Abstract
Aims
Low-density lipoprotein (LDL) particles cause atherosclerotic cardiovascular disease (ASCVD) through their retention, modification, and accumulation within the arterial intima. High plasma concentrations of LDL drive this disease, but LDL quality may also contribute. Here, we focused on the intrinsic propensity of LDL to aggregate upon modification. We examined whether inter-individual differences in this quality are linked with LDL lipid composition and coronary artery disease (CAD) death, and basic mechanisms for plaque growth and destabilization.
Methods and results
We developed a novel, reproducible method to assess the susceptibility of LDL particles to aggregate during lipolysis induced ex vivo by human recombinant secretory sphingomyelinase. Among patients with an established CAD, we found that the presence of aggregation-prone LDL was predictive of future cardiovascular deaths, independently of conventional risk factors. Aggregation-prone LDL contained more sphingolipids and less phosphatidylcholines than did aggregation-resistant LDL. Three interventions in animal models to rationally alter LDL composition lowered its susceptibility to aggregate and slowed atherosclerosis. Similar compositional changes induced in humans by PCSK9 inhibition or healthy diet also lowered LDL aggregation susceptibility. Aggregated LDL in vitro activated macrophages and T cells, two key cell types involved in plaque progression and rupture.
Conclusion
Our results identify the susceptibility of LDL to aggregate as a novel measurable and modifiable factor in the progression of human ASCVD.
Keywords: Low-density lipoprotein , Atherosclerosis , Cardiovascular death , Sphingomyelin , Lipidomics
Translational perspective
Accumulation of low-density lipoprotein (LDL)-derived cholesterol in the arterial wall causes atherosclerotic cardiovascular disease (ASCVD). High plasma concentrations of LDL drive this disease, but LDL quality may also contribute. Here, inter-individual differences in LDL lipidome were found to determine the susceptibility of LDL to aggregate during lipolysis by human recombinant secretory sphingomyelinase. The presence of aggregation-prone LDL in plasma predicted future cardiovascular death in coronary artery disease patients. Interventions in pre-clinical models to rationally alter LDL composition lowered its susceptibility to aggregate and slowed atherosclerosis development. Our results identify the susceptibility of LDL to aggregate as a novel measurable and modifiable factor in the progression of human ASCVD.
Introduction
An elevated plasma concentration of low-density lipoprotein cholesterol (LDL-C) is a primary causal factor in the development of atherosclerotic cardiovascular disease (ASCVD)1 and significantly contributes to the total cardiovascular risk.2 However, even after efficient LDL-C-lowering, a substantial residual risk for ASCVD events remains.3–5Atherosclerosis arises from subendothelial retention, or trapping, of LDL within the arterial intima, and several steps are required for plasma LDL-C to provoke normal arteries to become diseased. The retained lipoproteins become modified by arterial-wall enzymes and oxidants,6,7 tend to aggregate,8 and aggregated lipoprotein-derived particles are found both in human and in experimentally induced atherosclerotic lesions in animal models.9–12 The intimal processes triggering aggregation of LDL particles have been proposed to include lipid peroxidation and proteolytic and lipolytic digestion of LDL by local enzymes, such as the mast cell chymase having chymotrypsin-like activity,13 the group V secretory phospholipase A2 (PLA2),14 which is produced by macrophages, as well as the secretory sphingomyelinase (SMase), which is released by macrophages and endothelial cells.15 Aggregation enhances the binding of lipoproteins to the arterial extracellular matrix,8 and their large size makes egress back across the endothelium nearly impossible.7 Moreover, aggregated LDL induces the formation of foam cells, a hallmark of lesions at all stages of atherogenesis.14,16,17 Indeed, the development of an atherosclerotic lesion involves a series of maladaptive responses of innate and adaptive immune cells to the retained, modified, and aggregated lipoprotein-derived material.18,19
In this study, we focused on the intrinsic susceptibility of circulating LDL particles to aggregate upon modification, and, for the first time, examined inter-individual differences in this quality. This study aimed to determine whether the degree of aggregation susceptibility of LDL can predict future cardiovascular deaths and whether it can be modified by nutritional or medical treatment.
Materials and methods
Human plasma
Human blood plasma samples were obtained from healthy volunteers (Finnish Red Cross Blood Service, Helsinki, Finland), 100 samples derived from subjects participating in the Health 2000 Health Examination Survey,20 48 samples from Corogene survey,21 57 samples from SYSDIET (Systems biology in controlled dietary interventions and cohort studies) survey, 29 samples from the 18-week diet group and 28 samples from the 24-week diet group,22,23 and 40 samples from the EQUATOR study.24
The use of human material conformed to the principles outlined in the Declaration of Helsinki, and the studies were approved by the local ethics committees. Written informed consent was obtained from all participants.
Isolation of LDL and lipid and lipoprotein measurements
Lipid measurements
Fasting plasma total cholesterol and triglycerides were enzymatically measured (Roche Diagnostics, GmbH, Mannheim, Germany). ApoB-100 content was measured with ELISA-kit (MABTECH, Nacka, Sweden).
LDL isolation
LDL (d = 1.019 to 1.063 g/mL) was isolated from plasma by KBr-based sequential ultracentrifugation.25 LDL concentrations are expressed as their protein concentrations, which were determined by the BCA protein assay (Pierce, Rockford, USA) using bovine serum albumin as a standard, or as apoB-100 concentration determined by ELISA.
Measurement of LDL aggregation
Development of a method to quantify person-to-person variability in LDL aggregation-susceptibility is available in Supplementary material online, Methods and Figures S1–S3. In the assay we developed, LDL isolated from human plasma was extensively dialysed against 20 mM MES buffer, pH 5.5, containing 150 mM NaCl, 2 mM MgCl2, 2 mM CaCl2, and 50 µM ZnCl2. The sample was diluted with the same buffer to give a final concentration of 0.2 mg of apoB-100/mL, then human recombinant secretory sphingomyelinase (hrSMase) was added to to give a final enzyme concentration of 75 µg/mL, and the mixture was incubated at +37°C. The size of LDL was determined immediately and the hourly up to 6 h. An aliquot was taken at the same time points for phosphorylcholine measurement. The degree of sphingomyelins (SM) hydrolysis was determined by measuring the phosphorylcholine content from the samples with Amplex Red reagent (Invitrogen).
SM- PC-, and LPC-enrichment of LDL ex vivo
LDL was enriched ex vivo with SM, PC, or LPC using phospholipid vesicles, as described in detail in Supplementary material online, Methods.
Mice
Large ‘empty’ vesicle-treated mice
Human APOB100 transgenic, Ldlr−/−-mice on a chow diet, with food and water received ad libitum (13–19 weeks old for control with phosphate-buffered saline (PBS) and 19 weeks old for large ‘empty’ vesicle (LEV), weight 30–33g) (n = 16 per group) were injected once with PBS or with LEVs (also known as large unilamellar vesicles, 100-nm in diameter, dosed at 1000 mg PC/kg body weight). One hour after PBS or LEVs injection, plasma was collected, and the d < 1.063g/mL KBr fraction was isolated using ultracentrifugation. Because LEVs can appear in the LDL density range, we collected the supernatant fraction after ultracentrifugation, loaded it onto a Superose 6 HR column (size-exclusion FPLC), and eluted with 150 mM NaCl, 1 mM EDTA, pH 7.4. LDL fractions, which came out after the peak for LEVs and VLDL, were pooled and re-loaded onto the Superose 6 HR column, eluted with 150 mM NaCl, 1 mM EDTA pH 7.4, at which point no peak for LEVs or VLDL was detectable. LDL fractions were pooled in pairs (sample size, n = 16) and protein concentration was measured. The animal procedures were approved by the Animal Ethical Committee at the Gothenburg University.
Myriocin-treated mice
Male Ldlr−/−, Apob100/100 mice on the C57BL/6J genetic background were obtained from Jackson Laboratory (Bar Harbor, ME, USA) and housed in the National Laboratory Animal Center of the University of Eastern Finland. Mice were divided into control (n = 11) and myriocin (n = 11) groups. The mice were fed on an high-fat atherogenic diet (21% milk fat, 0.2% cholesterol; Harlan Teklad; TD88137; colloquially called the ‘Western’ diet) from 14 to 22 weeks of age onwards, with food and water received ad libitum. Myriocin (0.3 mg/kg) (Biomol Research Laboratories Inc.) or PBS as control was injected intraperitoneally (i.p.) three times per week for 10 weeks. After 10 weeks on high-fat diet with myriocin/PBS treatment, mice were fasted for 4 h and terminal anaesthetised with isoflurane inhalation (Vetflurane 1000 mg/g, Virbab animal health). Blood was collected by heart puncture. These animal procedures were approved by the National Animal Experiment Board of Finland and carried out in accordance with the guidelines of The Finnish Act on Animal Experimentation.
Soat2-/-mice
Human APOB100 transgenic, Ldlr−/−, Soat2−/−, and human APOB100 transgenic, Ldlr−/− expressing sterol O-acyltransferase 2 (SOAT2) mice were fed on a diet rich in cis-monounsaturated fat26 for 8 months, after which plasma was collected and LDL particles were isolated by ultracentrifugation. These animal procedures were approved by the Institutional Animal Care and Use Committee at Wake Forest University Health Sciences.
Assessment of atherosclerotic lesions
The degree of atherosclerosis was assessed by Sudan IV staining of the aortas as described in Supplementary material online.
Lipid mass spectrometry analyses
LDL lipids were analysed by mass spectrometry as described in Supplementary material online.
Circular dichroism analyses
The secondary structure of apoB-100 in LDL particles was determined by circular dichroism as described previously27 and in Supplementary material online.
Cell culture
Cell culture material and methods are available in Supplementary material online.
Mathematical modelling and statistical analyses
Mathematical modelling and statistical analyses are available in Supplementary material online.
Results
Measurement of the susceptibility of LDL to aggregate ex vivo
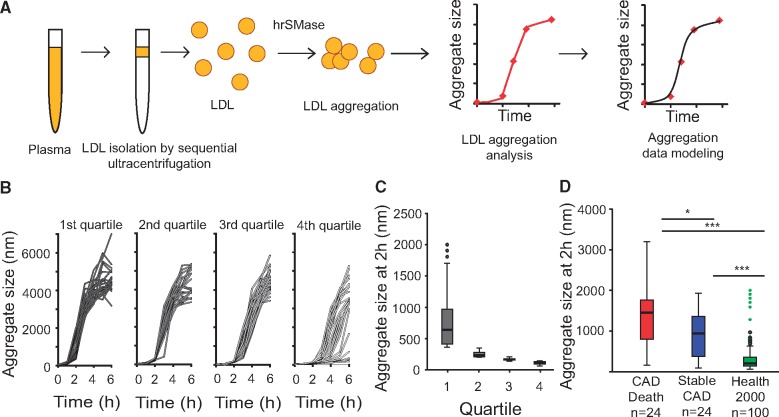
We developed a novel, reproducible method to quantify donor-to-donor variation in the susceptibility of LDL particles to aggregate (see Supplementary material online, Methods and Figures S1–S3). LDL was isolated from plasma samples by ultracentrifugation, aggregation of each LDL preparation was induced ex vivo by incubation with hrSMase, and the kinetics of aggregation were followed in real time by measuring the growth of the aggregates by dynamic light scattering (Figure 1A). Other agents to modify LDL ex vivo produced far smaller aggregates with negligible discrimination between individuals (see Supplementary material online, Figure S1a–e). In the absence of any modifying agent, LDL particles show no sign of aggregation (Supplementary material online, Methods).
Figure 1.
Measurement of the susceptibility of low-density lipoprotein (LDL) from healthy human subjects and from coronary artery disease patients to aggregate ex vivo. (A) LDL is isolated from blood plasma by ultracentrifugation and aggregation was induced by incubation with human recombinant secretory sphingomyelinase (hrSMase) at pH 5.5. The size of LDL particles was measured before hrSMase treatment (time = 0 h), and formation of LDL aggregates was followed in real time by measuring their size with dynamic light scattering. (B) LDL particles were isolated from 100 plasma samples collected from the Finnish Health 2000 Health Examination Survey and the aggregation susceptibility of the particles was analysed. Based on LDL aggregate size at 2 h, the particles were divided into quartiles. (C) Size distributions of LDL aggregates at the 2 h time point. The box encompasses the middle 50% of the measured values; the horizontal line within each box shows the median of the measured values; the whiskers encompass the most extreme data point that is still no further from the margins of the box than 1.5 times the interquartile range. (D) Patients (n = 48) from the Corogene study, having >50% stenosis in their coronary arteries were divided into two groups: (i) CAD death group, in which patients died of coronary events during an average 2.5-year follow-up period and (ii) stable CAD group, having no cardiovascular events during the follow-up period. The patients were matched for the conventional cardiovascular risk factors. LDL was isolated and LDL aggregation was induced by treatment with hrSMase. The box plot diagram shows the distribution of aggregate sizes after incubation for 2 h in the two groups from Corogene study and in 100 subjects from the Health 2000 study (all quartiles from C combined). Statistical differences between the groups were determined using Kruskal–Wallis test followed by Dunn’s test. P < 0.001 by Kruskal–Wallis test; *P < 0.05, ***P <0.001 by Dunn’s test.
We used our hrSMase-based assay to screen LDL aggregation susceptibility in samples derived from the Finnish Health 2000 Health Examination Survey,20 which comprised largely healthy individuals (n = 100, Table 1, Figure 1B). To quantify the inter-individual variation in the aggregation susceptibility of LDL, we first developed a population-based generalized mixed-effect model (see Supplementary material online, Figure S3a). As described in the Supplementary material online, Methods, these data revealed that the inflection points in the curves of aggregate size vs. incubation time readily distinguished the aggregation susceptibility of the different LDL samples. Further, we found that LDL aggregate size at the 2-h time point correlated tightly and significantly with the inflection point (see Supplementary material online, Figure S3b, rho = −0.961, P < 0.001). Importantly, the extent of aggregation at 2 h clearly identified subjects having extremely aggregation-prone LDL particles (Figure 1C, Quartile 1). Therefore, in further assays, aggregate size at this time point was used as a measure of LDL aggregation susceptibility.
Table 1.
Clinical characteristics of Health 2000 Health Examination survey participants assessed in this study
| Characteristics | Health 2000 |
|---|---|
| Number of subjectsa | 100 |
| Gender (male)a | 50 (50%) |
| Age (years)b | 40 (33–48) |
| Current smokera | 15 (15%) |
| Blood pressure: syst/diast (mmHg)b | 121/78 (110–132/68–85) |
| Body mass index (kg/m2)b | 24.2 (22.5–28.1) |
| Glucose (mmol/L)b | 5.2 (5.0–5.5) |
| Diabetesa | 1 (1%) |
| Statin (n = 91)a | 4 (4%) |
| Total cholesterol (mmol/L)b | 5.5 (4.8–6.5) |
| LDL-C (mmol/L)b | 3.1 (2.6–4.0) |
| HDL-C (mmol/L)b | 1.3 (1.1–1.6) |
| TG (mmol/L)b | 1.3 (0.9–1.9) |
| C-reactive protein (mg/L)b | 0.6 (0.2–1.8) |
Number of cases (%).
Median (interquartile range).
The susceptibility of LDL to aggregate predicts future cardiovascular deaths
We next measured the aggregation susceptibility of LDL isolated from plasma samples derived from patients with clinically diagnosed coronary artery disease (CAD). The samples were from a nested case–control study28 that had been designed using samples from the Finnish Corogene study.21 The cases in that study included all patients who had experienced coronary death within an average follow-up of 2.5 years. Control patients were selected from the group who had no cardiovascular events during the follow-up period (Stable CAD group), and they were pairwise matched based on conventional CAD risk factors, statin use, and coronary stenosis index. The plasma samples selected for this study (n = 48) were from non-diabetic males, all of whom had ≥50% coronary stenosis (Table 2).
Table 2.
Baseline characteristics of Corogene study patients assessed in this study
| Characteristics | CAD death | Stable CAD |
|---|---|---|
| Number of patients | 24 | 24 |
| Gender (male)a | 24 (100) | 24 (100) |
| Age (years)b | 66 (60–73) | 66 (60–73) |
| Current smokera | 8 (33) | 8 (33) |
| Hypertensiona | 18 (75) | 13 (54) |
| Body mass index (kg/m2)b | 26.1 (25.1–29.8) | 25.6 (24.8–27.4) |
| Diabetesa | 0 (0) | 0 (0) |
| Statina | 13 (54) | 13 (54) |
| Coronary stenosis indexb | 14 (3–28) | 15 (2–42) |
| Total cholesterol (mmol/L)b | 3.5 (2.9–4.1) | 3.8 (3.1–4.4) |
| LDL-C (mmol/L)b | 2.1 (1.6–2.5) | 2.1 (1.6–2.7) |
| HDL-C (mmol/L)b | 0.8 (0.7–1.0) | 0.9 (0.9–1.1) |
| TG (mmol/L)b | 2.5 (2.1–2.8) | 2.9 (1.9–3.7) |
| C-reactive protein (mg/L)b | 4.5 (2.3–12) | 0.8 (0.7–2.0) |
Number of cases (%).
Median (interquartile range).
We again observed substantial inter-individual differences in the aggregation susceptibility of the isolated LDL particles. Importantly, in the CAD Death group, LDL particles aggregated significantly faster than in the Stable CAD group, the median sizes of the aggregates after incubation for 2 h being 1500 nm (range 150–3200 nm) and 940 nm (range 90–1990 nm), respectively (Figure 1D). Moreover, the 2-h aggregate sizes of LDL samples in both CAD groups were significantly higher than in the 100 LDL samples obtained from the Health 2000 study; median 200 nm (range 60–2000 nm) (Figure 1D). LDL aggregation at 2 h was not associated with the initial sizes of LDL particles, nor did it associate significantly with plasma concentrations of LDL-C, apoB-100, C-reactive protein (hsCRP), or lipoprotein (a), nor with statin use, age or smoking, but showed a negative correlation with plasma triglyceride levels (see Supplementary material online, Tables S1 and S2).
The susceptibility of LDL particles to aggregate strongly associates with the particle lipid composition
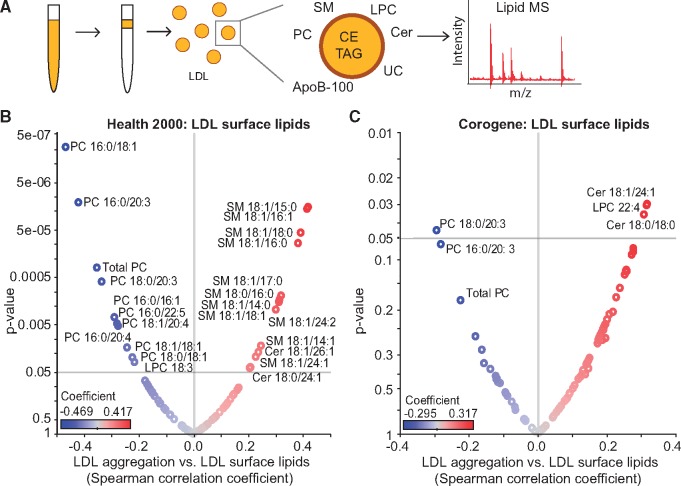
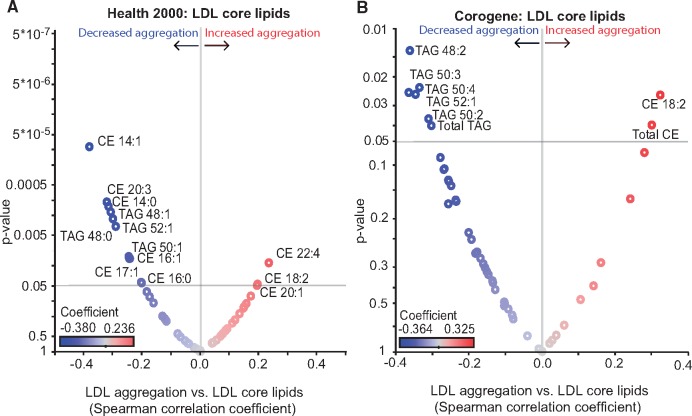
The surface monolayer of LDL particles comprises phospholipids, free cholesterol, ceramides (Cer), and a single copy of apoB-100 as the principal structural apolipoprotein of the particle (Figure 2A). Phosphatidylcholines (PC) are the major phospholipid class, followed by sphingomyelins (SM), and lysophosphatidylcholines (LPC). The surface also contains small amounts of other classes of phospholipids and ceramides. The particle core contains mainly cholesteryl esters (CE) and triacylglycerols (TAG). The compositions of isolated LDL preparations from the Health 2000 and Corogene studies described above were determined by quantitative mass spectrometry-based lipidomics. Volcano plots show the significant (P < 0.05) correlations between LDL aggregation and the molar percentages of specific lipids of the LDL surface (Figure 2B, C) and core (Figure 3A, B). Several sphingolipids (SMs and various forms of Cer) correlated positively (red) and various phosphatidylcholine (PC) species correlated negatively (blue) with LDL aggregation. Regarding the core lipids of LDL, the degree of LDL aggregation correlated negatively particularly with several 48–52 carbon TAGs in both cohorts (Figure 3A, B). Of note, of the lipids that significantly associate with LDL aggregation, the ceramides and TAG 50:4 differed significantly between the Stable CAD and CAD Death groups, ceramides being higher and TAG 50:4 lower in the case group.
Figure 2.
The susceptibility of low-density lipoprotein (LDL) to aggregate strongly correlates with the surface lipid composition of the particles. (A) LDL was isolated from plasma and LDL lipidome was analysed using mass spectrometry. Volcano plots showing Spearman correlation coefficients of LDL aggregate size at 2 h vs. LDL surface lipids in (B) Health 2000 samples and (C) in Corogene samples. Red circles indicate positive correlations, and blue circles indicate negative correlations. The identities of only those lipids with significance correlation values (P < 0.05) are indicated. Cer, ceramide; LPC, lysophosphatidylcholine; PC, phosphatidylcholine; SM, sphingomyelin.
Figure 3.
The susceptibility of low-density lipoprotein (LDL) to aggregate strongly correlates with the core lipid composition of the particles. Volcano plots showing Spearman correlation coefficients of LDL aggregate size at 2 h vs. LDL core lipids (A) in Health 2000 samples and (B) in Corogene samples. Red circles indicate positive correlations, and blue circles indicate negative correlations. The identities of only those lipids with significance correlation values (P < 0.05) are indicated. CE, cholesteryl ester; TAG, triacylglycerol.
Direct enrichment LDL with different phospholipids changes aggregation susceptibility of LDL and conformation of apoB-100
To determine causal effects of lipid composition on LDL aggregation, we next isolated LDL from four healthy volunteers and enriched the LDL with SM 18:1/16:0, PC 16:0/18:1, or LPC 16:0. Changes in the relative proportions of phospholipids in these lipid-enriched LDL particles were small (see Supplementary material online, Figure S4a) and the final compositions were well within ranges we observed in other human LDL samples. Although the aggregability of control LDL from the four donors varied considerably (see Supplementary material online, Figure S4b, black lines), in each case the LDL preparations enriched with SM became more susceptible to aggregation during incubation with hrSMase, while LDL preparations enriched with PC or LPC became less susceptible. We know from previous studies that SMase-treatment of LDL induces conformational changes in apoB-100 that expose otherwise hidden segments of apoB-100 that mediate particle aggregation.27 Here, we show that enrichment of LDL with SM enhanced SMase-induced conformational changes in apoB-100 (see Supplementary material online, Figure S4c, d).
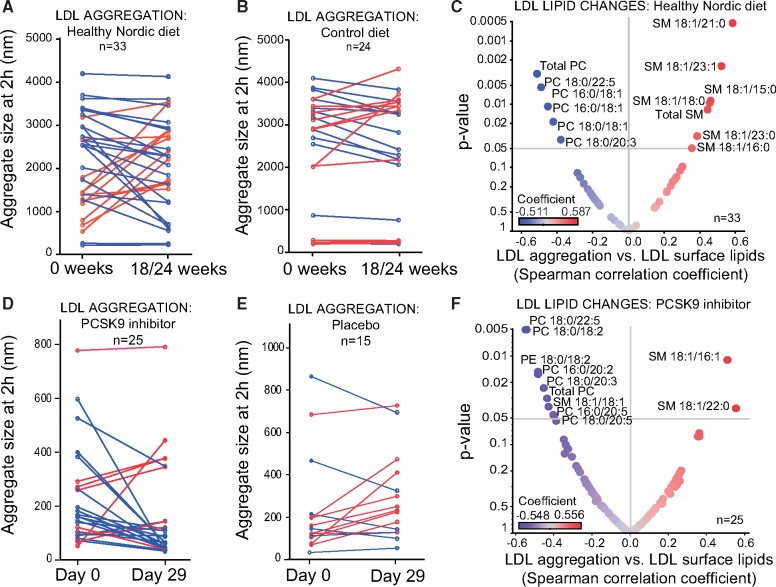
In human subjects, decreases in LDL-SM by dietary change, or by PCSK9 inhibition decreased LDL aggregation susceptibility
To determine if a change in LDL lipid composition changes the aggregation susceptibility of LDL, we analysed samples from two different interventions: (i) SYSDIET, a dietary intervention, in which healthy volunteers with features of the metabolic syndrome were randomly assigned to either a Healthy Nordic diet (n = 33) or to a Control diet (n = 24) for 18 or 24 weeks22,23 and (ii) EQUATOR, a randomised placebo-controlled phase II trial of a fully human monoclonal antibody RG7652 that inhibits the function of proprotein convertase subtilisin/kexin type 9 (PCSK9, n = 25; placebo, n = 15). Fasting plasma samples were obtained at baseline and 29 days after treatment.24
First, we analysed the aggregation of LDL from plasma samples that had been collected from the Finnish participants in the SYSDIET study. Supplementary material online, Table S3 shows the clinical characteristics of these subjects at baseline and the end of the study, and Supplementary material online, Figure S5a shows the changes in the macro- and micro-nutrient compositions of what each subject consumed based on food diaries at the beginning and at the end of the study.22,23 In the Healthy Nordic diet group, LDL aggregation decreased in two-thirds of the participants, whereas in the control group, only small changes in LDL aggregation were observed (Figure 4A, B). To estimate how much of the changes in LDL aggregation could be attributed to the dietary changes, we constructed a multivariate model using data from the subjects in the Healthy Nordic diet group. The best model included just two components from the food diaries: changes in dietary vitamin E and changes in dietary sucrose (Table 3). Decreased aggregation susceptibility was associated with increased dietary vitamin E and decreased dietary sucrose consumption. An increase in dietary vitamin E is considered a useful marker of increased consumption of vegetable oils rich in polyunsaturated fatty acids, and both were significantly associated with increased proportion of PCs and decreased proportion of SMs in plasma LDL particles (see Supplementary Figure S5b). These lipidomics changes were also associated with reduced LDL aggregation susceptibility in the Healthy Nordic diet group (Figure 4C and Supplementary material online, Figure S5b), but no significant associations were observed in the control group (see Supplementary material online, Figure S6a).
Table 3.
Multiple regression explaining the effect of dietary changes on changes in the 2 h aggregate size in the SYSDIET group
| Predictors | b | SE b | β | t | P-value | R2 | Adjusted R2 | F |
|---|---|---|---|---|---|---|---|---|
| Intercept | 2450.2 | 579.9 | 4.23 | <0.001 | 0.494 | 0.456 | 13.16 | |
| Δ Dietary Vitamin E | −1830.7 | 362.3 | −0.746 | −5.05 | <0.001 | |||
| Δ Dietary sucrose | 562.3 | 208.3 | 0.398 | 2.70 | 0.012 |
n = 31.
Figure 4.
A dietary intervention and PCSK9 inhibition in human subjects improves their low-density lipoprotein (LDL) composition and renders their particles less susceptible to aggregate. Plasma samples were obtained from the SYSDIET-study, where participants were placed on either an isocaloric healthy Nordic diet (n = 33) or a control diet (n = 25) for 18 or 24 weeks and from the EQUATOR study, a randomized placebo-controlled phase II trial of a monoclonal antibody inhibiting the function of PCSK9, RG7652, (n = 25), or placebo (n = 15) for 29 days. LDL was isolated, and aggregation analysed from samples before and after the diet/treatment period. (A and B) LDL aggregate sizes at the 2-h time point are shown in the diet group and control group before and after the diet period. Each line represents one subject and blue lines show a decrease and red lines an increase in aggregate size. (D and E) LDL aggregate sizes at the 2-h time point are shown in the PSCK9 inhibitor group and placebo group before and after the treatment period. Each line represents one subject and blue lines show a decrease and red lines an increase in aggregate size. (C and F) Volcano plot showing the Spearman correlation coefficients of LDL aggregate size at 2 h vs. LDL surface lipids in the SYSDIET study and in the EQUATOR study. PC, phosphatidylcholine; PE, phosphatidylethanolamine; SM, sphingomyelin.
Inhibition of PCSK9 is known to strikingly lower LDL-C, and we recently showed that PCSK9 inhibition also influences plasma and lipoprotein phospholipid composition.29 The clinical characteristics of EQUATOR trial subjects at baseline and the end of the study are descripted in Supplementary material online, Table 4. In the treatment group of the EQUATOR trial, LDL aggregation susceptibility decreased in two-thirds of the subjects, whereas in the placebo group, only small changes in LDL aggregation occurred during the trial (Figure 4D, E). In addition, the overall change in aggregation susceptibility between the groups was significantly different between the two treatment groups (P = 0.035). The decrease in LDL aggregation in the treatment group correlated with an increase in several PC species and a decrease in several SM species (Figure 4F). In the control group, only PC 16:0/18:2 correlated significantly with decreased LDL aggregation susceptibility (see Supplementary material online, Figure S6b).
Pharmacological and genetic interventions in vivo to render LDL resistant to aggregation
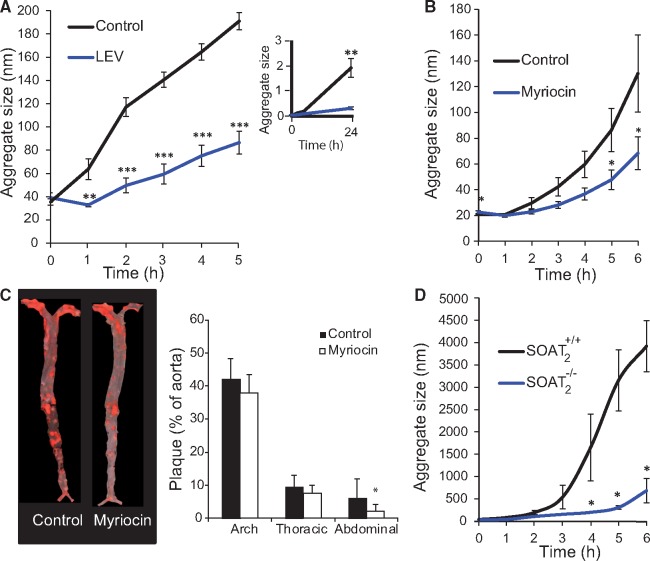
To further examine our model linking changes in LDL lipids with changes in the susceptibility of LDL to aggregate, we carried out three interventions that had previously been shown to induce changes in LDL lipid composition and to decrease atherosclerosis in hypercholesterolaemic animals, but, remarkably, without changing the plasma concentrations of LDL-C.30–32 In the first intervention, we administered a single intravenous bolus injection of large 100-nm ‘empty’ vesicles (LEVs, also known as large unilamellar vesicles) composed of a single lipid, PC 16:0/18:1, into human APOB100 transgenic/Ldlr−/− mice (n = 16) fed on a regular chow diet. Control APOB100 transgenic/Ldlr−/− littermates (n = 16) received an intravenous injection of an equivalent volume of PBS. Plasma was collected 1 h after the injection, and LDL was purified sequentially by ultracentrifugation and size-exclusion chromatography. Treatment with LEVs substantially altered LDL lipid composition in vivo (see Supplementary material online, Figure S7) and rendered LDL nearly completely resistant to aggregation ex vivo, even after 24 h of incubation with hrSMase (Figure 5A and inset).
Figure 5.
Pharmacological and genetic interventions that favourably alter low-density lipoprotein (LDL) lipid composition in vivo in hypercholesterolaemic mice render their LDL aggregation-resistant. (A) Human APOB transgenic/LDLr−/− mice were given a single intravenous injection of large ‘empty’ vesicles (LEVs) made from PC 16:0/18:1 or equivalent volume of PBS (control, n= 8 per group). After 1 h, plasma was collected, LDL isolated, and aggregation induced by treatment with hrSMase. LDL aggregation was followed by dynamic light scattering at the indicated time points. The insert shows LDL aggregation for up to 24 h. (B) LDLr−/−/Apob100/100 mice were simultaneously started on an atherogenic western-type diet and intraperitoneal injections three times per week of either myriocin, and inhibitor of SM biosynthesis, or PBS (n = 11 mice per group). Diet and injections continued for 10 weeks. LDL was isolated, then treated with hrSMase, and particle aggregation was followed by dynamic light scattering at the indicated time points. (C) Representative aortas from a control mouse and a myriocin-treated mouse stained with Sudan IV. The extent of atherosclerosis was determined by measuring the amounts of Sudan IV positive areas in the aortas. (D) LDL was isolated from the plasma of human APOB transgenic/LDLr−/−/Soat2−/− mice and human APOB transgenic/LDLr−/−/Soat2+/+ littermates. LDL was treated with hrSMase and LDL aggregation followed by dynamic light scattering at the indicated time points. The line and column graphs display averages ± standard deviations. *P <0.05, **P < 0.01, and ***P < 0.001 by Student’s t-test.
In the second intervention, we suppressed SM biosynthesis in Ldlr−/−/Apob100/100-mice using myriocin, an inhibitor of serine-palmitoyl transferase, the rate-limiting enzyme in SM biosynthesis. Inhibition of this enzyme in mice leads to the generation of SM-depleted lipoprotein particles.31 After 10 weeks of a high-fat atherogenic diet combined with three intraperitoneal injections/week of myriocin or PBS (n = 11 per group), the animals were sacrificed. Treatment with myriocin did not change plasma cholesterol or triglyceride levels, but LDL particles from the myriocin-injected mice contained a far lower proportion of SM (see Supplementary material online, Figure S8) and were less susceptible to hrSMase-induced aggregation than LDL from PBS-injected control littermates (Figure 5B). In addition, atherosclerotic lesion areas were smaller in myriocin-treated mice than in control mice, particularly in the region of the abdominal aorta (Figure 5C).
In the third intervention, we investigated the effect of the composition of the lipid core of LDL particles on their susceptibility to aggregate by using mice deficient in SOAT2, and enzyme also known as acyl-CoA: cholesterol acyltransferase-2 or ACAT2. LDL was isolated from the plasma of APOB100 transgenic/Ldlr−/−/Soat2−/− mice (n = 5) and APOB100 transgenic/Ldlr−/−/Soat2+/+ littermates (n = 3). LDL particles from SOAT2-deficient mice are characterized by enrichment in polyunsaturated CEs and TAGs, when compared with LDL from control mice.26 Here, we found that LDL particles from Soat2−/− mice were particularly aggregation-resistant (Figure 5D), consistent with our data from human LDL (Figure 3).
Aggregated LDL induces MMP-7 secretion from macrophage foam cells and activates T-cells in vitro
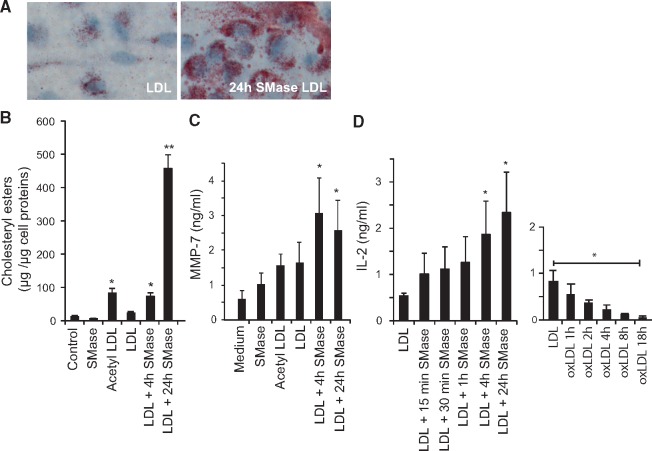
To study maladaptive cellular responses relevant to atherosclerosis, we examined the effects of hrSMase-aggregated LDL vs. native LDL on cultured human primary monocyte-derived macrophages and murine T-cell hybridomas that had been raised against oxidized LDL.33 Aggregated LDL induced accumulation of cholesteryl ester-rich lipid droplets in the human primary monocyte-derived macrophages (Figure 6A, B), consistent with prior literature.16 We also analysed the concentrations of several matrix metalloproteinases (MMPs) in the culture media and found that foam cells induced with hrSMase-treated LDL, but not with acetylated LDL, secrete increased amounts of MMP-7 (Figure 6C), a protease considered to be an important contributor to weakening, and ensuing rupture of atherosclerotic plaques.34
Figure 6.
The effect of aggregated low-density lipoprotein (LDL) on macrophages and T-cells. (A) Oil Red O-stained human monocyte-derived macrophages incubated for 20 h with 100 µg/mL of LDL or aggregated LDL (24-h treatment with hrSMase). (B) The amounts of cholesteryl esters in monocyte-derived macrophages incubated in the presence of 100 µg/mL of the variously treated LDL preparations for 20 h. (C) The amount of MMPs secreted from the cells was determined by a Multiplex array. Only secretion of MMP-7 was induced by SMase-treated LDL. (D) Activation of human apoB-100-specific T-cell hybridoma 48-5 measured by IL-2 secretion after 24 h co-culture with antigen presenting cells and 10 µg/mL LDL with increasing amount of sphingomyelinase modification (left panel) or oxidation (right panel). The column graphs show averages ± standard deviations. Statistical differences between the groups were determined using Kruskal–Wallis test followed by post hoc pairwise comparison using Dunn’s test. *P < 0.05 and **P < 0.01.
In parallel with macrophages, T cells are also recruited to atherosclerotic lesions. Triggered by LDL that is taken up and presented by antigen-presenting cells, T cells can accelerate atherosclerosis in hypercholesterolaemic animals and promote features associated with plaque instability.18 Paradoxically, T-cell hybridomas raised against oxidized LDL do not react to oxidized LDL.33 Nonetheless, T cells with a similar set of clonotypic T-cell receptors in hypercholesterolaemic mice have been shown to participate in the growth of their atherosclerotic plaques.18 Here, we found that the degree of LDL aggregation induced by SMase associated positively with the degree of T-cell activation, as measured by the secretion of interleukin-2 (Figure 6D, left panel). In contrast, consistent with earlier data,33 oxidized LDL inhibited the T-cell response (Figure 6D, right panel).
Discussion
In this study, we show that the susceptibility of LDL particles to aggregate in the presence of hrSMase varies significantly amongst human subjects and depends on the lipid composition of their LDL particles. The presence of aggregation-prone LDL was associated with future CAD deaths independently of conventional CAD risk factors including plasma LDL-C concentration, smoking, and hypertension. Importantly, we show that the susceptibility of LDL particles to aggregate can be favourably modified in humans by nutritional and medical interventions and in vivo in animals by altering LDL lipid composition.
Aggregated LDL has been suggested to promote atherogenesis by inducing lipid accumulation in the arterial wall both extra- and intra-cellularly.8,16,35 A major trigger for aggregation of LDL particles within the arterial wall is their digestion by the secretory SMase,15 and genetic deletion of this enzyme in hypercholesterolaemic animals dramatically retards atherosclerotic plaque development.36 The importance of SMase in human atherogenesis is reflected by the observation that large LDL aggregates isolated from human atherosclerotic lesions are enriched in ceramides, the lipolytic products of SMase action.15 Aggregated lipoprotein particles accumulating in human atherosclerotic lesions also show signs of other modifications, such as proteolysis, lipolysis of both the core and the surface lipids, and oxidation.12 Interestingly, studies in vitro have shown that these modifications promote sphingomyelinase-induced LDL modification.13,15
In this study, we extend these earlier findings and show that foam cells generated by incubating macrophages in the presence of SMase-aggregated LDL secreted MMP-7, a proteinase with a potential role in the rupture of human atherosclerotic plaques.37 Moreover, we show that LDL aggregated by SMase can trigger a potent T-cell response against apoB-100. This maladaptive immunological mechanism may accelerate the progression of atherosclerosis and the development of plaque instability.38 In fact, SMase-induced modification and aggregation of LDL represents the first pathophysiologically plausible modification of LDL ex vivo that enhances the ability of LDL to activate T-cell hybridomas linked with atherosclerosis.
The present data indicate that aggregation-prone human LDL particles are enriched in SM and ceramides, and that they contain less choline phospholipids (PC and LPC) and TAGs than aggregation-resistant LDL particles. Causality of these lipids in LDL aggregation susceptibility was established by altering their contents in isolated LDL in vitro, in humans by diet and by PCSK9 inhibition, and in three atherosclerotic mouse models in vivo. Thus, increase in vitamin E consumption was associated with decreased LDL aggregation, and, when the aggregation data were normalized for changes in the consumption of vitamin E, increased intake of sucrose was found to associate with accelerated aggregation. Interestingly, plasma levels of SM have been strongly influenced by genetic effects.39 Such influence could partly explain our observation that the aggregation susceptibility decreased in only two-thirds of the subjects in the healthy diet group. Similarly, inhibition of PCSK9 decreased LDL aggregation in two-thirds of the subjects.
SM-rich LDL from apoE−/− mice has previously been shown to be prone to the formation of large aggregates.15,40,41 Here, we used three atherosclerotic mouse models, in which we modified the composition of LDL particles in vivo. We demonstrate here that an increase in LDL-PC by intravenous injection of PC-containing LEVs, a pharmacologically induced decrease in LDL-SM with myriocin, an inhibitor of SM biosynthesis, and a genetically induced increase in polyunsaturated CEs and TAGs in LDL, each decreased the susceptibility of LDL particles to aggregate. Importantly, each of these treatments has been shown to reduce atherosclerosis in hypercholesterolaemic animals, notably without influencing plasma concentrations of cholesterol.26,30–32,42
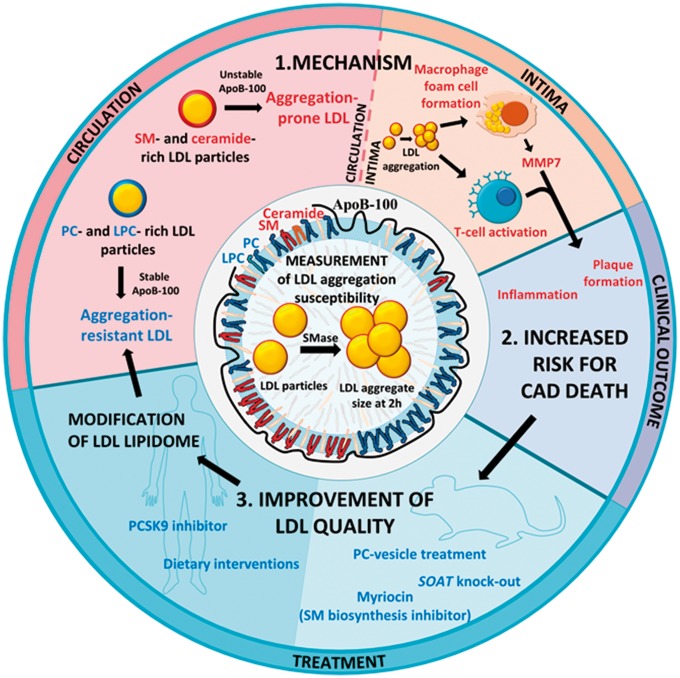
In most studies examining the relationship between lipid markers and ASCVD risk, the lipid composition of whole plasma, rather than that of circulating LDL particles, has been determined. In such studies, high levels of plasma SM43 and low levels of certain LPC-species44 were associated with increased risk for future development of clinically significant ASCVD. In addition, plasma levels of certain ceramide species have recently been found to predict future risk of cardiovascular death in patients with ASCVD.28 These results from whole plasma reflect the lipid composition of all plasma lipoprotein classes. Therefore, our present findings demonstrating that aggregation-prone LDL particles are SM-rich, ceramide-rich, PC-poor, and LPC-poor may explain the previously reported associations between the plasma levels of these lipids and future ASCVD risk. Moreover, our work supports a mechanistic chain from these compositional differences to differences in LDL quality that predispose it to SMase-induced aggregation, to plaque initiation, progression and, destabilization (Take home figure).
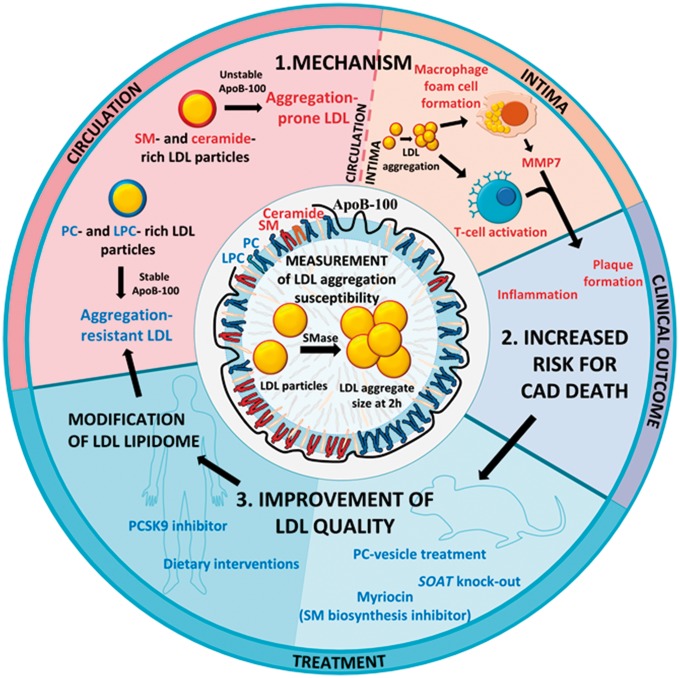
Take home figure.
Development of a measurement of LDL aggregation susceptibility revealed the importance of qualitative differences in LDL particles in ASCVD. The circulating LDL particles of patients succumbing in CAD have a high proportion of sphingomyelins (SM) and ceramides. When such aggregation-prone LDL particles enter the arterial intima, they easily aggregate upon modification. Intimal LDL aggregates promote atherogenesis, inflammation and plaque rupture by inducing foam cell formation, secretion of matrix metalloproteinase 7 (MMP7) and by activating T-cells, ultimately increasing the risk for fatal CAD. Therefore, decreasing the aggregation susceptibility of circulating LDL could reduce CAD risk. In vitro and in mouse models, aggregation-prone LDL particles can be rendered aggregation-resistant by reducing their sphingolipid content. In humans, a healthy diet or treatment with a PCSK9 inhibitor improves the quality of LDL particles resulting in aggregation-resistant phosphatidylcholine (PC)- and lysophosphatidylcholine (LPC)-rich LDL particles.
In summary, aggregation-prone LDL was found to predict death from cardiovascular causes independent of conventional atherosclerosis risk factors. Importantly, prior and current data indicate that aggregated LDL has the potential to promote multiple steps along the atherogenic pathway from LDL retention to maladaptive responses that include initiation and growth of atherosclerotic lesions, plaque destabilization, and plaque rupture. Any treatment that induces a favourable change in LDL lipid composition offers a means to attenuate LDL aggregation within the arterial wall and its deleterious consequences. These results emphasize the importance of LDL quality in human ASCVD. Moreover, measurement of the susceptibility of LDL to aggregate may serve as a predictive biomarker for the identification of patients at significant residual or unrecognized risk of cardiovascular morbidity and mortality and who might benefit from personalised, targeted interventions.
Supplementary material
Supplementary material is available at European Heart Journal online.
Supplementary Material
Acknowledgements
The authors are thankful to Genzyme for providing hrSMase. We also wish to thank Maija Atuegwu, Mari Jokinen, and Jari Metso for their excellent technical assistance.
Funding
Wihuri Research Institute is maintained by the Jenny and Antti Wihuri Foundation. This work was also supported by grants from the Finnish Foundation for Cardiovascular Research (to M.R., K.Ö.), from the Academy of Finland [265940 to K.Ö.] and [295504 to T.A.], from the Magnus Ehrnrooth Foundation (to K.Ö.), from Jane and Aatos Erkko Foundation (to M.J.), and from the Swedish Heart-Lung Foundation (to K.J.W.).
Conflict of interest: T.V., M.H., and R.L. are employed by Zora Biosciences and A.B. by Roche. K.J.W. has ownership interests in Gemphire Therapeutics, Inc., which is developing an orally administered lipoprotein-lowering agent, and in Hygieia, Inc., which provides insulin management services in Northern Ireland and in Michigan, USA. K.J.W. is also the inventor of a number of patents on the use of large empty vesicles (LEVs) in atherosclerotic cardiovascular disease. M.R., P.T.K., and K.Ö. have applied for a patent on analysis of LDL aggregation susceptibility using hrSMase. All other authors declared no conflict of interest.
Footnotes
See page 2574 for the editorial comment on this article (doi: 10.1093/eurheartj/ehy387)
References
- 1. Ference BA, Ginsberg HN, Graham I, Ray KK, Packard CJ, Bruckert E, Hegele RA, Krauss RM, Raal FJ, Schunkert H, Watts GF, Borén J, Fazio S, Horton JD, Masana L, Nicholls SJ, Nordestgaard BG, van de Sluis B, Taskinen M-R, Tokgözoğlu L, Landmesser U, Laufs U, Wiklund O, Stock JK, Chapman MJ, Catapano AL.. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J 2017;38:2459–2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, Cooney MT, Corra U, Cosyns B, Deaton C, Graham I, Hall MS, Hobbs FDR, Lochen ML, Lollgen H, Marques-Vidal P, Perk J, Prescott E, Redon J, Richter DJ, Sattar N, Smulders Y, Tiberi M, van der Worp HB, van Dis I, Verschuren WMM, Binno S. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J 2016;37:2315–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, Kuder JF, Wang H, Liu T, Wasserman SM, Sever PS, Pedersen TR.. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med 2017;376:1713–1722. [DOI] [PubMed] [Google Scholar]
- 4. Kaasenbrood L, Boekholdt SM, van der Graaf Y, Ray KK, Peters RJ, Kastelein JJ, Amarenco P, LaRosa JC, Cramer MJ, Westerink J, Kappelle LJ, de Borst GJ, Visseren FL.. Distribution of estimated 10-year risk of recurrent vascular events and residual risk in a secondary prevention population. Circulation 2016;134:1419–1429. [DOI] [PubMed] [Google Scholar]
- 5. Catapano AL, Graham I, De Backer G, Wiklund O, Chapman MJ, Drexel H, Hoes AW, Jennings CS, Landmesser U, Pedersen TR, Reiner Z, Riccardi G, Taskinen MR, Tokgozoglu L, Verschuren WMM, Vlachopoulos C, Wood DA, Zamorano JL, Cooney MT. 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias. Eur Heart J 2016;37:2999–3058. [DOI] [PubMed] [Google Scholar]
- 6. Williams KJ, Tabas I.. Lipoprotein retention—and clues for atheroma regression. Arterioscler Thromb Vasc Biol 2005;25:1536–1540. [DOI] [PubMed] [Google Scholar]
- 7. Borén J, Williams KJ.. The central role of arterial retention of cholesterol-rich apolipoprotein-B-containing lipoproteins in the pathogenesis of atherosclerosis: a triumph of simplicity. Curr Opin Lipidol 2016;27:473–483. [DOI] [PubMed] [Google Scholar]
- 8. Öörni K, Pentikäinen MO, Ala-Korpela M, Kovanen PT. Aggregation, fusion, and vesicle formation of modified low density lipoprotein particles: molecular mechanisms and effects on matrix interactions. J Lipid Res 2000;41:1703–1714. [PubMed] [Google Scholar]
- 9. Hoff HF, Morton RE.. Lipoproteins containing apo B extracted from human aortas. Structure and function. Ann N Y Acad Sci 1985;454:183–194. [DOI] [PubMed] [Google Scholar]
- 10. Aviram M, Maor I, Keidar S, Hayek T, Oiknine J, Barel Y, Adler Z, Kertzman V, Milo S.. Lesioned low density lipoprotein in atherosclerotic apolipoprotein E-deficient transgenic mice and in humans is oxidized and aggregated. Biochem Biophys Res Commun 1995;216:501–513. [DOI] [PubMed] [Google Scholar]
- 11. Schissel SL, Tweedie-Hardman J, Rapp JH, Graham G, Williams KJ, Tabas I.. Rabbit aorta and human atherosclerotic lesions hydrolyze the sphingomyelin of retained low-density lipoprotein. Proposed role for arterial-wall sphingomyelinase in subendothelial retention and aggregation of atherogenic lipoproteins. J Clin Invest 1996;98:1455–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lehti S, Nguyen SD, Belevich I, Vihinen H, Heikkilä HM, Soliymani R, Käkelä R, Saksi J, Jauhiainen M, Grabowski GA, Kummu O, Hörkkö S, Baumann M, Lindsberg PJ, Jokitalo E, Kovanen PT, Öörni K.. Extracellular lipid accumulates in human carotid arteries as distinct three-dimensional structures with proinflammatory properties. Am J Pathol 2018;188:525–538. [DOI] [PubMed] [Google Scholar]
- 13. Plihtari R, Hurt-Camejo E, Oörni K, Kovanen PT.. Proteolysis sensitizes LDL particles to phospholipolysis by secretory phospholipase A2 group V and secretory sphingomyelinase. J Lipid Res 2010;51:1801–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Öörni K, Kovanen PT.. PLA2-V: a real player in atherogenesis. Arterioscler Thromb Vasc Biol 2007;27:445–447. [DOI] [PubMed] [Google Scholar]
- 15. Schissel SL, Jiang X-C, Tweedie-Hardman J, Jeong T-S, Camejo EH, Najib J, Rapp JH, Williams KJ, Tabas I.. Secretory sphingomyelinase, a product of the acid sphingomyelinase gene, can hydrolyze atherogenic lipoproteins at neutral pH. Implications for atherosclerotic lesion development. J Biol Chem 1998;273:2738–2746. [DOI] [PubMed] [Google Scholar]
- 16. Tabas I, Li Y, Brocia RW, Xu SW, Swenson TL, Williams KJ.. Lipoprotein lipase and sphingomyelinase synergistically enhance the association of atherogenic lipoproteins with smooth muscle cells and extracellular matrix. A possible mechanism for low density lipoprotein and lipoprotein(a) retention and macrophage foam cell formation. J Biol Chem 1993;268:20419–20432. [PubMed] [Google Scholar]
- 17. Grosheva I, Haka AS, Qin C, Pierini LM, Maxfield FR.. Aggregated LDL in contact with macrophages induces local increases in free cholesterol levels that regulate local actin polymerization. Arterioscler Thromb Vasc Biol 2009;29:1615–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ketelhuth DF, Hansson GK.. Adaptive response of T and B cells in atherosclerosis. Circ Res 2016;118:668–678. [DOI] [PubMed] [Google Scholar]
- 19. Libby P, Hansson GK.. Inflammation and immunity in diseases of the arterial tree: players and layers. Circ Res 2015;116:307–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Aromaa A, Koskinen S.. Health and Functional Capacity in Finland: Baseline Results of the Health 2000 Health Examination Survey, Vol. B12/200. Publications of the National Public Health Institute; Helsinki, Finland: 2004. [Google Scholar]
- 21. Vaara S, Nieminen MS, Lokki ML, Perola M, Pussinen PJ, Allonen J, Parkkonen O., Sinisalo J.. Cohort Profile: the Corogene study. Int J Epidemiol 2012;41:1265–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Magnusdottir OK, Landberg R, Gunnarsdottir I, Cloetens L, Åkesson B, Önning G, Jonsdottir SE, Rosqvist F, Schwab U, Herzig K-H, Savolainen MJ, Brader L, Hermansen K, Kolehmainen M, Poutanen K, Uusitupa M, Thorsdottir I, Risérus U.. Plasma alkylresorcinols reflect important whole-grain components of a healthy Nordic diet. J Nutr 2013;143:1383–1390. [DOI] [PubMed] [Google Scholar]
- 23. Uusitupa M, Hermansen K, Savolainen MJ, Schwab U, Kolehmainen M, Brader L, Mortensen LS, Cloetens L, Johansson-Persson A, Onning G, Landin-Olsson M, Herzig K-H, Hukkanen J, Rosqvist F, Iggman D, Paananen J, Pulkki KJ, Siloaho M, Dragsted L, Barri T, Overvad K, Bach Knudsen KE, Hedemann MS, Arner P, Dahlman I, Borge GIA, Baardseth P, Ulven SM, Gunnarsdottir I, Jónsdóttir S, Thorsdottir I, Orešič M, Poutanen KS, Risérus U, Akesson B.. Effects of an isocaloric healthy Nordic diet on insulin sensitivity, lipid profile and inflammation markers in metabolic syndrome—a randomized study (SYSDIET). J Intern Med 2013;274:52–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Baruch A, Mosesova S, Davis JD, Budha N, Vilimovskij A, Kahn R, Peng K, Cowan KJ, Harris LP, Gelzleichter T, Lehrer J, Davis JC, Tingley WG.. Effects of RG7652, a monoclonal antibody against PCSK9, on LDL-C, LDL-C subfractions, and inflammatory biomarkers in patients at high risk of or with established coronary heart disease (from the Phase 2 EQUATOR Study). Am J Cardiol 2017;119:1576–1583. [DOI] [PubMed] [Google Scholar]
- 25. Havel RJ. Biology of cholesterol, lipoproteins and atherosclerosis. Clin Exp Hypertens A 1989;11:887–900. [DOI] [PubMed] [Google Scholar]
- 26. Melchior JT, Sawyer JK, Kelley KL, Shah R, Wilson MD, Hantgan RR, Rudel LL.. LDL particle core enrichment in cholesteryl oleate increases proteoglycan binding and promotes atherosclerosis. J Lipid Res 2013;54:2495–2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sneck M, Nguyen SD, Pihlajamaa T, Yohannes G, Riekkola ML, Milne R, Kovanen PT, Oörni K.. Conformational changes of apoB-100 in SMase-modified LDL mediate formation of large aggregates at acidic pH. J Lipid Res 2012;53:1832–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Laaksonen R, Ekroos K, Sysi-Aho M, Hilvo M, Vihervaara T, Kauhanen D, Suoniemi M, Hurme R, März W, Scharnagl H, Stojakovic T, Vlachopoulou E, Lokki M-L, Nieminen MS, Klingenberg R, Matter CM, Hornemann T, Jüni P, Rodondi N, Räber L, Windecker S, Gencer B, Pedersen ER, Tell GS, Nygård O, Mach F, Sinisalo J, Lüscher TF.. Plasma ceramides predict cardiovascular death in patients with stable coronary artery disease and acute coronary syndromes beyond LDL-cholesterol. Eur Heart J 2016;37:1967–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hilvo M, Simolin H, Metso J, Ruuth M, Öörni K, Jauhiainen M, Laaksonen R, Baruch A.. PCSK9 inhibition alters the lipidome of plasma and lipoprotein fractions. Atherosclerosis 2018;269:159–165. [DOI] [PubMed] [Google Scholar]
- 30. Rodrigueza WV, Mazany KD, Essenburg AD, Pape ME, Rea TJ, Bisgaier CL, Williams KJ.. Large versus small unilamellar vesicles mediate reverse cholesterol transport in vivo into two distinct hepatic metabolic pools. Implications for the treatment of atherosclerosis. Arterioscler Thromb Vasc Biol 1997;17:2132–2139. [DOI] [PubMed] [Google Scholar]
- 31. Hojjati MR, Li Z, Zhou H, Tang S, Huan C, Ooi E, Lu S, Jiang X-C.. Effect of myriocin on plasma sphingolipid metabolism and atherosclerosis in apoE-deficient mice. J Biol Chem 2005;280:10284–10289. [DOI] [PubMed] [Google Scholar]
- 32. Willner EL, Tow B, Buhman KK, Wilson M, Sanan DA, Rudel LL, Farese RV.. Deficiency of acyl CoA: cholesterol acyltransferase 2 prevents atherosclerosis in apolipoprotein E-deficient mice. Proc Natl Acad Sci USA 2003;100:1262–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hermansson A, Ketelhuth DFJ, Strodthoff D, Wurm M, Hansson EM, Nicoletti A, Paulsson-Berne G, Hansson GK.. Inhibition of T cell response to native low-density lipoprotein reduces atherosclerosis. J Exp Med 2010;207:1081–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Khokha R, Murthy A, Weiss A.. Metalloproteinases and their natural inhibitors in inflammation and immunity. Nat Rev Immunol 2013;13:649–665. [DOI] [PubMed] [Google Scholar]
- 35. Tabas I, Williams KJ, Boren J.. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation 2007;116:1832–1844. [DOI] [PubMed] [Google Scholar]
- 36. Devlin CM, Leventhal AR, Kuriakose G, Schuchman EH, Williams KJ, Tabas I.. Acid sphingomyelinase promotes lipoprotein retention within early atheromata and accelerates lesion progression. Arterioscler Thromb Vasc Biol 2008;28:1723–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Abbas A, Aukrust P, Russell D, Krohg-Sørensen K, Almås T, Bundgaard D, Bjerkeli V, Sagen EL, Michelsen AE, Dahl TB, Holm S, Ueland T, Skjelland M, Halvorsen B.. Matrix metalloproteinase 7 is associated with symptomatic lesions and adverse events in patients with carotid atherosclerosis. PLoS One 2014;9:e84935.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ketelhuth DFJ, Gisterå A, Johansson DK, Hansson GK.. T cell-based therapies for atherosclerosis. Curr Pharm Des 2013;19:5850–5858. [DOI] [PubMed] [Google Scholar]
- 39. Frahnow T, Osterhoff MA, Hornemann S, Kruse M, Surma MA, Klose C, Simons K, Pfeiffer AFH.. Heritability and responses to high fat diet of plasma lipidomics in a twin study. Sci Rep 2017;7:3750.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Deevska GM, Sunkara M, Morris AJ, Nikolova-Karakashian MN.. Characterization of secretory sphingomyelinase activity, lipoprotein sphingolipid content and LDL aggregation in ldlr-/- mice fed on a high-fat diet. Biosci Rep 2012;32:479–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jeong TS, Schissel SL, Tabas I, Pownall HJ, Tall AR, Jiang X.. Increased sphingomyelin content of plasma lipoproteins in apolipoprotein E knockout mice reflects combined production and catabolic defects and enhances reactivity with mammalian sphingomyelinase. J Clin Invest 1998;101:905–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Friedman M, Byers SO, Rosenman RH.. Resolution of aortic atherosclerotic infiltration in the rabbit by phosphatide infusion. Proc Soc Exp Biol Med 1957;95:586–588. [DOI] [PubMed] [Google Scholar]
- 43. Jiang XC, Paultre F, Pearson TA, Reed RG, Francis CK, Lin M, Berglund L, Tall AR.. Plasma sphingomyelin level as a risk factor for coronary artery disease. Arterioscler Thromb Vasc Biol 2000;20:2614–2618. [DOI] [PubMed] [Google Scholar]
- 44. Fernandez C, Sandin M, Sampaio JL, Almgren P, Narkiewicz K, Hoffmann M, Hedner T, Wahlstrand B, Simons K, Shevchenko A, James P, Melander O.. Plasma lipid composition and risk of developing cardiovascular disease. PLoS One 2013;8:e71846.. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.