, Chasman DI, Cooper MN, Jansson JO, Lawrence RW, Pellikka N, Prokopenko I, Shi J, Thiering E, Alavere H, Alibrandi MT, Almgren P, Arnold AM, Aspelund T, Atwood LD, Balkau B, Balmforth AJ, Bennett AJ, Ben-Shlomo Y, Bergman RN, Bergmann S, Biebermann H, Blakemore AI, Boes T, Bonnycastle LL, Bornstein SR, Brown MJ, Buchanan TA, Busonero F, Campbell H, Cappuccio FP, Cavalcanti-Proença C, Chen YD, Chen CM, Chines PS, Clarke R, Coin L, Connell J, Day IN, den Heijer M, Duan J, Ebrahim S, Elliott P, Elosua R, Eiriksdottir G, Erdos MR, Eriksson JG, Facheris MF, Felix SB, Fischer-Posovszky P, Folsom AR, Friedrich N, Freimer NB, Fu M, Gaget S, Gejman PV, Geus EJC, Gieger C, Gjesing AP, Goel A, Goyette P, Grallert H, Grässler J, Greenawalt DM, Groves CJ, Gudnason V, Guiducci C, Hartikainen A-L, Hassanali N, Hall AS, Havulinna AS, Hayward C, Heath AC, Hengstenberg C, Hicks AA, Hinney A, Hofman A, Homuth G, Hui J, Igl W, Iribarren C, Isomaa B, Jacobs KB, Jarick I, Jewell E, John U, Jørgensen T, Jousilahti P, Jula A, Kaakinen M, Kajantie E, Kaplan LM, Kathiresan S, Kettunen J, Kinnunen L, Knowles JW, Kolcic I, König IR, Koskinen S, Kovacs P, Kuusisto J, Kraft P, Kvaløy K, Laitinen J, Lantieri O, Lanzani C, Launer LJ, Lecoeur C, Lehtimäki T, Lettre G, Liu J, Lokki M-L, Lorentzon M, Luben RN, Ludwig B, Manunta P, Marek D, Marre M, Martin NG, McArdle WL, McCarthy A, McKnight B, Meitinger T, Melander O, Meyre D, Midthjell K, Montgomery GW, Morken MA, Morris AP, Mulic R, Ngwa JS, Nelis M, Neville MJ, Nyholt DR, O'Donnell CJ, O’Rahilly S, Ong KK, Oostra B, Paré G, Parker AN, Perola M, Pichler I, Pietiläinen KH, Platou CG, Polasek O, Pouta A, Rafelt S, Raitakari O, Rayner NW, Ridderstråle M, Rief W, Ruokonen A, Robertson NR, Rzehak P, Salomaa V, Sanders AR, Sandhu MS, Sanna S, Saramies J, Savolainen MJ, Scherag S, Schipf S, Schreiber S, Schunkert H, Silander K, Sinisalo J, Siscovick DS, Smit JH, Soranzo N, Sovio U, Stephens J, Surakka I, Swift AJ, Tammesoo M-L, Tardif J-C, Teder-Laving M, Teslovich TM, Thompson JR, Thomson B, Tönjes A, Tuomi T, van Meurs JB, van Ommen G-J, Vatin V, Viikari J, Visvikis-Siest S, Vitart V, Vogel CI, Voight BF, Waite LL, Wallaschofski H, Walters GB, Widen E, Wiegand S, Wild SH, Willemsen G, Witte DR, Witteman JC, Xu J, Zhang Q, Zgaga L, Ziegler A, Zitting P, Beilby JP, Farooqi IS, Hebebrand J, Huikuri HV, James AL, Kähönen M, Levinson DF, Macciardi F, Nieminen MS, Ohlsson C, Palmer LJ, Ridker PM, Stumvoll M, Beckmann JS, Boeing H, Boerwinkle E, Boomsma DI, Caulfield MJ, Chanock SJ, Collins FS, Cupples LA, Smith GD, Erdmann J, Froguel P, Grönberg H, Gyllensten U, Hall P, Hansen T, Harris TB, Hattersley AT, Hayes RB, Heinrich J, Hu FB, Hveem K, Illig T, Jarvelin MR, Kaprio J, Karpe F, Khaw KT, Kiemeney LA, Krude H, Laakso M, Lawlor DA, Metspalu A, Munroe PB, Ouwehand WH, Pedersen O, Penninx BW, Peters A, Pramstaller PP, Quertermous T, Reinehr T, Rissanen A, Rudan I, Samani NJ, Schwarz PE, Shuldiner AR, Spector TD, Tuomilehto J, Uda M, Uitterlinden A, Valle TT, Wabitsch M, Waeber G, Wareham NJ, Watkins H, Wilson JF, Wright AF, Zillikens MC, Chatterjee N, McCarroll SA, Purcell S, Schadt EE, Visscher PM, Assimes TL, Borecki IB, Deloukas P, Fox CS, Groop LC, Haritunians T, Hunter DJ, Kaplan RC, Mohlke KL, O’Connell JR, Peltonen L, Schlessinger D, Strachan DP, van Duijn CM, Wichmann HE, Frayling TM, Thorsteinsdottir U, Abecasis GR, Barroso I, Boehnke M, Stefansson K, North KE, McCarthy MI, Hirschhorn JN, Ingelsson E, Loos RJ.. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat Genet 2010;42:937–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Scott RA, Lagou V, Welch RP, Wheeler E, Montasser ME, Luan J, Mägi R, Strawbridge RJ, Rehnberg E, Gustafsson S, Kanoni S, Rasmussen-Torvik LJ, Yengo L, Lecoeur C, Shungin D, Sanna S, Sidore C, Johnson PC, Jukema JW, Johnson T, Mahajan A, Verweij N, Thorleifsson G, Hottenga JJ, Shah S, Smith AV, Sennblad B, Gieger C, Salo P, Perola M, Timpson NJ, Evans DM, Pourcain BS, Wu Y, Andrews JS, Hui J, Bielak LF, Zhao W, Horikoshi M, Navarro P, Isaacs A, O'Connell JR, Stirrups K, Vitart V, Hayward C, Esko T, Mihailov E, Fraser RM, Fall T, Voight BF, Raychaudhuri S, Chen H, Lindgren CM, Morris AP, Rayner NW, Robertson N, Rybin D, Liu CT, Beckmann JS, Willems SM, Chines PS, Jackson AU, Kang HM, Stringham HM, Song K, Tanaka T, Peden JF, Goel A, Hicks AA, An P, Müller-Nurasyid M, Franco-Cereceda A, Folkersen L, Marullo L, Jansen H, Oldehinkel AJ, Bruinenberg M, Pankow JS, North KE, Forouhi NG, Loos RJ, Edkins S, Varga TV, Hallmans G, Oksa H, Antonella M, Nagaraja R, Trompet S, Ford I, Bakker SJ, Kong A, Kumari M, Gigante B, Herder C, Munroe PB, Caulfield M, Antti J, Mangino M, Small K, Miljkovic I, Liu Y, Atalay M, Kiess W, James AL, Rivadeneira F, Uitterlinden AG, Palmer CN, Doney AS, Willemsen G, Smit JH, Campbell S, Polasek O, Bonnycastle LL, Hercberg S, Dimitriou M, Bolton JL, Fowkes GR, Kovacs P, Lindström J, Zemunik T, Bandinelli S, Wild SH, Basart HV, Rathmann W, Grallert H. DIAbetes Genetics Replication and Meta-analysis (DIAGRAM) Consortium Maerz W, Kleber ME, Boehm BO, Peters A, Pramstaller PP, Province MA, Borecki IB, Hastie ND, Rudan I, Campbell H, Watkins H, Farrall M, Stumvoll M, Ferrucci L, Waterworth DM, Bergman RN, Collins FS, Tuomilehto J, Watanabe RM, de Geus EJ, Penninx BW, Hofman A, Oostra BA, Psaty BM, Vollenweider P, Wilson JF, Wright AF, Hovingh GK, Metspalu A, Uusitupa M, Magnusson PK, Kyvik KO, Kaprio J, Price JF, Dedoussis GV, Deloukas P, Meneton P, Lind L, Boehnke M, Shuldiner AR, van Duijn CM, Morris AD, Toenjes A, Peyser PA, Beilby JP, Körner A, Kuusisto J, Laakso M, Bornstein SR, Schwarz PE, Lakka TA, Rauramaa R, Adair LS, Smith GD, Spector TD, Illig T, de Faire U, Hamsten A, Gudnason V, Kivimaki M, Hingorani A, Keinanen-Kiukaanniemi SM, Saaristo TE, Boomsma DI, Stefansson K, van der Harst P, Dupuis J, Pedersen NL, Sattar N, Harris TB, Cucca F, Ripatti S, Salomaa V, Mohlke KL, Balkau B, Froguel P, Pouta A, Jarvelin MR, Wareham NJ, Bouatia-Naji N, McCarthy MI, Franks PW, Meigs JB, Teslovich TM, Florez JC, Langenberg C, Ingelsson E, Prokopenko I, Barroso I.. Large-scale association analyses identify new loci influencing glycemic traits and provide insight into the underlying biological pathways. Nat Genet 2012;44:991–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ference BA, Robinson JG, Brook RD, Catapano AL, Chapman MJ, Neff DR, Voros S, Giugliano RP, Davey Smith G, Fazio S, Sabatine MS.. Variation in PCSK9 and HMGCR and risk of cardiovascular disease and diabetes. N Engl J Med 2016;375:2144–2153. [DOI] [PubMed] [Google Scholar]
- 33. Lotta LA, Sharp SJ, Burgess S, Perry JRB, Stewart ID, Willems SM, Luan J, Ardanaz E, Arriola L, Balkau B, Boeing H, Deloukas P, Forouhi NG, Franks PW, Grioni S, Kaaks R, Key TJ, Navarro C, Nilsson PM, Overvad K, Palli D, Panico S, Quirós J-R, Riboli E, Rolandsson O, Sacerdote C, Salamanca-Fernandez E, Slimani N, Spijkerman AMW, Tjonneland A, Tumino R, van der A DL, van der Schouw YT, McCarthy MI, Barroso I, O’Rahilly S, Savage DB, Sattar N, Langenberg C, Scott RA, Wareham NJ.. Association between low-density lipoprotein cholesterol-lowering genetic variants and risk of type 2 diabetes: a meta-analysis. JAMA 2016;316:1383–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schmidt AF, Swerdlow DI, Holmes MV, Patel RS, Fairhurst-Hunter Z, Lyall DM, Hartwig FP, Horta BL, Hyppönen E, Power C, Moldovan M, van Iperen E, Hovingh GK, Demuth I, Norman K, Steinhagen-Thiessen E, Demuth J, Bertram L, Liu T, Coassin S, Willeit J, Kiechl S, Willeit K, Mason D, Wright J, Morris R, Wanamethee G, Whincup P, Ben-Shlomo Y, McLachlan S, Price JF, Kivimaki M, Welch C, Sanchez-Galvez A, Marques-Vidal P, Nicolaides A, Panayiotou AG, Onland-Moret NC, van der Schouw YT, Matullo G, Fiorito G, Guarrera S, Sacerdote C, Wareham NJ, Langenberg C, Scott R, Luan J, Bobak M, Malyutina S, Pająk A, Kubinova R, Tamosiunas A, Pikhart H, Husemoen LL, Grarup N, Pedersen O, Hansen T, Linneberg A, Simonsen KS, Cooper J, Humphries SE, Brilliant M, Kitchner T, Hakonarson H, Carrell DS, McCarty CA, Kirchner HL, Larson EB, Crosslin DR, de Andrade M, Roden DM, Denny JC, Carty C, Hancock S, Attia J, Holliday E, O'Donnell M, Yusuf S, Chong M, Pare G, van der Harst P, Said MA, Eppinga RN, Verweij N, Snieder H. LifeLines Cohort study group Christen T, Mook-Kanamori DO, Gustafsson S, Lind L, Ingelsson E, Pazoki R, Franco O, Hofman A, Uitterlinden A, Dehghan A, Teumer A, Baumeister S, Dörr M, Lerch MM, Völker U, Völzke H, Ward J, Pell JP, Smith DJ, Meade T, Maitland-van der Zee AH, Baranova EV, Young R, Ford I, Campbell A, Padmanabhan S, Bots ML, Grobbee DE, Froguel P, Thuillier D, Balkau B, Bonnefond A, Cariou B, Smart M, Bao Y, Kumari M, Mahajan A, Ridker PM, Chasman DI, Reiner AP, Lange LA, Ritchie MD, Asselbergs FW, Casas JP, Keating BJ, Preiss D, Hingorani AD; UCLEB consortium, Sattar N.PCSK9 genetic variants and risk of type 2 diabetes: a mendelian randomisation study. Lancet Diabetes Endocrinol 2017;5:97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Org E, Blum Y, Kasela S, Mehrabian M, Kuusisto J, Kangas AJ, Soininen P, Wang Z, Ala-Korpela M, Hazen SL, Laakso M, Lusis AJ.. Relationships between gut microbiota, plasma metabolites, and metabolic syndrome traits in the METSIM cohort. Genome Biol 2017;18:70.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Würtz P, Wang Q, Soininen P, Kangas AJ, Fatemifar G, Tynkkynen T, Tiainen M, Perola M, Tillin T, Hughes AD, Mäntyselkä P, Kähönen M, Lehtimäki T, Sattar N, Hingorani AD, Casas JP, Salomaa V, Kivimäki M, Järvelin MR, Davey Smith G, Vanhala M, Lawlor DA, Raitakari OT, Chaturvedi N, Kettunen J, Ala-Korpela M.. Metabolomic profiling of statin use and genetic inhibition of HMG-CoA Reductase. J Am Coll Cardiol 2016;67:1200–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Colhoun HM, Ginsberg HN, Robinson JG, Leiter LA, Müller-Wieland D, Henry RR, Cariou B, Baccara-Dinet MT, Pordy R, Merlet L, Eckel RH.. No effect of PCSK9 inhibitor alirocumab on the incidence of diabetes in a pooled analysis from 10 ODYSSEY Phase 3 studies. Eur Heart J 2016;37:2981–2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sabatine MS, Leiter LA, Wiviott SD, Giugliano RP, Deedwania P, De Ferrari GM, Murphy SA, Kuder JF, Gouni-Berthold I, Lewis BS, Handelsman Y, Pineda AL, Honarpour N, Keech AC, Sever PS, Pedersen TR.. Cardiovascular safety and efficacy of the PCSK9 inhibitor evolocumab in patients with and without diabetes and the effect of evolocumab on glycaemia and risk of new-onset diabetes: a prespecified analysis of the FOURIER randomised controlled trial. Lancet Diabetes Endocrinol 2017; 5:941–950. [DOI] [PubMed] [Google Scholar]
- 39. Giugliano RP, Wiviott SD, Blazing MA, De Ferrari GM, Park JG, Murphy SA, White JA, Tershakovec AM, Cannon CP, Braunwald E.. Long-term safety and efficacy of achieving very low levels of low-density lipoprotein cholesterol a prespecified analysis of the IMPROVE-IT trial. JAMA Cardiol 2017;2:547–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Besseling J, Kastelein JJ, Defesche JC, Hutten BA, Hovingh GK.. Association between familial hypercholesterolemia and prevalence of type 2 diabetes mellitus. JAMA 2015;313:1029–1036. [DOI] [PubMed] [Google Scholar]
- 41. Xu H, Ryan KA, Jaworek TJ, Southam L, Reid JG, Overton JD, Baras A, Puurunen MK, Zeggini E, Taylor SI, Shuldiner AR, Mitchell BD.. Familial hypercholesterolemia and type 2 diabetes in the Old Order Amish. Diabetes 2017;66:2054–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. HPS3/TIMI55–REVEAL Collaborative Group, Bowman L, Hopewell JC, Chen F, Wallendszus K, Stevens W, Collins R, Wiviott SD, Cannon CP, Braunwald E, Sammons E, Landray MJ.. Effects of anacetrapib in patients with atherosclerotic vascular disease. N Engl J Med 2017;377:1217–1227. [DOI] [PubMed] [Google Scholar]
- 43. Ray KK, Seshasai SR, Wijesuriya S, Sivakumaran R, Nethercott S, Preiss D, Erqou S, Sattar N.. Effect of intensive control of glucose on cardiovascular outcomes and death in patients with diabetes mellitus: a meta-analysis of randomised controlled trials. Lancet 2009;373:1765–1772. [DOI] [PubMed] [Google Scholar]
- 44. Vallejo-Vaz AJ, Kondapally Seshasai SR, Kurogi K, Michishita I, Nozue T, Sugiyama S, Tsimikas S, Yoshida H, Ray KK.. Effect of pitavastatin on glucose, HbA1c and incident diabetes: a meta-analysis of randomized controlled clinical trials in individuals without diabetes. Atherosclerosis 2015;241:409–418. [DOI] [PubMed] [Google Scholar]
- 45. Yamazaki T, Kishimoto J, Ito C, Noda M, Odawara M, Terauchi Y, Shiba T, Kitazato H, Iwamoto Y, Akanuma Y, Kadowaki T; for the J-PREDICT study investigators. Japan Prevention Trial of Diabetes by Pitavastatin in Patients with Impaired Glucose Tolerance (the J-PREDICT study): rationale, study design, and clinical characteristics of 1269 patients. Diabetology Int 2011;2:134–140. [Google Scholar]
- 46. Kowa Pharmaceutical Europe Co. Ltd. Livazo Consolidated SmPC. Summary of Product Characteristics. http://www.kowapharmaceuticals.eu/de/assets/dl/Livazo_Consolidated_SmPC_05-12-16.pdf (29 March 2018).
- 47. European Medicines Authority. Pravastatin Sodium 40 mg Tablets. Summary of Product Characteristics. https://www.medicines.org.uk/emc/medicine/25732 (17 September 2017).
- 48. Simons M, Keller P, Dichgans J, Schulz JB.. Cholesterol and Alzheimer's disease: is there a link? Neurology 2001;57:1089–1093. [DOI] [PubMed] [Google Scholar]
- 49. Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, Myers RH, Pericak-Vance MA, Risch N, van Duijn CM.. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA 1997;278:1349–1356. [PubMed] [Google Scholar]
- 50. Salat D, Ribosa R, Garcia-Bonilla L, Montaner J.. Statin use before and after acute ischemic stroke onset improves neurological outcome. Expert Rev Cardiovasc Ther 2009;7:1219–1230. [DOI] [PubMed] [Google Scholar]
- 51. Elias PK, Elias MF, D'Agostino RB, Sullivan LM, Wolf PA.. Serum cholesterol and cognitive performance in the Framingham Heart Study. Psychosom Med 2005;67:24–30. [DOI] [PubMed] [Google Scholar]
- 52. Mahley RW. Central nervous system lipoproteins: apoE and regulation of cholesterol metabolism. Arterioscler Thromb Vasc Biol 2016;36:1305–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Song Y, Nie H, Xu Y, Zhang L, Wu Y.. Association of statin use with risk of dementia: a meta-analysis of prospective cohort studies. Ger Gerontol Int 2013;13:817–824. [DOI] [PubMed] [Google Scholar]
- 54. U.S. Food and Drug Administration. FDA Drug Safety Communication: Important safety label changes to cholesterol-lowering statin drugs. 2012. https://www.fda.gov/drugs/drugsafety/ucm293101.htm (14 September 2017).
- 55. Richardson K, Schoen M, French B, Umscheid CA, Mitchell MD, Arnold SE, Heidenreich PA, Rader DJ, deGoma EM.. Statins and cognitive function: a systematic review. Ann Intern Med 2013;159:688–697. [DOI] [PubMed] [Google Scholar]
- 56. McGuinness B, Craig D, Bullock R, Malouf R, Passmore P.. Statins for the treatment of dementia. Cochrane Database Syst Rev 2014;7:CD007514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ott BR, Daiello LA, Dahabreh IJ, Springate BA, Bixby K, Murali M, Trikalinos TA.. Do statins impair cognition? A systematic review and meta-analysis of randomized controlled trials. J Gen Intern Med 2015;30:348–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Giugliano RP, Pedersen TR, Park JG, De Ferrari GM, Gaciong ZA, Ceska R, Toth K, Gouni-Berthold I, Lopez-Miranda J, Schiele F, Mach F, Ott BR, Kanevsky E, Pineda AL, Somaratne R, Wasserman SM, Keech AC, Sever PS, Sabatine MS; FOURIER Investigators. Clinical efficacy and safety of achieving very low LDL-cholesterol concentrations with the PCSK9 inhibitor evolocumab: a prespecified secondary analysis of the FOURIER trial. Lancet 2017; 390:1962–1971. [DOI] [PubMed] [Google Scholar]
- 59. Giugliano RP, Mach F, Zavitz K, Kurtz C, Im K, Kanevsky E, Schneider J, Wang H, Keech A, Pedersen TR, Sabatine MS, Sever PS, Robinson JG, Honarpour N, Wasserman SM, Ott BR; EBBINGHAUS Investigators. Cognitive function in a randomized trial of evolocumab. N Engl J Med 2017;377:633–643. [DOI] [PubMed] [Google Scholar]
- 60. Benn M, Frikke-Schmidt R, Nordestgaard BG, Tybjærg-Hansen A.. Low LDL cholesterol, PCSK9 and HMGCR genetic variation, and risk of Alzheimer’s disease and Parkinson’s disease: mendelian randomisation study. BMJ 2017;357:j1648.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Singh-Manoux A, Gimeno D, Kivimaki M, Brunner E, Marmot MG.. Low HDL cholesterol is a risk factor for deficit and decline in memory in midlife: the Whitehall II study. Arterioscler Thromb Vasc Biol 2008;28:1556–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Brunner EJ, Welch CA, Shipley MJ, Ahmadi-Abhari S, Singh-Manoux A, Kivimäki M.. Midlife risk factors for impaired physical and cognitive functioning at older ages: a cohort study. J Gerontol A Biol Sci Med Sci 2017;72:237–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kesse-Guyot E, Andreeva VA, Touvier M, Jeandel C, Ferry M, Hercberg S, Galan P; SU.VI.MAX 2 Research Group. Overall and abdominal adiposity in midlife and subsequent cognitive function. J Nutr Health Aging 2015;19:183–189. [DOI] [PubMed] [Google Scholar]
- 64. Zhong G, Wang Y, Zhang Y, Guo JJ, Zhao Y.. Smoking is associated with an increased risk of dementia: a meta-analysis of prospective cohort studies with investigation of potential effect modifiers. PLoS One 2015;10:e0118333.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20536 high-risk individuals: a randomised placebo controlled trial. Lancet 2002;360:7–22.12114036 [Google Scholar]
- 66. Trompet S, van Vliet P, de Craen AJ, Jolles J, Buckley BM, Murphy MB, Ford I, Macfarlane PW, Sattar N, Packard CJ, Stott DJ, Shepherd J, Bollen EL, Blauw GJ, Jukema JW, Westendorp RG.. Pravastatin and cognitive function in the elderly. Results of the PROSPER study. J Neurol 2010;257:85–90. [DOI] [PubMed] [Google Scholar]
- 67. Healy D, Morgan R, Chinnaswamy S.. Transient global amnesia associated with statin intake. BMJ Case Rep 2009; doi:10.1136/bcr.06.2008.0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Koren MJ, Sabatine MS, Giugliano RP, Langslet G, Wiviott SD, Kassahun H, Ruzza A, Ma Y, Somaratne R, Raal FJ.. Long-term low-density lipoprotein cholesterol–lowering efficacy, persistence, and safety of evolocumab in treatment of hypercholesterolemia. Results up to 4 years from the open-label OSLER-1 Extension Study. JAMA Cardiol 2017;2:598–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Robinson JG, Rosenson RS, Farnier M, Chaudhari U, Sasiela WJ, Merlet L, Miller K, Kastelein JJ.. Safety of very low low-density lipoprotein cholesterol levels with alirocumab: pooled data from randomized trials. J Am Coll Cardiol 2017;69:471–482. [DOI] [PubMed] [Google Scholar]
- 70. Saunders NL, Summers MJ.. Longitudinal deficits to attention, executive, and working memory in subtypes of mild cognitive impairment. Neuropsychol 2011;25:237–248. [DOI] [PubMed] [Google Scholar]
- 71. Wanner C, Tonelli M; Kidney Disease: Improving Global Outcomes Lipid Guideline Development Work Group M. KDIGO Clinical Practice Guideline for Lipid Management in CKD: summary of recommendation statements and clinical approach to the patient. Kidney Int 2014;85:1303–1309. [DOI] [PubMed] [Google Scholar]
- 72. Cholesterol Treatment Trialists C, Herrington WG, Emberson J, Mihaylova B, Blackwell L, Reith C, Solbu MD, Mark PB, Fellström B, Jardine AG, Wanner C, Holdaas H, Fulcher J, Haynes R, Landray MJ, Keech A, Simes J, Collins R, Baigent C.. Impact of renal function on the effects of LDL cholesterol lowering with statin-based regimens: a meta-analysis of individual participant data from 28 randomised trials. Lancet Diabetes Endocrinol 2016;4:829–839. [DOI] [PubMed] [Google Scholar]
- 73. Ridker PM, MacFadyen J, Cressman M, Glynn RJ.. Efficacy of rosuvastatin among men and women with moderate chronic kidney disease and elevated high-sensitivity C-reactive protein: a secondary analysis from the JUPITER (Justification for the Use of Statins in Prevention-an Intervention Trial Evaluating Rosuvastatin) trial. J Am Coll Cardiol 2010;55:1266–1273. [DOI] [PubMed] [Google Scholar]
- 74. Hou W, Lv J, Perkovic V, Yang L, Zhao N, Jardine MJ, Cass A, Zhang H, Wang H.. Effect of statin therapy on cardiovascular and renal outcomes in patients with chronic kidney disease: a systematic review and meta-analysis. Eur Heart J 2013;34:1807–1817. [DOI] [PubMed] [Google Scholar]
- 75. Palmer SC, Navaneethan SD, Craig JC, Johnson DW, Perkovic V, Hegbrant J, Strippoli GF.. HMG CoA reductase inhibitors (statins) for people with chronic kidney disease not requiring dialysis. Cochrane Database Syst Rev 2014;CD007784. [DOI] [PubMed] [Google Scholar]
- 76. Fellström BC, Jardine AG, Schmieder RE, Holdaas H, Bannister K, Beutler J, Chae DW, Chevaile A, Cobbe SM, Grönhagen-Riska C, De Lima JJ, Lins R, Mayer G, McMahon AW, Parving HH, Remuzzi G, Samuelsson O, Sonkodi S, Sci D, Süleymanlar G, Tsakiris D, Tesar V, Todorov V, Wiecek A, Wüthrich RP, Gottlow M, Johnsson E, Zannad F; AURORA Study Group. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med 2009;360:1395–1407. [DOI] [PubMed] [Google Scholar]
- 77. Palmer SC, Navaneethan SD, Craig JC, Johnson DW, Perkovic V, Hegbrant J, Strippoli GF.. HMG CoA reductase inhibitors (statins) for kidney transplant recipients. Cochrane Database Syst. Rev. 2014;1:CD005019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Wanner C, Krane V, März W, Olschewski M, Mann JF, Ruf G, Ritz E; German Diabetes and Dialysis Study Investigators. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med 2005;353:238–248. [DOI] [PubMed] [Google Scholar]
- 79. Baigent C, Landray MJ, Reith C, Emberson J, Wheeler DC, Tomson C, Wanner C, Krane V, Cass A, Craig J, Neal B, Jiang L, Hooi LS, Levin A, Agodoa L, Gaziano M, Kasiske B, Walker R, Massy ZA, Feldt-Rasmussen B, Krairittichai U, Ophascharoensuk V, Fellström B, Holdaas H, Tesar V, Wiecek A, Grobbee D, de Zeeuw D, Grönhagen-Riska C, Dasgupta T, Lewis D, Herrington W, Mafham M, Majoni W, Wallendszus K, Grimm R, Pedersen T, Tobert J, Armitage J, Baxter A, Bray C, Chen Y, Chen Z, Hill M, Knott C, Parish S, Simpson D, Sleight P, Young A, Collins R; SHARP Investigators. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet 2011;377:2181–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Davidson MH. Rosuvastatin safety: lessons from the FDA review and post-approval surveillance. Expert Opin Drug Safety 2004;3:547–557. [DOI] [PubMed] [Google Scholar]
- 81. Vidt DG, Cressman MD, Harris S, Pears JS, Hutchinson HG.. Rosuvastatin-induced arrest in progression of renal disease. Cardiology 2004;102:52–60. [DOI] [PubMed] [Google Scholar]
- 82. Sidaway JE, Davidson RG, McTaggart F, Orton TC, Scott RC, Smith GJ, Brunskill NJ.. Inhibitors of 3-hydroxy-3-methylglutaryl-CoA reductase reduce receptor-mediated endocytosis in opossum kidney cells. J Amn Soc Nephrol 2004;15:2258–2265. [DOI] [PubMed] [Google Scholar]
- 83. Verhulst A, D'Haese PC, De Broe ME.. Inhibitors of HMG-CoA reductase reduce receptor-mediated endocytosis in human kidney proximal tubular cells. J Am Soc Nephrol 2004;15:2249–2257. [DOI] [PubMed] [Google Scholar]
- 84. Dormuth CR, Hemmelgarn BR, Paterson JM, James MT, Teare GF, Raymond CB, Lafrance JP, Levy A, Garg AX, Ernst P; Canadian Network for Observational Drug Effect Studies. Use of high potency statins and rates of admission for acute kidney injury: multicenter, retrospective observational analysis of administrative databases. BMJ 2013;346:f880.. [DOI] [PubMed] [Google Scholar]
- 85. Hippisley-Cox J, Coupland C.. Unintended effects of statins in men and women in England and Wales: population based cohort study using the QResearch database. BMJ 2010;340:c2197.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Acharya T, Huang J, Tringali S, Frei CR, Mortensen EM, Mansi IA.. Statin use and the risk of kidney disease with long-term follow-up (8.4-year study). Am J Cardiol 2016;117:647–655. [DOI] [PubMed] [Google Scholar]
- 87. Bangalore S, Fayyad R, Hovingh GK, Laskey R, Vogt L, DeMicco DA, Waters DD; Treating to New Targets Steering Committee and Investigators. Statin and the risk of renal-related serious adverse events: analysis from the IDEAL, TNT, CARDS, ASPEN, SPARCL, and other placebo-controlled trials. Am J Cardiol 2014;113:2018–2020. [DOI] [PubMed] [Google Scholar]
- 88. Sanguankeo A, Upala S, Cheungpasitporn W, Ungprasert P, Knight EL.. Effects of statins on renal outcome in chronic kidney disease patients: a systematic review and meta-analysis. PLoS One 2015;10:e0132970.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Zhang Z, Wu P, Zhang J, Wang S, Zhang G.. The effect of statins on microalbuminuria, proteinuria, progression of kidney function, and all-cause mortality in patients with non-end stage chronic kidney disease: a meta-analysis. Pharmacol Res 2016;105:74–83. [DOI] [PubMed] [Google Scholar]
- 90. Collins R, Armitage J, Parish S, Sleight P, Peto R; Heart Protection Study Collaborative G. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet 2003;361:2005–2016. [DOI] [PubMed] [Google Scholar]
- 91. Tonelli M, Moyé L, Sacks FM, Cole T, Curhan GC; Cholesterol and Recurrent Events Trial Investigators. Effect of pravastatin on loss of renal function in people with moderate chronic renal insufficiency and cardiovascular disease. J Am Soc Nephrol 2003;14:1605–1613. [DOI] [PubMed] [Google Scholar]
- 92. Athyros VG, Mikhailidis DP, Papageorgiou AA, Symeonidis AN, Pehlivanidis AN, Bouloukos VI, Elisaf M.. The effect of statins versus untreated dyslipidaemia on renal function in patients with coronary heart disease. A subgroup analysis of the Greek atorvastatin and coronary heart disease evaluation (GREACE) study. J Clin Pathol 2004;57:728–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Nikolic D, Banach M, Nikfar S, Salari P, Mikhailidis DP, Toth PP, Abdollahi M, Ray KK, Pencina MJ, Malyszko J, Rysz J, Rizzo M; Lipid and Blood Pressure Meta-Analysis Collaboration Group. A meta-analysis of the role of statins on renal outcomes in patients with chronic kidney disease. Is the duration of therapy important? Int J Cardiol 2013;168:5437–5447. [DOI] [PubMed] [Google Scholar]
- 94. de Zeeuw D, Anzalone DA, Cain VA, Cressman MD, Heerspink HJ, Molitoris BA, Monyak JT, Parving HH, Remuzzi G, Sowers JR, Vidt DG.. Renal effects of atorvastatin and rosuvastatin in patients with diabetes who have progressive renal disease (PLANET I): a randomised clinical trial. Lancet Diabetes Endocrinol 2015;3:181–190. [DOI] [PubMed] [Google Scholar]
- 95. Su X, Zhang L, Lv J, Wang J, Hou W, Xie X, Zhang H.. Effect of statins on kidney disease outcomes: a systematic review and meta-analysis. Am J Kidney Dis 2016;67:881–888. [DOI] [PubMed] [Google Scholar]
- 96. Corsini A, Ganey P, Ju C, Kaplowitz N, Pessayre D, Roth R, Watkins PB, Albassam M, Liu B, Stancic S, Suter L, Bortolini M.. Current challenges and controversies in drug-induced liver injury. Drug Saf 2012;35:1099–1117. [DOI] [PubMed] [Google Scholar]
- 97. Food and Drug Administration. Guidance for Industry. Drug-Induced Liver Injury: Premarketing Clinical Evaluation. 2009. https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM174090.pdf (29 March 2018).
- 98. Zimmerman H, Drug-induced liver disease In: Zimmerman H, ed. Hepatotoxicity, the Adverse Effects of Drugs and Other Chemicals on the Liver, 2nd ed Philadelphia: Lippincott Williams & Wilkins; 1999. pp. 428–433. [Google Scholar]
- 99. Corsini A, Bortolini M.. Drug-induced liver injury: the role of drug metabolism and transport. J Clin Pharmacol 2013;53:463–474. [DOI] [PubMed] [Google Scholar]
- 100. Lammert C, Bjornsson E, Niklasson A, Chalasani N.. Oral medications with significant hepatic metabolism at higher risk for hepatic adverse events. Hepatology 2010;51:615–620. [DOI] [PubMed] [Google Scholar]
- 101. Tolman KG. The liver and lovastatin. Am J Cardiol 2002;89:1374–1380. [DOI] [PubMed] [Google Scholar]
- 102. Naci H, Brugts J, Ades T.. Comparative tolerability and harms of individual statins: a study-level network meta-analysis of 246 955 participants from 135 randomized, controlled trials. Circ Cardiovasc Qual Outcomes 2013;6:390–399. [DOI] [PubMed] [Google Scholar]
- 103. Newman C, Tsai J, Szarek M, Luo D, Gibson E.. Comparative safety of atorvastatin 80 mg versus 10 mg derived from analysis of 49 completed trials in 14, 236 patients. Am J Cardiol 2006;97:61–67. [DOI] [PubMed] [Google Scholar]
- 104. Björnsson E, Jacobsen EI, Kalaitzakis E.. Hepatotoxicity associated with statins: reports of idiosyncratic liver injury post-marketing. J Hepatol 2012;56:374–380. [DOI] [PubMed] [Google Scholar]
- 105. Clarke AT, Johnson PC, Hall GC, Ford I, Mills PR.. High dose atorvastatin associated with increased risk of significant hepatotoxicity in comparison to simvastatin in UK GPRD Cohort. PLoS One 2016;11:e0151587.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Pastori D, Polimeni L, Baratta F, Pani A, Del Ben M, Angelico F.. The efficacy and safety of statins for the treatment of non-alcoholic fatty liver disease. Dig Liver Dis 2015;47:4–11. [DOI] [PubMed] [Google Scholar]
- 107. Sorokin A, Brown JL, Thompson PD.. Primary biliary cirrhosis, hyperlipidemia, and atherosclerotic risk: a systematic review. Atherosclerosis 2007;194:293–299. [DOI] [PubMed] [Google Scholar]
- 108. Athyros VG, Tziomalos K, Gossios TD, Griva T, Anagnostis P, Kargiotis K, Pagourelias ED, Theocharidou E, Karagiannis A, Mikhailidis DP; GREACE Study Collaborative Group. Safety and efficacy of long-term statin treatment for cardiovascular events in patients with coronary heart disease and abnormal liver tests in the Greek Atorvastatin and Coronary Heart Disease Evaluation (GREACE) Study: a post-hoc analysis. Lancet 2010;376:1916–1922. [DOI] [PubMed] [Google Scholar]
- 109. Kim RG, Loomba R, Prokop LJ, Singh S.. Statin use and risk of cirrhosis and related complications in patients with chronic liver diseases: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2017;15:1521–1530.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Herrick C, Litvin M, Goldberg AC.. Lipid lowering in liver and chronic kidney disease. Best Pract Res Clin Endocrinol Metab 2014;28:339–352. [DOI] [PubMed] [Google Scholar]
- 111. Andrade RJ, Robles M, Ulzurrun E, Lucena MI.. Drug‐induced liver injury: insights from genetic studies. Pharmacogenomics 2009;10:1467–1487. [DOI] [PubMed] [Google Scholar]
- 112. Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J; Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology 2008;135:1924–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Bays H, Cohen DE, Chalasani N, Harrison SA.. The National Lipid Association's Statin Safety Task Force. An assessment by the statin liver safety task force: 2014 update. J Clin Lipidol 2014;8(Suppl 3): S47–S57. [DOI] [PubMed] [Google Scholar]
- 114. Russo MW, Hoofnagle JH, Gu J, Fontana RJ, Barnhart H, Kleiner DE, Chalasani N, Bonkovsky HL.. Spectrum of statin hepatotoxicity: experience of the drug-induced liver injury network. Hepatology 2014;60:679–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Perdices EV, Medina-Cáliz I, Hernando S, Ortega A, Martín-Ocaña F, Navarro JM, Peláez G, Castiella A, Hallal H, Romero-Gómez M, González-Jiménez A, Robles-Díaz M, Lucena MI, Andrade RJ.. Hepatotoxicity associated with statin use: analysis of the cases included in the Spanish Hepatotoxicity Registry. Rev Esp Enferm Dig 2014;106:246–254. [PubMed] [Google Scholar]
- 116. Mancini GB, Baker S, Bergeron J, Fitchett D, Frohlich J, Genest J, Gupta M, Hegele RA, Ng D, Pearson GJ, Pope J, Tashakkor AY.. Diagnosis, prevention, and management of statin adverse effects and intolerance: Canadian Consensus Working Group Update. Can J Cardiol 2016;32:S35–S65. [DOI] [PubMed] [Google Scholar]
- 117. Cholesterol Treatment Trialists’ (CTT) Collaboration, Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, Peto R, Barnes EH, Keech A, Simes J, Collins R.. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170, 000 participants in 26 randomised trials. Lancet 2010;376:1670–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Sturgeon JD, Folsom AR, Longstreth WT, Shahar E, Rosamond WD, Cushman M.. Risk Factors for Intracerebral Hemorrhage in a Pooled Prospective Study. Stroke 2007;38:2718–2725. [DOI] [PubMed] [Google Scholar]
- 119. Vergouwen MD, de Haan RJ, Vermeulen M, Roos YB.. Statin treatment and the occurrence of hemorrhagic stroke in patients with a history of cerebrovascular disease. Stroke 2008;39:497–502. [DOI] [PubMed] [Google Scholar]
- 120. Amarenco P, Bogousslavsky J, Callahan A 3rd, Goldstein LB, Hennerici M, Rudolph AE, Sillesen H, Simunovic L, Szarek M, Welch KM, Zivin JA; Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Investigators High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med 2006;355:549–559. [DOI] [PubMed] [Google Scholar]
- 121. Goldstein LB, Amarenco P, Szarek M, Callahan A 3rd, Hennerici M, Sillesen H, Zivin JA, Welch KM; SPARCL Investigators Hemorrhagic stroke in the Stroke Prevention by Aggressive Reduction in Cholesterol Levels study. Neurology 2008;70:2364–2370. [DOI] [PubMed] [Google Scholar]
- 122. Boekholdt SM, Hovingh GK, Mora S, Arsenault BJ, Amarenco P, Pedersen TR, LaRosa JC, Waters DD, DeMicco DA, Simes RJ, Keech AC, Colquhoun D, Hitman GA, Betteridge DJ, Clearfield MB, Downs JR, Colhoun HM, Gotto AM Jr, Ridker PM, Grundy SM, Kastelein JJ.. Very low levels of atherogenic lipoproteins and the risk for cardiovascular events: a meta-analysis of statin trials. J Am Coll Cardiol 2014;64:485–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Hackam DG, Woodward M, Newby LK, Bhatt DL, Shao M, Smith EE, Donner A, Mamdani M, Douketis JD, Arima H, Chalmers J, MacMahon S, Tirschwell DL, Psaty BM, Bushnell CD, Aguilar MI, Capampangan DJ, Werring DJ, De Rango P, Viswanathan A, Danchin N, Cheng CL, Yang YH, Verdel BM, Lai MS, Kennedy J, Uchiyama S, Yamaguchi T, Ikeda Y, Mrkobrada M.. Statins and intracerebral hemorrhage: collaborative systematic review and meta-analysis. Circulation 2011;124:2233–2242. [DOI] [PubMed] [Google Scholar]
- 124. McKinney JS, Kostis WJ.. Statin therapy and the risk of intracerebral hemorrhage: a meta-analysis of 31 randomized controlled trials. Stroke 2012;43:2149–2156. [DOI] [PubMed] [Google Scholar]
- 125. Casula M, Soranna D, Corrao G, Merlino L, Catapano AL, Tragni E.. Statin use and risk of cataract: a nested case-control study within a healthcare database. Atherosclerosis 2016;251:153–158. [DOI] [PubMed] [Google Scholar]
- 126. Desai CS, Martin SS, Blumenthal RS.. Non-cardiovascular effects associated with statins. BMJ 2014;349:g3743.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Hockwin O, Evans M, Roberts SA, Stoll RE.. Post-mortem biochemistry of beagle dog lenses after treatment with Fluvastatin (Sandoz) for 2 years at different dose levels. Lens Eye Toxic Res 1990;7:563–575. [PubMed] [Google Scholar]
- 128. Leuschen J, Mortensen EM, Frei CR, Mansi EA, Panday V, Mansi I.. Association of statin use with cataracts: a propensity score-matched analysis. JAMA Ophthalmol 2013;131:1427–1434. [DOI] [PubMed] [Google Scholar]
- 129. Yusuf S, Bosch J, Dagenais G, Zhu J, Xavier D, Liu L, Pais P, López-Jaramillo P, Leiter LA, Dans A, Avezum A, Piegas LS, Parkhomenko A, Keltai K, Keltai M, Sliwa K, Peters RJ, Held C, Chazova I, Yusoff K, Lewis BS, Jansky P, Khunti K, Toff WD, Reid CM, Varigos J, Sanchez-Vallejo G, McKelvie R, Pogue J, Jung H, Gao P, Diaz R, Lonn E; HOPE-3 Investigators. Cholesterol lowering in intermediate-risk persons without cardiovascular disease. N Engl J Med 2016;374:2021–2031. [DOI] [PubMed] [Google Scholar]
- 130. Wise SJ, Nathoo NA, Etminan M, Mikelberg FS, Mancini GB.. Statin use and risk for cataract: a nested case-control study of 2 populations in Canada and the United States. Can J Cardiol 2014;30:1613–1619. [DOI] [PubMed] [Google Scholar]
- 131. Laties AM, Shear CL, Lippa EA, Gould AL, Taylor HR, Hurley DP, Stephenson WP, Keates EU, Tupy-Visich MA, Chremos AN.. Expanded clinical evaluation of lovastatin (EXCEL) study results. II. Assessment of the human lens after 48 weeks of treatment with lovastatin. Am J Cardiol 1991;67:447–453. [DOI] [PubMed] [Google Scholar]
- 132. Harris ML, Bron AJ, Brown NA, Keech AC, Wallendszus KR, Armitage JM, MacMahon S, Snibson G, Collins R.. Absence of effect of simvastatin on the progression of lens opacities in a randomised placebo controlled study. Oxford Cholesterol Study Group. Br J Ophthalmol 1995;79:996–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Bang CN, Greve AM, La Cour M, Boman K, Gohlke-Bärwolf C, Ray S, Pedersen T, Rossebø A, Okin PM, Devereux RB, Wachtell K.. Effect of randomized lipid lLowering with simvastatin and ezetimibe on cataract development (from the Simvastatin and Ezetimibe in Aortic Stenosis Study). Am J Cardiol 2015;116:1840–1844. [DOI] [PubMed] [Google Scholar]
- 134. Yu S, Chu Y, Li G, Ren L, Zhang Q, Wu L.. Statin use and the risk of cataracts: a systematic review and meta‐Analysis. J Am Heart Assoc 2017;6:e004180.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Kostis JB, Dobrzynski JM.. Prevention of cataract by statins. Am J Cardiol 2016;117:1196.. [DOI] [PubMed] [Google Scholar]
- 136. Lim S, Barter P.. Antioxidant effects of statins in the management of cardiometabolic disorders. J Atheroscler Thromb 2014;21:997–1010. [DOI] [PubMed] [Google Scholar]