Abstract
Carbendazim is nowadays widely used to control fungus in various nectariferous crops. Little is known about how honey bees, Apis mellifera L. (Hymenoptera: Apidae), respond to carbendazim exposure. In this study, the effects of field-realistic concentrations of carbendazim (4.516, 0.4516, and 0.04516 ppm) on the survival, biomarker enzyme activity (AChE, GST, CarE, and P450), and four antimicrobial peptide gene expression (hymenoptaecin, defensin, apidaecin, and abaecin) in forager honey bees were evaluated. The forager bees were fed with the pesticides for 10 d. The results showed that the field-realistic concentrations of carbendazim did not affect survival; activities of AChE, GST, and CarE; and expression levels of defensin and abaecin in forager bees. However, 4.516, 0.4516, and 0.04516 ppm of carbendazim all significantly inhibited the expression of hymenoptaecin and apidaecin (P < 0.01), while P450 (7-ethoxycoumarin-O-deethylase) activity was downregulated by 4.516 ppm of carbendazim (P < 0.05). Our results indicate that the field-realistic concentrations of carbendazim may alter the immune response and P450-mediated detoxification of honey bees. Thus, carbendazim should be discreetly used on nectariferous crops during florescence.
Keywords: Apis mellifera L, forager bee, carbendazim, survival, physiology
Honey bees (Hymenoptera: Apidae) are essential economic insect, and they provide humans with a rich assortment of bee products, like honey, pollen, royal jelly, and propolis. In addition, they also play a key role in maintaining the biodiversity of ecosystems (Breeze et al. 2011). Nevertheless, in the past decades, honey bee colonies have suffered severe losses which has been cause for global concern (Potts et al. 2010, Chauzat et al. 2013). Although the specific reasons for the decline of honey bee population are inconclusive, many experts declare that the use of pesticides in agriculture is one of the important factors that lead to population decline (Goulson et al. 2015, Schmuck and Lewis 2016). In recent years, the use of low-toxicity pesticides in agriculture has alleviated the acute deaths of bees caused by high-toxicity pesticides, but its sublethal effects on bees cannot be neglected.
Numerous studies have demonstrated the sublethal effects of pesticides on tissue development, physiology, biochemistry, and behavior in honey bees. Neonicotinoids are the most widely used insecticides across the world. Sublethal doses of the neonicotinoids imidacloprid, thiamethoxam, clothianidin, and thiacloprid not only can cause harm to bees brain and midgut development (Catae et al. 2014, Oliveira et al. 2014, Peng and Yang 2016), but also reduce their learning, foraging, and homing abilities (Henry et al. 2012, Sandrock et al. 2014, Alkassab and Kirchner 2016, Tison et al. 2016). In addition, many other commonly used insecticides, such as pyrethroids and organophosphorus pesticides, have also been reported to negatively affect bee tissue development and various behaviors (Chaimanee et al. 2016, Li et al. 2017a, Liao et al. 2017, Wang et al. 2017a). Compared with insecticides, the effects of fungicides and herbicides routinely used in agriculture on bee health were less studied. In recent years, many studies have found fungicides in bee products (Tong et al. 2016, Calatayud-Vernich et al. 2017, Tosi et al. 2018); it is necessary to study the effects of fungicides on honey bees.
Carbendazim is an effective, low-toxicity, broad-spectrum fungicide, with remarkable control effects on the diseases caused by fungi on crops. In China, carbendazim is frequently used on camellia and seedrape crops, which constitute a large part of nectar resources (Li et al. 2017b). Tong et al. (2016) have surveyed the pesticide residues of 48 bee pollens in eight provinces in mainland China, and their results showed that carbendazim was the highest detectable pesticide, and its maximum residue was as high as 4,516 ng/g. Although carbendazim is not acutely lethal at field levels (Cang et al., 2012), the subchronic effects on honey bees’ health remain to be further explored.
To evaluate honey bee health, some biomarkers are usually used to evaluate physiological effects of environmental stressors (Boily et al. 2013, Carvalho et al. 2013, Badawy et al. 2015). Acetylcholinesterase (AChE) is an enzyme that controls the neuronal activity of cholinergic synapses (Badiou et al. 2008). Cytochrome P450 monooxygenase (P450), glutathione-S-transferase (GST), and carboxylesterases (CarE) are important enzymes involved in the detoxification and endocrine systems (Maxwell 1992, Diao et al. 2006, Gottardi et al. 2015). Moreover, previous reviews have declared that pesticide-induced immune stress is also a possible mechanism of toxicity in insects (James and Xu 2012), and antimicrobial peptides are a key component of honey bee innate immunity (Danihlík et al. 2015). Forager honey bees mainly collect nectar, pollen, and water for the colonies (Robinson 2002). Compared with the nurse bees, larvae, and queen, they are more likely to have greater and more frequent exposure to pesticides. In this study, the effects of field-realistic concentration of carbendazim (4.516, 0.4516, and 0.04516 ppm) on survival, biomarkers (AChE, P450, GST, and CarE), and immune-related antimicrobial peptide genes (hymenoptaecin, defensin, apidaecin, and abaecin) expression in forager bees were investigated.
Material and Methods
Honey Bee Foragers
Two frames with sealed brood were taken from a healthy colony at the Institute of Apiculture Research of Anhui Agricultural University (Hefei, China). The frames were held in an incubator under the following conditions: 34°C, a relative humidity (RH) of 60%, and in darkness. After 12 h, about 3,000 newly emerged bees were obtained and marked on the thorax with red paint. Then bees were placed back to the original colony. After 24 d, we collected the marked forager bees and put them into plastic cages.
Pesticide Exposure
The residues of carbendazim range from 3.2 to 4,516 ng/g in trapped pollen in China (Tong et al. 2016). Based on this, 4.516, 0.4516, and 0.04516 ppm of carbendazim were selected as the field-realistic concentrations. Carbendazim (50% wettable powder; ZhB, Beijing, China) was dissolved in 50% (w/v) sucrose–water solution, and three doses of carbendazim (50% wettable powder), 9.032, 0.9032, and 0.09032 ppm (active ingredients: 4.516, 0.4516, and 0.04516 ppm), were prepared. Forager bees were provided with enough 50% sucrose–water solution containing certain concentrations of pesticide or only sucrose–water solution as control. During feeding, wettable powder might have fallen out of solution, resulting in exposure to higher or lower concentration of carbendazim than intended. Thirty bees were counted into each cage (replicate), and each treatment has three replicates. The cages were maintained at 28°C, an RH of 70%, and in darkness. We recorded and removed the dead bees daily while replaced the food every 2 or 3 d. After 10 d, all living bees were gathered and stored at −80°C.
Measurement of Enzyme Activity
Five bee midguts from each replicate were pooled to measure the activities of P450, GST, and CarE, while five bee heads were used for AChE activity. The tissue samples were put in a precooled 15-ml centrifuge tube and then 1 ml of 0.1 mol/liter phosphate buffer (containing 0.1% Triton X-100, pH 8.0) was added. The mixtures were homogenized with an electric pestle in an ice bath and then centrifuged at 12,000 × g for 30 min at 4°C. The supernatants were collected as enzyme sources for enzyme activity assays. Total protein concentration of each enzyme source was measured by BCA protein assay kit (Bestbio, Shanghai, China). The activities of GST and AChE were measured using the procedures previously described by Yu (1982) and Ellman (1961), respectively. CarE activity was measured as previously described, and α-naphthyl acetate was used as the substrate (van Asperern 1962). 7-Ethoxycoumarin was used as the substrate to measure P450 7-ethoxycoumarin-O-deethylase (7-ECOD) activity following the method of Aitio (1978).
Real-Time Quantitative PCR
A total of five bee midguts from each replicate were pooled for total RNA extraction using RNAiso Plus reagent (Takara, Dalian, China). Then 0.5 μg of total RNA from each sample was prepared to obtain the cDNA using ReverTra Ace qPCR RT Master Mix Kit (Toyobo, Osaka, Japan) according to the manufacturer’s protocol. SuperReal PreMix Plus (SYBR Green) Kit (Tiangen, Beijing, China) was used to perform real-time quantitative PCR assay. RpS5 was used as reference gene for normalizing the expression levels of the target genes. All the primers used are listed in Table 1. The relative expression levels of the target genes were calculated using the comparative 2−ΔCt method (Schmittgen and Livak 2008).
Table 1.
Primer sequences
| Genes | Primer sequence (5′–3′) | Amplification efficiency |
|---|---|---|
| apidaecin | F: TTTTGCCTTAGCAATTCTTGTTG | 0.949 |
| R: GAAGGTCGAGTAGGCGGATCT | ||
| abaecin | F: TGTCGGCCTTCTCTTCATGG | 0.95 |
| R: TGACCTCCAGCTTTACCCAAA | ||
| hymenoptaecin | F: ATATCCCGACTCGTTTCCGA | 0.973 |
| R: TCCCAAACTCGAATCCTGCA | ||
| defensin | F: TGTCGGCCTTCTCTTCATGG | 0.946 |
| R:TGACCTCCAGCTTTACCCAAA | ||
| RpS5 | F: AATTATTTGGTCGCTGGAATTG | 0.954 |
| R: TAACGTCCAGCAGAATGTGGTA |
Data Analysis
Log-rank Kaplan–Meier survival analyses were used to compare survival among the four groups. Significant differences among all groups for enzymes activities and genes expression were evaluated using one-way analysis of variance (ANOVA) followed by Duncan’s multiple range test. All statistical analyses were conducted by IBM SPSS Statistics 24 software.
Results
Survival of the Forager Bees
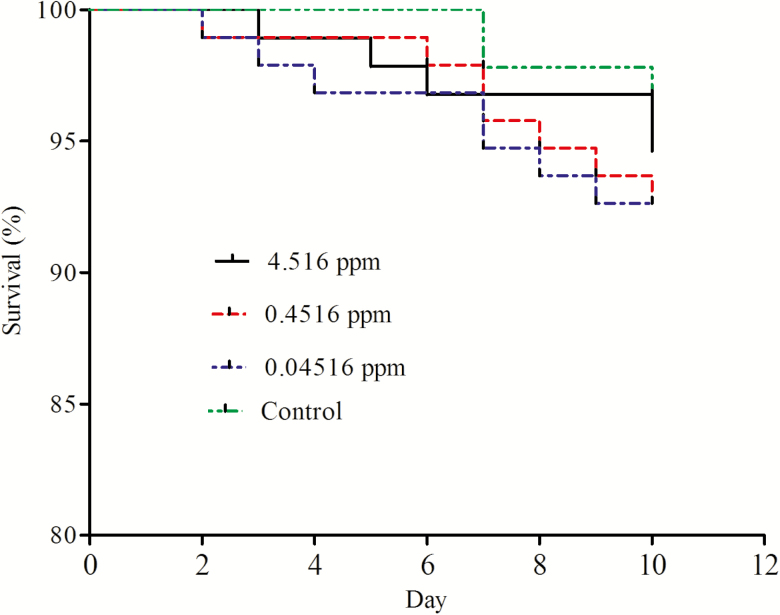
As shown in Fig. 1, the average survival rate on day 10 for the forager bees exposed to 4.516, 0.4516, 0.04516 ppm carbendazim, and control of 50% sucrose–water solution were 94.5, 92.2, 92.2, and 96.7%, respectively. There were no significant differences among all treatments (Log-rank χ2 = 1.914, df = 3, P = 0.594; Fig. 1).
Fig. 1.
Survival of forager bees subjected to chronic exposure to field-realistic concentrations of carbendazim over 10 d. Survival among the four groups was compared by log-rank Kaplan–Meier survival analyses.
Enzyme Activities
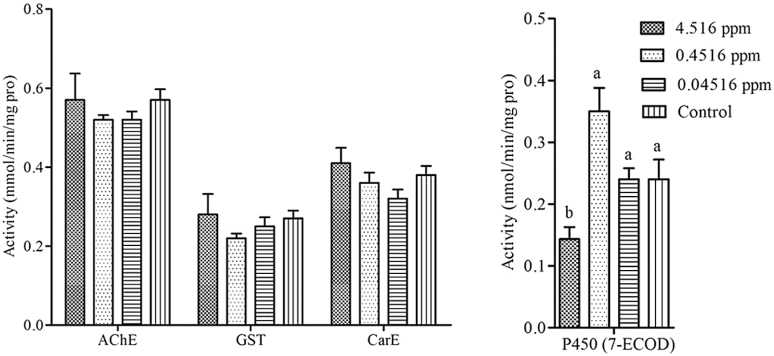
After 10 d of feeding, the field-realistic concentrations of carbendazim (4.516, 0.4516, and 0.04516 ppm) did not significantly affect the activities of AChE (F = 0.543, df = 3, P = 0.666), GST (F = 0.572, df = 3, P = 0.649), and CarE (F = 1.642, df = 3, P = 0.255) in forager bees (Fig. 2A). However, the activity of P450 (7-ECOD) in forager bees was downregulated by 4.516 ppm of carbendazim (F = 6.644, df = 3, P < 0.05; Fig. 2B).
Fig. 2.
Activities of acetylcholinesterase, glutathione-S-transferase, carboxylesterase (A), and cytochrome P450 monooxygenase (B) in forager bees after exposed to field-realistic concentrations of carbendazim over 10 d. Data are mean ± SE. Different lowercase letters on the bars indicate significant difference among groups based on Duncan’s multiple range test (P < 0.05). AChE = acetylcholinesterase; GST = glutathione-S-transferase; CarE = carboxylesterases; P450 (7-ECOD) = 7-ethoxycoumarin-O-deethylase.
Expression of Antimicrobial Peptide Genes
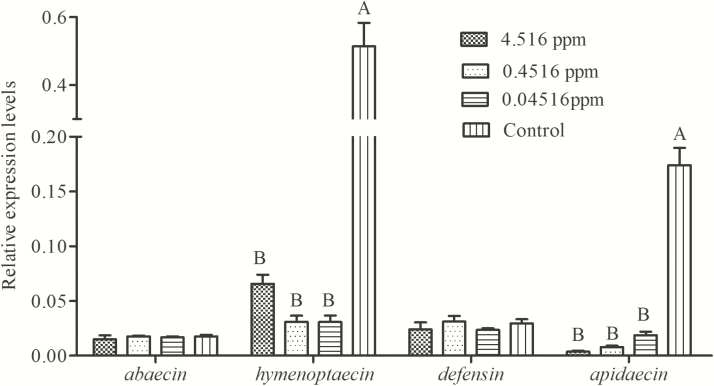
The relative expression levels of four antimicrobial peptide genes in forager bees fed on different sucrose–water solution are shown in Fig. 3. There were no significant differences in the expression levels of defensin (F = 0.693, df = 3, P = 0.581) and abaecin (F = 0.392, df = 3, P = 0.762) among all treatments (Fig. 3). While groups treated at 4.516, 0.4516, and 0.04516 ppm of carbendazim, all significantly inhibited hymenoptaecin (F = 46.081, df = 3, P < 0.01) and apidaecin (F = 101.359, df = 3, P < 0.01) expression in forager bees.
Fig. 3.
Relative expression levels of four antimicrobial peptide genes in forager bees after exposed to field-realistic concentrations of carbendazim over 10 d. Data are mean ± SE. Different capital letters on the bars indicate significant difference among groups (P < 0.01, Duncan’s multiple range test).
Discussion
Carbendazim is nowadays widely used to control fungus in various nectariferous crops (Li et al. 2017b). Recently, many studies have detected carbendazim residue in bee products, e.g., honey and pollen (Tong et al. 2016, Tosi et al. 2018). Honey bees are important pollination insects (Breeze et al. 2011), and they can be exposed to carbendazim by consuming contaminated nectar and pollen. In this study, the effects of field-realistic concentrations of carbendazim (4.516, 0.4516, and 0.04516 ppm) on the survival, biomarker enzyme activity, and antimicrobial peptide genes expression in forager bees were evaluated. Our results indicated that field-realistic concentrations of carbendazim had no risk on the survival of forager bees during 10-d oral exposure. This finding is similar with the report of Wang et al. (2017) that sublethal concentrations of carbendazim (0.25 and 0.75 mg/g) do not affect the eclosion rate of honey bee larvae.
In regard to biomarkers, AChE is an enzyme that controls the neuronal activity of cholinergic synapses in insect (Badiou et al. 2008). P450, GST, and CarE are important detoxification enzymes in honey bees. P450 and CarE play major roles in phaseⅠmetabolism or detoxification of toxins, while GST is mainly involved in phase II detoxification (Gong and Diao 2017). In the present study, carbendazim had no obvious effects on the activities of AChE, GST, and CarE in forager bees, but P450 (7-ECOD) activity was significantly inhibited by 4.516 ppm of carbendazim (P < 0.05). Previous studies showed that inhibiting P450 activity can greatly increase toxicity of several pyrethroids (Johnson et al. 2006, 2012) and neonicotinoid insecticides (Iwasa et al. 2004, Manjon et al. 2018) to honey bees. The ergosterol biosynthesis inhibitor (EBI) fungicides are known to suppress P450 activity (Hassold et al. 2009), and the presence of some EBI fungicides can also enhance neonicotinoids toxicity to honey bees (Sgolastra et al. 2017, Robinson et al. 2017, Tsvetkov et al. 2017).
Antimicrobial peptides are a key component of the honey bee innate humoral immunity (Danihlík et al. 2015). Four antimicrobial peptides, apidaecin (Casteels et al. 1989), abaecin (Casteels et al. 1990), hymenoptaecin (Casteels et al. 1993), and defensin (Casteels and Tempst 1994) have been confirmed in honey bees, and they display a wide spectrum activity against protozoa, bacteria, and fungi. In this study, two antimicrobial peptide genes, hymenoptaecin and apidaecin, were significantly inhibited by field-realistic concentrations of carbendazim (P < 0.01). Apidaecin belongs to the prolin-rich apidaecin family and shows higher efficiency against G-bacteria, while hymenoptaecin is a member of glycin-rich peptide family and restrains the growth of both G+ and G− bacteria (Casteels et al. 1989, 1993; Chan et al. 2009). The carbendazim-induced reduction of hymenoptaecin and apidaecin here suggests adverse impact on immune systems in forager bees (Christen et al. 2016, Shi et al. 2017).
In summary, the field-realistic concentrations of carbendazim (4.516, 0.4516, and 0.04516 ppm) showed no negative effect on the survival of forager honey bees, but can disturb immune system and P450-mediated detoxification. This is likely to increase the risk of honey bees to other environment stressors, such as pathogens and insecticides, and cause potential harm to the development of the colony. However, in this study, we used formulation-grade carbendazim (50% wettable powder) instead of technical-grade carbendazim. Because the 50% wettable powder is composed of 50% active carbendazim ingredient and the other ingredient. Then, we may just surmise that the formulation-grade carbendazim (50% wettable powder) shows negative impacts on honey bees. Future studies should focus on the effects of technical-grade carbendazim in honey bees.
Acknowledgments
This work was supported by the National Special Construction Apiculture Technology System of China (No. CARS-45-KXJ10).
References Cited
- Aitio A. 1978. A simple and sensitive assay of 7-ethoxycoumarin deethylation. Anal. Biochem. 85: 488–491. [DOI] [PubMed] [Google Scholar]
- Alkassab A. T. and Kirchner W. H.. 2016. Impacts of chronic sublethal exposure to clothianidin on winter honeybees. Ecotoxicology. 25: 1000–1010. [DOI] [PubMed] [Google Scholar]
- van Asperen K. 1962. A study of housefy esterase by means of a sensitive colorimetric method. J. Insect. Physiol. 8: 401–416. [Google Scholar]
- Badawy M. E. I., Nasr H. M., and Rabea E. I.. 2015. Toxicity and biochemical changes in the honey bee Apis mellifera exposed to four insecticides under laboratory conditions. Apidologie. 46: 177e193. [Google Scholar]
- Badiou A., M. Meled, and Belzunces L. P.. 2008. Honeybee Apis mellifera acetylcholinesterase—a biomarker to detect deltamethrin exposure. Ecotoxicol. Environ. Saf. 69: 246–253. [DOI] [PubMed] [Google Scholar]
- Boily M., B. Sarrasin C. Deblois P. Aras, and Chagnon M.. 2013. Acetylcholinesterase in honey bees (Apis mellifera) exposed to neonicotinoids, atrazine and glyphosate: laboratory and field experiments. Environ. Sci. Pollut. Res. Int. 20: 5603–5614. [DOI] [PubMed] [Google Scholar]
- Breeze T. D., Bailey A. P., Balcombe K. G., and Potts S. G.. 2011. Pollination services in the UK: how important are honeybees?Agric. Ecosyst. Environ. 142: 137–143. [Google Scholar]
- Calatayud-Vernich P., F. Calatayud E. Simó, and Picó Y.. 2017. Occurrence of pesticide residues in Spanish beeswax. Sci. Total Environ. 605-606: 745–754. [DOI] [PubMed] [Google Scholar]
- Cang T., Y. H. Wang R. X. Yu C. X. Wang, Chen L. P., Wu S. G., and Zhao X. P.. 2012. The acute toxicity and risk assessment of 25 pesticides used in nectar plant to Apis mellifera L. Acta. Agriculture Zhejiangensis. 24: 853–859. [Google Scholar]
- Carvalho S. M., L. P. Belzunces G. A. Carvalho J. L. Brunet, and Badiou-Beneteau A.. 2013. Enzymatic biomarkers as tools to assess environmental quality: a case study of exposure of the honeybee Apis mellifera to insecticides. Environ. Toxicol. Chem. 32: 2117–2124. [DOI] [PubMed] [Google Scholar]
- Casteels P. and Tempst P.. 1994. Apidaecin-type peptide antibiotics function through a non-poreforming mechanism involving stereospecificity. Biochem. Biophys. Res. Commun. 199: 339–345. [DOI] [PubMed] [Google Scholar]
- Casteels P., C. Ampe F. Jacobs M. Vaeck, and Tempst P.. 1989. Apidaecins: antibacterial peptides from honeybees. Embo J. 8: 2387–2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels P., C. Ampe L. Riviere J. Van Damme C. Elicone M. Fleming F. Jacobs, and Tempst P.. 1990. Isolation and characterization of abaecin, a major antibacterial response peptide in the honeybee (Apis mellifera). Eur. J. Biochem. 187: 381–386. [DOI] [PubMed] [Google Scholar]
- Casteels P., C. Ampe F. Jacobs, and Tempst P.. 1993. Functional and chemical characterization of Hymenoptaecin, an antibacterial polypeptide that is infection-inducible in the honeybee (Apis mellifera). J. Biol. Chem. 268: 7044–7054. [PubMed] [Google Scholar]
- Catae A. F., T. C. Roat R. A. De Oliveira R. C. Nocelli, and Malaspina O.. 2014. Cytotoxic effects of thiamethoxam in the midgut and malpighian tubules of Africanized Apis mellifera (Hymenoptera: Apidae). Microsc. Res. Tech. 77: 274–281. [DOI] [PubMed] [Google Scholar]
- Chaimanee V., J. D. Evans Y. Chen C. Jackson, and Pettis J. S.. 2016. Sperm viability and gene expression in honey bee queens (Apis mellifera) following exposure to the neonicotinoid insecticide imidacloprid and the organophosphate acaricide coumaphos. J. Insect Physiol. 89: 1–8. [DOI] [PubMed] [Google Scholar]
- Chan Q. W., A. P. Melathopoulos S. F. Pernal, and Foster L. J.. 2009. The innate immune and systemic response in honey bees to a bacterial pathogen, Paenibacillus larvae. BMC Genomics. 10: 387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauzat M. P., L. Cauquil L. Roy S. Franco P. Hendrikx, and Ribière-Chabert M.. 2013. Demographics of the European apicultural industry. PLos One. 8: e79018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christen V., F. Mittner, and Fent K.. 2016. Molecular effects of neonicotinoids in honey bees (Apis mellifera). Environ. Sci. Technol. 50: 4071–4081. [DOI] [PubMed] [Google Scholar]
- Danihlík J., Aronstein K., and Petřivalský M.. 2015. Antimicrobial peptides: a key component of honey bee innate immunity. J. Apicult. Res. 54: 1–14. [Google Scholar]
- Diao Q., Yuan K., Liang P., and Gao X.. 2006. Tissue distribution and properties of glutathione-S-transferases in Apis cerana cerana Fabricius and Apis mellifera ligustica Spinola. J. Apic. Res. 45: 145e152. [Google Scholar]
- Ellman G. L., K. D. Courtney V. Andres Jr, and Feather-Stone R. M.. 1961. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 7: 88–95. [DOI] [PubMed] [Google Scholar]
- Gong Y. H., and Diao Q. Y.. 2017. Current knowledge of detoxification mechanisms of xenobiotic in honey bees. Ecotoxicology. 26: 1–12. [DOI] [PubMed] [Google Scholar]
- Gottardi M., A. Kretschmann, and Cedergreen N.. 2015. Measuring cytochrome P450 activity in aquatic invertebrates: a critical evaluation of in vitro and in vivo methods. Ecotoxicology. 25: 419–430. [DOI] [PubMed] [Google Scholar]
- Goulson D., E. Nicholls C. Botías, and Rotheray E. L.. 2015. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science. 347: 1255957. [DOI] [PubMed] [Google Scholar]
- Hassold E. and Backhaus T.. 2009. Chronic toxicity of five structurally diverse demethylase-inhibiting fungicides to the crustacean Daphnia magna: a comparative assessment. Environ. Toxicol. Chem. 28: 1218–1226. [DOI] [PubMed] [Google Scholar]
- Henry M., M. Béguin F. Requier O. Rollin J. F. Odoux P. Aupinel J. Aptel S. Tchamitchian, and Decourtye A.. 2012. A common pesticide decreases foraging success and survival in honey bees. Science. 336: 348–350. [DOI] [PubMed] [Google Scholar]
- Iwasa T., Motoyama N., Ambrose J. T., and Michael Roe R.. 2004. Mechanism for the differential toxicity of neonicotinoid insecticides in the honey bee, Apis mellifera.Crop. Prot. 23: 371–378. [Google Scholar]
- James R. R. and Xu J.. 2012. Mechanisms by which pesticides affect insect immunity. J. Invertebr. Pathol. 109: 175–182. [DOI] [PubMed] [Google Scholar]
- Johnson R. M., Z. Wen M. A. Schuler, and Berenbaum M. R.. 2006. Mediation of pyrethroid insecticide toxicity to honey bees (Hymenoptera: Apidae) by cytochrome P450 monooxygenases. J. Econ. Entomol. 99: 1046–1050. [DOI] [PubMed] [Google Scholar]
- Johnson R. M., W. Mao H. S. Pollock G. Niu M. A. Schuler, and Berenbaum M. R.. 2012. Ecologically appropriate xenobiotics induce cytochrome P450s in Apis mellifera. PLos One. 7: e31051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z. G., Li M., Huang J. N., Ma C. S., Xiao L. C., Huang Q., Zhao Y. Z., Nie H. Y., and Su S. K.. 2017a. Effects of sublethal concentrations of chlorpyrifos on olfactory learning and memory performances in two bee species, Apis mellifera and Apis cerana. Sociobiology. 64: 174–181. [Google Scholar]
- Li Y. H., B. L. Zhou M. R. Qian Q. Wang, and Zhang H.. 2017b. Transfer assessment of carbendazim residues from rape flowers to apicultural products. J. Anal. Methods Chem. 2017: 6075405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao C. H., Wu J., Wang Z. L., Zeng Z. J., and Wu X. B.. 2017. Effect of fenpropathrin on the viability and homing ability of worker bees Apis mellifera. J. Asia-Pac. Entomol. 20: 1063–1066. [Google Scholar]
- Manjon C., B. J., Troczka M., Zaworra K., Beadle E., Randall G., Hertlein K. S., Singh C. T., Zimmer R. A., Homem B., Lueke, et al. 2018. Unravelling the molecular determinants of bee sensitivity to neonicotinoid insecticides. Curr. Biol. 28: 1137–1143.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell D. M. 1992. The specificity of carboxylesterase protection against the toxicity of organophosphorus compounds. Toxicol. Appl. Pharmacol. 114: 306–312. [DOI] [PubMed] [Google Scholar]
- Oliveira R. A., T. C. Roat S. M. Carvalho, and Malaspina O.. 2014. Side-effects of thiamethoxam on the brain andmidgut of the Africanized honeybee Apis mellifera (Hymenopptera: Apidae). Environ. Toxicol. 29: 1122–1133. [DOI] [PubMed] [Google Scholar]
- Peng Y. C. and Yang E. C.. 2016. Sublethal dosage of imidacloprid reduces the microglomerular density of honey bee mushroom bodies. Sci. Rep. 6: 19298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potts S. G., J. C. Biesmeijer C. Kremen P. Neumann O. Schweiger, and Kunin W. E.. 2010. Global pollinator declines: trends, impacts and drivers. Trends Ecol. Evol. 25: 345–353. [DOI] [PubMed] [Google Scholar]
- Robinson G. E. 2002. Genomics and integrative analyses of division of labor in honeybee colonies. Am. Nat. 160 (Suppl 6): S160–S172. [DOI] [PubMed] [Google Scholar]
- Robinson A., H. Hesketh E. Lahive A. A. Horton C. Svendsen A. Rortais J. L. Dorne J. Baas M. S. Heard, and Spurgeon D. J.. 2017. Comparing bee species responses to chemical mixtures: common response patterns?PLoS One. 12: e0176289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandrock C., M. Tanadini L. G. Tanadini A. Fauser-Misslin S. G. Potts, and Neumann P.. 2014. Impact of chronic neonicotinoid exposure on honeybee colony performance and queen supersedure. PLos One. 9: e103592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmittgen T. D. and Livak K. J.. 2008. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 3: 1101–1108. [DOI] [PubMed] [Google Scholar]
- Schmuck R. and Lewis G.. 2016. Review of field and monitoring studies investigating the role of nitro-substituted neonicotinoid insecticides in the reported losses of honey bee colonies (Apis mellifera). Ecotoxicology. 25: 1617–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sgolastra F., P. Medrzycki L. Bortolotti M. T. Renzi S. Tosi G. Bogo D. Teper C. Porrini R. Molowny-Horas, and Bosch J.. 2017. Synergistic mortality between a neonicotinoid insecticide and an ergosterol-biosynthesis-inhibiting fungicide in three bee species. Pest Manag. Sci. 73: 1236–1243. [DOI] [PubMed] [Google Scholar]
- Shi T. F., Y. F. Wang F. Liu L. Qi, and Yu L. S.. 2017. Sublethal effects of the neonicotinoid insecticide thiamethoxam on the transcriptome of the honey bees (Hymenoptera: Apidae). J. Econ. Entomol. 110: 2283–2289. [DOI] [PubMed] [Google Scholar]
- Tison L., M. L. Hahn S. Holtz A. Rößner U. Greggers G. Bischoff, and Menzel R.. 2016. Honey bees’ behavior is impaired by chronic exposure to the neonicotinoid thiacloprid in the field. Environ. Sci. Technol. 50: 7218–7227. [DOI] [PubMed] [Google Scholar]
- Tong Z., Wu Y. C., Liu Q. Q., Shi Y. H., Zhou L. J., Liu Z. Y., Yu L. S., and Cao H. Q.. 2016. Multi-residue analysis of pesticide residues in crude pollens by UPLC-MS/MS. Molecules. 21: 1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosi S., C. Costa U. Vesco G. Quaglia, and Guido G.. 2018. A 3-year survey of Italian honey bee-collected pollen reveals widespread contamination by agricultural pesticides. Sci. Total Environ. 615: 208–218. [DOI] [PubMed] [Google Scholar]
- Tsvetkov N., O. Samson-Robert K. Sood H. S. Patel D. A. Malena P. H. Gajiwala P. Maciukiewicz V. Fournier, and Zayed A.. 2017. Chronic exposure to neonicotinoids reduces honey bee health near corn crops. Science. 356: 1395–1397. [DOI] [PubMed] [Google Scholar]
- Wang Q., Q. Diao P. Dai Y. Chu Y. Wu T. Zhou, and Cai Q.. 2017a. Exploring poisonous mechanism of honeybee, Apis mellifera ligustica Spinola, caused by pyrethroids. Pestic. Biochem. Physiol. 135: 1–8. [DOI] [PubMed] [Google Scholar]
- Wang K., Pang Q., Zhang W.W., and Ji T.. 2017b. Effects of sublethal doses of carbendazim on the growth and detoxifying enzyme activities of honeybee (Apis mellifera ligustica) larvae. Acta. Entomologica. Sinica. 60: 642–649. [Google Scholar]
- Yu S. J. 1982. Host plant induction of glutathione S-transferases in the fall armyworm. Pestic. Biochem. Physiol. 18: 101–106. [Google Scholar]