Brazilian Aedes aegypti from Amazonas is highly permissive to monoinfection and coinfection with dengue virus and Zika virus (ZIKV) and capable of cotransmitting both arboviruses by bite to vertebrate hosts. Coinfection strongly influences vector competence, favoring transmission of ZIKV.
Keywords: Coinfection, Aedes aegypti, Zika virus, dengue virus, preferential transmission, vector bite
Abstract
Background
Several tropical cities are permissive to Aedes aegypti and dengue virus (DENV) endemicity and have allowed for invasion and circulation of Zika virus (ZIKV) in the same areas. People living in arbovirus-endemic regions have been simultaneously infected with ≥2 arboviruses.
Methods
A. aegypti mosquitoes from Manaus, the capital city of Amazonas State in Brazil, were coinfected with circulating strains of DENV and ZIKV. The coinfected vectors were allowed to bite BALB/c mice.
Results
A. aegypti from Manaus is highly permissive to monoinfection and coinfection with DENV and ZIKV and is capable of cotransmitting both pathogens by bite. Coinfection strongly influences vector competence, favoring transmission of ZIKV to the vertebrate host.
Conclusions
This finding suggests that A. aegypti is an efficient vector of ZIKV and that ZIKV would be preferentially transmitted by coinfected A. aegypti. Coinfection in the vector population should be considered a new critical epidemiological factor and may represent a major public health challenge.
Dengue virus (DENV) is considered to be the most important arbovirus, owing to the number and severity of human infections worldwide [1]. However, Zika virus (ZIKV) has emerged as a global health threat. To date, 563168 suspected cases of Zika have been reported from Latin America and the Caribbean [2]. In contrast to this newly introduced pathogen, DENV was introduced into the Americas in the 1600s [3]. It is estimated that DENV infects 390 million people worldwide every year, of whom approximately 96 million present with mild-to-severe symptoms [4]. In the Americas alone, 2338848 suspected dengue cases were reported in the 2016 [5].
ZIKV and DENV are primarily spread via the bite of infected mosquitoes. Aedes aegypti is widespread in the Americas and is responsible for most autochthonous transmission. To become a vector, the virus must replicate in a series of mosquito tissues and disseminate to and cross several biological barriers, including target organs, such as the midgut and salivary gland [6, 7]. These dynamics will also determine the intensity of viral infection in the salivary glands, which is related to the amount of virus that can be inoculated by vector bite into human skin.
Sociological, ecological, and epidemiological conditions in Latin American cities are permissive to A. aegypti and DENV endemicity and have allowed for invasion and circulation of ZIKV into the same large geographic areas. Consequently, as the distribution of ZIKV expands, vectors will have an increasing opportunity to acquire simultaneous and/or mixed infections with >1 arbovirus. This will occur by either a single infectious blood meal from a viremic human concurrently infected with DENV and ZIKV or by sequential blood meals from 2 individuals, each carrying a single arboviral infection.
There are reports of DENV and ZIKV coinfection [8, 9] and ZIKV and chikungunya virus (CHIKV) coinfection [10] among humans in the Americas and of DENV and CHIKV coinfection among those in other regions [11–14]. Rare multiple coinfections with all 3 arboviruses—CHIKV, DENV, and ZIKV—have also been reported [15, 16]. Concurrent infections of humans with DENV and ZIKV or even other arboviruses are not so uncommon and may have epidemiologic implications, including more-severe disease with overlapping symptoms, a situation where the diagnosis and management of such patients could be even more challenging than for a patient infected with a single arbovirus.
This study was performed in Manaus, capital city of the state of Amazonas, Brazil. The city is located in the middle of the Amazon forest and, with population growth, has undergone unplanned sprawl, which inadvertently contributes to the proliferation of A. aegypti and consequently, the endemicity of arboviruses, including ZIKV and DENV [17]. With a realistic potential that vector coinfection could occur in this area of ZIKV and DENV endemicity, a local population of A. aegypti was assessed to determine whether it was susceptible to infection with strains of DENV and ZIKV circulating in Brazil, as well as whether coinfection with both arboviruses will modulate replication or interfere with the vector competence of either virus. We found that almost all A. aegypti mosquitoes evaluated were susceptible to coinfection with the 2 arboviruses and that coinfection strongly influenced vector competence, with preferential transmission of ZIKV by A. aegypti bite to the vertebrate host.
METHODS
Mosquitoes
A. (Stegomyia) aegypti eggs were collected with oviposition traps placed during 4 weeks in 5 regional districts of Manaus. A total of 2501 eggs were collected and used to start a colony. Eggs were allowed to hatch, and resulting larvae were reared to adults, allowed to feed on blood, and reared through 3 successive generations as a single locality colony. However, parental generation of mosquitoes derived from the field-collected eggs were evaluated by quantitative polymerase chain reaction (qPCR) as described below, to ensure that they were free of ZIKV and DENV.
Viruses
ZIKV (ZikaSPH2015) [18] and DENV-2 (GenBank accession number KP188569) are currently circulating in Brazil and were used in these vector competence experiments. Virus stocks were propagated in an Aedes albopictus cell line (C6/36) growing in Leibowitz L-15 medium supplemented with 2% inactivated fetal bovine serum, 20 μg/mL gentamicin, 5 μg/mL amphotericin B, and 200 U/mL penicillin (all from Sigma Aldrich, St Louis). Virus titration followed the 50% tissue culture infectious dose method [19, 20]
Monoinfection and Coinfection of A. aegypti
Six hundred 3–5-day-old adult female mosquitoes were divided in 3 groups and infected with ZIKV and/or DENV via a membrane feeding assay [20, 21] with blood meals containing either 1 virus (monoinfection) or both viruses (coinfection). Identical titers of ZIKV and DENV were used in single or coinfection experiments. Virus titers of 1 × 105 plaque-forming units per mL from C6/36 cell culture supernatants of each virus were mixed with fresh mouse blood (ratio, 2:1) and offered to the mosquitoes as described elsewhere [20, 21]. Three groups of exposed A. aegypti were obtained: (1) DENV monoinfection, (2) ZIKV monoinfection, and (3) DENV/ZIKV coinfection. The blood-fed mosquitoes were maintained on 10% sucrose ad libitum. Seven and 14 days after blood meal, 30 infected mosquitoes from each experimental group were randomly chosen from each group to be analyzed by qPCR.
Transmission by Bite of Coinfected A. aegypti
Fourteen days after infection, 80 ZIKV/DENV-coinfected A. aegypti were separated into 10 groups of 8 mosquitoes and placed in small plastic vials (11.1-mL volume, 4.8-cm height, and 1.8-cm diameter) covered at one end with a 0.25-mm nylon mesh [20]. Mice were immediately euthanized following mosquito bite exposure, and the mosquito-exposed region of each ear was biopsied with a 4-mm tissue punch. All fully engorged mosquitoes were killed quickly via cold exposure, removed from the vials, and dissected as detailed below.
The mosquito tissues, as well as the mouse ears, were macerated after vector bite assays and processed for RNA extraction using the QIAamp Viral RNA Mini Kit (Qiagen) according to the manufacturer’s protocols.
Real-Time qPCR for Quantification of ZIKV and DENV Complementary DNA (cDNA) in Mosquitoes and Mouse Ears
Mosquito heads and salivary glands (heads/SGs) were dissected from the bodies of the 3 experimental groups (described above). These mosquito tissues, as well as the mouse ear biopsy specimens obtained after vector bite exposures, were macerated and processed for RNA extraction. Primer and probe sets specific for DENV and ZIKV were designed as previously described [22, 23]. Primers were synthesized by Integrated DNA Technologies and by probes with 5-FAM as the reporter dye (ThermoFisher). All real-time assays were performed by using the TaqMan RNA-to-CT 1-Step Kit, with amplification in the 7500 Fast and 7500 Real-Time PCR System, according the manufacturer’s protocol. The infection rate (IR) was calculated as the proportion (ie, percentage) of all experimentally blood-fed mosquitoes in which the 2 arboviruses were detected in the mosquito. The intensity of the infection was estimated by determining the number of viral cDNA copies present in the sample. The disseminated infection rate (DIR) was calculated as the proportion of DENV- or ZIKV-infected mosquito heads/SGs among all infected mosquitoes.
Infectivity of ZIKV and DENV in A. aegypti SG
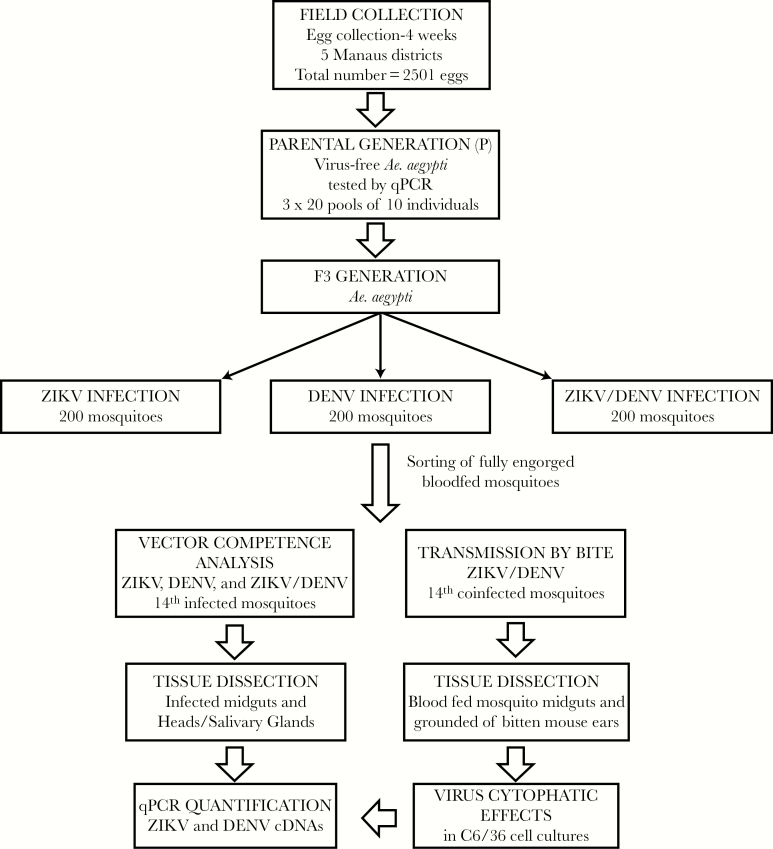
To verify viral the viability and the infectivity of ZIKV and DENV isolates, 10 A. aegypti from each group of mosquitoes for which blood meals were either monoinfected (DENV or ZIKV) or coinfected (ZIKV+DENV) were randomly selected from the cage 14 days after feeding, and their SGs were dissected and macerated individually. Each SG supernatant was divided into 2 subsets: one directly tested for cDNA copies of the 2 arboviruses by qPCR and the other cultivated in C6/36 cells for 3–4 days at 27°C to develop infection and observe viral cytopathic effects as describe elsewhere [24]. These infected C6/36 cell supernatants were also processed by qPCR 4 days after inoculation to confirm the presence of ZIKV and DENV cDNA copies, respectively. Figure 4 shows the main steps of the experimental protocol.
Figure 4.
Diagram showing the main steps of the experimental protocol of Aedes aegypti coinfection with Zika virus and dengue virus. Abbreviations: cDNA, complementary DNA; DENV, dengue virus; qPCR, quantitative polymerase chain reaction; ZIKV, Zika virus
Statistical Analyses
Shapiro Wilk and Wilcoxon–Mann-Whitney tests were used to evaluate significance among groups in relation median amounts or virus. Two-tailed χ2 or Fisher exact tests were used to evaluate differences between IRs and the intensity of the infection (ie, the number of viral cDNA copies) present in the mosquito tissues of the experimental infected groups. The Spearman nonparametric r test was used to test for a statistically significant correlation between the numbers of DENV and ZIKV cDNA copies. All statistical analyses were performed in GraphPad Prism, version 7.00 (La Jolla, CA), and P values ≤.05 were considered statistically significant.
Ethics Approval
This study was conducted in accordance with the Manual for the Use of Animals, Oswaldo Cruz Foundation, Ministry of Health of Brazil (national decree 3179). It was approved by the Ethics Committee for the Use of Animals, Oswaldo Cruz Foundation (number L-1715), and by the Animal Research Ethics Committee of Tropical Medicine, Foundation Dr. Heitor Vieira Dourado (002380/2016).
RESULTS
Susceptibility of A. aegypti to DENV and ZIKV Monoinfection and Coinfection
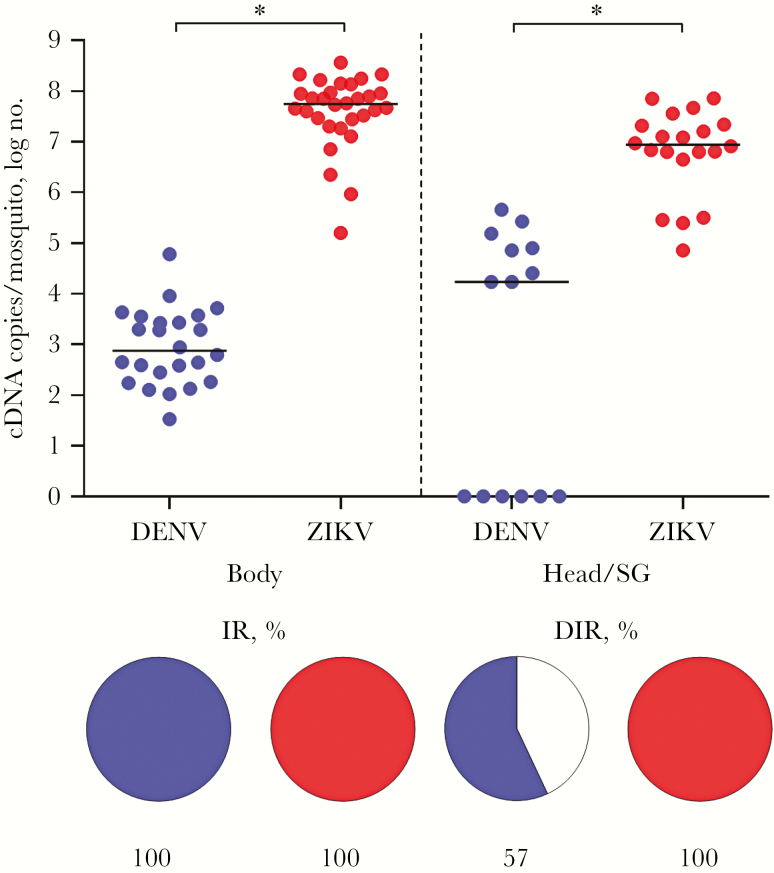
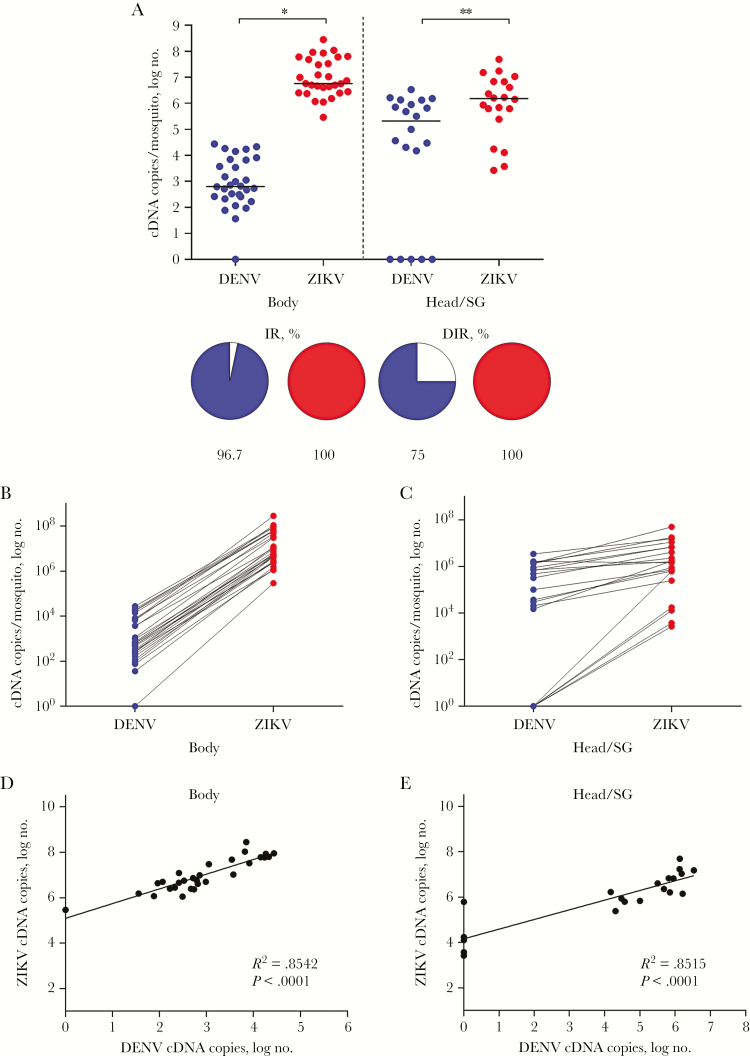
Fourteen days after infection, the monoinfected and coinfected mosquitoes were evaluated for infections. Coinfected mosquitoes were also used to test for virus transmission to a vertebrate host [20]. The IR for monoinfection, calculated as the percentage of individual A. aegypti infected with either DENV or ZIKV, was 100%. The DIR for these single infections, reported as the percentage of individual A. aegypti monoinfected with DENV or ZIKV in the head/SG, was 57% and 100%, respectively (Figure 1). In the coinfected A. aegypti, the IR for DENV and ZIKV was 96.7% and 100%, respectively. The DIR for these same coinfected mosquitoes for DENV and ZIKV was 75% and 100%, respectively (Figure 2).
Figure 1.
Analysis of monoinfection of Aedes aegypti with dengue virus (DENV; blue) and Zika virus (ZIKV; red). The intensity of infection of each experimental group is presented as in the graph as the number of complementary DNA (cDNA) copies per mosquito (top). The infection rate (IR) and the disseminated infection rate (DIR) are represented in the pie charts as the percentage of infected mosquito bodies and heads/salivary glands (SGs). The DIR of DENV was significantly different from that of ZIKV (P = .0022). The intensity of infection for ZIKV was significantly different from that of DENV in the body and head/SG (P < .001).
Figure 2.
Comparative analysis of dengue virus (DENV; blue) and Zika virus (ZIKV; red) presence in coinfected Aedes aegypti. A, The intensity of infection of each experimental group is presented as in the graph as the number of complementary DNA (cDNA) copies per mosquito (top). The infection rate (IR) and the disseminated infection rate (DIR) are represented in the pie charts as the percentage of infected mosquito bodies and heads/salivary glands (SGs). The intensity of infection in the coinfected mosquitoes was significantly greater for ZIKV than for DENV in the bodies (P < .001) and heads/SGs (P = .01). The DIR of DENV was significantly lower than that of ZIKV (P = .0475) in these same mosquitoes. B and C, Relationship between the number of cDNA copies, representing the intensity of infection of DENV and ZIKV, in the body and head/SG of each coinfected A. aegypti, with the intensity of ZIKV infection greater in every instance. D and E, Significantly positive correlation between the intensity of infection of DENV and ZIKV in the bodies and heads/SGs of coinfected A. aegypti.
Quantification of DENV and ZIKV cDNA Copies in Single and Coinfected A. aegypti
In A. aegypti with DENV or ZIKV monoinfection, 4.5 × 102 or 4.6 × 107 cDNA copies, respectively, were detected in the body. The heads/SGs of these same monoinfected mosquitoes contained 1.7 × 104 and 8.8 × 106 DENV and ZIKV cDNA copies, respectively. Comparatively, in the monoinfected mosquitoes, the number of ZIKV cDNA copies was significantly greater than the number of DENV cDNA copies in both the bodies and heads/SGs (P < .001; Figure 1).
In the coinfected A. aegypti, there were 5.8 × 102 DENV cDNA copies and 5.0 × 106 ZIKV cDNA copies in the mosquito bodies and 2.1 × 105 DENV cDNA copies and 1.5 × 106 ZIKV cDNA copies in the heads/SGs. Similar to monoinfections, the number of viral cDNA copies in the coinfected mosquitoes was significantly greater for ZIKV than for DENV in both the bodies (P < .001) and the heads/SGs (P = .01; Figure 2A). This remained true for comparisons within individual mosquitoes, although the numbers of DENV and ZIKV cDNA copies were much more similar in the heads/SGs as compared to the bodies of coinfected mosquitoes (Figure 2B and 2C).
In addition, the number of ZIKV cDNA copies was higher in monoinfected mosquitoes as compared to coinfected mosquitoes in both the body (P = .002) and head/SG (P = .0144). However, there was no significant difference in the number of DNV cDNA copies between monoinfected and coinfected mosquito bodies, and in contrast to ZIKV, the number DENV cDNA copies in mosquito heads was greater in coinfected mosquitoes than in monoinfected individuals (P = .0443).
There is a direct positive correlation between the number of DENV and ZIKV cDNA copies in the body (R2 = 0.8542; P < .0001) and head/SG (R2 = 0.8515; P < .0001) of each coinfected A. aegypti (Figure 2D and 2E). For example, a high number of ZIKV cDNA copies in the body correspond to a high number of ZIKV cDNA copies in the head/SG of individual mosquitoes. This positive relationship was stronger in the bodies of coinfected mosquitoes. Four mosquitoes were negative for DENV (1 body and 3 head/SG). Interestingly, these negative DENV mosquitoes were positive for ZIKV, but at relatively low cDNA values (Figure 2B and 2C). The data also revealed a direct, positive correlation between the quantity of the DENV and ZIKV cDNA copies in the body (R2 = 0.8542, P < .0001) and head/SG (R2 = 0.8515, P < .0001; Figure 2D and 2E). In this comparison, high ZIKV cDNA levels in the body correlated with high DENV cDNA levels in body samples from the same mosquito.
Preferential Transmission of ZIKV by Coinfected A. aegypti Bite
Ten groups of 8 coinfected A. aegypti were evaluated for arbovirus transmission by bite to BALB/c mice (Figure 3). At least 1 mosquito placed on mouse ears in all 10 groups fed to visible engorgement. This transmission experiment resulted in 3 sample types: postbite A. aegypti bodies and dissected SGs, and BALB/c mouse ear punch biopsy specimens. Samples were pooled by type within each of the 10 feeding groups (Table 1). All 10 pools of A. aegypti bodies from the transmission experiment were positive for both DENV and ZIKV, with cDNA levels ranging from 1.0 × 102 to 4.41 × 104 cDNA copies and from 1.23 × 106 to 4.26 × 108 cDNA copies, respectively. DENV was only detected in 8 SG pools, whereas ZIKV was detected in all 10 SG pools, with values ranging from 3.88 × 103 to 1.53 × 106 DENV cDNA copies and from 1.42 × 106 to 1.73 × 107 ZIKV cDNA copies.
Figure 3.
Dengue virus and Zika virus transmission by bites of coinfected Aedes aegypti. A group of 8 coinfected mosquitoes were placed in a vial with the nylon mesh side against the ears of an anesthetized BALB/c mouse. Mosquitoes can be seen probing on the right ear of the mouse. The mosquito-exposed region of the left ear is visible after removing the plastic feeding vial (white arrows).
Table 1.
Number of Complementary DNA (cDNA) Copies of Zika Virus (ZIKV) and Dengue Virus (DENV) Transmitted by Coinfected Aedes aegypti to BALB/c Mouse Ears
| Pool No. | Engorged Mosquitoes, No./Poola | cDNA Copies | |
|---|---|---|---|
| DENV | ZIKV | ||
| 1 | 1b | 4.55 × 102 | 5.00 × 103 |
| 2 | 2 | 0 | 2.00 × 103 |
| 3 | 2 | 0 | 1.36 × 1010 |
| 4 | 4 | 0 | 1.76 × 1010 |
| 5 | 5 | 0 | 4.88 × 109 |
| 6 | 5 | 0 | 4.65 × 108 |
| 7 | 3 | 0 | 2.74 × 102 |
| 8 | 4b | 1.83 × 103 | 1.92 × 102 |
| 9 | 8 | 0 | 6.50 × 103 |
| 10 | 1 | 0 | 1.20 × 108 |
aNumber of fully engorged mosquitoes per pool after bite exposure.
bPool of mosquitoes positive for DENV cDNA copies.
The ZIKV transmission rate by bite from coinfected A. aegypti to mouse ears was 100%. ZIKV cDNA was detected in all mouse ears, with a wide range of viral cDNA levels (1.92 × 102–1.76 × 1010 copies) and a median value of 6.00 × 107 cDNA copies. In contrast, the DENV transmission rate was only 20%. DENV cDNA was detected in only 2 of 10 mouse ears, with low numbers of DENV cDNA copies (4.55 × 102 and 1.83 × 103 copies). These 2 mouse ears were also positive for ZIKV, illustrating that simultaneous transmission of ZIKV and DENV is possible (Table 1).
Viability of DENV and ZIKV in A. aegypti SGs
Viability of DENV and ZIKV from SGs was verified 14 days after infectious blood feed by determining cytopathic effect on monolayers of C6/36 cells [24]. DENV and ZIKV from monoinfected SGs were infectious to C6/36 cells. Ten of 10 SGs from ZIKV-exposed mosquitoes and 7 of 10 SGs from DENV-exposed mosquitoes caused cytopathic effect in C6/36 cells and were positive for viral cDNA by qPCR. Viral quantification by qPCR analysis of culture supernatants showed that the median numbers of DENV and ZIKV cDNA copies were 5.1 × 104 and 1.33 × 104 copies, respectively. Nine of 10 SGs from mosquitoes coinfected with DENV and ZIKV were infectious and caused cytopathic effect in C6/36 cells. However, of these 9 SGs, 5 SG supernatants were positive for DENV, and all 9 were positive for ZIKV by qPCR analysis. qPCR quantitation revealed a mean number of 1.08 × 104 cDNA copies in the 5 DENV-positive SGs and 1.05 × 105 cDNA copies in the 9 ZIKV-positive SGs. These complementary assays confirmed the presence and infectivity of ZIKV and DENV, their ability to be cotransmitted by A. aegypti bite, and their potential to initiate coinfections in a host vertebrate.
DISCUSSION
People living in arbovirus-endemic regions have presented complex arbovirus infections involving simultaneous infection with ≥2 arboviruses, including DENV, ZIKV, and CHIKV [8–16]. Coinfection with ZIKV and CHIKV has already been identified in regions of Brazil where both viruses are endemic [25, 26]. In Brazilian cities like Manaus, where DENV is already highly endemic, the introduction of ZIKV puts thousands of people at risk for coinfection with these 2 potentially highly pathogenic arboviruses.
Studies evaluating potential simultaneous transmission of coinfected mosquitoes support our results, although they observed different mosquito species. One study could not illustrate coinfection with DENV and CHIKV in A. aegypti [27], but a second detected both arboviruses in saliva specimens from A. albopictus [28]. Potential cotransmission of CHIKV and DENV was also reported at low rates in A. aegypti and A. albopictus from the same region [29]. A.aegypti from Mexico were experimentally infected with CHICK, DENV, and ZIKV, resulting in monoinfection and coinfection involving 2 or 3 viruses. These mosquitoes can potentially transmit all combinations of these 3 viruses simultaneously, based on findings from qPCR analysis and cell culture of saliva specimens [30]. Another study evaluated coinfection of ZIKV and CHIKV in colonized A. aegypti and found that 84.4% of mosquitoes were coinfected but that only 11.5% had both viruses in saliva [31]. The number of ZIKA cDNA copies was higher than the number of CHIKV cDNA copies, similar to what we have observed, but in contrast there was no apparent impact of coinfection when they compared ZIKV/CHIKV-coinfected mosquitoes to mosquitoes with ZIKV or CHIKV monoinfection [31]. Another study involving A. aegypti reported that rates of neither DENV nor ZIKV infection were significantly affected by DENV/ZIKV coinfection, compared with DENV nor ZIKV monoinfection [30]. However, it is important to note that all of these cotransmission reports are based on forced salivation and equate this fact to the “potential transmission” by the vector. In contrast, our study is the first to evaluate arbovirus transmission by a natural route, the bite from ZIKV/DENV-coinfected A. aegypti in a mouse ear model, to provide irrefutable evidence of a cotransmission event. Our study also paired A. aegypti recently colonized from the field (F3) with DENV and ZIKV strains that are currently cocirculating in Brazil.
The Amazonian A. aegypti population demonstrated high permissiveness and competence for monoinfection with DENV or ZIKV, resulting in high infection rates and disseminated infections. The extrinsic incubation period [32], defined as interval between ingestion of the infective blood meal and the ability of the vector to transmit the acquired virus to a vertebrate, is 7–10 days for ZIKV [22] and 7–14 days for DENV [23]. Therefore, we opted to evaluate A. aegypti infection 14 after infection, to verify the potential for dual arbovirus transmission. Fourteen days after infection, these Amazonian A. aegypti were efficiently coinfected with ZIKV and DENV, with both viruses capable of being transmitted by mosquito bite. However, in these coinfections, ZIKV grew to higher titers and more efficiently infected the SGs. Despite an apparent difference in the replication efficiency of ZIKV and DENV in this A. aegypti population, there was a positive correlation between replication and dissemination of the 2 viruses in coinfected mosquitoes. The analysis of each individual coinfected mosquito showed a robust direct positive relationship between the amount of DENV and ZIKV in the mosquito body and head/SG. This is the first demonstration of a positive correlation of the permissiveness of individual mosquitoes to infection with 2 different arboviruses. Individual mosquitoes that were more permissive to DENV localized and disseminated infection were also more permissive to ZIKV localized and disseminated infection. However, of those evaluated from this A. aegypti population, a few individual mosquitoes that did not develop SG infection with DENV, which suggests that there is a potential barrier to DENV invasion that should be more thoroughly evaluated. This was even true in the coinfection experiments, in which the same individual mosquitoes had both the body and the head/SG invaded by ZIKV.
ZIKV replicated to greater titers and was preferentially transmitted to mouse tissue during the bite experiments. In fact, ZIKV replicated to higher titers (defined as the number of cDNA copies) than DENV in both monoinfection and coinfection, with infection and dissemination rates of 100% in all experiments. The lower infection and dissemination rates for DENV reported here as compared to other studies may be attributable to the innate permissiveness of the Amazonian A. aegypti population to DENV. However, it should be reinforced that this mosquito colony was only the third generation from the field and should be representative of the vector population in Manaus, which, although highly permissive to DENV, appears to be completely permissive to ZIKV.
In conclusion, A. aegypti from Manaus is highly permissive to monoinfection and coinfection with DENV and ZIKV and is capable of cotransmitting both arboviruses, as demonstrated through a transmission-by-bite model. We provide the first evidence of a positive correlation in permissiveness for 2 different arboviruses in a mosquito population. Individual mosquitoes with relatively high or low DENV titers had similarly high or low ZIKV titers when coinfected. Although ZIKV had efficient replication and tissue invasion, with high IRs, DIRs in both the monoinfections and coinfections were lower in both body and head/SG tissues during coinfection. However, despite somewhat suppressed viral titers, all infection, dissemination, and transmission rates were 100% for ZIKV. This important finding suggests that ZIKV would be efficiently transmitted via this population of mosquito vectors and would be preferentially transmitted if these mosquitoes were coinfected and, therefore, had the potential to cotransmit both viruses.
Notes
Financial support. This work was supported by the Oswaldo Cruz Foundation, the Brazilian Council for Scientific and Technological Development, Science Without Borders, the Instituto Nacional de Ciência e Tecnologia (INCT)—Entomologia Molecular, the Minas Gerais State Research Support Foundation (FAPEMIG), the Federal University of Bahia Support Program for Young PhD Professors, the Amazonas State Research Support Foundation (FAPEAM), the Brazilian Comissão de Aperfeiçoamento de Pessoal de Nivel Superior (CAPES) (support to B. A. C., T. B. C., and R. N.-P.), and the Conselho Nacional de Desenvolvimento Cientifico e Tecnologico (CNPq) (support to N. R. B., LV, A. S. O., N. F. C. S., M. V. G. d. L., and P. F. P. P.).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Gubler DJ. Resurgent vector-borne diseases as a global health problem. Emerg Infect Dis 1998; 4:442–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. PAHO/WHO. Zika cases and congenital syndrome associated with Zika virus reported by countries and territories in the Americas, 2015–2017—cumulative cases. 2017. http://www.paho.org/hq/index.php?option=com_docman&task=doc_view&Itemid=270&gid=39894&lang=en. Accessed 16 May 2017. [Google Scholar]
- 3. Brathwaite Dick O, San Martín JL, Montoya RH, del Diego J, Zambrano B, Dayan GH. The history of dengue outbreaks in the Americas. Am J Trop Med Hyg 2012; 87:584–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bhatt S, Gething PW, Brady OJ, et al. The global distribution and burden of dengue. Nature 2013; 496:504–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. PAHO/WHO. Number of reported cases of dengue and severe dengue (SD) in the Americas, by Country. 2017. http://www.paho.org/hq/index.php?option=com_docman&task=doc_download&Itemid=&gid=37782&lang=pt. Accessed 21 May 2017. [Google Scholar]
- 6. Hardy J. Susceptibility and resistance of vector mosquitoes. In: Monath T, ed. Arboviruses Epidemiol Ecol. Boca Raton, FL: CRC Press, 1988:87–126. [Google Scholar]
- 7. Bennett KE, Olson KE, Muñoz Mde L, et al. Variation in vector competence for dengue 2 virus among 24 collections of Aedes aegypti from Mexico and the United States. Am J Trop Med Hyg 2002; 67:85–92. [DOI] [PubMed] [Google Scholar]
- 8. Dupont-Rouzeyrol M, O’Connor O, Calvez E, et al. Co-infection with Zika and dengue viruses in 2 patients, New Caledonia, 2014. Emerg Infect Dis 2015; 21:381–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Iovine NM, Lednicky J, Cherabuddi K, et al. Coinfection with Zika and dengue-2 viruses in a traveler returning from Haiti, 2016: clinical presentation and genetic analysis. Clin Infect Dis 2017; 64:72–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zambrano H, Waggoner JJ, Almeida C, Rivera L, Benjamin JQ, Pinsky BA. Zika virus and chikungunya virus coinfections: a series of three cases from a single center in Ecuador. Am J Trop Med Hyg 2016; 95:894–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Furuya-Kanamori L, Liang S, Milinovich G, et al. Co-distribution and co-infection of chikungunya and dengue viruses. BMC Infect Dis 2016; 16:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ratsitorahina M, Harisoa J, Ratovonjato J,. et al. Outbreak of dengue and chikungunya fevers, Toamasina, Madagascar, 2006. Emerg Infect Dis 2008; 14:1135–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Caron M, Paupy C, Grard G, et al. Recent introduction and rapid dissemination of Chikungunya virus and Dengue virus serotype 2 associated with human and mosquito coinfections in Gabon, central Africa. Clin Infect Dis 2012; 55:e45–53. [DOI] [PubMed] [Google Scholar]
- 14. Martin S, Omarjee R, Prat CM, et al. Importance of case definition to monitor ongoing outbreak of chikungunya virus on a background of actively circulating dengue virus. Euro Surveill 2013; 19. [DOI] [PubMed] [Google Scholar]
- 15. Villamil-Gómez WE, Rodríguez-Morales AJ, Uribe-García AM, et al. Zika, dengue, and chikungunya co-infection in a pregnant woman from Colombia. Int J Infect Dis 2016; 51:135–8. [DOI] [PubMed] [Google Scholar]
- 16. Waggoner JJ, Gresh L, Vargas MJ, et al. Viremia and clinical presentation in Nicaraguan patients infected with zika virus, chikungunya virus, and dengue virus. Clin Infect Dis 2016; 63:1584–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brazilian Ministry of Health. Monitoramento dos casos de dengue, febre de chikungunya e febre pelo vírus Zika até a Semana Epidemiológica 4, 2017. 2017, 1–9. http://combateaedes.saude.gov.br/images/pdf/2017-Dengue_Zika_Chikungunya-SE4.pdf. Accessed 7 June 2017. [Google Scholar]
- 18. Cunha MS, Esposito DLA, Rocco IM, et al. First complete genome sequence of zika virus (Flaviviridae, Flavivirus) from an autochthonous transmission in Brazil. Genome Announc. 2016; 4:e00032–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. White LA. Susceptibility of Aedes albopictus C6/36 cells to viral infection. J Clin Microbiol 1987; 25:1221–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. LaBarre DD, Lowy RJ. Improvements in methods for calculating virus titer estimates from TCID50 and plaque assays. J. Virol. Methods 2001; 96:107–26. [DOI] [PubMed] [Google Scholar]
- 21. Gonçalves CM, Melo FF, Bezerra JM, et al. Distinct variation in vector competence among nine field populations of Aedes aegypti from a Brazilian dengue-endemic risk city. Parasit Vectors 2014; 7:320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Leparc-Goffart I, Baragatti M, Temmam S, et al. Development and validation of real-time one-step reverse transcription-PCR for the detection and typing of dengue viruses. J Clin Virol 2009; 45:61–6. [DOI] [PubMed] [Google Scholar]
- 23. Lanciotti RS, Kosoy OL, Laven JJ, et al. Genetic and serologic properties of zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis 2008; 14:1232–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Esposito DLA, Fonseca BAL da. Zika and chikungunya infections in Brazil: reviewing the epidemic and treatment options. Rev Soc Bras Med Trop 2016; 49:535–536. [DOI] [PubMed] [Google Scholar]
- 25. Rohani A, Potiwat R, Zamree I, Lee HL. Refractoriness of Aedes aegypti (Linnaeus) to dual infection with dengue and chikungunya virus. Southeast Asian J Trop Med Public Health 2009; 40:443–8. [PubMed] [Google Scholar]
- 26. Vazeille M, Mousson L, Martin E, Failloux AB. Orally co-infected Aedes albopictus from La Reunion Island, Indian Ocean, can deliver both dengue and chikungunya infectious viral particles in their saliva. PLoS Negl Trop Dis 2010; 4:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nuckols JT, Huang YJ, Higgs S, et al. Evaluation of simultaneous transmission of chikungunya virus and dengue virus type 2 in infected Aedes aegypti and Aedes albopictus (Diptera: Culicidae). J Med Entomol 2015; 52:447–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rückert C, Weger-Lucarelli J, Garcia-Luna SM, et al. Impact of simultaneous exposure to arboviruses on infection and transmission by Aedes aegypti mosquitoes. Nat Commun 2017; 8:15412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Göertz GP, Vogels CBF, Geertsema C, Koenraadt CJM, Pijlman G. Mosquito co-infection with Zika and chikungunya virus allows simultaneous transmission without affecting vector competence of Aedes aegypti. PLoS Negl Trop Dis 2017:1–22. doi:10.1371/.0005654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Salazar MI, Richardson JH, Sánchez-Vargas I, Olson KE, Beaty BJ. Dengue virus type 2: replication and tropisms in orally infected Aedes aegypti mosquitoes. BMC Microbiol 2007; 7:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Boorman JPT, Porterfield JS. A simple technique for infection of mosquitoes with viruses; transmission of Zika virus. Trans R Soc Trop Med Hyg 1956; 50:238–242. [DOI] [PubMed] [Google Scholar]
- 32. Secundino NFC, Chaves BA, Orfano AS, et al. Zika virus transmission to mouse ear by mosquito bite: a laboratory model that replicates the natural transmission process. Parasit Vectors 2017; 10:346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. World Health Organization (WHO). WHO Director-General summarizes the outcome of the Emergency Committee regarding clusters of microcephaly and Guillain-Barré syndrome. Geneva: WHO, 2016. http://www.who.int/mediacentre/news/statements/2016/emergency-committee-zika-microcephaly/en/. Accessed 18 April 2018. [Google Scholar]
- 34. Bonaldo MC, Ribeiro IP, Lima NS, et al. Isolation of infective zika virus from urine and saliva of patients in Brazil. PLoS Negl Trop Dis 2016; 10(6):e0004816. doi: 10.1371/journal.pntd.0004816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sardi SI, Somasekar S, Naccache SN, et al. Coinfections of zika and chikungunya viruses in Bahia, Brazil, identified by metagenomic next-generation sequencing. J Clin Microbiol 2016; 54:2348–2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Carrington LB, Armijos MV, Lambrechts L, Scott TW. Fluctuations at a low mean temperature accelerate dengue virus transmission by Aedes aegypti. PLoS Negl Trop Dis 2013; 7:e2190. [DOI] [PMC free article] [PubMed] [Google Scholar]