Abstract
This editorial refers to ‘Cardiovascular disease risk associated with elevated lipoprotein(a) attenuates at low low-density lipoprotein cholesterol levels in a primary prevention setting’†, by R. Verbeek et al., on page 2589.
In this issue of the European Heart Journal, Verbeek et al. investigated the risk pattern of Lipoprotein(a) [Lp(a)] across a wide range of values among two large community cohorts, as well as potential interactions with LDL-C levels.1 Data were derived from the well-known Copenhagen City Heart Study (CCHS), consisting of 9448 individuals, and the European Prospective Investigation of Cancer (EPIC)-Norfolk study, consisting of 16 654 individuals, in the primary prevention setting with available measurements for Lp(a) and LDL-C. The clinical primary outcome of cardiovascular disease (CVD) events was defined as coronary heart disease death, non-fatal myocardial infarction, and fatal or non-fatal stroke. Authors first categorized Lp(a) values at baseline into high (≥ 80th percentile) and normal (<80th percentile) groups using 50 mg/dL as a threshold, as recommended by the guidelines for clinical implications.2 In the EPIC-Norfolk cohort, patients’ CVD risk was lowest when associated with Lp(a) values <80th percentile and LDL-C <2.5 mmol/L, while patients’ CVD risk increased with higher LDL-C levels, reaching hazard ratios (HRs) of 1.61 [95% confidence interval (CI) 1.29–2.00, P < 0.001] for LDL-C ≥ 5.5 mmol/L. For patients with Lp(a) values ≥ 80th percentile, the risk for CVD events followed the same pattern, with an HR of 2.17 (95% CI 1.58–2.98, P < 0.001) for LDL-C values ≥ 5.5 mmol/L. These results corroborated with those of the CCHS study: HR for CVD was 1.42 (95% CI 1.15–1.74) for patients with Lp(a) values < 80th percentile and LDL-C ≥ 5.5 mmol/L, and increased to HR 2.34 (95% CI 1.65–3.35, P < 0.001) for patients with Lp(a) values ≥ 80th percentile and LDL-C ≥ 5.5 mmol/L. HR for CVD risk demonstrated the highest level of significance (P < 0.001) for patients with Lp(a) values ≥ 80th percentile and LDL-C ≥ 5.5 mmol/L, compared with patients with Lp(a) values < 80th percentile and LDL-C < 2.5 mmol/L. Risk estimates followed the same pattern when using the threshold of 50 mg/dL: for patients with Lp(a) ≥ 50 mg/dL and LDL-C < 2.5 mmol/L, HR was 2.56 (95% 1.94–3.39) and 1.71 (95% CI 1.32–2.22) in the EPIC-Norfolk and the CCHS cohort, respectively, when compared with subjects with Lp(a) < 50 mg/dL and LDL-C < 2.5 mmol/L. Of note, for a same given level of LDL-C, CVD risk increased by 40–50% when Lp(a) values were high (≥ 50 mg/dL or ≥ 80th percentile).
Verbeek et al.’s article further highlights that high Lp(a) levels are also closely associated with adverse clinical outcomes in subjects with low LDL-C values. For instance, HR was 1.44 (95% CI 1.16–1.78, P < 0.01) in the group with LDL-C values ranging between 2.5–3.49 mmol/L in the EPIC-Norfolk cohort. On the other hand, in the relatively small group of patients with LDL-C < 2.5 mmol/L (< 10% of the cohort sample size) and high Lp(a), the association of CVD risk with Lp(a) levels was strongly attenuated: HR 1.11 (95% CI 0.77–1.59) and 1.08 (95% CI 0.85–1.38) in the EPIC-Norfolk and the CCHS cohort, respectively. However, the rate of CVD events did increase by more than 100% when high levels of Lp(a) were associated with higher LDL-C values (≥ 5.5 mmol/L). The findings of this large observational study add new evidence to the role of Lp(a) as a potential independent CVD risk factor, and suggest that the risk is highest when both Lp(a) and LDL-C values are elevated. HRs are, on the other hand, modest when taken individually for Lp(a). The clinical implications of these findings for medical practice remain still to be assessed, and it is also unclear whether Lp(a) can be added as an incremental value for CVD risk prediction beyond traditional risk factors.
Previous studies, including a large meta-analysis, have suggested that the risk of CVD increased according to Lp(a) concentration, after adjustment for sex and age.3,4 In addition, Mendelian randomization data have shown that genetic mutations for Lp(a) increase the risk of CVD, reinforcing the likely causal association between the two.5 A prospective cohort study including data from 46 200 individuals from the Copenhagen General Population Study showed that for patients with familial hypercholesterolemia (FH), the highest risk of CVD was associated with Lp(a) values ≥ 50 mg/dL (HR 5.3, 95% CI 3.6–7.6), and CVD risk remained high with normal Lp(a) values (HR 3.2, 95% CI 2.5–4.1) when compared with the reference group of subjects without FH and Lp(a) values ≤ 50 mg/dL.6 In the GENdEr and Sex determinantS of cardiovascular disease: from bench to beyond-Premature Acute Coronary Syndrome (GENESIS-PRAXY) study, including 939 individuals with premature acute coronary syndromes, patients with high Lp(a) were more likely to have high LDL-C values, with a significant synergistic interaction and an increasingly strong association for LDL-C thresholds > 3.5 and 4.5 mmol/L.7 The strength of Verbeek et al.’s study is that it expressly assessed CVD risk associated with Lp(a) levels according to different LDL-C cut-offs, with adequately powered sample sizes across all strata of the cohorts.
The results reported in Verbeek et al.’s study are valid for the primary prevention setting. Evidence is more controversial in secondary prevention and for patients on statins. In the Rosuvastatin Versus Atorvastatin (SATURN) trial, baseline and follow-up Lp(a) levels were not associated with changes in per cent atheroma volume as measured with ultrasound.8 LDL-C decreased from 114 mg/dL to 60 mg/dL, while Lp(a) remained unchanged (17.4 mg/dL and 16.5 mg/dL, respectively) with statin therapy. The cut-off of 50 mg/dL for Lp(a) was neither protective nor a risk factor for disease progression.8 These data suggest that Lp(a) is not a good marker for the estimation of residual CV risk after initiation of statin therapy. A second subanalysis of the dal-Outcomes trial, including 4139 acute coronary syndrome patients treated with statins, did not show any association between Lp(a) concentrations and major adverse outcomes: for a doubling of the dose of Lp(a), the level of risk for CVD was stagnant (HR 1.01, 95% CI 0.96–1.06, P=0.66).9 Similar results were also reported in three different pooled studies with 6708 subjects known for coronary artery disease: odds ratios (ORs) were 1.03 (95% CI 0.96–1.11) for each increase in log-transformed SD of Lp(a) or by quintile (highest vs. lowest OR 1.05, 95% CI 0.83–1.34).10
Function of Lp(a)
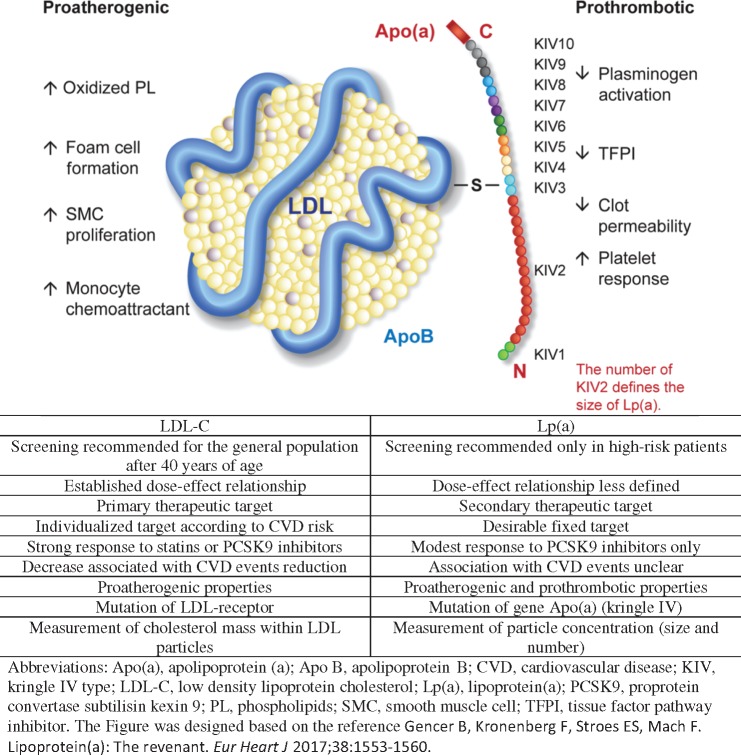
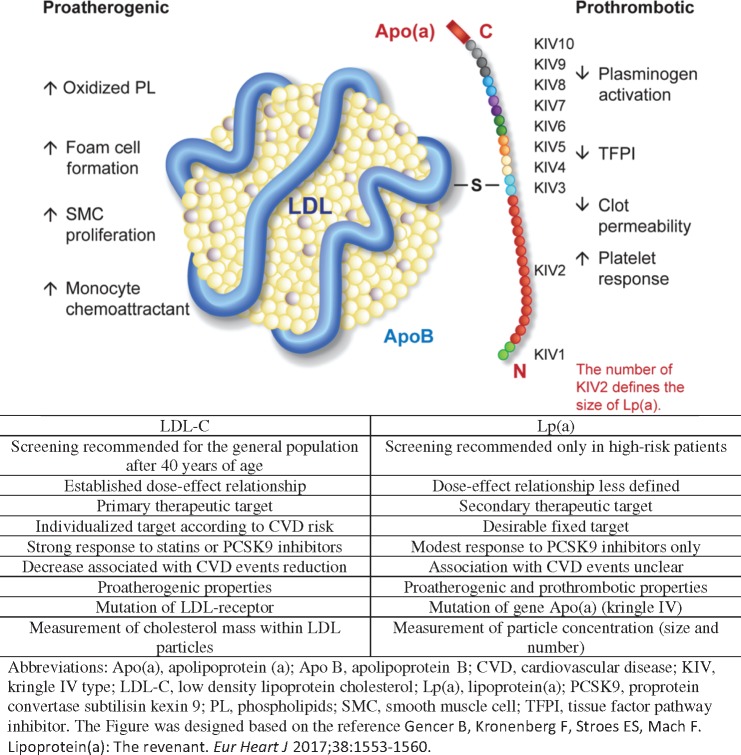
What is the difference between Lp(a) and LDL-C? This key question is addressed in Take home figure. Lp(a) particles have two major and distinct components: (i) a structure similar to an LDL particle containing Apolipoprotein (Apo) B and (ii) a glycoprotein [Apo(a)] that is similar to plasminogen.3,11 The gene variations of Lp(a) mainly determine the size of Apo(a) via the number of kringle IV repetitions and not the LDL part. Besides a pro-atherogenic effect similar to that of LDL-C, which generates inflammation or cholesterol deposition in vessel walls,12 Lp(a) has additional pro-thrombotic properties that could potentially explain the associated increase in CVD risk for the same level of LDL-C in primary prevention.3 It is unclear at which point antithrombotic treatments could attenuate this phenomenon. For this reason, and as previously mentioned, Lp(a) is not a good surrogate marker in secondary prevention, or for patients treated with statin or antithrombotic therapy.
Take home figure.
Comparison between LDL-C and lipoprotein(a).
Lp(a): what do guidelines tell us?
The 2010 consensus document on Lp(a) from the European Society of Cardiology proposed a linear association between Lp(a) levels and CVD events,13 which might be too simplistic given the level of interaction and modification that LDL-C levels appear to have on cardiovascular risk. Measurement of Lp(a) is recommended in clinical practice in the following settings: (i) premature CVD, (ii) FH, (iii) a family history of CVD or elevated Lp(a), and (iv) recurrent CVD despite optimal statin therapy and ≥ 5% 10 year risk of fatal CVD according to Systematic COronary Risk Evaluation (SCORE).2 The treatment goal for CVD patients is first to lower LDL-C levels and reach desirable Lp(a) levels < 50 mg/dL. However, to date, there is no evidence that lowering Lp(a) really does improve clinical outcomes or that this molecule is a critical target to decrease residual risk. Statin treatment is not associated with a reduction of Lp(a), as opposed to PCSK9 inhibitors, which have been demonstrated to significantly reduce Lp(a) by 20–30%. In the case of proprotein convertase subtilisin kexin 9 (PCSK9) inhibitors, Lp(a) reduction is systematically correlated with LDL-C reduction, and it is most probably the increased levels of LDL-receptor expression typically induced by PCSK9 inhibitors that lead to subsequent lowering of circulating Lp(a) levels.14 The beneficial effect of PCSK9 inhibition on clinical events therefore seems to be mediated by reductions in LDL-C levels, while it remains unclear what benefit the concomitant decrease of Lp(a) might have.15 Recently, treatment with antisense oligonucleotides targeting Apo(a) in persons with elevated Lp(a) has shown very impressive results in lowering Lp(a) levels (≥ 70–80% decrease).16,17 A clinical study of cardiovascular outcomes is now required to evaluate the impact of specifically reducing Lp(a). A phase 2B trial will start to recruit patients with elevated Lp(a), defined as Lp(a) ≥ 60 mg/dL, and established CVD to test the efficacy and safety of ISIS 681257 administered subcutaneously with a target sample size of 270 participants (ClinicalTrials.gov identifier NCT03070782).
In conclusion, for more than three decades now, Lp(a) has been explored in various mechanistic and clinical studies, but continues to take the role of perpetual supporting actor as a secondary or exploratory target, since no therapy has yet succeeded in specifically lowering Lp(a) without at the same time lowering LDL-C. Recent developments might finally change the scenario by highlighting Lp(a)’s independent role in cardiovascular risk reduction. Lp(a) might yet receive a leading actor Oscar nomination after all.
Acknowledgements
Special gratitude is expressed to Aliki Buhayer (Prism Scientific Sarl) for medical writing support. Full acknowledgement for the permission of original figure to European Heart Journal.
Funding
F.M. and B.G.’s research is supported by the Swiss National Science Foundation (SPUM 33CM30-124112 and SPUM 33CM30-140 336 ‘Inflammation and acute coronary syndromes (ACS)-Novel strategies for prevention and clinical management’, and 32473B_163271 ‘Long-term benefit of the multi-center, multi-dimensional secondary prevention program in patients with acute coronary syndromes). B.G.’s research is supported by grants from Geneva University Hospitals, the Swiss Heart Foundation, the de Reuter Foundation, the Gerbex-Bourget Foundation, and the Gustave-Prevot and Schmidheiny Foundation.
Conflict of interest: F.M. has received research grants to the institution from Amgen, AstraZeneca, Boston Scientific, Biotronik, Medtronic, MSD, Eli Lilly, Sanofi, Pfizer, and St. Jude Medical, including speaker or consultant fees.
Footnotes
The opinions expressed in this article are not necessarily those of the Editors of the European Heart Journal or of the European Society of Cardiology.
† doi:10.1093/eurheartj/ehy334.
References
- 1. Verbeek R, Hoogeveen RM, Langsted A, Stiekema LCA, Verweij SL, Hovingh GK, Wareham NJ, Khaw K-T, Boekholdt SM, Nordestgaard BG, Stroes ESG. Cardiovascular disease risk associated with elevated lipoprotein(a) attenuates at low low-density lipoprotein cholesterol levels in a primary prevention setting. Eur Heart J 2018;39:2589–2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Catapano AL, Graham I, De Backer G, Wiklund O, Chapman MJ, Drexel H, Hoes AW, Jennings CS, Landmesser U, Pedersen TR, Reiner Z, Riccardi G, Taskinen MR, Tokgozoglu L, Verschuren WMM, Vlachopoulos C, Wood DA, Zamorano JL, Cooney MT.. 2016 ESC/EAS guidelines for the management of dyslipidaemias. Eur Heart J 2016;37:2999–3058. [DOI] [PubMed] [Google Scholar]
- 3. Gencer B, Kronenberg F, Stroes ES, Mach F.. Lipoprotein(a): The revenant. Eur Heart J 2017;38:1553–1560. [DOI] [PubMed] [Google Scholar]
- 4. Erqou S, Kaptoge S, Perry PL, Di Angelantonio E, Thompson A, White IR, Marcovina SM, Collins R, Thompson SG, Danesh J.. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA 2009;302:412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kamstrup PR, Tybjaerg-Hansen A, Steffensen R, Nordestgaard BG.. Genetically elevated lipoprotein(a) and increased risk of myocardial infarction. JAMA 2009;301:2331–2339. [DOI] [PubMed] [Google Scholar]
- 6. Langsted A, Kamstrup PR, Benn M, Tybjaerg-Hansen A, Nordestgaard BG.. High lipoprotein(a) as a possible cause of clinical familial hypercholesterolaemia: A prospective cohort study. Lancet Diabetes Endocrinol 2016;4:577–587. [DOI] [PubMed] [Google Scholar]
- 7. Afshar M, Pilote L, Dufresne L, Engert JC, Thanassoulis G.. Lipoprotein(a) interactions with low-density lipoprotein cholesterol and other cardiovascular risk factors in premature acute coronary syndrome (ACS). J Am Heart Assoc 2016;5:e003012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Puri R, Ballantyne CM, Hoogeveen RC, Shao M, Barter P, Libby P, Chapman MJ, Erbel R, Arsenault BJ, Raichlen JS, Nissen SE, Nicholls SJ.. Lipoprotein(a) and coronary atheroma progression rates during long-term high-intensity statin therapy: Insights from saturn. Atherosclerosis 2017;263:137–144. [DOI] [PubMed] [Google Scholar]
- 9. Schwartz GG, Ballantyne CM, Barter PJ, Kallend D, Leiter LA, Leitersdorf E, McMurray JJV, Nicholls SJ, Olsson AG, Shah PK, Tardif JC, Kittelson J.. Association of lipoprotein(a) with risk of recurrent ischemic events following acute coronary syndrome: Analysis of the dal-outcomes randomized clinical trial. JAMA Cardiol 2018;3:164–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. O'Donoghue ML, Morrow DA, Tsimikas S, Sloan S, Ren AF, Hoffman EB, Desai NR, Solomon SD, Domanski M, Arai K, Chiuve SE, Cannon CP, Sacks FM, Sabatine MS.. Lipoprotein(a) for risk assessment in patients with established coronary artery disease. J American Coll Cardiol 2014;63:520–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schmidt K, Noureen A, Kronenberg F, Utermann G.. Structure, function, and genetics of lipoprotein (a). J Lipid Res 2016;57:1339–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. van der Valk FM, Bekkering S, Kroon J, Yeang C, Van den Bossche J, van Buul JD, Ravandi A, Nederveen AJ, Verberne HJ, Scipione C, Nieuwdorp M, Joosten LA, Netea MG, Koschinsky ML, Witztum JL, Tsimikas S, Riksen NP, Stroes ES.. Oxidized phospholipids on lipoprotein(a) elicit arterial wall inflammation and an inflammatory monocyte response in humans. Circulation 2016;134:611–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nordestgaard BG, Chapman MJ, Ray K, Boren J, Andreotti F, Watts GF, Ginsberg H, Amarenco P, Catapano A, Descamps OS, Fisher E, Kovanen PT, Kuivenhoven JA, Lesnik P, Masana L, Reiner Z, Taskinen MR, Tokgozoglu L, Tybjaerg-Hansen A.. Lipoprotein(a) as a cardiovascular risk factor: Current status. Eur Heart J 2010;31:2844–2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Raal FJ, Giugliano RP, Sabatine MS, Koren MJ, Blom D, Seidah NG, Honarpour N, Lira A, Xue A, Chiruvolu P, Jackson S, Di M, Peach M, Somaratne R, Wasserman SM, Scott R, Stein EA.. PCSK9 inhibition-mediated reduction in Lp(a) with evolocumab: An analysis of 10 clinical trials and the LDL receptor's role. J Lipid Res 2016;57:1086–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, Kuder JF, Wang H, Liu T, Wasserman SM, Sever PS, Pedersen TR.. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med 2017;376:1713–1722. [DOI] [PubMed] [Google Scholar]
- 16. Tsimikas S, Viney NJ, Hughes SG, Singleton W, Graham MJ, Baker BF, Burkey JL, Yang Q, Marcovina SM, Geary RS, Crooke RM, Witztum JL.. Antisense therapy targeting apolipoprotein(a): A randomised, double-blind, placebo-controlled phase 1 study. Lancet 2015;386:1472–1483. [DOI] [PubMed] [Google Scholar]
- 17. Viney NJ, van Capelleveen JC, Geary RS, Xia S, Tami JA, Yu RZ, Marcovina SM, Hughes SG, Graham MJ, Crooke RM, Crooke ST, Witztum JL, Stroes ES, Tsimikas S.. Antisense oligonucleotides targeting apolipoprotein(a) in people with raised lipoprotein(a): Two randomised, double-blind, placebo-controlled, dose-ranging trials. Lancet 2016;388:2239–2253. [DOI] [PubMed] [Google Scholar]