Abstract
BACKGROUND
The use of intraoperative navigation during microscope cases can be limited when attention needs to be divided between the operative field and the navigation screens. Heads-up display (HUD), also referred to as augmented reality, permits visualization of navigation information during surgery workflow.
OBJECTIVE
To detail our initial experience with HUD.
METHODS
We retrospectively reviewed patients who underwent HUD-assisted surgery from April 2016 through April 2017. All lesions were assessed for accuracy and those from the latter half of the study were assessed for utility.
RESULTS
Seventy-nine patients with 84 pathologies were included. Pathologies included aneurysms (14), arteriovenous malformations (6), cavernous malformations (5), intracranial stenosis (3), meningiomas (27), metastasis (4), craniopharygniomas (4), gliomas (4), schwannomas (3), epidermoid/dermoids (3), pituitary adenomas (2) hemangioblastoma (2), choroid plexus papilloma (1), lymphoma (1), osteoblastoma (1), clival chordoma (1), cerebrospinal fluid leak (1), abscess (1), and a cerebellopontine angle Teflon granuloma (1). Fifty-nine lesions were deep and 25 were superficial. Structures identified included the lesion (81), vessels (48), and nerves/brain tissue (31). Accuracy was deemed excellent (71.4%), good (20.2%), or poor (8.3%). Deep lesions were less likely to have excellent accuracy (P = .029). HUD was used during bed/head positioning (50.0%), skin incision (17.3%), craniotomy (23.1%), dural opening (26.9%), corticectomy (13.5%), arachnoid opening (36.5%), and intracranial drilling (13.5%). HUD was deactivated at some point during the surgery in 59.6% of cases. There were no complications related to HUD use.
CONCLUSION
HUD can be safely used for a wide variety of vascular and oncologic intracranial pathologies and can be utilized during multiple stages of surgery.
Keywords: Augmented reality, Image-guided surgery, Neuronavigation
ABBREVIATIONS
- AR
augmented reality
- AVM
arteriovenous malformation
- CSF
cerebrospinal fluid
- CTA
computed tomography angiography
- EC-IC
extracranial–intracranial
- HUD
heads-up display
- IC-IC
intracranial–intracranial
- MRI
magnetic resonance imaging
Intraoperative navigation and microscope integration are very useful, but can be limited when attention needs to be divided between the operative field and the navigation screens. A heads-up display (HUD), available in the aviation industry for many years, is a recent addition to the neurosurgery toolkit. HUD provides visualization of navigation information during the surgery workflow. There has been a limited use of HUD across the surgical field thus far. This study details our early use of HUD in skull base and vascular cases. To our knowledge, this is the largest series utilizing HUD for intracranial surgery.
METHODS
Institutional Review Board approval was obtained to perform this study. A waiver of consent was obtained to perform this retrospective review. This is a retrospective review of all patients who underwent intracranial surgery using HUD from April 2016 through April 2017. There were no other inclusion or exclusion criteria. Intraoperative navigation was performed with Brain Lab Curve™ Image Guided Surgery (Brainlab, Munich, Germany). The Zeiss Pentero 900 (Carl Zeiss Meditec Inc, Dublin, California) was used for the majority of cases, and the Leica OH6 (Leica Microsystems Inc, Buffalo Grove, Illinois) was used for only a small number of cases. Prior to surgery, preoperative imaging, usually contrast-enhanced MRI and CTA, were reviewed by the senior author (JB) and team. Using the Brainlab platform, the lesions of interest, surrounding vessels, and/or surrounding nerves/brain tissue were painted using the Brainlab Smartbrush function by a member of the surgical team and then approved by the senior author, JB. Patient registration and microscope integration were performed in the standard fashion. The operating room setup included the operating microscope, Brainlab Navigation, and Surgical Theater imaging (Surgical Theater, Mayfield, Ohio; Figure 1).
FIGURE 1.
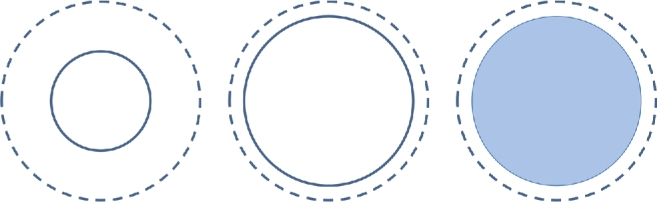
HUD convention. Dashed lines represent the maximum dimension of the painted lesion. Solid lines represent the dimension of the lesion that is in focus at any given time. Therefore, dashed lines will not change in size, but solid lines will change depending on focal length. In addition, the object can be displayed as outline-only or filled in with different degrees of transparency.
The HUD could be overlaid at multiple time points during the surgery (ie, during exposure of skin, bone, dura, cortex, and/or lesion). Microscope integration was typically performed after dural opening at the time of first use, but could be done earlier in the operation if the surgeon wanted to use HUD for phases such as head/bed positioning, skin incision, craniotomy, or dural opening. By convention, HUD objects have either a solid or dashed outline (Figure 1). The dashed outline represents the greatest dimension of the object projected in the surgeon's point of view, irrespective of microscope focus depth. A solid outline represents the object dimension at the current focal depth. Objects could also be outlined or filled with different degrees of opacity.
Pathologies were grouped together as to their vascular, oncologic, or other origin. Lesions were labeled as superficial if they came to within 1 cm of the surface of the brain or calvarium and all others were labeled deep. Structures painted included the lesion itself as well as surrounding vessels, nerves, or brain/brain tissue. HUD accuracy was subjectively determined based on the visualized overlap of painted structures with the location of the actual structures. Accuracy was determined retrospectively by authors JB and JM and was graded as excellent (perfect overlay), good (minimal overlay displacement), or poor (significant overlay displacement). This assessment was an estimate, not a measurement. Accuracy was assessed when the painted objects first came into view. Other patient data were recorded by reviewing the medical record. The chi-squared test was used to compare accuracy at different depths with a significance level of 0.05.
HUD utility during phases other than lesion localization/resection was assessed for patients in the second half of the study by recording the other phases of surgery when HUD was used. Other phases of surgery included bed/head positioning, skin incision, craniotomy, dural opening, corticectomy, arachnoid incision, and intracranial drilling. Head positioning could only be performed prior to the start of surgery, but bed positioning could be performed either prior to or during surgery. In order to be counted for utility, the HUD had to be actively used during that phase of surgery (ie, not just be turned on). The decision to use HUD during a certain phase of surgery, however, was operator dependent. Additionally, it was recorded whether HUD was turned off during the case and the reason for deactivation.
RESULTS
Seventy-nine patients with 84 intracranial lesions were included in the study. One patient had 3 meningiomas, 1 patient had both an aneurysm and a meningioma, 1 patient underwent both an intracranial–intracranial bypass and an indirect bypass during a separate surgery, and 1 patient had both an aneurysm and an arteriovenous malformation (AVM). All other patients had a single lesion/procedure. Average patient age was 53.2 yr and 53.2% were female.
A wide variety of both cerebrovascular (28) and oncologic (53) lesions were evaluated (Table 1). Vascular lesions included aneurysms (14), AVMs (6), cavernous malformations (5), and intracranial stenosis (3). Oncologic lesions included meningioma (27), metastasis (4), craniopharygnioma (4), glioma (4), schwannoma (3), epidermoid/dermoid (3), pituitary adenoma (2), hemangioblastoma (2), choroid plexus papilloma (1), lymphoma (1), osteoblastoma (1), and clival chordoma (1). Other lesions included a frontotemporal abscess, an inferior frontal cerebrospinal fluid (CSF) leak, and a cerebellopontine angle Teflon granuloma years following a microvascular decompression. A transcranial surgical approach was used for 77 lesions, and a microscopic transphenoidal approach was used for 7 lesions (craniopharyngioma (4), tuberculum meningioma, pituitary metastasis, and clival chordoma).
TABLE 1.
Breakdown of Pathologies
| N | % | |
|---|---|---|
| Cerebrovascular | 28 | 33.3% |
| Aneurysms | 14 | 16.7% |
| ICA | 5 | 6.0% |
| ACA | 3 | 3.6% |
| MCA | 3 | 3.6% |
| PICA | 2 | 2.4% |
| SCA | 1 | 1.2% |
| AVMs | 6 | 7.1% |
| CPA | 2 | 2.4% |
| Cerebellar | 1 | 1.2% |
| Parietoccipital | 1 | 1.2% |
| Temporoparietal | 1 | 1.2% |
| Lateral ventricular | 1 | 1.2% |
| Cavernous malformations | 5 | 6.0% |
| Temoral | 3 | 3.6% |
| Cerebellar | 1 | 1.2% |
| 4th ventricular | 1 | 1.2% |
| Intracranial stenosis | 3 | 3.6% |
| Oncologic | 53 | 63.1% |
| Meningioma | 27 | 32.1% |
| Sphenorbital/clinoidal | 10 | 11.9% |
| Convexity | 9 | 10.7 |
| Tuberculum | 2 | 2.4% |
| Foramen magnum | 2 | 2.4% |
| Tentorial | 2 | 2.4% |
| CPA | 1 | 1.2% |
| Torcular | 1 | 1.2% |
| Metastasis | 4 | 4.8% |
| Craniopharyngioma | 4 | 4.8% |
| Glioma | 4 | 4.8% |
| Schwannoma | 3 | 3.6% |
| Epidermoid/dermoid | 3 | 3.6% |
| Pituitary adenoma | 2 | 2.4% |
| Hemangioblastoma | 2 | 2.4% |
| Choroid plexus papilloma | 1 | 1.2% |
| Lymphoma | 1 | 1.2% |
| Osteoblastoma | 1 | 1.2% |
| Clival chordoma | 1 | 1.2% |
| Other | 3 | 3.6% |
| Abscess | 1 | 1.2% |
| CSF leak | 1 | 1.2% |
| CPA Teflon granuloma | 1 | 1.2% |
There were 59 deep and 25 superficial lesions (Table 2). Structures identified with HUD included the lesion itself in 81 cases, surrounding vessels in 48 cases, and surrounding nerves or brain tissue in 31 cases. HUD accuracy was deemed to be excellent in 71.4% of cases, good in 20.2% of cases, and poor in 8.3% of cases. Deep lesions were less likely have excellent accuracy compared to superficial lesions (64.4% vs 88.0%, P = .029; Figure 2).
TABLE 2.
HUD Results
| Location | ||
| Deep | 59 | 70.2% |
| Superficial | 25 | 29.8% |
| Accuracy | ||
| Excellent | 60 | 71.4% |
| Good | 17 | 20.2% |
| Poor | 7 | 8.3% |
| Structures | ||
| Lesion | 81 | 96.4% |
| Vessels | 48 | 57.1% |
| Nerves | 31 | 36.9% |
FIGURE 2.
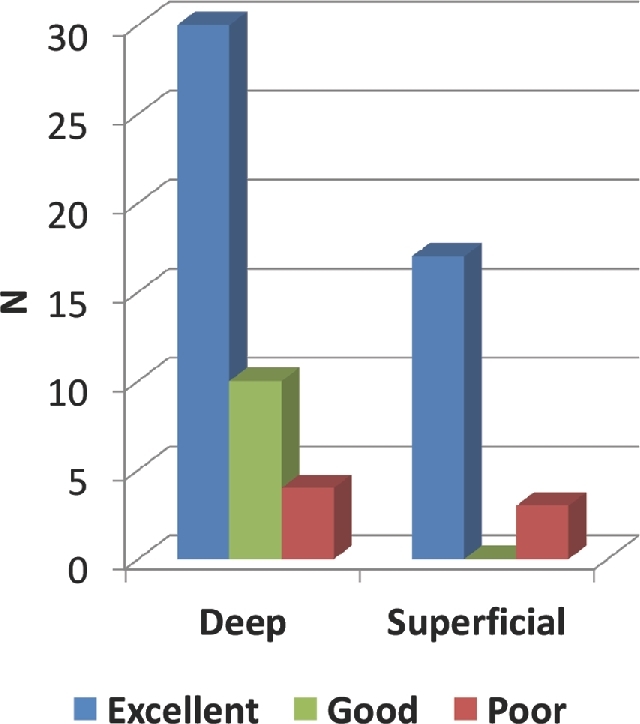
Accuracy analysis by depth. Deep lesions were less likely have excellent accuracy compared to superficial lesions (64.4% vs 88.0%, P = .029).
Utility was assessed for 52 (61.9%) lesions (Table 3). HUD was actively used during bed/head positioning (50.0%), skin incision (17.3%), craniotomy (23.1%), dural opening (26.9%), corticectomy (13.5%), arachnoid opening (36.5%), and intracranial drilling (13.5%). HUD was disabled in 59.6% of cases for a variety of reasons. Reasons to turn HUD off included that it was no longer useful (48.4%), distracting (38.7%), inaccurate (9.7%), or there was a technical difficulty (3.2%). We found associations between certain pathologies and measures of utility (Table 4). Examples of each measure of utility are demonstrated in Figures 3-12 and Videos, Supplemental Digital Content 1 and 2. There were no complications related to HUD inaccuracy or over-reliance.
TABLE 3.
Measure of Utility
| Utility | ||
| Lesions Evaluated | 52 | 61.9% |
| Bed positioning | 26 | 50.0% |
| Skin incision | 9 | 17.3% |
| Craniotomy | 12 | 23.1% |
| Dural opening | 14 | 26.9% |
| Cortical incision | 7 | 13.5% |
| Arachnoid dissection | 19 | 36.5% |
| Intracranial drilling | 7 | 13.5% |
| Turned off | ||
| Yes | 31 | 59.6% |
| No | 21 | 40.4% |
| Reason to turn off | ||
| No longer needed | 15 | 48.4% |
| Distracting | 12 | 38.7% |
| Inaccurate | 3 | 9.7% |
| Technical difficulty | 1 | 3.2% |
HUD utility during phases other than lesion localization/resection was assessed for patients in the second half of the study by recording the other phases of surgery when HUD was used.
TABLE 4.
Utility of HUD by Pathology/Surgical Procedure
| Pathology/surgical procedure | Measure of utility |
|---|---|
| Intra-axial/superficial lesions | Skin incision |
| Craniotomy | |
| Dural opening | |
| Corticectomy | |
| Skull base lesions | Head/bed positioning |
| Extra and intradural drilling | |
| Vascular lesions | Arachnoid opening |
| Transphenoidal approach | Head/bed positioning |
| Extradural drilling | |
| Dural opening |
FIGURE 3.
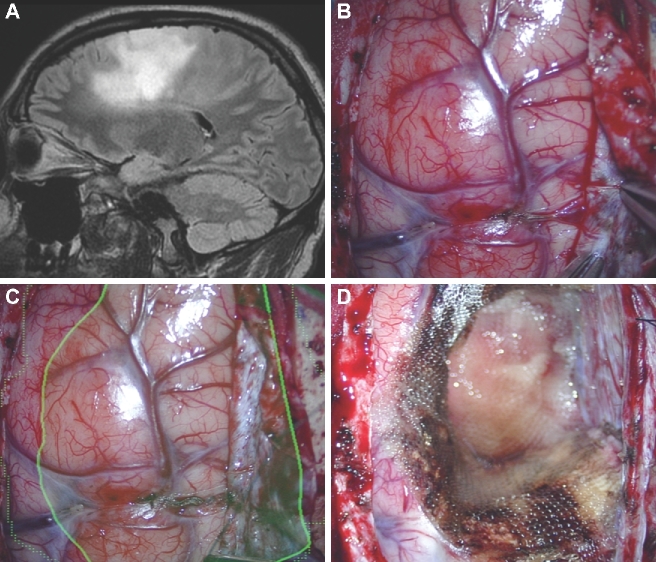
Bed/head positioning. A, A patient with a third ventricular hemangioblastoma. An interhemispheric and subfrontal approach was planned. HUD was activated prior to surgery. B, The patient's head was positioned with too much flexion, at first, and the HUD demonstrated that the approach to the lesion would transgress the frontal lobe. C, By extending the patient's head, the lesion was translated to a corridor along the skull base.
FIGURE 12.
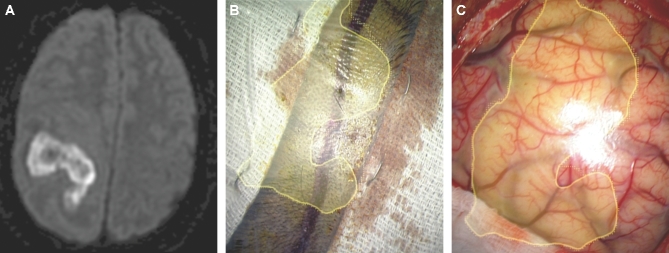
Identification of normal appearing pathological tissue. A, A patient with a first-time grand mal seizure was found to have a nonenhancing, flair positive left frontal lesion as seen in this sagittal flair MRI. In this case, the flair positive area was painted (green). B, After dural opening, normal appearing cortex was encountered. C, The HUD was turned on and outlined the flair positive lesion. D, The resection of normal-appearing tissue was guided by the HUD.
Figure 4.
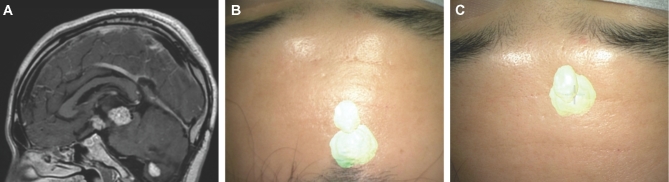
Skin incision. A, A patient with diabetes presenting with hemiplegia had a MRI with diffusion weighted imaging sequences performed, which demonstrated an extensive frontoparietal abscess. B, The HUD was used to outline the abscess (yellow) and was activated early to help tailor the correct skin incision. C, The HUD was then projected onto the cortical surface to understand the abscess location beneath the cortical surface.
FIGURE 5.
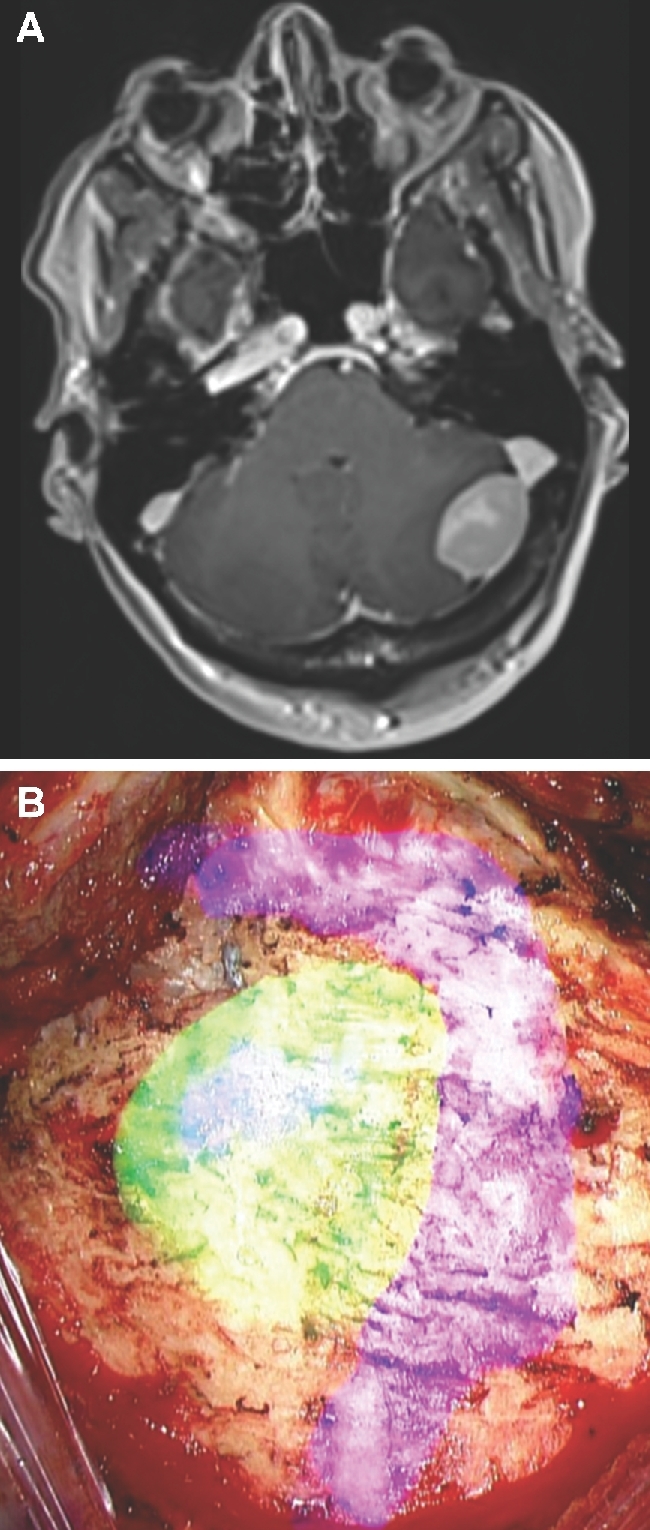
Craniotomy/craniectomy. A, A patient with a cerebellar meningioma at the junction of the transverse and sigmoid sinuses. B, The HUD was activated prior to performing the craniectomy and was used to demonstrate the tumor (green), dural sinuses (purple), and guide the craniectomy.
FIGURE 6.
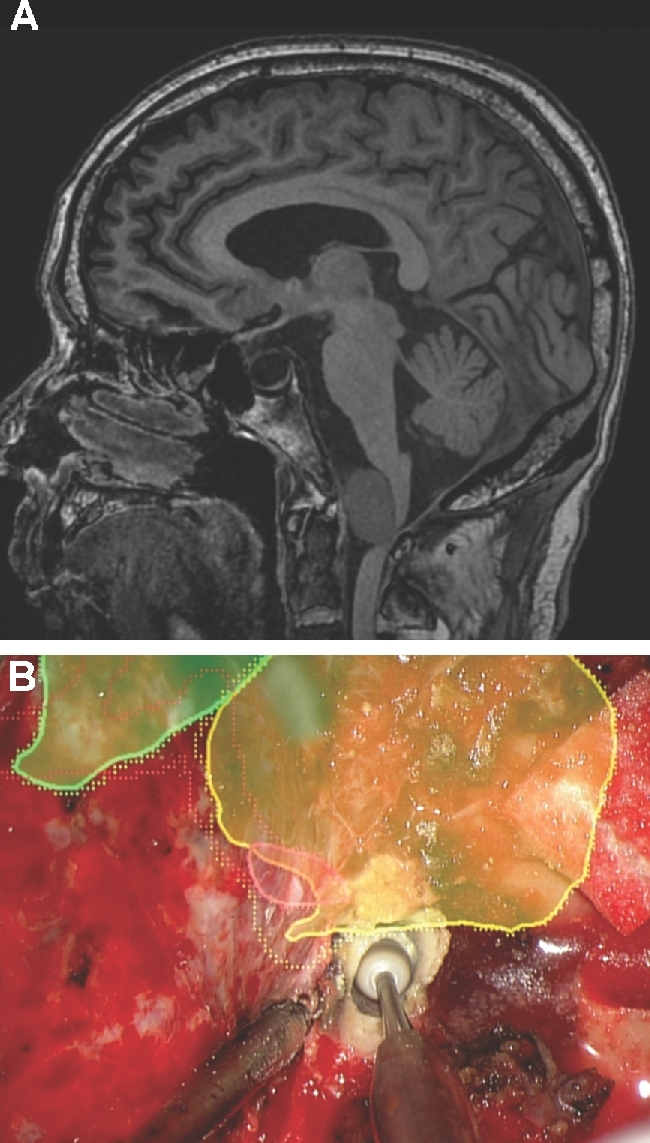
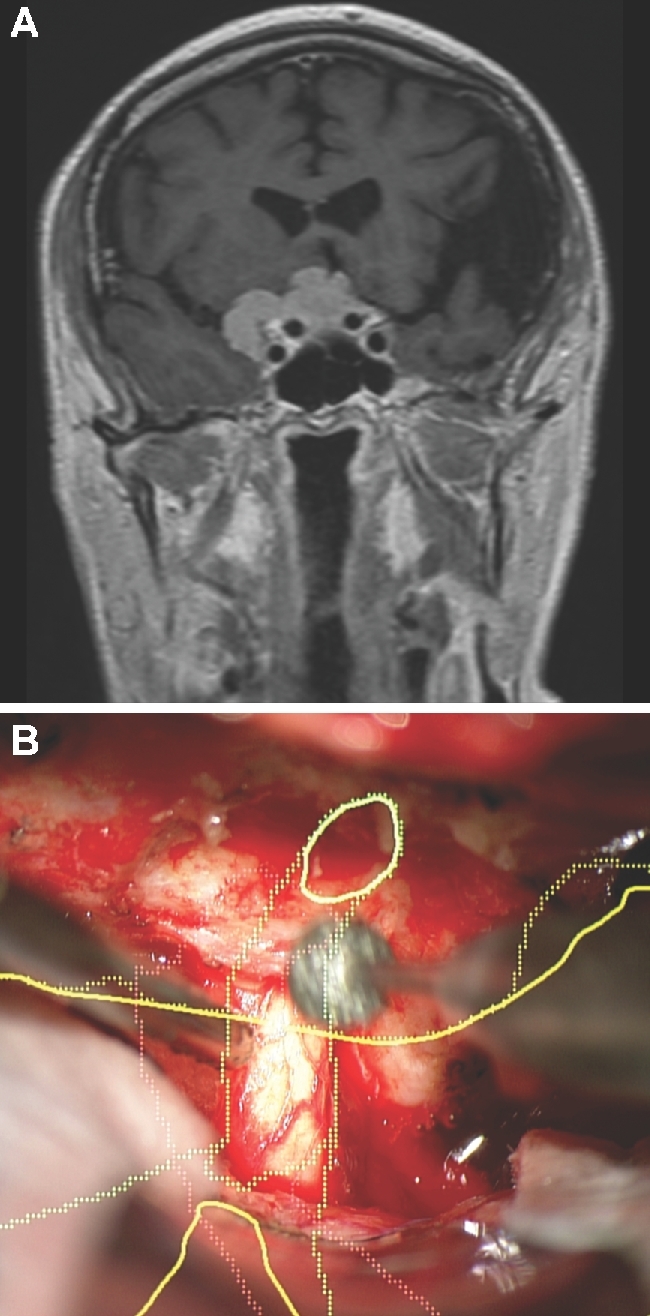
Extradural drilling. A, A patient with ataxia and hemiparesis was found to have large anterior foramen magnum meningioma, as seen here in this noncontrast sagittal MRI. The patient underwent a left far lateral craniectomy. B, The HUD was used to outline the tumor (yellow), vertebral artery (red), and brainstem (green). A C1 laminectomy was performed to reach the bottom of the tumor and drilling of the occipital condyle was tailored to reach the lateral aspect of the tumor as well as the intracranial vertebral artery.
FIGURE 7.
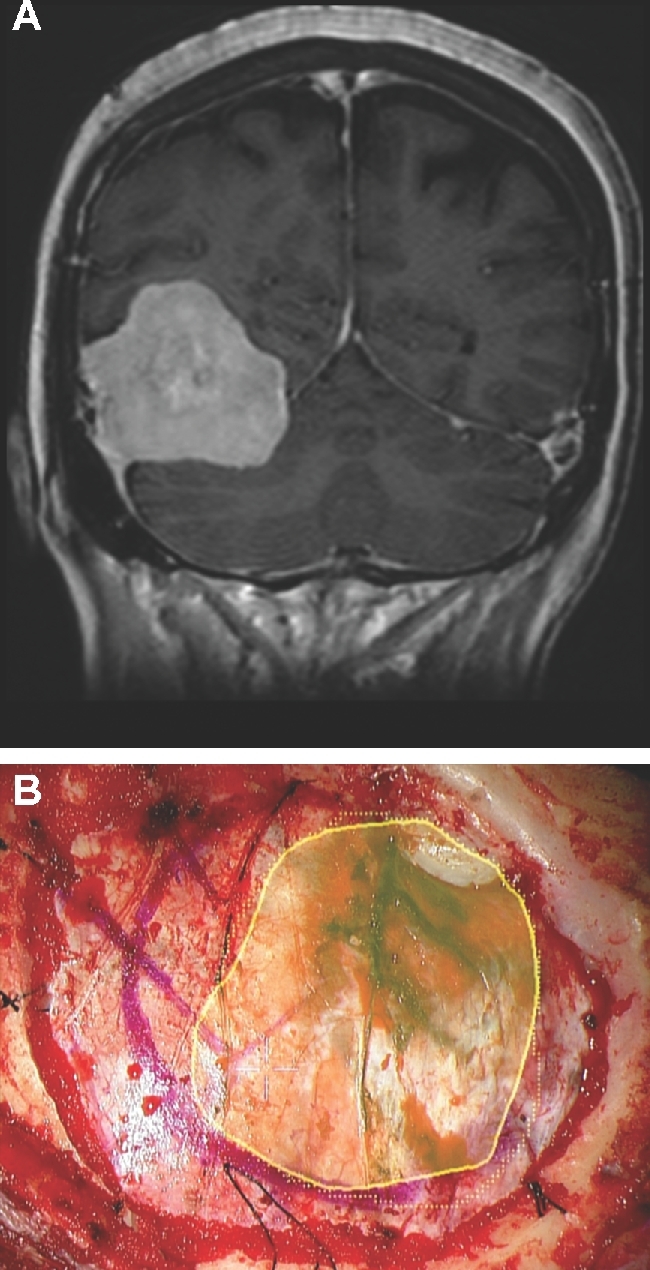
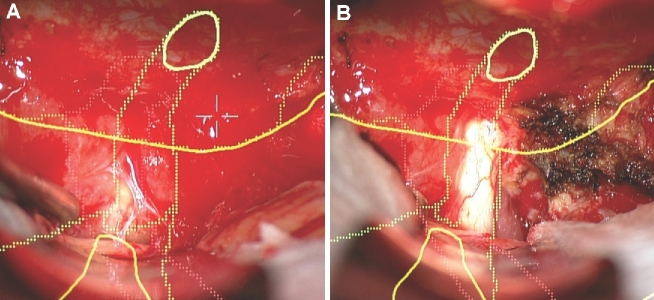
Dural opening. A, A patient with headaches was found to have a large right-sided tentorial meningioma, as seen here in a contrast enhanced coronal MRI. B, The patient underwent a temporal craniotomy. The HUD was used to outline the tumor (yellow), and was used to help guide the dural opening.
FIGURE 8.
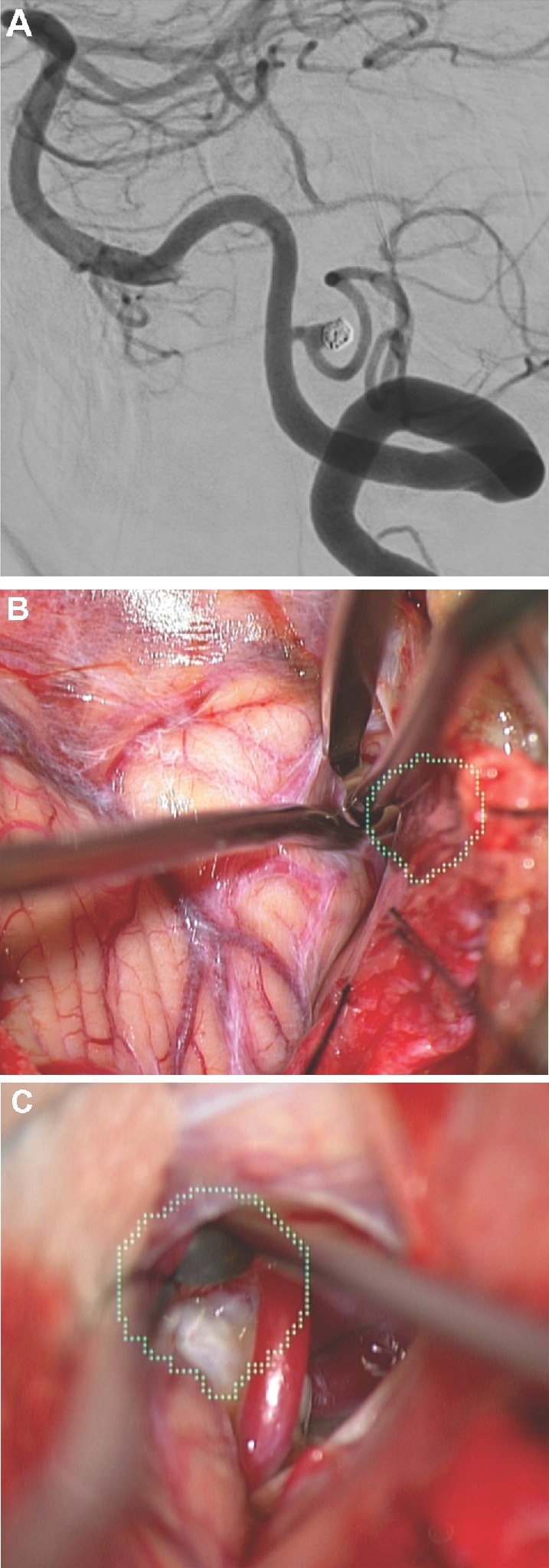
Arachnoid opening. A, A patient with previous subarachnoid hemorrhage from a ruptured posterior inferior cerebellar artery aneurysm with aneurysm recurrence following coiling, as seen here on a lateral digital subtraction angiography. B and C, In this case, the aneurysm was painted (green). The HUD was used to tailor a focused arachnoid opening directly over the aneurysm.
FIGURE 9.
Corticectomy. A, A patient with a lateral ventricular AVM that had previously undergone radiation, but was not obliterated, had undergone cystic change as seen in this contrast-enhanced coronal MRI with a planned temporal trajectory. In this case, the AVM was painted (red). The HUD was activated after dural opening and was used to choose a precise temporal cortisectomy (B) to reach this deep lesion (C). The HUD allowed for visualization of an accurate, narrow, and safe trajectory to a deep location.
FIGURE 10.
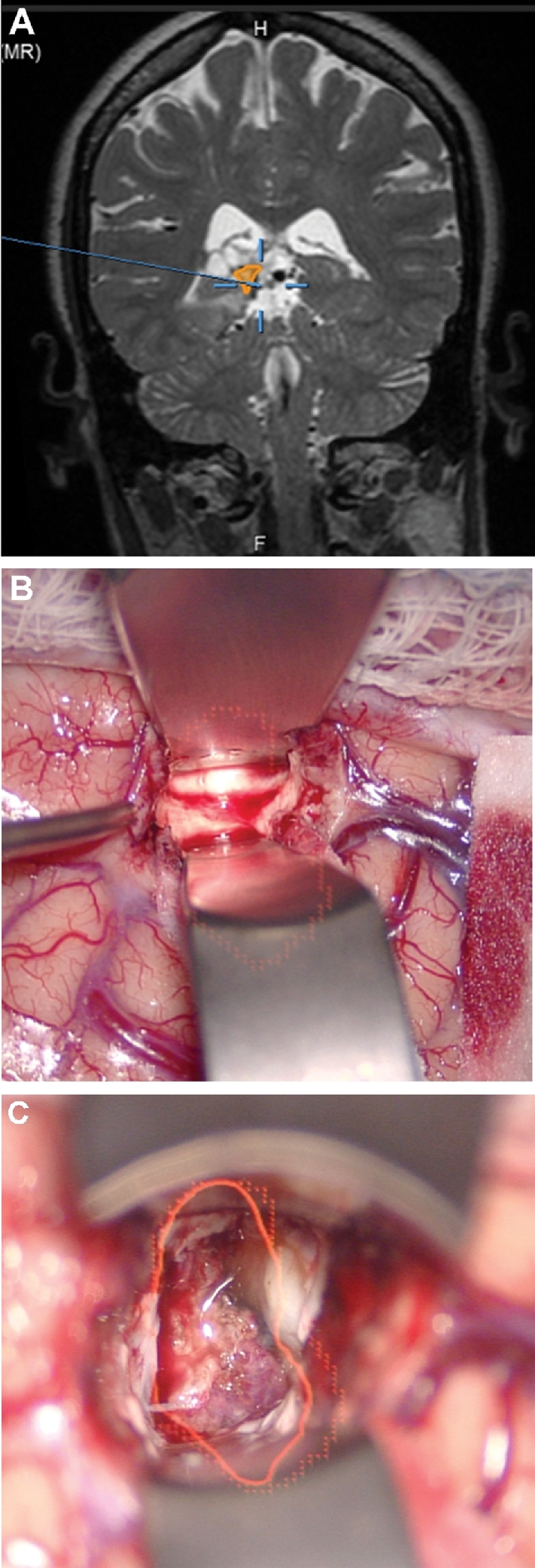
Intradural drilling. A, A patient with a tuberculum meningioma with a lateral extent, as seen on contrast enhanced coronal MRI. Given the lateral extent, the patient was selected for a transcranial approach, specifically a bifrontal craniectomy and subfrontal approach to the tumor. In this case, the tumor (yellow), optic nerves (green), and carotid arteries (red) were painted. The optic nerve can be seen entering the optic canal and then taking a normal slightly lateral trajectory. B, The HUD is used here to understand the course of the optic nerve within the optic canal while drilling the orbital roof.
FIGURE 11.
Identification of nerves/vessels within tumor. A, In the same patient shown in Figure 10, the optic nerve is first embedded within the tumor and difficult to visualize. B, The HUD provides guidance as to its location, and once a portion of the tumor has been removed, the optic nerve is better visualized.
DISCUSSION
Main Findings
This series is, to our knowledge, the largest experience using HUD to assist with intracranial surgery. We have shown here that HUD can be used for a wide variety of both vascular and oncologic pathologies both on the surface and in the depths of the brain. Excellent or good accuracy was maintained in the majority of cases (91.6%). Deep lesions were less likely to have excellent accuracy. The overlay can be used to outline not only the pathological lesion, but also surrounding vessels and nervous tissue that must be anticipated, identified, and preserved.
We have also shown that HUD has potential value during multiple stages of surgery (other than the lesion localization/resection) from as early as the skin incision/positioning to arachnoid dissection and intracranial drilling. Our experience was that HUD utility varied depending on pathology. We found that for intra-axial and superficial lesions, HUD was more useful for skin incision, craniotomy, dural opening, and corticectomy. On the other hand, for skull base lesions, HUD was more useful for bed/head positioning as well as extradural/intradural bone removal. These findings are intuitive, as intra-axial lesions require more unique operative plans, whereas skull base lesions generally follow a more typical surgical approach and depend on bone removal for adequate lesion exposure. HUD used during bed positioning, skin incision, craniotomy, and dural opening represents a deviation from normal microscope workflow. We have demonstrated here that HUD can be potentially used during these phases, but only if the operator deems that it would be useful.
During the tranphenoidal approach, HUD can be useful for choosing the correct trajectory to the sellar region, defining the carotid arteries and optic nerves from the nasal cavity, and in turn guiding the craniectomy, dural opening, and tumor resection. Although navigation is typically not utilized during aneurysm and intracranial stenosis surgery, HUD proved to have some utility in these cases. During aneurysm surgery, HUD can be used to visualize the aneurysm and tailor the arachnoid dissection. In addition, we used HUD to identify the target recipient vessel during the bypass surgery. Finally, based on our small experience with low-grade gliomas, we postulate that HUD can be useful for guiding the resection of lesions that do not appear abnormal to the naked eye.
HUD Limitations
HUD itself has 2 major limitations (Figure 13). First, HUD relies on navigation accuracy and any loss of accuracy can potentially lead to false reliance on HUD. HUD accuracy can be affected by poor intraoperative navigation, inaccurate object painting, brain shift, brain retraction, and may also deteriorate as the surgery progresses. Navigation accuracy should be confirmed throughout each procedure to assure validity of information provided by the HUD. This involves vigilance from members of the operating team including surgeons, circulating and scrub staff, anesthesia, and intraoperative neurophysiology monitoring teams to assure that the navigation star with fiducials is not moved during preparation or other phases of the operation. Further, loss of accuracy has different implications for different pathologies. For instance, imperfect accuracy can be tolerated for lesions such as extra-axial tumors or aneurysms, where HUD may serve as a guide to the general vicinity of the lesion, but the lesion itself is obvious thereafter. On the other hand, excellent HUD accuracy is essential for normal-appearing lesions, such as low-grade gliomas, and deep intra-axial lesions that have no other landmarks. It is essential that the operator understands the importance of accuracy for each case.
Figure 13.
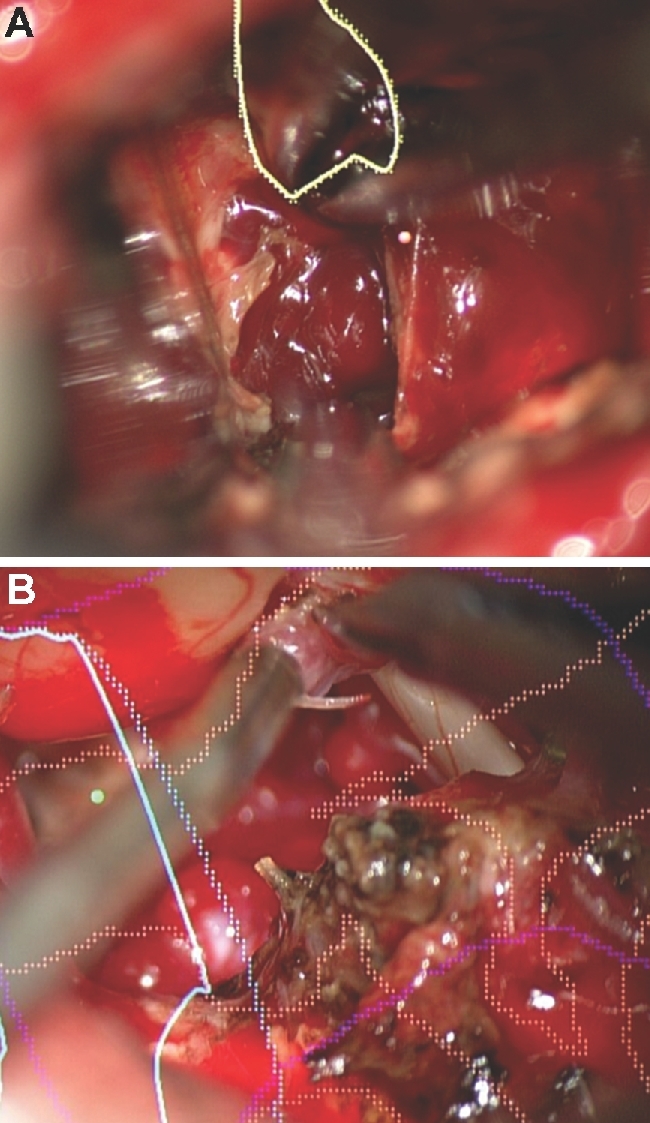
HUD limitations. The main limitations of HUD are inaccuracy as seen here with the HUD not accurately overlaying a cerebellar cavernous malformation (A) and distractibility as seen here during resection of a cerebellopontine angle AVM (B).
The second major HUD limitation is that the painted objects that are injected into the microscope can become distracting from the normal anatomy. There is a learning curve for visualizing normal anatomy while the HUD is active and for integrating information provided by the HUD graphical overlay. Distraction was cited as the reason for disabling the HUD in 38.7% of cases for which HUD was disabled during a case. There is certainly room for improvement in terms of seamlessly integrating HUD without disruption.
Previous Literature
There have been very few reports of HUD use in surgery. In 2016, Yoon et al1 reported the use of HUD for placement of 40 pedicle screws. The authors used Medtronic StealthStation (Medtronic, Dublin, Republic of Ireland) with the HUD injected into Google Glass (Google, Mountain View, California) in this instance. They found the HUD to be useful, especially when the neuronavigation monitor was placed behind the surgeon so that the surgeon did not have to turn his or her head 180°. The surgeons felt they could maintain focus on the operative task without having to move their head or shift focus. HUD has been used in ophthalmologic procedures,2,3 diabetic limb salvage surgery,4 orthopedic procedures,5 and bedside procedures such as central line placements.6 Anesthesiologists have used HUD to view vital signs.7 Many of these approaches utilize Google Glass. HUD has been used in the aviation industry for many years to project data on to the window in front of the pilots’ eyes. Similarly, automated surgical trajectories utilizing microscope-navigation integration have been described.8
The concept of augmented reality (AR) in neurosurgery has been explored as early as the 1990s,9,10 including reports of injecting overlays into the operative microscope.11 AR has been used in endoscopic transsphenoidal surgery with virtual images of the tumor and nearby structures overlaid onto the endoscopic tower view.12 Kockro et al13 described the Dex-Ray system in which a handheld probe on the skin surface integrated with projections on an adjacent screen. Deng et al14 described an easy-to-use AR neuronavigation system using a tablet PC to view the virtual image. Cabrilo et al15 described AR use for 28 patients with 39 unruptured aneurysms in which preoperative imaging was injected into the operative microscope. In this work, Brainlab was integrated with Zeiss, as it was in our study. The authors showed examples of bony anatomy projected onto the skin to tailor the incision, vessel anatomy projected onto the bony surface to tailor a craniotomy, as well as aneurysm projection onto the arachnoid to tailor the final dissection. The authors also describe its utility in positioning the head (10%), tailoring the craniotomy (63.3%), minimizing arachnoid dissection (66.7%), choosing clip position (92.3%), and its overall major impact (16.7%). The same authors also describe the use of AR in the treatment of AVMs and during bypass surgery.16,17 They found it to be less useful for obtaining relevant information regarding feeding arteries during the AVM surgery, but helpful in identifying donor and recipient vessels during the bypass surgery, especially outlining the superficial temporal artery on the skin beforehand.
Limitations
Our study is limited first by its retrospective nature. A prospective assessment of accuracy and utility would improve the strength of the study. Secondly, our assessments of accuracy and utility are entirely subjective and therefore it is difficult to truly quantify the accuracy and utility of HUD. Our assessment of accuracy was subjective (not objective). Further, we only assessed accuracy once during a given case, rather than at multiple time points to demonstrate if there is accuracy deterioration. Finally, we did not record the source of lost accuracy (eg, brain shift vs poor registration vs poor painting), which is an important factor to understand. Although we reported utility by describing the phases of surgery in which HUD was used, the decision to use HUD in a given phase of surgery was entirely operator dependent. Finally, we have not demonstrated its use in comparison to non-HUD cases and we have not demonstrated an impact on outcome. In future investigations, it would be useful to assess operative time, surgical approach, extent of resection, and patient outcome in HUD and non-HUD cases.
CONCLUSION
Our early experience with HUD technology demonstrates that it can be safely used for a wide variety of vascular and oncologic intracranial pathologies and has potential value during multiple stages of surgery. A prospective assessment of the technology with predetermined endpoints is needed.
Disclosure
The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
Supplementary Material
Supplemental digital content is available for this article at www.operativeneurosurgery-online.com.
COMMENTS
This paper reports a series of 60 patients who underwent treatment of 64 lesions using a ‘heads up display’ (HUD) navigation platform. The series is a reasonable size for the authors to describe the areas of utility for different pathologies and they have clearly devoted time to understanding the application of this new tool. However, much of the data is subjective and therefore it is difficult to quantify the true utility of HUD technology.
This study is unable to report actual system accuracy. Measurements were not taken, but rather retrospective estimates were made by the authors. This is a relatively flawed method in an already subjective paper. Inaccuracy due to shift is another critical factor in different phases of surgery. This was also not measured or estimated. Accuracy is apparently rated at very different parts of the case and no attempt to capture or report sources of error or loss of accuracy through the case is made.
HUD was apparently used for bed positioning and skin incision. Using a microscope during this phase reflects a dramatic change in workflow which could be cumbersome and is not well detailed nor of clear utility. The fact that HUD was not used throughout the case for most cases seems to be a sign of a potential workflow issue or lack of utility. Finally, ‘painting’ is required to use this technology and may be another source of inaccuracy which is not well detailed.
In the end, the fact that HUD was inactivated in over half of the cases does indicate that there is still work to be done on this technology to make it seamlessly integrated and not disruptive. The authors have done a good job describing the areas on which they used it, but there is a significant lack of objective data to support any conclusions. Future studies must better evaluate the accuracy and reliability of this system.
Paul A. Gardner
Pittsburgh, Pennsylvania
This is an interesting retrospective study of 79 patients who were operated upon with the use of HUD during some part of the procedure. Pathologies spanned the entire gamut of intracranial conditions. In the majority of cases the operators found the HUD to be useful at least for some part of the procedure and in 59% of the cases the HUD was at some point turned off. Not surprising HUD co-registered with the navigation system was useful from positioning, to the actual exposure of the pathology. Although the methodology used is suspect with respect to robustness of their findings, and despite all the shortcomings and limitations of the study that the authors describe, I find this to be an interesting addition to our literature as a preview of what surgical technique may look into the near and certainly further out future. Facility with technology that removes the operator a bit further away from direct access to the patient will be very important and studies such as this will help us at least raise the relevant questions we will need to focus on as we continue on this accelerated technological journey.
Philip Theodosopoulos
San Francisco, California
REFERENCES
- 1. Yoon JW, Chen RE, Han PK, Si P, Freeman WD, Pirris SM. Technical feasibility and safety of an intraoperative head-up display device during spine instrumentation. Int J Med Robot. 2016. doi:10.1002/rcs.1770. [DOI] [PubMed] [Google Scholar]
- 2. Ehlers JP, Srivastava SK, Feiler D et al. . Integrative advances for OCT-guided ophthalmic surgery and intraoperative OCT: microscope integration, surgical instrumentation, and heads-Up display surgeon feedback PLoS One. 2014;9(8):e105224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Eckardt C, Paulo EB. Heads-Up surgery for vitreoretinal procedures Retina. 2016;36(1):137-147. [DOI] [PubMed] [Google Scholar]
- 4. Armstrong DG, Rankin TM, Giovinco NA, Mills JL, Matsuoka Y. A heads-up display for diabetic limb salvage surgery J Diabetes Sci Technol. 2014;8(5):951-956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chimenti PC, Mitten DJ. Google glass as an alternative to standard fluoroscopic visualization for percutaneous fixation of hand fractures Plast Reconstr Surg. 2015;136(2):328-330. [DOI] [PubMed] [Google Scholar]
- 6. Wu TS, Dameff CJ, Tully JL. Ultrasound-guided central venous access using google glass J Emerg Med. 2014;47(6):668-675. [DOI] [PubMed] [Google Scholar]
- 7. Liu D, Jenkins SA, Sanderson PM, Fabian P, Russell WJ. Monitoring with head-mounted displays in general anesthesia Anesth Analg. 2010;110(4):1032-1038. [DOI] [PubMed] [Google Scholar]
- 8. Oppenlander ME, Chowdhry SA, Merkl B, Hattendorf GM, Nakaji P, Spetzler RF. Robotic autopositioning of the operating microscope. Neurosurgery. 2014;10(suppl 2):214-219; discussion 219. [DOI] [PubMed] [Google Scholar]
- 9. Wagner A, Ploder O, Enislidis G, Truppe M, Ewers R. Image-guided surgery. Int J Oral Maxillofac Surg. 1996;25(2):147-151. [DOI] [PubMed] [Google Scholar]
- 10. Iseki H, Masutani Y, Iwahara M. Volumegraph (overlaid three-dimensional image-guided navigation). Clinical application of augmented reality in neurosurgery. Stereotact Funct Neurosurg. 1997;68(1-4 pt 1):18-24. PMID: 9711690 [DOI] [PubMed] [Google Scholar]
- 11. King AP, Edwards PJ, Maurer CR Jr et al. . A system for microscope-assisted guided interventions. Stereotact Funct Neurosurg. 1999;72(2-4):107-111. [DOI] [PubMed] [Google Scholar]
- 12. Kawamata T, Iseki H, Shibasaki T, Hori T. Endoscopic augmented reality navigation system for endonasal transsphenoidal surgery to treat pituitary tumors: technical note. Neurosurgery. 2002;50(6):1393-1397. [DOI] [PubMed] [Google Scholar]
- 13. Kockro RA, Tsai YT, Ng I et al. . DEX-RAY. Neurosurgery. 2009;65(4):795-808; discussion 807-798. [DOI] [PubMed] [Google Scholar]
- 14. Deng W, Li F, Wang M, Song Z. Easy-to-use augmented reality neuronavigation using a wireless tablet PC Stereotact Funct Neurosurg. 2014;92(1):17-24. [DOI] [PubMed] [Google Scholar]
- 15. Cabrilo I, Bijlenga P, Schaller K. Augmented reality in the surgery of cerebral aneurysms. Neurosurgery. 2014;10(suppl 2):252-261; discussion 260-251. [DOI] [PubMed] [Google Scholar]
- 16. Cabrilo I, Bijlenga P, Schaller K. Augmented reality in the surgery of cerebral arteriovenous malformations: technique assessment and considerations. Acta Neurochir. 2014;156(9):1769-1774. [DOI] [PubMed] [Google Scholar]
- 17. Cabrilo I, Schaller K, Bijlenga P. Augmented reality-assisted bypass surgery: embracing minimal invasiveness. World Neurosurg. 2015;83(4):596-602. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.