Abstract
Hypomorphic mutations in six different genes involved in the glycosylphosphatidylinositol (GPI) biogenesis pathway are linked to Mabry syndrome (hyperphosphatasia with mental retardation syndrome, HPMRS). This report on the third affected family with a HPMRS phenotype caused by mutations in PIGL, confirming the seventh GPI biogenesis gene linked to HPMRS. Two siblings presented with the main features of HPMRS; developmental delay, cognitive impairment, seizure disorder, skeletal deformities, and high alkaline phosphatase. We identified two heterozygous mutations in the PIGL gene (P.Trp20Ter and p.Arg88Cys). PIGL mutations have been linked to another distinctive neuroectodermal disorder: CHIME syndrome. The clinical picture of our patients expands the spectrum of PIGL-related phenotypes.
Keywords: GPI biogenesis, Hyperphosphatasia mental retardation syndrome (HPMRS), Mabry syndrome, PIGL gene, CHIME syndrome
Abbreviations: GPI, glycosylphosphatidylinositol; HPMRS, hyperphosphatasia with mental retardation syndrome; CHIME, ocular colobomas, heart defect, ichthyosis, mental retardation, and abnormal ears or epilepsy; PIGL, phosphatidylinositol glycan anchor biosynthesis class L; ALP, alkaline phosphatase; CSS, Coffin-Siris syndrome
1. Introduction
Glycosylphosphatidylinositol (GPI) glycolipids play a critical role in the post-translation modification and cell membrane anchoring of >150 eukaryotic proteins which ultimately affect the sorting, trafficking, and dynamics of those proteins. Also, they participate in the process of embryogenesis, neurogenesis, immune responses, and fertilization. The biogenesis of mature GPI involves >20 genes and occurs in eukaryotes via a conserved post-translational pathway [1].
In animal studies, complete deletion of the GPI pathway results in embryonic lethality. However, complete deletion has not been reported in humans. Hypomorphic mutations in the genes encoding the GPI biogenesis pathway result in partial reduction of GPI-anchored proteins, which leads to phenotypically heterogeneous clinical presentations, with global developmental delay a common feature. This element supports the notion that GPI-anchored proteins play a vital role in neurogenesis [2,3].
PIGL encodes the endoplasmic phosphatidylinositol glycan anchor biosynthesis class L, which is involved in the second step of GPI biosynthesis: the de-n-acetylation of n-acetylglucosaminyl-phosphatidylinositol (GluNAc-PI), generating glucosaminyl-phosphatidylinositol (GlcN-PI) on the cytoplasmic side of the endoplasmic reticulum [4].
Mutations in PIGL have been linked to two rare distinctive syndromes: CHIME syndrome (Zunich neuroectodermal syndrome) and Mabry syndrome (hyperphosphatasia with mental retardation syndrome, HPMRS) [5,6].
2. Clinical data
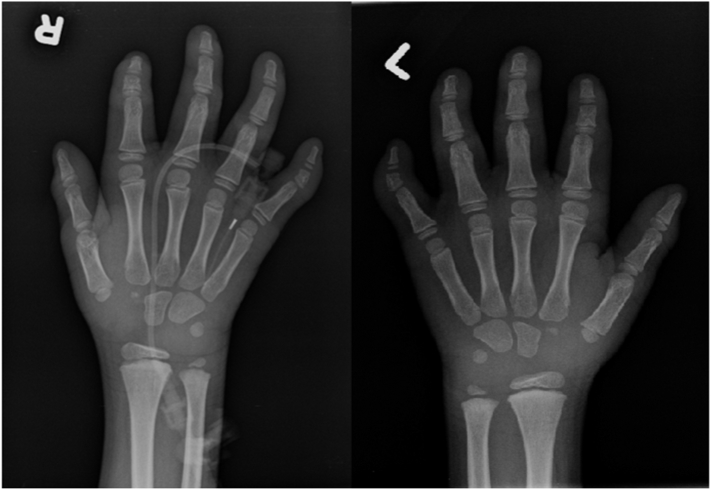
The proband is a 10-year-old West African male who is the first offspring of a non-consanguineous couple. He was delivered spontaneously at term after an uneventful pregnancy weighing 2665 g (5th percentile) with a head circumference of 32 cm (5th percentile) and Apgar score of 9. At the age of 6 months, he presented with myoclonic seizures that were partially controlled with medications. By the age of 12 months, he had surgical correction of inguinal hernia, hydrocele, and strabismus. His developmental milestones were globally delayed, including cognitive, language, gross and fine motor functions, with no major improvement despite intensive rehabilitation. His first available baseline physical examination was documented at 4 years of age. Growth parameters were normal with weight, height and head circumference on the 75th percentile. The facial features were coarsely dysmorphic, including prominent forehead, high arched eyebrows and sparse on the outer third, nystagmus, mild telecanthus, uplifted ear lobes, and open mouth with intermittent drooling. In addition, he had mild pectus excavatum and clinodactyly involving the fifth digits, 4th and 5th toes bilaterally. (Fig. 1, hand X-ray). No skin abnormalities were noted. Other features included reducible umbilical hernia, mild hepatomegaly, and ataxic gait. Audiology and heart evaluations were normal. Brain MRI showed symmetrical bilateral patchy signal abnormalities in the periventricular zones in the parietal, occipital and frontal regions, white matter loss, and thin corpus callosum. Liver enzymes showed mildly elevated transaminases with significantly elevated ALP 968 U/L (normal: 135–320 U/L). Vitamin E and alpha-fetoprotein were normal. All metabolic tests were unremarkable, including but not limited to purines/pyrimidines, glycosaminoglycans, creatine panel, carbohydrate transferrin, homocysteine, sterol profiles, and creatine kinase. Array CGH and Coffin-Siris molecular testing were normal.
Fig. 1.
X-ray hands showed deformity of the middle phalanx of the 5th finger bilaterally with the lateral aspect of the phalanx being shorter than the medial aspect.
Evaluation of the patient's younger 4-year-old brother revealed a very similar presentation. He had unremarkable antenatal and perinatal history. His developmental milestones were globally delayed. He developed his first onset of partially controlled tonic-clonic seizures at the age of 4 months. Strabismus was surgically corrected at the age of 1 year. On examination, he was inattentive with coarse facial features including high hairlines, hypertelorism, epicanthal folds, horizontal nystagmus, depressed nasal bridge, delayed teeth eruption, everted and partially bifid lower lip, folded ears, and irregular hypopigmented skin margins surrounding the eyes and the nose. His head circumference was 51 cm (75th percentile). Systemic examination revealed spastic lower limbs, bilateral brachydactyly, severe clinodactyly of both fifth fingers and toes and dry eczematous skin. No chest deformity or organomegaly was noted. His ALP was 454 U/L.
3. Molecular data
A commercially available multigene panel for intellectual disability (GeneDx) was performed in the proband and revealed two variants in PIGL gene, a nonsense (c.60G > A; p.Trp20Ter; W20X) pathogenic variant and missense (c.262C > T; p.Arg88Cys; R88C) variant. Neither variant was observed in approximately 65,000 individuals of European and African American ancestry in the NHLBI Exome Sequencing Project. The first variant was interpreted as pathogenic as it causes loss of normal protein function through truncation or nonsense-mediated mRNA decay. For the second variant, in silico analysis predicted it as likely damaging the protein structure and function. Parental analyses were done by Sanger sequencing and confirmed in trans mutations. Targeted genetic testing for the brother detected the same variants.
4. Discussion
In 1970, Mabry et al. described a unique syndrome comprised of severe cognitive impairment, seizures, various neurologic deficits, and elevated serum ALP [6]. This syndrome was acronymed HPMRS-1(OMIM: 239300) [7] and linked to mutations in the PIGV gene, which is involved in the early steps of GPI anchor assembly [8]. Subsequently, other pathogenic defects in genes involved in either GPI anchor assembly or maturation were linked to the same phenotype. [Table 1].
Table 1.
The clinical feature of the previously reported patients with Hyperphosphatasia with Mental Retardation syndrome types (HPMRS).
| Type |
HPMRS1 |
HPMRS2 |
HPMRS3 |
HPMRS4 |
HPMRS5 |
HPMRS6 |
HPMRS7⁎ |
|---|---|---|---|---|---|---|---|
| Gene | PIGV | PIGO | PGAP2 | PGAP3 | PIGW | PIGY | PIGL |
| No. of reported cases | 29 | 3 | 13 | 5 | 1 | 4 | 4(2 in this report) |
| Cognitive impairment | + | + | + | + | + | + | + |
| Seizure | + | + | + | + | + | + | + |
| High ALP | + | + | + | + | + | + | + |
| Growth | NA | Poor growth | NA | Poor growth | NA | Poor growth | Normal |
| Dysmorphic features | + | + | NA | + | + | + | + |
| Heart defects | VSD | ASD | NA | NA | NA | NA | No |
| GI/GU anomalies | Feeding problem Anorectal anomalies | Anal stenosis/atresia vesicoureteral reflux | NA | NA | NA | Poor feeding renal dilatation increased renal parenchyma echogenicity | Hepatomegaly mild elevated transaminases |
| Brian, skull anomalies | Plagio-cephaly | Microcephaly enlarged ventricles plagiocephaly coronal synostosis | Micro-cephaly cerebral atrophy | Micro-cephaly | NA | Microcephaly | Thin corpus callosum |
| Skeletal anomalies | Hypoplastic terminal phalanges tapered fingers hypoplastic toes bilateral adducted forefoot hypoplastic/curved nails | Brachy-telephalangy broad halluces hypoplastic/absent nails | NA | NA | NA | Joint contractures osteopenia Hip dysplasia proximal limb shortening brachyphalangy clinodactyly | Bilateral brachydactly severe clinodactly of fifth figures bilateral clinodactly (4th and 5th toes) |
Abbreviations: *the proposed 7th type of HPMRS in this report, MR: Mental retardation, ALP: alkaline phosphatase, NA: not available, GI/GU: gastrointestinal and genitourinary systems.
The PIGL gene is responsible for the second step of GPI biosynthesis. Mutations in this gene were initially reported in seven cases presenting with CHIME syndrome. CHIME (OMIM: 280000, ocular Colobomas, Heart defect, Ichthyosis, Mental Retardation, and abnormal Ears or Epilepsy) was first described clinically by Zunich et al. in 1983, acronymed by Shashi et al. in 1995, and linked to PIGL mutations by Ng et al. in 2012. All patients presented the main features of CHIME syndrome plus a variable combination of facial dysmorphism such as brachycephaly, flat and broad nasal root, short philtrum, hypertelorism, cloudy corneas, overfolded helices, wide mouth, full lips, cleft palate, and abnormal dentation. Mildly elevated alkaline phosphatase was reported in only one patient who presented with a clinical phenotype of CHIME syndrome (not molecularly confirmed) in the setting of acute lymphocytic leukemia [5,[9], [10], [11], [12]].
Recently, in addition to its association with CHIME syndrome, mutations in PIGL have been linked to HPMRS in two cases [13,14]. We are reporting the third family with a HPMRS phenotype secondary to PIGL mutations supported by the clinical presentations of cognitive delay, seizures, skeletal deformities, and elevated ALP. Our patients had some overlapping features between CHIME and HPMRS, particularly in the characteristic facial features, seizures, and global developmental delay. However, the absence of coloboma, heart defect and hearing impairment, in addition to the distinctive skeletal phenotype of short terminal phalanges and the high ALP made the presentation more suggestive of HPMRS.
The phenotypic difference between HPMRS and CHIME syndromes could be related to the type of PIGL mutation. All the reported cases with a HPMRS phenotype, including our patients, presented at least one PIGL mutation leading to complete loss of the protein's function [13,14]. On the other hand, all reported cases of CHIME shared the same p.Leu176Pro mutation in a compound heterozygous state with another pathogenic variant. Homozygous p.Leu176Pro mutations have never been reported. The p.Leu176Pro alteration has been shown to preserve some of the protein's function, thus allowing a milder phenotype [10,12].
The craniofacial features that our patients share with the first reported case of HPMRS secondary to PIGL mutation include hypertelorism, high palate, strabismus, ear anomalies, and the skeletal deformities of the hands. However, the additional minor facial traits described by Fujiwara et al. were lacking in our patients [13]. Interestingly, the coarse facial features observed in our patients were previously reported in patients with HPMRS where there was initial suspicion of a lysosomal storage disorder [7]. All of the HPMRS types share the phenotype of global developmental delay, seizure disorder, and high ALP [15]. The hypoplastic terminal phalanges were reported in HPMRS type 1 and 2 only. This skeletal finding could lead to suspicion of Coffin-Siris syndrome given the neurological findings in both syndromes [Table 1] [[16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28]].
The elevated ALP in HPMRS is the nonspecific ALPL isoform (liver/bone/kidney). Defects in GPI biogenesis affect the expression of the anchored proteins, including ALP. The exact mechanism of ALP elevation in HPMRS is not well-explained. Experimental studies on mutant CHO cells showed that defects in the genes involved in the later steps of the pathway efficiently secrete ALP into the medium and consequently give high ALP levels. In comparison, mutations in the early steps will degrade the ALP in the cells and result in normal ALP levels. Additionally, GPI transamidase, which is activated through binding with a mannose-containing GPI intermediate before the enzyme complex processes the precursor proteins for release, plays a major role in expressing the proteins [29]. This could explain the elevated ALP levels in mutations involved in some of the late steps of GPI anchor synthesis including PIGV (fifth step) and PIGO (eighth step). However, elevated ALP levels were not observed in other late steps of GPI synthesis including PIGN in the sixth step and PIGT in the tenth step. On the other hand, mutations in the final step of GPI-anchor fatty acid remodeling, mainly in PGAP2 and PGAP3, resulted in an elevated ALP level [30]. Mutation in PIGL, which involves the second step of GPI synthesis, is another exception for the hypothesized theory. Further studies are needed to understand the factors affecting the expression of GPI-anchored proteins.
ALP is essential for dephosphorylation of the circulating pyridoxal 5-phosphate to pyridoxal. Defects in anchoring ALP could predispose to B6-responsive seizures in HPMRS in a similar manner as in hypophosphatasia (OMIM: 241500) secondary to ALPL (alkaline phosphatase, liver/bone/kidney) mutations as both might affect the function of the ALP enzyme. Pyridoxine supplements in two patients with HPMRS, one with PIGO mutations and one with unknown underlying gene mutations, showed a good clinical and electroencephalographic response [31,32].
Most of the GPI biogenesis syndromes are diagnosed by whole exome/genome sequencing due to the rarity of those disorders, the heterogeneity in the clinical phenotypes, and the lack of clear biochemical markers. Even if very rare (<0.5% of developmental disorders), including ALP as a simple, sensitive screening test in patients presenting with unexplained developmental delay would allow detecting this sub-type of GPI biogenesis disorders [14]. Further studies are emerging to correlate elevated ALP to GPI biogenesis disorders and to potential therapies for those disorders i.e. the role of pyridoxine for seizure control.
In conclusion, our clinical, biochemical and molecular findings support the previous reports of HPMRS caused by PIGL mutations. PIGL should be considered in the differential diagnosis of the known PIG classes (PIGV, PIGO, and PIGW) and the post-GPI attachment to proteins genes (PGAP2, PGAP3) that are currently linked to HPMRS.
Acknowledgments
Acknowledgment
We thank Dr. Sara Aldekhyl and Andrea Secord for their help in editing the manuscript, and we thank the patients' family who kindly agreed for sharing their children's cases.
Conflict of interest
Ruqaiah Altassan declares no conflict of interest.
Stephanie Fox declares no conflict of interest.
Chantal Poulin declares no conflict of interest.
Daniela Buhas declares no conflict of interest.
References
- 1.Ferguson M.A.J., Kinoshita T., Hart G.W. Glycosylphosphatidylinositol anchors. In: Varki A., Cummings R.D., Esko J.D., editors. Essentials of Glycobiology. 2nd ed. Cold Spring Harbor Laboratory Press; Cold Spring Harbor (NY): 2009. https://www.ncbi.nlm.nih.gov/books/NBK1966/ Chapter 11. (Available from:) [Google Scholar]
- 2.Kinoshita T. Biosynthesis and deficiencies of glycosylphosphatidylinositol. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2014;90(4):130–143. doi: 10.2183/pjab.90.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Freeze H.H., Chong J.X., Bamshad M.J., Ng B.G. Solving glycosylation disorders: fundamental approaches reveal complicated pathways. Am. J. Hum. Genet. 2014 Feb 6;94(2):161–175. doi: 10.1016/j.ajhg.2013.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.OMIM (Online Mendelian Inheritance in Man).
- 5.Zunich J., Kaye C.I. New syndrome of congenital ichthyosis with neurologic abnormalities. Am. J. Med. Genet. 1983 Jun;15(2):331–333. doi: 10.1002/ajmg.1320150217. [DOI] [PubMed] [Google Scholar]
- 6.Mabry C.C., Bautista A., Kirk R.F.H., Dubilier L.D., Braunstein H., Koepke J.A. Familial hyper phosphatase with mental retardation, seizures, and neurologic deficits. J. Pediatr. 1970 Jul;77(1):74–85. doi: 10.1016/s0022-3476(70)80047-6. [DOI] [PubMed] [Google Scholar]
- 7.Thompson M.D., Nezarati M.M., Gillessen-Kaesbach G., Meinecke P., Mendoza-Londono R., Mornet E., Brun-Heath I., Squarcioni C.P., Legeai-Mallet L., Munnich A., Cole D.E. Hyperphosphatasia with seizures, neurologic deficit, and characteristic facial features: five new patients with Mabry syndrome. Am. J. Med. Genet. A. 2010 Jul;152A(7):1661–1669. doi: 10.1002/ajmg.a.33438. [DOI] [PubMed] [Google Scholar]
- 8.Krawitz P.M., Schweiger M.R., Rödelsperger C., Marcelis C., Kölsch U., Meisel C., Stephani F., Kinoshita T., Murakami Y., Bauer S., Isau M., Fischer A., Dahl A., Kerick M., Hecht J., Köhler S., Jäger M., Grünhagen J., de Condor B.J., Doelken S., Brunner H.G., Meinecke P., Passarge E., Thompson M.D., Cole D.E., Horn D., Roscioli T., Mundlos S., Robinson P.N. Identity-by-descent filtering of exome sequence data identifies PIGV mutations in hyperphosphatasia mental retardation syndrome. Nat. Genet. 2010 Oct;42(10):827–829. doi: 10.1038/ng.653. [DOI] [PubMed] [Google Scholar]
- 9.Shashi V., Zunich J., Kelly T.E., Fryburg J.S. Neuroectodermal (CHIME) syndrome: an additional case with long term follow up of all reported cases. J. Med. Genet. 1995 Jun;32(6):465–469. doi: 10.1136/jmg.32.6.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ng B.G., Hackmann K., Jones M.A., Eroshkin A.M., He P., Wiliams R., Bhide S., Cantagrel V., Gleeson J.G., Paller A.S., Schnur R.E., Tinschert S., Zunich J., Hegde M.R., Freeze H.H. Mutations in the glycosylphosphatidylinositol gene PIGL cause CHIME syndrome. Am. J. Hum. Genet. 2012 Apr 6;90(4):685–688. doi: 10.1016/j.ajhg.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.RE1 Schnur, Greenbaum B.H., Heymann W.R., Christensen K., Buck A.S., Reid C.S. Acute lymphoblastic leukemia in a child with the CHIME neuroectodermal dysplasia syndrome. Am. J. Med. Genet. 1997 Oct 3;72(1):24–29. [PubMed] [Google Scholar]
- 12.Knight Johnson A., Schaefer G.B., Lee J., Hu Y., Del Gaudio D. Alu-mediated deletion of PIGL in a patient with CHIME syndrome. Am. J. Med. Genet. A. 2017 May;173(5):1378–1382. doi: 10.1002/ajmg.a.38181. [DOI] [PubMed] [Google Scholar]
- 13.Fujiwara I., Murakami Y., Niihori T., Kanno J., Hakoda A., Sakamoto O., Okamoto N., Funayama R., Nagashima T., Nakayama K., Kinoshita T., Kure S., Matsubara Y., Aoki Y. Mutations in PIGL in a patient with Mabry syndrome. Am. J. Med. Genet. A. 2015 Apr;167A(4):777–785. doi: 10.1002/ajmg.a.36987. [DOI] [PubMed] [Google Scholar]
- 14.Pagnamenta A.T., Murakami Y., Taylor J.M., Anzilotti C., Howard M.F., Miller V., Johnson D.S., Tadros S., Mansour S., Temple I.K., Firth R., Rosser E., Harrison R.E., Kerr B., Popitsch N., Study D.D.D., Kinoshita T., Taylor J.C., Kini U. Analysis of exome data for 4293 trios suggests GPI-anchor biogenesis defects are a rare cause of developmental disorders. Eur. J. Hum. Genet. 2017 Jun;25(6):669–679. doi: 10.1038/ejhg.2017.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cole D.E., Thompson M.D. Neurogenetic aspects of Hyperphosphatasia in Mabry syndrome. Subcell. Biochem. 2015;76:343–361. doi: 10.1007/978-94-017-7197-9_16. [DOI] [PubMed] [Google Scholar]
- 16.Horn D., Schottmann G., Meinecke P. Hyperphosphatasia with mental retardation, brachytelephalangy, and a distinct facial gestalt: delineation of a recognizable syndrome. Eur. J. Med. Genet. 2010 Mar-Apr;53(2):85–88. doi: 10.1016/j.ejmg.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 17.Horn D., Krawitz P., Mannhardt A., Korenke G.C., Meinecke P. Hyperphosphatasia-mental retardation syndrome due to PIGV mutations: expanded clinical spectrum. Am. J. Med. Genet. A. 2011 Aug;155A(8):1917–1922. doi: 10.1002/ajmg.a.34102. [DOI] [PubMed] [Google Scholar]
- 18.Horn D., Wieczorek D., Metcalfe K., Barić I., Paležac L., Cuk M., Petković Ramadža D., Krüger U., Demuth S., Heinritz W., Linden T., Koenig J., Robinson P.N., Krawitz P. Delineation of PIGV mutation spectrum and associated phenotypes in hyperphosphatasia with mental retardation syndrome. Eur. J. Hum. Genet. 2014 Jun;22(6):762–767. doi: 10.1038/ejhg.2013.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xue J., Li H., Zhang Y., Yang Z. Clinical and genetic analysis of two Chinese infants with Mabry syndrome. Brain Dev. 2016 Oct;38(9):807–818. doi: 10.1016/j.braindev.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 20.Nakamura K., Osaka H., Murakami Y., Anzai R., Nishiyama K., Kodera H., Nakashima M., Tsurusaki Y., Miyake N., Kinoshita T., Matsumoto N., Saitsu H. PIGO mutations in intractable epilepsy and severe developmental delay with mild elevation of alkaline phosphatase levels. Epilepsia. 2014 Feb;55(2):e13–7. doi: 10.1111/epi.12508. [DOI] [PubMed] [Google Scholar]
- 21.Krawitz P.M., Murakami Y., Hecht J., Krüger U., Holder S.E., Mortier G.R., Delle Chiaie B., De Baere E., Thompson M.D., Roscioli T., Kielbasa S., Kinoshita T., Mundlos S., Robinson P.N., Horn D. Mutations in PIGO, a member of the GPI-anchor-synthesis pathway, cause hyperphosphatasia with mental retardation. Am. J. Hum. Genet. 2012 Jul 13;91(1):146–151. doi: 10.1016/j.ajhg.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krawitz P.M., Murakami Y., Rieß A., Hietala M., Krüger U., Zhu N., Kinoshita T., Mundlos S., Hecht J., Robinson P.N., Horn D. PGAP2 mutations, affecting the GPI-anchor-synthesis pathway, cause hyperphosphatasia with mental retardation syndrome. Am. J. Hum. Genet. 2013 Apr;4(92):584–589. doi: 10.1016/j.ajhg.2013.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Naseer M.I., Rasool M., Jan M.M., Chaudhary A.G., Pushparaj P.N., Abuzenadah A.M., Al-Qahtani M.H. A novel mutation in PGAP2 gene causes developmental delay, intellectual disability, epilepsy and microcephaly in consanguineous Saudi family. J. Neurol. Sci. 2016 Dec 15;371:121–125. doi: 10.1016/j.jns.2016.10.027. [DOI] [PubMed] [Google Scholar]
- 24.Howard M.F., Murakami Y., Pagnamenta A.T., Daumer-Haas C., Fischer B., Hecht J., Keays D.A., Knight S.J., Kölsch U., Krüger U., Leiz S., Maeda Y., Mitchell D., Mundlos S., Phillips J.A., III, Robinson P.N., Kini U., Taylor J.C., Horn D., Kinoshita T., Krawitz P.M. Mutations in PGAP3 impair GPI-anchor maturation, causing a subtype of hyperphosphatasia with mental retardation. Am. J. Hum. Genet. 2014 Feb 6;94(2):278–287. doi: 10.1016/j.ajhg.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knaus A., Awaya T., Helbig I., Afawi Z., Pendziwiat M., Abu-Rachma J., Thompson M.D., Cole D.E., Skinner S., Annese F., Canham N., Schweiger M.R., Robinson P.N., Mundlos S., Kinoshita T., Munnich A., Murakami Y., Horn D., Krawitz P.M. Rare noncoding mutations extend the mutational Spectrum in the PGAP3 subtype of Hyperphosphatasia with mental retardation syndrome. Hum. Mutat. 2016 Aug;37(8):737–744. doi: 10.1002/humu.23006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chiyonobu T., Inoue N., Morimoto M., Kinoshita T., Murakami Y. Glycosylphosphatidylinositol (GPI) anchor deficiency caused by mutations in PIGW is associated with west syndrome and hyperphosphatasia with mental retardation syndrome. J. Med. Genet. 2014 Mar;51(3):203–207. doi: 10.1136/jmedgenet-2013-102156. [DOI] [PubMed] [Google Scholar]
- 27.Ilkovski B., Pagnamenta A.T., O'Grady G.L., Kinoshita T., Howard M.F., Lek M., Thomas B., Turner A., Christodoulou J., Sillence D., Knight S.J., Popitsch N., Keays D.A., Anzilotti C., Goriely A., Waddell L.B., Brilot F., North K.N., Kanzawa N., Macarthur D.G., Taylor J.C., Kini U., Murakami Y., Clarke N.F. Mutations in PIGY: expanding the phenotype of inherited glycosylphosphatidylinositol deficiencies. Hum. Mol. Genet. 2015 Nov 1;24(21):6146–6159. doi: 10.1093/hmg/ddv331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rabe P., Haverkamp F., Emons D., Rosskamp R., Zerres K., Passarge E. Syndrome of developmental retardation, facial and skeletal anomalies, and hyperphosphatasia in two sisters: nosology and genetics of the coffin-Siris syndrome. Am. J. Med. Genet. 1991 Dec 1;41(3):350–354. doi: 10.1002/ajmg.1320410317. [DOI] [PubMed] [Google Scholar]
- 29.Murakami Y., Kanzawa N., Saito K., Krawitz P.M., Mundlos S., Robinson P.N., Karadimitris A., Maeda Y., Kinoshita T. Mechanism for release of alkaline phosphatase caused by glycosylphosphatidylinositol deficiency in patients with hyperphosphatasia mental retardation syndrome. J. Biol. Chem. 2012 Feb 24;287(9):6318–6325. doi: 10.1074/jbc.M111.331090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ng B.G., Freeze H.H. Human genetic disorders involving glycosylphosphatidylinositol (GPI) anchors and glycosphingolipids (GSL) J. Inherit. Metab. Dis. 2015 Jan;38(1):171–178. doi: 10.1007/s10545-014-9752-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thompson M.D., Killoran A., Percy M.E., Nezarati M., Cole D.E., Hwang P.A. Hyperphosphatasia with neurologic deficit: a pyridoxine-responsive seizure disorder? Pediatr. Neurol. 2006 Apr;34(4):303–307. doi: 10.1016/j.pediatrneurol.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 32.Kuki I., Takahashi Y., Okazaki S., Kawawaki H., Ehara E., Inoue N., Kinoshita T., Murakami Y. Vitamin B6-responsive epilepsy due to inherited GPI deficiency. Neurology. 2013 Oct 15;81(16):1467–1469. doi: 10.1212/WNL.0b013e3182a8411a. [DOI] [PubMed] [Google Scholar]