Abstract
Lymphocytic choriomeningitis virus (LCMV) poses a substantial risk to immunocompromised individuals. The case fatality rate in recent clusters of LCMV infection in immunosuppressed organ transplantation recipients has exceeded 70%. In the present study, we demonstrate potent antiviral activity of favipiravir against acute, disseminated LCMV infection in NZB mice. Treatment resulted in complete protection against mortality and dramatic reductions in viral loads. In contrast, ribavirin, the current antiviral of choice, was mostly ineffective. Our findings, and the high lethality associated with LCMV infection in transplant recipients, support the consideration of favipiravir as a first-line therapeutic option.
Keywords: lymphocytic choriomeningitis virus, arenavirus, favipiravir, antiviral
Using a mouse model of acute, disseminated lymphocytic choriomeningitis virus infection (LCMV), we demonstrate highly protective antiviral intervention with favipiravir. Our findings, and the lethality of LCMV infection in transplant recipients, support the potential use of favipiravir as a first-line therapeutic.
(See the Editorial commentary by de la Torre, on pages 509–11).
Lymphocytic choriomeningitis virus (LCMV; family Arenaviridae, genus Mammarenavirus) is a rodent-borne, negative-sense, single-stranded RNA virus for which the natural host is the common house mouse (Mus musculinus). Transmitted congenitally though transplacental infection and through direct contact or inhalation of virus-containing rodent excrement, seroprevalence of LCMV ranges from 2% to 5% in human populations [1]. LCMV infections are often asymptomatic; however a small portion of cases progress to a range of diseases including aseptic meningitis and meningoencephalitis.
Recent clusters of disseminated LCMV infection in immunosuppressed organ transplant recipients highlight the risk of transmission from donors with underlying infections [1–3]. These individuals often develop severe disease with prevalent hemorrhagic manifestations and case fatality rates exceeding 70%. Therapeutic options are limited to intravenous ribavirin and/or intravenous immunoglobulin with undefined virus neutralization capacity [1, 2]. The benefit of ribavirin is unclear and treatment is associated with toxicity, underscoring the need for development of new therapeutics for severe LCMV infections.
Favipiravir, a new antiviral therapeutic that is currently approved as an anti-influenza drug in Japan and under review by the US Food and Drug Administration for the same application in the United States, has been shown to be broadly active against a wide range of RNA viruses including arenaviruses [4–8]. To date, favipiravir has not been evaluated against LCMV. In the present study, we demonstrate the efficacy of favipiravir in a mouse model of acute, disseminated LCMV infection and hemorrhagic disease.
METHODS
Biosafety
All work with LCMV was conducted in biosafety level 3 (BSL-3) laboratories at Utah State University (USU; Logan, UT) following established standard operating procedures approved by the USU Biohazards Committee.
Ethics Statement
All animal procedures were performed by trained personnel in the Association for Assessment and Accreditation of Laboratory Animal Care accredited Laboratory Animal Research Center on the USU campus and complied with guidelines set by the United States Department of Agriculture and USU Animal Care and Use Committee.
Animals
Male and female 4-week-old NZB mice (The Jackson Laboratory, Bar Harbor, ME) were used for both experiments. The animals were acclimated for 1 week prior to virus challenge and fed Harlan Lab Block and tap water ad libitum.
Virus
LCMV Clone 13 (LCMV-Cl13) was provided by Maria Salvato (University of Maryland School of Medicine, Baltimore, MD). The virus used was from a stock prepared following a single passage in BHK-21 cells. The stock was diluted in minimal essential medium (MEM; Hyclone, Logan, UT) prior to challenge.
Compounds
Favipiravir (T-705) was obtained from the Toyama Chemical Co. (Toyama, Japan) and ribavirin was from ICN Pharmaceuticals (Costa Mesa, CA). Both compounds were prepared in sterile water supplemented with 74.6 mg/mL meglumine excipient.
In Vitro Antiviral Activity
Antiviral activity of favipiravir and ribavirin against LCMV-Cl13 was evaluated in vitro in Vero cells by a previously described virus yield reduction assay [8]. Briefly, subconfluent cells in 96-well microplates were infected at a multiplicity of infection of 0.001 and treated with half-log dilutions of drugs dissolved in culture medium supplemented with 2% fetal bovine serum. Plates were incubated for 7 days and cell lysates were titrated for LCMV by endpoint dilution. The 50% cell cytotoxic dose (CC50) was determined by neutral red dye uptake in uninfected, drug-treated cells. The 90% effective concentration (EC90) was determined by regression analysis and represents the concentration of drug that reduced the virus yield by one log10. The selectivity index (SI) was calculated using the formula: SI = CC50/EC90.
Mouse Studies
For the initial study, mice (n = 34) were weighed and sorted by sex and weight prior to infection to minimize differences between treatment groups. Mice (n = 8/treatment group) were challenged by intraperitoneal (IP) injection with 4 × 104 plaque-forming units (PFU) of LCMV-Cl13 and treated IP, twice daily with favipiravir (300 or 150 mg/kg/day), ribavirin (100 mg/kg/day), or vehicle placebo. The 8-day treatment regimens were initiated 1 day post LCMV challenge. Whole blood was collected via submandibular puncture on day 8 postinfection from predetermined subsets of animals (n = 3/treatment group) for analysis of serum viral titers. The mice were observed for morbidity and mortality for 21 days. Sham-infected controls (n = 2) were included for comparison.
To assess the efficacy of favipiravir against advanced LCMV infection, a second study was designed in which initiation of treatment was delayed until days 2 and 6 postinfection. Mice (n = 33) were sorted by sex and weight (n = 10/treatment group) prior to IP challenge with 4 × 104 PFU of LCMV-Cl13 and treated IP, twice daily with favipiravir (300 mg/kg/day) or placebo. The 8-day treatment regimens were initiated on day 2 or 6 postinfection with favipiravir or day 2 postinfection for the placebo group. Predetermined subsets of animals (n = 3/treatment group) were sacrificed on day 7 to assess viremia and liver, spleen, and brain tissue viral loads. The remaining animals were observed for morbidity and mortality until day 21 postinfection when the surviving mice were sacrificed for end-of-study serum and tissue viral titer analysis. Sham-infected controls (n = 3) were included for comparison.
Virus Titrations
Presence and concentration of virus in serum and select tissues was determined using an infectious cell culture assay as previously described [8]. Briefly, a specific volume of serum or tissue homogenate was serially diluted and added to triplicate wells of Vero cell monolayers in 96-well microtiter plates. The viral cytopathic effect was determined 11 days after plating from which the 50% endpoints were calculated. The lower limits of detection (LLD) for serum was 1.67 log10 50% cell culture infectious dose (CCID50)/mL, and tissues were 2.2–3.2 log10 CCID50/g. Samples presenting with undetectable virus were assigned the LLD value for statistical analyses.
Statistical Analysis
The Mantel-Cox log-rank test was used for analysis of Kaplan-Meier survival curves. A 1-way analysis of variance with Dunnett’s posttest to correct for multiple comparisons was used to compare differences in viral titers. All statistical evaluations were done using Prism 7 (GraphPad Software, La Jolla, CA).
RESULTS
Prior to initiating in vivo studies, we assessed the antiviral activity of favipiravir against LCMV-Cl13 in cell culture. Consistent with our findings from a previous LCMV replicon-based assay [6], the EC90 of favipiravir versus LCMV-Cl13 was 35 µM with an SI = 182 (Supplementary Figure 1). Ribavirin was substantially less potent with an EC90 of 196 µM and SI = 21.
To evaluate the therapeutic efficacy of favipiravir, we designed a treatment regimen in which 4-week-old NZB mice were challenged IP with LCMV-Cl13 and received 300 or 150 mg/kg/day of favipiravir, 100 mg/kg/day of ribavirin, or placebo by IP route beginning 24 hours postinfection (Figure 1A). All of the animals that received favipiravir survived the infection, whereas only 2 of 8 ribavirin-treated mice, and 1 of 8 placebo-treated mice recovered (Figure 1B). On day 8 postinfection, the presence of LCMV-CL13 in the serum in subsets of mice (n = 3) from each treatment group was determined. As shown in Figure 1C, there was no detectable virus in the 300 mg/kg/day favipiravir group and significantly reduced serum titers in the lower-dose favipiravir group, while all of the animals in the ribavirin-treated group had viral loads comparable to the placebo group. To assess morbidity, individual animal weights were measured during the study and reported as the percent change in body weight (Figure 1D). Favipiravir-treated animals exhibited mild weight loss in the first week followed by weight gain at a trajectory comparable to the sham-infected control animals. In contrast, all of the ribavirin-treated mice lost weight similarly to placebo-treated animals and the 2 survivors recovered very slowly.
Figure 1.
Study design and efficacy of favipiravir in lymphocytic choriomeningitis virus (LCMV)-infected NZB mice: (A) experimental design; (B) survival outcomes; (C) analysis of serum viral titers on day 8 of the infection (the x-axis represents the lower limit of detection); and (D) percent change in body weight represented as the group mean with the standard error of the mean of the surviving animals compared to their starting weights on the day of infection (sham-infected controls, n = 2, are included for comparison and the bars represent the weight range of the 2 mice). ***P < 0.001 compared to animals receiving placebo; b P < 0.001 compared to animals receiving ribavirin. Abbreviations: CCID50, 50% cell culture infectious dose; IP, intraperitoneal; PFU, plaque-forming unit; Tx, treatment.
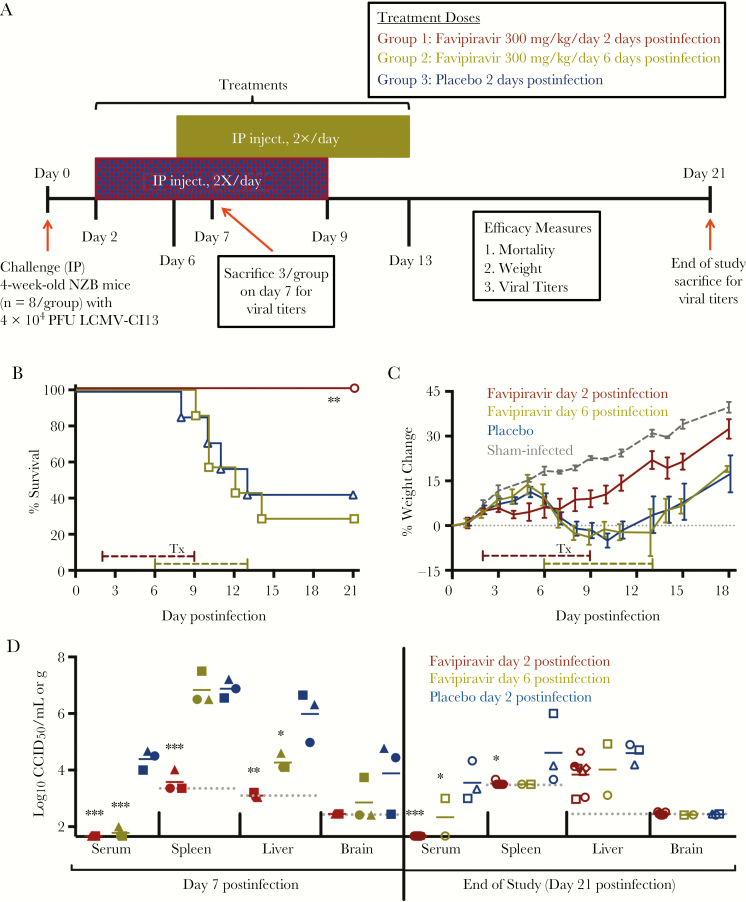
To evaluate the potential of favipiravir in treating more advanced LCMV infection in mice, a second study employing a delayed treatment regimen was conducted (Figure 2A). Cohorts of mice infected with LCMV-Cl13 were treated with 300 mg/kg/day of favipiravir for 8 days beginning 2 or 6 days postinfection or placebo starting 2 days postinfection. Notably, all of the animals receiving favipiravir beginning 2 days postinfection survived, while only 2 of 7 mice treated with favipiravir beginning day 6 postinfection and 3 of 7 mice treated with the placebo survived (Figure 2B). Thus, delaying initiation of favipiravir treatment until after the onset of clinical signs (weight loss; Figure 2C) did not improve survival outcome. The day 2 postinfection favipiravir treatment group failed to gain weight from day 3 to 7 postinfection, but resumed and was comparable to the sham-infected control group through the remainder of the experiment. Moreover, LCMV-Cl13 was largely undetectable in serum and tissues from animals sacrificed on day 7 postinfection (Figure 2D, left).
Figure 2.
Study design and efficacy of delayed favipiravir treatment initiation in lymphocytic choriomeningitis virus (LCMV)-infected mice: (A) experimental design; (B) survival outcomes; (C) percent change in body weight represented as a group mean with standard error of the mean of the surviving animals compared to their starting weights on the day of infection (sham-infected controls, n = 3, are included for comparison); and (D) analysis of serum and tissue viral titers in subsets of animals sacrificed on day 7 postinfection (left) and the surviving animals on day 21 postinfection (right). The x-axis represents the lower limit of detection for serum viral titers. Unique symbols in each treatment group represent values for the same animal across all viral titrations. Dashed line represents the respective tissue assay detection limit. *P < 0.05, **P < 0.01, ***P < 0.001 compared to animals receiving placebo. Abbreviations: CCID50, 50% cell culture infectious dose; IP, intraperitoneal; PFU, plaque-forming unit; Tx, treatment.
To assess whether the delayed favipiravir treatments could completely clear LCMV-Cl13 from the body, all survivors at the conclusion of the study (day 21 postinfection) were humanly euthanized and samples collected for determination of serum and tissue viral titers (Figure 2D, right). Virus was undetectable in the serum of all mice that received favipiravir beginning 2 days postinfection, while 1 of 2 mice in the day 6 postinfection treatment group and the 3 placebo-treated animals were still viremic on day 21. Spleen viral burden was also absent in favipiravir-treated mice, but present in 2 of the 3 placebo-treated survivors (Figure 2D, right). However, virus was detected in the liver of all survivors suggesting that the liver may serve as a reservoir tissue for chronic infection despite the effectiveness of favipiravir in preventing mortality due to acute infection and disease.
Discussion
Previous studies have reported that when challenged with high doses of LCMV-Cl13 inoculated via tail vein injection [9, 10], NZB mice are susceptible to an acute, lethal systemic disease that resembles overwhelming disseminated infections that occur in immunosuppressed organ transplant recipients [1, 2]. For the present studies, we used a lower virus challenge dose inoculated by IP injection to produce a more protracted disease course and a mortality rate (70%–80%) similar to that observed in the organ transplant LCMV cases that we were modeling. Using this model, we demonstrated highly effective treatment of acute, severe LCMV infection with favipiravir, a clinically experienced broad-spectrum antiviral approved for human use in Japan.
When administered within 2 days of LCMV-Cl13 challenge, treatment with 300 mg/kg/day favipiravir resulted in 100% protection from mortality across 2 experiments, and significantly limited viral replication to mostly undetectable levels during the acute infection. In contrast, treatment with 100 mg/kg/day of ribavirin resulted in a slight, but not statistically significant, delay in time of death, and had no effect in reducing serum LCMV-Cl13 titers. Published clinical reports reviewing the use of ribavirin in the treatment of LCMV seem to be consistent with these findings, as ribavirin therapy has had mixed results in controlling LCMV infection in transplant recipients [1, 2]. Ribavirin-treated mice also appeared to struggle with a more symptomatic chronic infection state as indicated by a very slow recovery and lethargy observed through much of the third week postinfection. Higher doses of ribavirin are associated with greater toxicity in mice and therefore were not used. The toxicity of ribavirin also presents challenges in a clinical setting with LCMV-infected organ transplant recipients. On the other hand, previous studies have shown favipiravir to be well tolerated in patients at doses in the range of 40 mg/kg/day [11, 12], which is considerably higher than the 24 mg/kg/day (body surface area-translated) human equivalent dose for a mouse treated with 300 mg/kg/day of drug [13].
With limited therapeutic options for treating severe LCMV infections in humans, the development of more effective and safer antivirals is urgently needed. This is especially imperative for immunocompromised individuals that are more vulnerable to fulminant LCMV infections. Our in vitro and mouse efficacy study findings, coupled with recent studies demonstrating efficacious treatment of lethal infections with the related Old World Lassa arenavirus [4, 5], provide the necessary supporting data to move forward with testing favipiravir in the cynomolgus macaque model of LCMV infection [14]. Disease in macaques recapitulates many of the features observed with infected immunocompromised individuals [15]. Because clinical trials are not likely to be feasible, demonstrating efficacy in mouse and nonhuman primate models may be the path forward for consideration under the Food and Drug Administration (FDA) “animal rule”. Due to the severity of LCMV infections in transplant recipients, with case fatality rates exceeding 70%, favipiravir should be considered for emergency use for future clusters of transplant-associated LCMV infections.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank Dr. Maria Salvato (University of Maryland School of Medicine, Baltimore, MD) for providing the LCMV (Clone 13 strain) and we are grateful for the technical support provided by Jason D. Fairbourn, Brittney Downs, Jean Maxwell, Devon Pfister, Angela Clyde, Trevor Truex, Michael Bertolio, Brennen McEwen, Taylor Redding, Jared Bennett, and Tyler Dahl.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases, National Institutes of Health (grant numbers HHSN272201000039I and HHSN272201100019I).
Potential conflicts of interest. T. K. and Y. F. are employees of the Toyama Chemical Co., Ltd., the manufacturer of favipiravir. All other authors report no potential conflicts of interests. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: International Conference on Antiviral Research, May 2017, Atlanta, Georgia (Abstract number 110).
References
- 1. Mathur G, Yadav K, Ford B, et al. . High clinical suspicion of donor-derived disease leads to timely recognition and early intervention to treat solid organ transplant-transmitted lymphocytic choriomeningitis virus. Transpl Infect Dis 2017; 19:e12707. [DOI] [PubMed] [Google Scholar]
- 2. Macneil A, Stroher U, Farnon E, et al. . Solid organ transplant-associated lymphocytic choriomeningitis, United States, 2011. Emerg Infect Dis 2012; 18:1256–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fischer SA, Graham MB, Kuehnert MJ, et al. ; LCMV in Transplant Recipients Investigation Team Transmission of lymphocytic choriomeningitis virus by organ transplantation. N Engl J Med 2006; 354:2235–49. [DOI] [PubMed] [Google Scholar]
- 4. Safronetz D, Rosenke K, Westover JB, et al. . The broad-spectrum antiviral favipiravir protects guinea pigs from lethal Lassa virus infection post-disease onset. Sci Rep 2015; 5:14775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Oestereich L, Rieger T, Lüdtke A, et al. . Efficacy of favipiravir alone and in combination with ribavirin in a lethal, immunocompetent mouse model of Lassa fever. J Infect Dis 2016; 213:934–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mendenhall M, Russell A, Juelich T, et al. . T-705 (favipiravir) inhibition of arenavirus replication in cell culture. Antimicrob Agents Chemother 2011; 55:782–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Furuta Y, Gowen BB, Takahashi K, Shiraki K, Smee DF, Barnard DL. Favipiravir (T-705), a novel viral RNA polymerase inhibitor. Antiviral Res 2013; 100:446–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gowen BB, Wong MH, Jung KH, et al. . In vitro and in vivo activities of T-705 against arenavirus and bunyavirus infections. Antimicrob Agents Chemother 2007; 51:3168–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Baccala R, Welch MJ, Gonzalez-Quintial R, et al. . Type I interferon is a therapeutic target for virus-induced lethal vascular damage. Proc Natl Acad Sci U S A 2014; 111:8925–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Puglielli MT, Browning JL, Brewer AW, et al. . Reversal of virus-induced systemic shock and respiratory failure by blockade of the lymphotoxin pathway. Nat Med 1999; 5:1370–4. [DOI] [PubMed] [Google Scholar]
- 11. Raabe VN, Kann G, Ribner BS, et al. ; Emory Serious Communicable Diseases Unit Favipiravir and ribavirin treatment of epidemiologically linked cases of lassa fever. Clin Infect Dis 2017; 65:855–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sissoko D, Laouenan C, Folkesson E, et al. ; JIKI Study Group Experimental treatment with favipiravir for Ebola virus disease (the JIKI Trial): a historically controlled, single-arm proof-of-concept trial in Guinea. PLoS Med 2016; 13:e1002009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J 2008; 22:659–61. [DOI] [PubMed] [Google Scholar]
- 14. Lukashevich IS, Djavani M, Rodas JD, et al. . Hemorrhagic fever occurs after intravenous, but not after intragastric, inoculation of rhesus macaques with lymphocytic choriomeningitis virus. J Med Virol 2002; 67:171–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zapata JC, Pauza CD, Djavani MM, et al. . Lymphocytic choriomeningitis virus (LCMV) infection of macaques: a model for Lassa fever. Antiviral Res 2011; 92:125–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.