The modified vaccinia virus Ankara (MVA) H5 influenza vaccine induced immune responses that display remarkable cross-reactivity with a variety of H5 viruses. We hypothesize that this MVA-H5 vaccine could provide immunity to protect against a large variety of antigenically distinct influenza viruses of the H5 subtype.
Keywords: Modified vaccinia virus Ankara, influenza virus, cross-reactive, immunity, correlates of protection
Abstract
Background
High-pathogenicity avian influenza viruses continue to circulate in poultry and wild birds and occasionally infect humans, sometimes with fatal outcomes. Development of vaccines is a priority to prepare for potential pandemics but is complicated by antigenic variation of the surface glycoprotein hemagglutinin. We report the immunological profile induced by human immunization with modified vaccinia virus Ankara (MVA) expressing the hemagglutinin gene of influenza A(H5N1) virus A/Vietnam/1194/04 (rMVA-H5).
Methods
In a double-blinded phase 1/2a clinical trial, 79 individuals received 1 or 2 injections of rMVA-H5 or vector control. Twenty-seven study subjects received a booster immunization after 1 year. The breadth, magnitude, and properties of vaccine-induced antibody and T-cell responses were characterized.
Results
rMVA-H5 induced broadly reactive antibody responses, demonstrated by protein microarray, hemagglutination inhibition, virus neutralization, and antibody-dependent cellular cytotoxicity assays. Antibodies cross-reacted with antigenically distinct H5 viruses, including the recently emerged subtypes H5N6 and H5N8 and the currently circulating subtype H5N1. In addition, the induction of T cells specific for H5 viruses of 2 different clades was demonstrated.
Conclusions
rMVA-H5 induced immune responses that cross-reacted with H5 viruses of various clades. These findings validate rMVA-H5 as vaccine candidate against antigenically distinct H5 viruses.
Clinical Trials Registration
NTR3401.
High-pathogenicity avian influenza A viruses (IAVs) of the H5 subtype continue to cause outbreaks in poultry in various countries. Sporadically, these viruses, particularly subtypes H5N1 and H5N6, infect humans, resulting in disease of variable severity. Since the first case of human infection with IAV subtype H5N1, in 1997 [1], 859 human cases have been reported as of July 2017, of which 453 had a fatal outcome [2]. In addition, several other H5 IAV subtypes have emerged, including H5N2 [3], H5N6 [4], and H5N8 [5]. Recently, mainly the H5N6 and H5N8 subtypes have caused outbreaks in wild birds and poultry in Asia, Europe, and North America. To date, no human infections with H5N8 have been reported, but there is evidence of H5N2 infections in humans [3]. Furthermore, since 2015, severe H5N6 infections in humans have been reported sporadically [4].
Although IAVs of the H5N1, H5N2 and H5N6 subtypes can infect humans, they are not efficiently transmitted from human to human. However, studies in ferrets have shown that only a limited number of mutations in the genes encoding the viral proteins hemagglutinin (HA) and polymerase subunit PB2 are required to facilitate airborne transmission of H5N1 [6, 7]. If these viruses acquire the capacity to be transmitted from human to human, they can potentially cause a pandemic, because virus-neutralizing (VN) antibodies to these viruses are virtually absent in the human population.
Vaccination is the most important measure to prevent IAV infection. The availability of an effective H5 vaccine would be pivotal in preventing severe disease and mortality in case of a pandemic. A rapid vaccination response could interrupt the transmission chain of H5 viruses; however, the latest IAV pandemic, in 2009, showed that this remains a challenge. Although a mock-up dossier that allows for fast-track licensing of inactivated influenza vaccines has been drafted in Europe [8], vaccines became available too late in most countries during this pandemic [9]. In addition to problems with production and distribution of sufficient vaccine doses during the earliest stages of an outbreak, conventional H5N1 vaccine formulations suffer from intrinsically low immunogenicity, compared with seasonal influenza vaccines. To overcome this problem, repeated vaccinations, increased antigen doses or use of adjuvants are required [10]. Furthermore, H5 vaccine development is complicated by the genetic diversification of H5 viruses into various clades and subclades, leading to increasing antigenic diversity of HA [11]. Collectively, this illustrates the need for novel (pre-)pandemic H5 vaccines that can be produced rapidly on a large scale and ideally confer broad protection against antigenically distinct H5 viruses.
Vector-based H5 influenza vaccines, such as those based on modified vaccinia virus Ankara (MVA), potentially fulfill the needs described above. Since generation of recombinant MVA (rMVA) by insertion of genes encoding antigens of interest into the viral genome is relatively easy, rMVA vaccine candidates have been developed for numerous infectious diseases, including influenza [12]. rMVA drives endogenous antigen production in cells infected by the vector, leading to efficient antigen processing and presentation and, subsequently, to induction of antigen-specific B- and T-cell responses. Furthermore, many viral vectors, including MVA, allow for rapid and large-scale production of vaccine doses. MVA has an excellent safety record, as was demonstrated in various animal models [12] and in >100000 individuals [13].
Previously, rMVA expressing the HA gene of the clade 1 IAV A/Vietnam/1194/04 (VN/04; rMVA-H5) was generated and proved to be safe and immunogenic in preclinical studies [14, 15], offering protection from either a lethal homologous or heterologous challenge infection [14]. Subsequently, rMVA-H5 was assessed in a double-blinded phase 1/2a clinical trial and proved to be safe in humans [16]. The immunogenicity of rMVA-H5 was solely assessed by assays to detect hemagglutination-inhibiting (HI) and VN antibodies against the homologous virus and the following 2 heterologous viruses: clade 2.1 A/Indonesia/5/05 (IND/05) and clade 2.3.4.4 H5N8 A/chicken/Netherlands/EMC-3/14 (ch/H5N8/14) [16, 17]. To achieve broadly protective immunity by vaccination, induction of correlates of protection other than HI and VN antibodies are considered essential [18]. Here, we report a detailed analysis of (non)neutralizing antibody and T-cell responses induced by immunization of humans with rMVA-H5, with emphasis on cross-reactivity with H5 viruses of currently circulating antigenically distinct clades.
METHODS
Ethics Statement
The study design was reviewed and approved by the Dutch Central Committee on Research Involving Human Subjects, as described previously [16].
Study Design
The randomized, double-blind phase 1/2a study was conducted at the Erasmus MC, Rotterdam, the Netherlands [16]. Seventy-nine healthy adult volunteers were included in the study. Individuals were randomly assigned to 1 of 8 groups on the basis of the number of immunizations (1 or 2), the immunization dose (107 or 108 plaque-forming units [PFU]), and the vaccine administered (rMVA-H5 or rMVA-F6 [empty vector]). Each group consisted of 10 individuals. The group receiving a single dose of 108 PFU of rMVA-H5 consisted of 9 individuals, owing to discontinuation of the study by a single individual. Immunizations were administered as intramuscular injections during the first (week 0) and second (week 4) visit. Blood samples were obtained before and 4 and 8 weeks after the initial immunization. To assess whether the H5-specific immune response could be boosted by an additional immunization, 27 individuals who received the rMVA-H5 vaccine during the main study received an additional immunization 1 year after the first immunization (ie, during week 52). Blood specimens were collected at weeks 52, 56, and 72. Serum and peripheral blood mononuclear cells (PBMCs) were obtained as described previously [16].
Protein Array Assay
Sera were probed for the presence of antibodies directed to a large panel of antigens by a protein array assay as described previously [19]. Protein array titers against 18 different H5 antigens were determined, in addition to an H1 and H3 antigen, using recombinant HA1 protein (containing mainly the globular head and part of the stem) from various IAVs (Table 1).
Table 1.
Antigens Used in Various Assays
| IAV Strain | Abbreviation | IAV Subtype | Clade | Pathogenicity | Assay(s)a |
|---|---|---|---|---|---|
| A/NewCaledonia/20/1999 | sH1N1 | H1N1 | … | Seasonal | PA |
| A/Wyoming/3/03 | sH3N2 | H3N2 | … | Seasonal | PA |
| A/duck/Hokkaido/167/07 | H5N3/07 | H5N3 | … | LPAI | PA |
| A/duck/NY/191255–59/02 | H5N8/02 | H5N8 | … | LPAI | PA |
| A/HongKong/156/97 | HK/97 | H5N1 | 0 | HPAI | PA |
| A/Vietnam/1194/04 | VN/04 | H5N1 | 1 | HPAI | PA, HI, VN, ADCC, T cell |
| A/Cambodia/R0405050/07 | CAM/07 | H5N1 | 1.1 | HPAI | PA |
| A/Indonesia/5/05 | IND/05 | H5N1 | 2.1 | HPAI | PA, HI, VN, ADCC, T cell |
| A/duck/Hunan/795/02 | HUN/02 | H5N1 | 2.1.3.2 | HPAI | PA |
| A/Turkey/15/06 | TUR/06 | H5N1 | 2.2 | HPAI | PA |
| A/Egypt/N03072/10 | Egypt/10 | H5N1 | 2.2.1 | HPAI | PA |
| A/chicken/Egypt/0879-NLQP/08 | Egypt/08 | H5N1 | 2.2.1.1 | HPAI | PA |
| A/Egypt/N01753/14 | Egypt/14 | H5N1 | 2.2.1.2 | HPAI | HI and VN |
| A/Hubei/1/10 | Hubei/10 | H5N1 | 2.3.2.1 | HPAI | PA |
| A/duck/Bangladesh/19097/13 | BANG/13 | H5N1 | 2.3.2.1 | HPAI | HI and VN |
| A/Anhui/1/05 | Anhui/05 | H5N1 | 2.3.4 | HPAI | PA |
| A/Vietnam/HN31432M/08 | VN/08 | H5N1 | 2.3.4.2 | HPAI | PA |
| A/turkey/Germany-MV/R2472/14 | tu/H5N8/14 | H5N8 | 2.3.4.4 | HPAI | PA |
| A/Guangzhou/39715/14 | H5N6/14 | H5N6 | 2.3.4.4 | HPAI | HI and VN |
| A/chicken/Netherlands/EMC-3/14 | ch/H5N8/14 | H5N8 | 2.3.4.4 | HPAI | HI and VN |
| A/chicken/Yamaguchi/7/04 | YAM/04 | H5N1 | 2.5 | HPAI | PA |
| A/goose/Guiyang/337/06 | GUI/06 | H5N1 | 4 | HPAI | PA |
| A/chicken/Vietnam/NCVD-016/08 | ch/VN/08 | H5N1 | 7 | HPAI | PA |
| A/chicken/Jilin/9/04 | JI/04 | H5N1 | … | HPAI | PA |
Abbreviations: ADCC, antibody-dependent cellular cytotoxicity; HI, hemagglutination inhibition; HPAI, high-pathogenicity avian influenza virus; IAV, influenza A virus; LPAI, low-pathogenicity avian influenza virus; PA, protein array; VN, virus neutralization.
aHemagglutinin subunit 1 (HA1) was used in PA analysis, viruses created using reverse-genetics techniques were used for HI/VN assays, and full-length HA was used for ADCC and T-cell assays.
Phylogenetic Analysis
Phylogenetic trees of IAVs were constructed on the basis of nucleotide sequences encoding the HA1 unit of HA. Multiple sequence alignment was performed using the MUSCLE algorithm integrated into MEGA 6.06. The phylogenetic tree was drawn according to the maximum likelihood method, using the best-fit model, in MEGA 6.06. Bootstrap analyses with 1000 resamplings were performed to determine confidence values for groupings within the phylogenetic tree. The tree was visualized in FigTree, version 1.3.1.
Detection of HI and VN Antibodies
Sera were thawed and HI and VN antibody titers against 6 different viruses (VN/04, IND/05, BANG/13, Egypt/14, H5N6/14, and ch/H5N8/14; Table 1) were determined as described previously [16, 20]. All VN assays were performed simultaneously, with the exception of analysis of VN antibodies to ch/H5N8/14, which was performed separately. H5 viruses used were obtained by reverse genetics and contained the HA gene segment only (Egypt/14 and BANG/13) or the HA and the neuraminidase gene segments with the remaining 7 or 6 gene segments of IAV A/Puerto Rico/8/34. None of the viruses contained a multibasic cleavage site. Sera from uninfected ferrets or ferrets inoculated with the homologous H5 viruses were included as negative and positive controls, respectively.
Antibody-Dependent Cellular Cytotoxicity (ADCC) Assay
A solid-phase ADCC assay was performed as described previously [21], using full-length recombinant HA to detect the presence of ADCC-mediating antibodies specific for VN/04 and IND/05 (Table 1).
Interferon γ (IFN-γ) Enzyme-Linked Immunospot (ELISpot) Assay
VN/04 and ID/05 HA-specific IFN-γ responses were assessed in PBMCs collected at week 0, 4, 8, 52, and 56 by performing an ELISpot assay according to the manufacturer’s instructions (Mabtech). Briefly, PBMCs were thawed, seeded into 96-well round-bottomed plates at 125000 cells/well (fewer cells were used if necessary), and stimulated overnight with 500 ng of full-length recombinant HA per well (Protein Sciences; Table 1). These antigen-presenting cells were subsequently cocultured with autologous, unstimulated PBMCs in a 1:1 ratio for 22–24 hours. The average number of spots per 125000 responder cells cultured in triplicate was determined using a CTL Immunospot reader with CTL Biospot software.
CD137 Expression on T Cells
PBMCs collected at weeks 0, 4, 8, 52, and 56 were thawed and seeded into 96-well round-bottomed plates at 200000 cells/well. Cells were stimulated for 20 hours with 1 μg of full-length recombinant HA from IAV VN/04 or IND/05 per well (Table 1). Cells were stained with CD3APC-Cy7 (Biolegend), CD4PerCP (Becton Dickinson), CD8PE-Cy7 (eBioscience), and CD137PE (Miltenyi Biotec); acquired on a FACS Canto II; and analyzed using DIVA software (BD Biosciences).
Statistical Analysis
All rMVA-H5 vaccinated individuals were initially analyzed as a single group, despite different dosing regimens. For ELISpot analysis and assays to detect HI and VN antibodies, ADCC, and CD137 expression, a normal distribution was not demonstrated by the D’Agostino Pearson normality test. The Friedman test was therefore used to evaluate paired samples. Additionally, different treatment groups were compared using a Kruskal-Wallis test (distributions were nonnormal among HI and VN antibody, ELISpot, and ADCC data for weeks 52, 56, and 72) or a 1-way analysis of variance (distributions of ADCC data were normal for weeks 0, 4, and 8).
RESULTS
H5-Specific Antibodies Induced by rMVA-H5 Immunization Showed Broad Reactivity
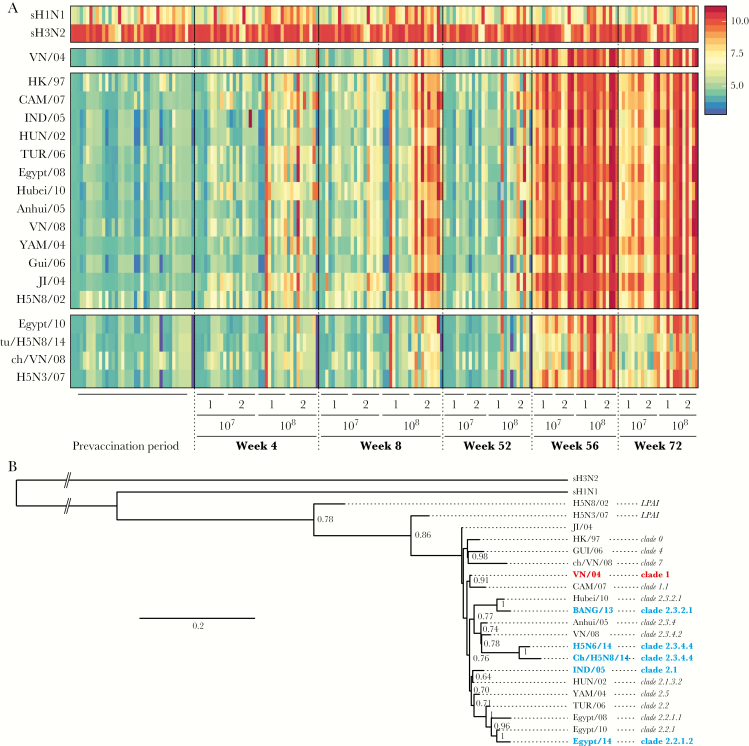
The profile of antibody binding to HA1 derived from 18 different H5 IAVs of various clades was determined by a protein array assay. HA1 derived from sH1N1 and sH3N2 IAVs were included on the array as controls, and reactivity to these antigens was similar between all groups and time points (Figure 1A). H5-specific antibodies were only detected at a background level before vaccination, but H5-specific antibodies were readily detected at week 8, particularly in subjects who received 2 high-dose immunizations (108 PFU each). H5-specific antibodies were still detectable at week 52, albeit at lower titers and only in recipients of 108 PFU of rMVA-H5. Interestingly, 4 weeks after the booster immunization, H5-binding antibodies were detected in all subjects (Figure 1A). In subjects who received the low vaccine dose (ie, 107 PFU), antibody levels waned but remained detectable 20 weeks after the booster (ie, on week 72). In contrast, high levels of H5-specific antibodies were detected in subjects immunized with a high dose (Figure 1A). Antibodies induced by rMVA-H5 bound not only to HA1 of the homologous IAV strain VN/04, but also to virtually all other H5 antigens tested (Figure 1A). In general, the H5 antibody binding profiles could be subdivided into 2 clusters; antibody responses against 13 H5 antigens from various clades showed a binding profile similar to that of the homologous VN/04 antigen. Weaker antibody binding to the HA1 from VN/08, tu/H5N8/14, H5N3/07, and Egypt/08 was observed (Figure 1A).
Figure 1.
Vaccination with modified vaccinia virus Ankara (MVA) expressing the hemagglutinin gene of influenza A(H5N1) virus A/Vietnam/1194/04 (rMVA-H5) induced a broadly reactive antibody response profile. A, Protein array analysis was used to determine titers against hemagglutinin subtype 1 (HA1) from 18 antigenically distinct H5 influenza A viruses in sera obtained at weeks 0, 4, 8, 52, 56, and 72 after vaccination. The seasonal influenza A viruses sH1N1 and sH3N2 were included as a control. Each column represents an individual serum sample (individual samples are ordered identically within each time point), and each row represents an antigen. The key indicates log2-transformed titers from protein array analysis, with blue indicating a low titer and red indicating a high titer. B, Phylogenetic tree of influenza virus antigens used in this study based on the HA1 domain of the HA gene. The maximum-likelihood tree was constructed using the best-fit model (HKY+G) in MEGA 6.06 and visualized in FigTree, version 1.3.1 (available at: http://tree.bio.ed.ac.uk/software/figtree). Bootstrap values were calculated from 1000 replicates; only values >0.6 are shown. Values indicate numbers of nucleotide substitutions per site. Red indicates the homologous virus strain, and blue indicates viruses used in follow-up experiments.
Genetic relationships between the antigens used in various assays were determined by performing a phylogenetic analysis based on the HA1 domain of the gene encoding IAV HA (Figure 1B). Among the H5 viruses, the 2 low-pathogenicity avian influenza viruses clustered separately, whereas all high-pathogenicity avian influenza H5 viruses clustered together as expected to their respective clades. Notably, the 2 antigenic clusters determined by a protein array assay (Figure 1A) were not necessarily defined by differences in genetic clades (Figure 1B). Collectively, antibody responses induced by rMVA-H5 immunization showed strong cross-reactivity to H5 viruses from antigenically and genetically diverse clades.
rMVA-H5 Induced Cross-reactive HI and VN Antibodies Against H5N1, H5N6, and H5N8 IAVs
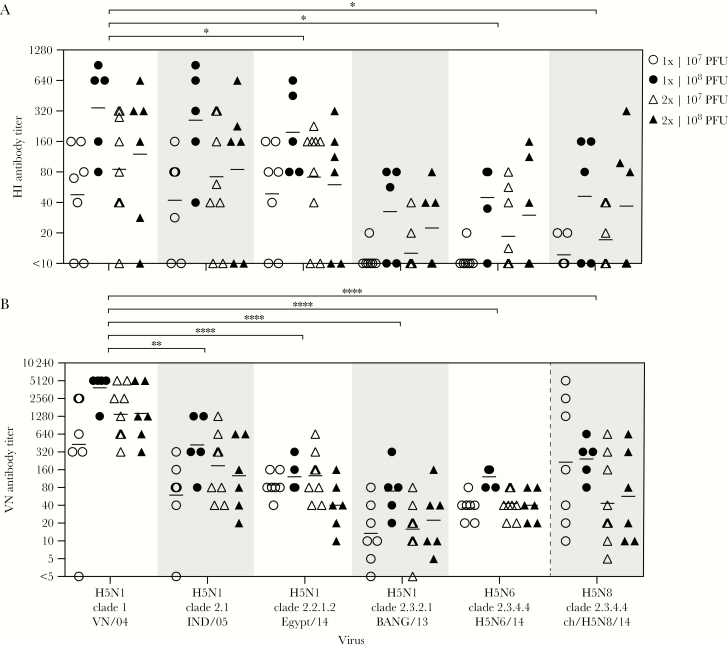
To test the functionality of rMVA-H5-induced cross-reactive antibodies, sera collected at week 56 were used in HI (Figure 2A) and VN (Figure 2B) antibody assays. Antibody titers were never detected using control serum from an uninfected ferret, whereas control sera from ferrets infected with the homologous virus showed a positive response (data not shown). Antibody reactivity with the homologous strain, VN/04, and the heterologous strains, IND/05 and ch/H5N8/14, was tested previously [16, 17] and was confirmed in the present study. In addition, the activity against Egypt/14 and BANG/13, which are among the H5N1 vaccine strains selected by the World Health Organization on the basis of current circulation data, and H5N6/14 (recently causing human infections) was tested.
Figure 2.
Vaccination with modified vaccinia virus Ankara (MVA) expressing the hemagglutinin gene of influenza A(H5N1) virus A/Vietnam/1194/04 (rMVA-H5) induced hemagglutination-inhibiting (HI) and virus-neutralizing (VN) antibodies reactive with homologous and antigenically distinct H5 viruses. Sera (from 7 individuals who received 1 dose of 107 plaque-forming units [PFU], 5 who received 1 dose of 108 PFU, 9 who received 2 doses of 107 PFU each, and 6 who received 2 doses of 108 PFU each) obtained 4 weeks after the booster immunization (ie, during week 56) were tested for the presence of HI (A) and VN (B) antibodies reactive with the homologous VN/04 virus and 5 antigenically distinct H5 viruses. A, Reciprocal HI antibody titers obtained by a 2-fold serum dilution series. Sera without HI antibody activity or a HI antibody titer of 10 were assigned a titer of <10. *P < .0001 as compared to the reference virus, VN/04 and BANG/13. B, Reciprocal VN antibody titers obtained by a 2-fold serum dilution series, incubated with 1000 PFU of influenza virus. Sera without VN antibody activity were set at a titer of <5. **P = .0036 and ****P < 0001, compared with the reference virus, VN/04. The key indicates the treatment groups; geometric mean titers are displayed as bars. Assays were performed at least 2 times, and representative results of the assays are shown.
HI antibodies specific for the homologous VN/04 virus were detected, which displayed considerable cross-reactivity with the IND/05 and Egypt/14 viruses. Cross-reactivity with the viruses BANG/13, H5N6/14, and ch/H5N8/14 was observed to a lesser extent (Figure 2A). Although differences between treatment groups were not statistically significant, study subjects who received a high vaccine dose displayed the highest HI antibody titers.
Similar findings were obtained in the VN antibody assay performed with the same 6 IAVs (Figure 2B). Serum antibodies efficiently neutralized the homologous VN/04 virus and cross-neutralized the clade 2.1, 2.2.1.2, 2.3.2.1, and 2.3.4.4 viruses. In accordance with the HI antibody responses, higher cross-neutralizing antibody titers were observed against IND/05 and Egypt/14 as compared to BANG/13, H5N6/14, and ch/H5N8/14 (Figure 2B). Taken together, serum antibodies induced by immunization with rMVA-H5 are broadly reactive and display cross-neutralizing capacity.
rMVA-H5 Immunization Induced ADCC-Mediating Antibodies
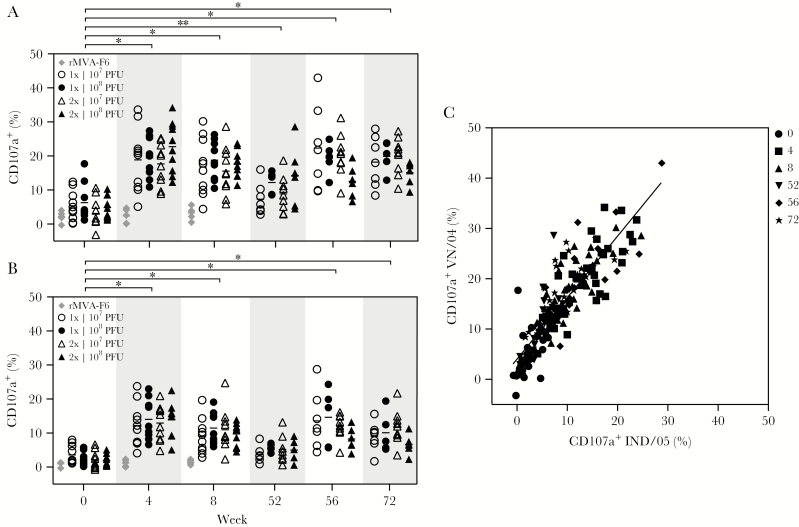
Sera from vaccinated individuals were tested for the presence of ADCC-mediating antibodies. ADCC activity was never observed, based on analysis of control sera obtained from rMVA-F6 immunized individuals (Figure 3A and 3B). A single immunization with rMVA-H5 induced a significant rise in ADCC activity against HA of the homologous virus, VN/04 (Figure 3A), and also the heterologous virus IND/05 (Figure 3B), albeit to a lower extent. The level of ADCC-mediating antibodies was not boosted by a second immunization and was maintained until week 8. Levels of ADCC-mediating antibodies waned at week 52 but were still significantly higher than those at week 0 for the homologous strain, VN/04. Four weeks after the booster immunization (ie, during week 56), ADCC activity substantially increased and showed limited waning over the following 20 weeks (ie, through week 72; Figure 3A). The ADCC-mediating antibody responses were comparable between the vaccine groups, regardless of the dose and number of immunizations the subjects received. The ADCC response to HA of VN/04 and IND/05 correlated significantly, confirming the cross-reactivity of the ADCC antibody response (Figure 3C).
Figure 3.
Presence of antibody-dependent cellular cytotoxicity (ADCC)–mediating antibodies reactive with a homologous and an antigenically distinct H5N1 virus. A and B, Presence of ADCC-mediating antibodies is expressed as a percentage of degranulating natural killer cells at a set serum dilution. Sera obtained at the indicated time points were tested against VN/04 (A) and IND/05 (B). *P < 0001 and **P = .0342, compared with values at the reference time point, week 0. Keys indicate the treatment groups; geometric mean titers are displayed as bars. C, Correlation between ADCC-mediating antibodies specific for VN/04 and IND/05. The key indicates weeks during which specimens were obtained for analysis. Linear regression r2 = 0.7584. PFU, plaque-forming units.
rMVA-H5 Immunization Induced HA-specific T-cell Responses
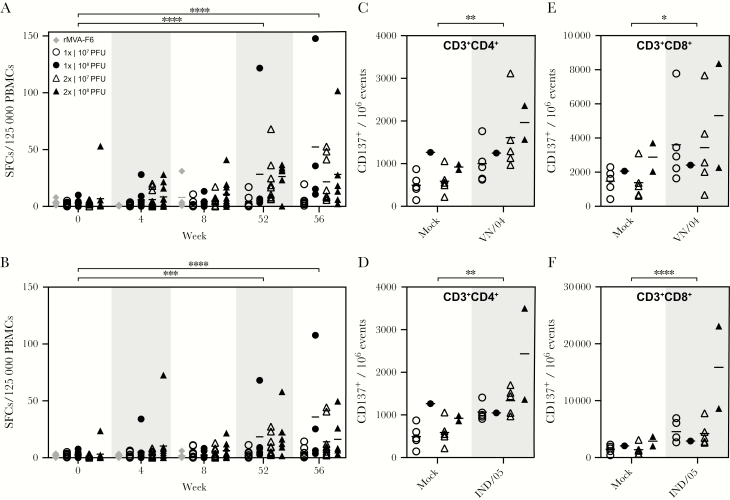
Induction of HA-specific T-cell responses was assessed by an IFN-γ ELISpot assay, using PBMCs obtained at specified time points. As expected, H5-specific T cells were not detected prior to immunization with rMVA-H5, with the exception of 1 subject (Figure 4A and 4B). At all time points after immunization, T cells stimulated with full-length HA from VN/04 or IND/05 showed significantly higher numbers of IFN-γ SFCs as compared to mock-stimulated cells (data not shown). Four weeks after the first immunization, T cells specific for HA derived from the strain used in the vaccine were detected, particularly in study subjects who received a high vaccine dose (P = not significant). The number of HA-specific T cells did not increase after a second immunization (week 8), and only limited cross-reactivity with the heterologous IND/05 was observed (Figure 4A and 4B). Interestingly, significantly higher numbers of T cells specific for VN/04 or IND/05 were detected prior to the booster immunization at week 52, compared with values at week 0 or after mock stimulation. After the booster immunization, the number of H5-specific T cells increased, and substantial cross-reactivity was observed. A trend toward stronger T-cell responses in study subjects who received the high dose of rMVA-H5 was detected at weeks 52 and 56 (Figure 4A and 4B).
Figure 4.
Vaccination with modified vaccinia virus Ankara (MVA) expressing the hemagglutinin gene of influenza A(H5N1) virus A/Vietnam/1194/04 (rMVA-H5) induced HA-specific T-cell responses specific for homologous and an antigenically distinct H5 virus. A and B, The presence of hemagglutinin (HA)–specific T cells among peripheral blood mononuclear cells (PBMCs) obtained at specified time points was tested by an interferon γ (IFN-γ) enzyme-linked immunospot assay. PBMCs were stimulated with purified HA from VN/04 (A) or IND/05 (B). Results are shown as number of spot-forming cells (SFCs) per 125 000 PBMCs. PFU, plaque-forming units. ***P = .0005 and ****P < 0001. C–F, CD3+CD4+ (C and D) or CD3+CD8+ (E and F) T-cell activation at week 56 was measured by determining CD137 expression after mock treatment or stimulation with full-length HA from VN/04 or IND/05. The key indicates the treatment groups; mean values are displayed as bars. *P = .0181, **P = .0098, and ****P < 0001.
In addition to detection of IFN-γ production by an ELISpot assay, antigen-specific T-cell activation at week 56 was measured by flow cytometry after in vitro stimulation, to determine the magnitude of CD137 induction. Compared with mock-stimulated PBMCs, a significant increase in CD137-expressing CD4+ and CD8+ T cells was detected after stimulation with HA from either VN/04 or IND/05 (Figure 4C–F).
DISCUSSION
In this study, we performed a comprehensive immunological analysis of samples obtained during a phase 1/2a clinical trial [16] of a (pre-)pandemic H5 vaccine based on the replication-deficient viral vector MVA. Broad reactivity of antibody responses induced by rMVA-H5 was initially demonstrated by a protein array assay. This technique is valuable, since it allows detection of antibody responses against a large panel of antigens in a high-throughput fashion with minute amounts of serum. However, the assay was not designed to detect antibodies to the conserved HA stalk, since only the HA1 subunit was used for coating slides and therefore, antibodies to the variable head domain were predominantly detected. Nevertheless, induction of cross-(sub)clade antibodies recognizing the HA head of the homologous vaccine strain (clade 1) and of viruses belonging to clades 0, 1.1, 2.1, 2.1.3.2, 2.2, 2.2.1, 2.2.1.1, 2.3.2.1, 2.3.4, 2.3.4.2, 2.3.4.4, 2.5, 4, and 7 was detected by a protein array assay. The reactivity detected by the protein array assay did not necessarily reflect genetic H5 clades, indicating that there is a clear distinction between antigenic and genetic differences. Broadly reactive antibodies were predominantly detected after the booster immunization. These results correspond well to data from the original study [16], which showed that low levels of HI and VN antibodies were induced after the first and second immunization and that the booster immunization at week 52 resulted in a major increase in antibody titers.
The functional activity of rMVA-H5-induced antibodies was confirmed using a selection of viruses. For conventional inactivated influenza vaccines, an HI titer of ≥40 is considered to provide protection in vivo [22]. In this study, induction of neutralizing antibodies against the homologous VN/04 virus and the heterologous IND/05 and ch/H5N8/14 viruses by rMVA-H5 immunization was observed, as was described previously [16, 17]. In addition, cross-neutralization against a recently emerged H5N6 virus and 2 H5N1 vaccine strains selected by the World Health Organization on the basis of current circulation data was observed here. Collectively, these data demonstrate that rMVA-H5 immunization induces antibodies that are capable of neutralizing recently emerged high-pathogenicity avian influenza A virus subtypes H5N1, H5N6, and H5N8.
Antibodies directed to antigenic sites in proximity of the receptor-binding site have the capacity to directly neutralize IAVs. In addition to neutralization, virus-specific antibodies, particularly IgG1 and IgG3, can have other modes of action, such as ADCC [23, 24]. The ADCC response against IAV is mainly mediated by the interaction between the Fc region of virus-specific antibodies and Fcγ-receptor IIIα (FcγRIIIα [CD16]), present on natural killer cells. It was recently shown that particularly antibodies specific to the HA stalk are capable of mediating ADCC [25–27]. Since the HA stalk is conserved among different subtypes of IAVs, ADCC-mediating antibodies may contribute to broadly protective immunity [28, 29]. Here, ADCC-mediating antibodies against the homologous virus were detected. Although sera were tested against only a single heterologous H5N1 IAV, cross-reactive ADCC-mediating antibodies were detected in this study after a single vaccination with rMVA-H5. This rapid onset, compared with that of HI or VN antibodies, can be explained by the boosting of preexisting ADCC-mediating antibodies specific to the HA stalk of seasonal IAVs.
In addition to antibody responses, efficient induction of T-cell responses specific to the homologous VN/04 and a single heterologous IND/05 IAV by rMVA-H5 immunization was observed by IFN-γ ELISpot analysis and CD137 flow cytometry. H5-specific T-cell responses were low after the initial immunizations, but they were readily detected directly before and after the booster immunization 1 year later, particularly in subjects who received 2 previous immunizations and/or a vaccine dose of 108 PFU.
In summary, we showed that rMVA-H5 efficiently induced humoral and cellular immune responses in humans that displayed remarkable cross-reactive responses to H5 viruses of various clades. Cross-reactivity was especially demonstrated by protein array, HI antibody, and VN antibody assays, but cross-reactive ADCC-mediating antibodies and T-cell responses recognizing a limited number of heterologous viruses were also observed. One or 2 immunizations with rMVA-H5 at a 4-week interval induced moderate immune responses, but primed individuals showed potent cross-reactive H5-specific antibody and T-cell responses upon booster immunization administered after 1 year. Therefore, priming of immunologically naive individuals by prepandemic immunization with rMVA-H5 and subsequent boosting after the emergence of a pandemic H5 virus seems an attractive approach in the face of a pandemic outbreak with these viruses.
Notes
Financial support. This work was supported by the European Research Council (grant FLUPLAN; project 250136); the European Union (grant FLUNIVAC [project 602604]); the Biomedical Advanced Research and Development Authority, Office of the Assistant Secretary for Preparedness and Response, Department of Health and Human Services (contract HHSO100201500033C); and the National Institute of Allergy and Infectious Diseases, National Institutes of Health (contract HHSN272201400008C).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. de Jong JC, Claas EC, Osterhaus AD, Webster RG, Lim WL. A pandemic warning?Nature 1997; 389:554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization (WHO). Cumulative number of confirmed human cases for avian influenza A(H5N1) reported to WHO, 2003–2017. Geneva: WHO, 2017. [Google Scholar]
- 3. Wu HS, Yang JR, Liu MT, Yang CH, Cheng MC, Chang FY. Influenza A(H5N2) virus antibodies in humans after contact with infected poultry, Taiwan, 2012. Emerg Infect Dis 2014; 20:857–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yang ZF, Mok CK, Peiris JS, Zhong NS. Human Infection with a Novel Avian Influenza A(H5N6) Virus. N Engl J Med 2015; 373:487–9. [DOI] [PubMed] [Google Scholar]
- 5. Zhao K, Gu M, Zhong L, et al. . Characterization of three H5N5 and one H5N8 highly pathogenic avian influenza viruses in China. Vet Microbiol 2013; 163:351–7. [DOI] [PubMed] [Google Scholar]
- 6. Herfst S, Schrauwen EJ, Linster M, et al. . Airborne transmission of influenza A/H5N1 virus between ferrets. Science 2012; 336:1534–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Linster M, van Boheemen S, de Graaf M, et al. . Identification, characterization, and natural selection of mutations driving airborne transmission of A/H5N1 virus. Cell 2014; 157:329–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. European Medicines Agency. Authorization procedures. http://www.ema.europa.eu/ema/index.jsp?curl=pages/special_topics/q_and_a/q_and_a_detail_000080.jsp. Accessed 17 March 2014.
- 9. Skowronski DM, Janjua NZ, De Serres G, et al. . Effectiveness of AS03 adjuvanted pandemic H1N1 vaccine: case-control evaluation based on sentinel surveillance system in Canada, autumn 2009. BMJ 2011; 342:c7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Couch RB, Decker WK, Utama B, et al. . Evaluations for in vitro correlates of immunogenicity of inactivated influenza a H5, H7 and H9 vaccines in humans. PLoS One 2012; 7:e50830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Smith GJ, Donis RO, World Health Organization/World Organisation for Animal Health/Food and Agriculture Organization (WHO/OIE/FAO) H5 Evolution Working Group Nomenclature updates resulting from the evolution of avian influenza A(H5) virus clades 2.1.3.2a, 2.2.1, and 2.3.4 during 2013–2014. Influenza Other Respir Viruses 2015; 9:271–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Altenburg AF, Kreijtz JH, de Vries RD, et al. . Modified vaccinia virus ankara (MVA) as production platform for vaccines against influenza and other viral respiratory diseases. Viruses 2014; 6:2735–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stickl HA. Smallpox vaccination and its consequences: first experiences with the highly attenuated smallpox vaccine “MVA”. Prev Med 1974; 3:97–101. [DOI] [PubMed] [Google Scholar]
- 14. Kreijtz JH, Suezer Y, van Amerongen G, et al. . Recombinant modified vaccinia virus Ankara-based vaccine induces protective immunity in mice against infection with influenza virus H5N1. J Infect Dis 2007; 195:1598–606. [DOI] [PubMed] [Google Scholar]
- 15. Kreijtz JH, Suezer Y, de Mutsert G, et al. . Preclinical evaluation of a modified vaccinia virus Ankara (MVA)-based vaccine against influenza A/H5N1 viruses. Vaccine 2009; 27:6296–9. [DOI] [PubMed] [Google Scholar]
- 16. Kreijtz JH, Goeijenbier M, Moesker FM, et al. . Safety and immunogenicity of a modified-vaccinia-virus-Ankara-based influenza A H5N1 vaccine: a randomised, double-blind phase 1/2a clinical trial. Lancet Infect Dis 2014; 14:1196–207. [DOI] [PubMed] [Google Scholar]
- 17. de Vries RD, De Gruyter HL, Bestebroer TM, et al. . Induction of influenza (H5N8) antibodies by modified vaccinia virus Ankara H5N1 vaccine. Emerg Infect Dis 2015; 21:1086–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Paules CI, Marston HD, Eisinger RW, Baltimore D, Fauci AS. The pathway to a universal influenza vaccine. Immunity 2017; 47:599–603. [DOI] [PubMed] [Google Scholar]
- 19. Koopmans M, de Bruin E, Godeke GJ, et al. . Profiling of humoral immune responses to influenza viruses by using protein microarray. Clin Microbiol Infect 2012; 18:797–807. [DOI] [PubMed] [Google Scholar]
- 20. McCullers JA, Van De Velde LA, Allison KJ, Branum KC, Webby RJ, Flynn PM. Recipients of vaccine against the 1976 “swine flu” have enhanced neutralization responses to the 2009 novel H1N1 influenza virus. Clin Infect Dis 2010; 50:1487–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. de Vries RD, Nieuwkoop NJ, Pronk M, et al. . Influenza virus-specific antibody dependent cellular cytoxicity induced by vaccination or natural infection. Vaccine 2017; 35:238–47. [DOI] [PubMed] [Google Scholar]
- 22. Hobson D, Curry RL, Beare AS, Ward-Gardner A. The role of serum haemagglutination-inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. J Hyg (Lond) 1972; 70:767–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bruhns P, Iannascoli B, England P, et al. . Specificity and affinity of human Fcgamma receptors and their polymorphic variants for human IgG subclasses. Blood 2009; 113:3716–25. [DOI] [PubMed] [Google Scholar]
- 24. Jegaskanda S, Laurie KL, Amarasena TH, et al. . Age-associated cross-reactive antibody-dependent cellular cytotoxicity toward 2009 pandemic influenza A virus subtype H1N1. J Infect Dis 2013; 208:1051–61. [DOI] [PubMed] [Google Scholar]
- 25. He W, Tan GS, Mullarkey CE, et al. . Epitope specificity plays a critical role in regulating antibody-dependent cell-mediated cytotoxicity against influenza A virus. Proc Natl Acad Sci U S A 2016; 113:11931–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Leon PE, He W, Mullarkey CE, et al. . Optimal activation of Fc-mediated effector functions by influenza virus hemagglutinin antibodies requires two points of contact. Proc Natl Acad Sci U S A 2016; 113:E5944–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. de Vries RD, Nieuwkoop NJ, van der Klis FRM, Koopmans MPG, Krammer F, Rimmelzwaan GF. Primary human influenza B virus infection induces cross-lineage HA-stalk-specific antibodies mediating antibody dependent cellular cytoxicity. J Infect Dis 2017; 217:3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. de Vries RD, Rimmelzwaan GF. Viral vector-based influenza vaccines. Hum Vaccin Immunother 2016; 12:2881–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Krammer F, Palese P. Influenza virus hemagglutinin stalk-based antibodies and vaccines. Curr Opin Virol 2013; 3:521–30. [DOI] [PMC free article] [PubMed] [Google Scholar]