Abstract
Extending two-dose recommendations of HPV vaccine to girls between 15 and 18 years will reduce program cost and improve compliance. Immunogenicity and vaccine targeted HPV infection outcomes were compared between 1795 girls aged 15–18 years receiving two (1–180 days) and 1515 girls of same age receiving three (1–60–180 days) doses. Immunogenicity outcomes in 15–18 year old two-dose recipients were also compared with the 10–14 year old three-dose (N = 2833) and two-dose (N = 3184) recipients. The 15–18 year old two-dose recipients had non-inferior L1-binding antibody titres at seven months against vaccine-targeted HPV types compared to three-dose recipients at 15–18 years and three-dose recipients at 10–14 years of age. Neutralizing antibody titres at 18 months in 15–18 year old two-dose recipients were non-inferior to same age three-dose recipients for all except HPV 18. The titres were inferior to those in the 10–14 year old three-dose recipients for all targeted types. Frequency of incident infections from vaccine-targeted HPV types in the 15–18 year old two-dose recipients was similar to the three dose recipients. None of the girls receiving two or three doses had persistent infection from vaccine-targeted types. These findings support that two doses of HPV vaccine can be extended to girls aged 15–18 years.
Keywords: Human papillomavirus, Quadrivalent vaccine, Two doses, age 15–18 years, Immunogenicity, Incident infections, Persistent infections
1. Introduction
The knowledge that persistent infection with a high-risk human papillomavirus (HPV) is the necessary cause of cervical cancer [1], [2] led to the development of prophylactic HPV vaccines. The proportion of cervical cancers attributed to HPV 16 and 18 ranges from 68% to 82% globally [3], [4] and the first generation of vaccines targeted these two types in a bivalent or quadrivalent format, containing recombinant virus-like particles (VLP) assembled from the L1 capsid proteins of HPV 16 and 18 or HPV 6, 11, 16 and 18 respectively. Either of these vaccines is used in the national immunization programs of more than 70 countries, although a nonavalent vaccine is now available [5]. Vaccine efficacy of three doses against high-grade cervical intraepithelial neoplasia (CIN) caused by vaccine-targeted HPV was close to 100% in HPV-naive populations and exceeded 55% in intention-to-treat populations [6], [7], [8]. The World Health Organisation (WHO) accepted in the year 2014 that two doses administered at six months interval were sufficient for healthy pre-adolescents aged less than 15 years at the time of the first dose [9], [10]. The high immune response among pre-adolescents indicates the potential of reduced doses in preventing cervical neoplasia [11], [12].
In 2009, the International Agency for Research on Cancer (IARC) initiated a cluster-randomized trial in India to evaluate the effectiveness of two doses of quadrivalent vaccine (Gardasil™, Merck) in preventing cervical neoplasia compared to three doses [13]. The vaccination was suspended prematurely due to reasons unrelated to our study. As a result the study lost its randomized nature and became an observational cohort study with participants having received a single dose, two doses or three doses of the vaccine. In this manuscript, we compare the immunogenicity outcomes of L1 binding antibody titres, neutralizing antibody titres and antibody avidity against HPV16, HPV18, HPV6 and HPV11 in cohorts of girls aged 15–18 years receiving two doses of the vaccine with the 15–18 year old three dose recipients (standard of care) and the 10–14 year old three dose recipients (best response group) in order to determine whether the benefit of the reduced dose regime can be extended to older girls as well. This immunogenicity analysis was based on participants in the trial who were randomly recruited prior to the break in enrollment, and that this represents 52.6% of the entire cohort. We have also reported the frequency of incident and persistent HPV infections in the different age and dose cohorts as the early efficacy end points.
2. Methods
2.1. Study design and participants
The study design, methods, and study participants’ characteristics have been described previously [13]. Briefly, the primary objective of the cluster randomized trial initiated at nine locations in India was to evaluate whether two doses of the quadrivalent HPV vaccine administered on days 1 and 180 would be effective in inducing non-inferior immune response and in preventing persistent vaccine-targeted HPV infection and cervical neoplasia compared to three doses administered on days 1, 60 and 180. The study recruited unmarried girls who were between 10 and 18 years of age on the date of recruitment. The ethical review committees of the participating centres and IARC approved the study. Written informed consent was obtained from one of the parents, or legal guardian, along with the assent of the participating girl. At follow-up, a fresh consent was obtained from the girls when they completed 18 years of age. A data safety monitoring board was constituted to regularly monitor the safety and outcomes of the study. The study is registered as “Trial of Two Versus Three Doses of Human Papillomavirus (HPV) Vaccine in India” with ISRCTN (registration number ISRCTN98283094) and with ClinicalTrials.gov (registration number NCT00923702).
Recruitment and vaccination of the eligible girls were initiated in September 2009 and progressed satisfactorily until April 2010, with more than 95% participation of the invited girls, when the Indian authorities suspended further vaccination of subjects in all HPV vaccination trials in India, due to events unrelated to our study.
As a result of the suspension, our study had four groups of vaccinated girls: those vaccinated on days 1, 60 and 180 or more (three-dose group); on days 1 and 180 or more (two-dose group); on days 1 and 60 by default (two-dose/D group); and those who received one dose only by default (one-dose/D group). We continued to follow-up these cohorts annually to evaluate outcomes in terms of immunogenicity, frequency of HPV infection and cervical neoplasia.
2.2. Collection and analysis of blood samples for immunological studies
To assess sero-conversion, immunogenicity, antibody levels and durability of the immune response, blood samples were collected at baseline, one month after the last dose and yearly thereafter from a sample of the study population. Due to the suspension of vaccination a sample was collected at 18 months from all the participants.
Three immunogenicity outcomes were measured: L1 genotype-specific binding antibody titres (measured as median fluorescence intensity (MFI)); antibody avidity for the vaccine-targeted HPV types; and geometric mean neutralization EC50 titres (GMT) of targeted HPV antibodies.
The plasma samples collected at baseline and at 7, 12, 18, 24 and 36 months from a sub-set of each cohort of vaccinated girls were analysed to estimate the HPV-L1 genotype specific binding antibody titres at the Rajiv Gandhi Centre for Biotechnology (RGCB), Thiruvananthapuram, India, where a dedicated laboratory was established with technology-transfer and external quality assurance from the German Cancer Research Center (DKFZ), Heidelberg, Germany. The presence of binding antibodies against the major capsid protein L1 of vaccine types HPV 16, 18, 6 and 11 was assessed using Luminex-based multiplex serology assay, which has been broadly used in epidemiological studies [14], [15]. The immunogenicity measure was the geometric mean of MFI. Sero-positivity cut-offs were calculated for each HPV type based on the MFI values of serum samples obtained from the participants at baseline. The cut-off values were defined after allowing for 5% sero-positivity among the total baseline samples.
HPV-L1 genotype specific binding antibody avidity, which reflects the degree of affinity maturation in the B cells, was done with a modified version of the assay described above to assess the quality of the antibody responses following the different dose regimes [13]. The modification involved analysing antibody avidities using an additional washing step with chaotropic agent after incubation of sera with antigen-loaded beads. The treatment with the chaotropic agent-urea at 5 M concentration for 15 min was included between first and second wash. Final antigen specific net MFI values were generated by subtraction of GST-tag and individual bead background values. The antibody avidity index was reported as the MFI values of urea-treated samples divided by the MFI values of the untreated samples and multiplied by 100%.
Neutralizing antibodies specific for neutralization-epitopes in HPV-L1 protein were measured using an automated and high-throughput pseudovirion-based neutralization assay (PBNA), an EC50 titre reflecting half maximum activity was determined from the titration curves [16]. The Lower Limit of Quantitation (LLOQ) for the HPV-PBNA was a reciprocal dilution of 40. Bovine papillomavirus (BPV) pseudovirion assays were run as controls. A sample was classified as sero-negative if the PBNA titre was < 50; seropositive if the PBNA titre was ≥ 50 and ≥ 2 times the BPV titre; or sero-status indeterminate if the PBNA titre was ≥ 50 and < 2 times the BPV titre.
2.3. Collection and analysis of cervical samples for HPV genotyping
Cervical samples for HPV genotyping were collected from the participants initially at 18 months after marriage or 6 months after first child-birth (whichever was earlier) and yearly thereafter for at least 3 years. HPV type-specific E7 PCR bead-based multiplex genotyping was performed at RGCB laboratory to detect 19 high-risk or probable high-risk types (16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68a, 68b, 70, 73, and 82), and two low-risk HPV types (6 and 11). Persistent infection was defined as the detection of the same HPV type in two consecutive cervical samples collected at an interval of 12 months.
All the scientists and technicians performing various assays and genotyping were blinded to the group distribution of the participants. For laboratory quality control a subset of serum samples were taken to DKFZ, for serological assessment and the cervical samples from a selected number of participants were taken to IARC for repeat analysis to validate the technology-transferred assays in India. Good correlations were observed for both the assays between the results from the RGCB and those from the reference laboratories (data not shown).
2.4. Statistical Analysis
In the present report we analysed the data on immunogenicity and incident and persistent HPV infection only from the three-dose (at 1, 60 and 180 days or more) and two-dose (at 1 and 180 days or more) cohorts stratified by age. Antibody titres at months 0, 7, 36 and 48 were compared in two ways: (i) level of HPV L1 antibody in the three-dose 10–14 year age cohort of each HPV type was taken as the comparator for the antibody titres against corresponding HPV type in the other cohorts; (ii) level of HPV L1 antibody titre for each HPV type in the three-dose 15–18 year age (standard of care) group was taken as the comparator for the two-dose 15–18 year age cohort. Non-inferiority was inferred when the lower bound of the confidence interval (CI) of the ratio of the immunogenicity measures exceeded 0.5. HPV infection outcomes were reported as frequencies of the detection of incident and persistent infections of vaccine-targeted and non-targeted high-risk HPV types accumulated during the follow-up.
3. Results
As a result of the study suspension, 4348 girls received quadrivalent HPV vaccine in a three-dose schedule and 4979 in a two-dose schedule. The number of blood samples collected from each group was 10,508 and 11,708 respectively. Analyses included 4785 plasma samples collected at baseline and at months 7, 18, 36, and 48 months after the first dose.
The socio-demographic characteristics of the participants by the age at first vaccination and the number of doses received are described in Table 1. The age and dose groups are comparable for all these variables. The participants of the different age and dose groups were followed up using the same protocol and had similar follow up duration. The median follow up duration (with the interquartile range in parenthesis) of the 10–14 year old participants receiving 3 doses was 6.3 years (5.8–6.5) and those receiving two doses was 6.3 years (6.0–6.5). The 15–18 year old participants receiving three doses and two doses had the median duration of follow up of 6.1 years (5.1–6.4) and 6.1 years (5.3–6.4) respectively.
Table 1.
Women baseline characteristics by dose received and age at first vaccination.
| Characteristics | 3-dose | 2-dose | ||||||
|---|---|---|---|---|---|---|---|---|
|
(Days 1, 60 and 180+) |
(Days 1 and 180+) |
|||||||
| 10–14 yrsa | 15–18 yrsa | 10–14 yrsa | 15–18 yrsa | |||||
| n (%) | n (%) | n (%) | n (%) | |||||
| Number recruited | 2833 | 1515 | 3184 | 1795 | ||||
| Site | ||||||||
| Ambillikai | 947 | (33.4) | 499 | (32.9) | 983 | (30.9) | 549 | (30.6) |
| Barshi | 465 | (16.4) | 279 | (18.4) | 503 | (15.8) | 321 | (17.9) |
| Delhi | 260 | (9.2) | 156 | (10.3) | 260 | (8.2) | 220 | (12.3) |
| Mumbai | 0 | (0.0) | 0 | (0.0) | 333 | (10.5) | 157 | (8.7) |
| Pune | 839 | (29.6) | 427 | (28.2) | 789 | (24.8) | 393 | (21.9) |
| Sikkim | 162 | (5.7) | 71 | (4.7) | 148 | (4.6) | 82 | (4.6) |
| Mizoram | 160 | (5.6) | 83 | (5.5) | 168 | (5.3) | 73 | (4.1) |
| Type of houseb | ||||||||
| Thatched | 270 | (9.7) | 129 | (8.7) | 248 | (8.1) | 113 | (6.5) |
| Tiled | 1864 | (67.2) | 980 | (65.9) | 2126 | (69.6) | 1173 | (67.7) |
| Concrete | 641 | (23.1) | 377 | (25.4) | 682 | (22.3) | 447 | (25.8) |
| Average monthly household income (in Rupees)b | ||||||||
| < 2000 | 966 | (34.1) | 485 | (32.0) | 953 | (29.9) | 501 | (27.9) |
| 2000–4999 | 1171 | (41.3) | 637 | (42.0) | 1343 | (42.2) | 760 | (42.3) |
| 5000–9999 | 482 | (17.0) | 294 | (19.4) | 613 | (19.3) | 396 | (22.1) |
| 10,000+ | 213 | (7.5) | 99 | (6.5) | 274 | (8.6) | 138 | (7.7) |
| Religion | ||||||||
| Hindu | 2544 | (89.8) | 1381 | (91.2) | 2761 | (86.7) | 1583 | (88.2) |
| Muslim | 48 | (1.7) | 22 | (1.5) | 98 | (3.1) | 63 | (3.5) |
| Christian | 207 | (7.3) | 101 | (6.7) | 272 | (8.5) | 120 | (6.7) |
| Other | 34 | (1.2) | 11 | (0.7) | 53 | (1.7) | 29 | (1.6) |
| Participant's educationb | ||||||||
| Nil | 22 | (0.8) | 24 | (1.6) | 13 | (0.4) | 22 | (1.2) |
| Primary | 315 | (11.1) | 49 | (3.2) | 387 | (12.2) | 56 | (3.1) |
| Middle | 1769 | (62.4) | 249 | (16.4) | 2022 | (63.5) | 361 | (20.1) |
| High | 715 | (25.2) | 749 | (49.4) | 740 | (23.2) | 816 | (45.5) |
| College | 12 | (0.4) | 444 | (29.3) | 21 | (0.7) | 540 | (30.1) |
Age at first vaccination.
Numbers do not add up to total because of missing information.
The comparison of the HPV L1 binding antibody titres between the 10–14 year and 15–18 year age cohorts for different doses at the various time points is shown in Table 2. At baseline, the antibody titres for all four HPV types were comparable between the two age groups. The antibody titres at month 7 against all four HPV types in the older age group receiving two doses were non-inferior to the antibody titres against the corresponding HPV types in the young adolescents receiving three doses of the vaccine. The ratios of the geometric mean MFI values of the 15–18 year age group receiving two doses compared to the 10–14 year age group receiving three doses were 0.94 (95% CI: 0.84–1.04) for HPV 16; 0.75 (95% CI: 0.64–0.87) for HPV 18; 0.91 (95% CI: 0.82–1.00) for HPV 6; and 0.81 (95% CI: 0.75–0.88) for HPV 11. At month 36, the antibody titres of the 15–18 year old two-dose cohort was inferior to those of the 10–14 year old three-dose cohort for all HPV types except HPV 11 [Geometric mean MFI ratios for HPV 16, HPV 18, HPV 6 and HPV 11 were 0.50 (95% CI: 0.40–0.62), 0.37 (95% CI: 0.30–0.47), 0.51 (95% CI: 0.41–0.65) and 0.66 (95% CI: 0.52–0.83) respectively]. However, at 48 months only the antibody titre against HPV 18 in the 15–18 year old two-dose cohort was inferior compared to the 10–14 year old three-dose cohort. The antibody titre ratios at 48 months between the older age two dose group and the younger age three dose group were 0.72 (95% CI: 0.53–0.98), 0.57 (95% CI: 0.43–0.77), 0.77 (95% CI: 0.58–1.03) and 1.02 (95% CI: 0.75–1.37) for HPV types 16, 18, 6 and 11 respectively. When compared to the 15–18 year old girls receiving three doses of the vaccine (standard of care), the L1 antibody titres in the 15–18 year old girls receiving two doses were non-inferior for all four HPV types at all time points. The MFI ratios between the 2 dose recipients and the 3 dose recipients belonging to the 15–18 year age group were 1.05 (95% CI: 0.92–1.19), 0.92 (95% CI: 0.76–1.11), 0.99 (95% CI: 0.89–1.11) and 1.10 (95% CI: 1.01–1.21) for HPV 16, 18, 6 and 11 respectively at 7 month; 0.82 (95% CI: 0.67–1.01), 0.69 (95% CI: 0.55–0.87), 0.87 (95% CI: 0.70–1.08) and 1.20 (95% CI: 0.97–1.49) for HPV 16, 18, 6 and 11 respectively at 36 month and 1.07 (95% CI: 0.77–1.50), 0.94 (95% CI: 0.67–1.31), 0.95 (95% CI: 0.68–1.34) and 1.40 (95% CI: 1.01–1.94) for HPV 16, 18, 6 and 11 respectively at 48 month.
Table 2.
Mean MFI values of HPV 16, 18, 6 and 11 L1 antibodies at 0, 7, 18, 36 and 48 months after the first dose among girls who received three doses (at 1, 60 and 180+ days) and two doses (at 1 and 180+ days) by age.
| HPV type/Vaccine dose received | Age group (years) | Number of samples | Geometric mean MFI (95% CI) | MFI ratio (95% CI)a[2-dose/3-dose, 10–14 age group] | MFI ratio (95% CI)b[2-dose, 15–18 age group/3-does, 15–18 age group] | |||
|---|---|---|---|---|---|---|---|---|
| Day 1c | ||||||||
| HPV 16 L1 | ||||||||
| 10–14 | 345 | 13 | (11–15) | |||||
| 15–18 | 246 | 11 | (9–13) | |||||
| HPV 18 L1 | ||||||||
| 10–14 | 345 | 6 | (5–7) | |||||
| 15–18 | 246 | 5 | (4–6) | |||||
| HPV 6 L1 | ||||||||
| 10–14 | 345 | 26 | (22–30) | |||||
| 15–18 | 246 | 25 | (21–30) | |||||
| HPV 11 L1 | ||||||||
| 10–14 | 345 | 8 | (7–9) | |||||
| 15–18 | 246 | 7 | (6–8) | |||||
| Month 7 | ||||||||
| HPV 16 L1 | ||||||||
| 3-dose (Days 1, 60, 180) | 10–14 | 161 | 5758 | (5380–6163) | 1.00 | |||
| 15–18 | 147 | 5150 | (4790–5537) | 0.89 | (0.80–0.99) | 1.00 | ||
| 2-dose (Days 1, 180) | 10–14 | 167 | 6876 | (6554–7214) | 1.19 | (1.08–1.32) | ||
| 15–18 | 150 | 5384 | (4847–5982) | 0.94 | (0.84–1.04) | 1.05 | (0.92–1.19) | |
| HPV 18 L1 | ||||||||
| 3-dose (Days 1, 60, 180) | 10–14 | 161 | 3252 | (2955–3579) | 1.00 | |||
| 15–18 | 147 | 2636 | (2357–2948) | 0.81 | (0.69–0.95) | 1.00 | ||
| 2-dose (Days 1, 180) | 10–14 | 167 | 3792 | (3482–4130) | 1.17 | (1.00–1.36) | ||
| 15–18 | 150 | 2423 | (2086–2815) | 0.75 | (0.64–0.87) | 0.92 | (0.76–1.11) | |
| HPV 6 L1 | ||||||||
| 3-dose (Days 1, 60, 180) | 10–14 | 161 | 4910 | (4579–5265) | 1.00 | |||
| 15–18 | 147 | 4509 | (4196–4846) | 0.92 | (0.83–1.02) | 1.00 | ||
| 2-dose (Days 1, 180) | 10–14 | 167 | 5376 | (5067–5704) | 1.09 | (0.99–1.21) | ||
| 15–18 | 150 | 4461 | (4100–4854) | 0.91 | (0.82–1.00) | 0.99 | (0.89–1.11) | |
| HPV 11 L1 | ||||||||
| 2-dose (Days 1, 180) | 10–14 | 167 | 7284 | (6933–7653) | 1.00 | |||
| 15–18 | 150 | 6506 | (6074–6968) | 0.89 | (0.82–0.97) | 1.00 | ||
| 3-dose (Days 1, 60, 180) | 10–14 | 161 | 6413 | (6042–6807) | 0.88 | (0.81–0.95) | ||
| 15–18 | 147 | 5899 | (5561–6258) | 0.81 | (0.75–0.88) | 1.10 | (1.01–1.21) | |
| Month 18 | ||||||||
| HPV 16 L1 | ||||||||
| 3-dose (Days 1, 60, 180) | 10–14 | 167 | 1447 | (1281–1635) | 1.00 | |||
| 15–18 | 146 | 985 | (868–1117) | 0.68 | (0.55–0.84) | 1.00 | ||
| 2-dose (Days 1, 180) | 10–14 | 166 | 1423 | (1280–1582) | 0.98 | (0.80–1.21) | ||
| 15–18 | 148 | 1030 | (888–1195) | 0.71 | (0.57–0.88) | 1.05 | (0.86–1.27) | |
| HPV 18 L1 | ||||||||
| 3-dose (Days 1, 60, 180) | 10–14 | 167 | 477 | (410–556) | 1.00 | |||
| 15–18 | 146 | 288 | (247–337) | 0.60 | (0.47–0.77) | 1.00 | ||
| 2-dose (Days 1, 180) | 10–14 | 166 | 329 | (288–375) | 0.69 | (0.54–0.87) | ||
| 15–18 | 148 | 214 | (181–254) | 0.45 | (0.35–0.58) | 0.74 | (0.59–0.94) | |
| HPV 6 L1 | ||||||||
| 3-dose (Days 1, 60, 180) | 10–14 | 167 | 1156 | (1020–1310) | 1.00 | |||
| 15–18 | 146 | 822 | (723–934) | 0.71 | (0.58–0.88) | 1.00 | ||
| 2-dose (Days 1, 180) | 10–14 | 166 | 888 | (784–1006) | 0.77 | (0.63–0.94) | ||
| 15–18 | 148 | 770 | (669–886) | 0.67 | (0.54–0.82) | 0.94 | (0.78–1.13) | |
| HPV 11 L1 | ||||||||
| 3-dose (Days 1, 60, 180) | 10–14 | 167 | 1619 | (1447–1811) | 1.00 | |||
| 15–18 | 146 | 1058 | (930–1203) | 0.65 | (0.53–0.80) | 1.00 | ||
| 2-dose (Days 1, 180) | 10–14 | 166 | 1382 | (1238–1543) | 0.85 | (0.70–1.04) | ||
| 15–18 | 148 | 1270 | (1120–1440) | 0.78 | (0.64–0.96) | 1.20 | (1.00–1.44) | |
| Month 36 | ||||||||
| HPV 16 L1 | ||||||||
| 3-dose (Days 1, 60, 180) | 10–14 | 144 | 266 | (228–310) | 1.00 | |||
| 15–18 | 127 | 179 | (152–210) | 0.67 | (0.54–0.84) | 1.00 | ||
| 2-dose (Days 1, 180) | 10–14 | 149 | 197 | (173–224) | 0.74 | (0.60–0.91) | ||
| 15–18 | 129 | 132 | (113–154) | 0.50 | (0.40–0.62) | 0.82 | (0.67–1.01) | |
| HPV 18 L1 | ||||||||
| 3-dose (Days 1, 60, 180) | 10–14 | 144 | 232 | (196–273) | 1.00 | |||
| 15–18 | 127 | 141 | (118–168) | 0.61 | (0.48–0.77) | 1.00 | ||
| 2-dose (Days 1, 180) | 10–14 | 149 | 151 | (130–176) | 0.65 | (0.52–0.82) | ||
| 15–18 | 129 | 87 | (72–104) | 0.37 | (0.30–0.47) | 0.69 | (0.55–0.87) | |
| HPV 6 L1 | ||||||||
| 3-dose (Days 1, 60, 180) | 10–14 | 144 | 749 | (640–876) | 1.00 | |||
| 15–18 | 127 | 506 | (431–594) | 0.68 | (0.53–0.86) | 1.00 | ||
| 2-dose (Days 1, 180) | 10–14 | 149 | 563 | (485–654) | 0.75 | (0.60–0.94) | ||
| 15–18 | 129 | 385 | (322–460) | 0.51 | (0.41–0.65) | 0.87 | (0.70–1.08) | |
| HPV 11 L1 | ||||||||
| 3-dose (Days 1, 60, 180) | 10–14 | 144 | 855 | (737–992) | 1.00 | |||
| 15–18 | 127 | 528 | (447–625) | 0.62 | (0.49–0.78) | 1.00 | ||
| 2-dose (Days 1, 180) | 10–14 | 149 | 745 | (647–859) | 0.87 | (0.69–1.09) | ||
| 15–18 | 129 | 561 | (473–665) | 0.66 | (0.52–0.83) | 1.20 | (0.97–1.49) | |
| Month 48 | ||||||||
| HPV 16 L1 | ||||||||
| 3-dose (Days 1, 60, 180) | 10–14 | 127 | 227 | (185–279) | 1.00 | |||
| 15–18 | 112 | 156 | (129–187) | 0.66 | (0.49–0.90) | 1.00 | ||
| 2-dose (Days 1, 180) | 10–14 | 131 | 222 | (186–265) | 0.99 | (0.73–1.33) | ||
| 15–18 | 112 | 164 | (134–201) | 0.72 | (0.53–0.98) | 1.07 | (0.77–1.50) | |
| HPV 18 L1 | ||||||||
| 3-dose (Days 1, 60, 180) | 10–14 | 127 | 157 | (126–196) | 1.00 | |||
| 15–18 | 112 | 99 | (80–122) | 0.60 | (0.45–0.81) | 1.00 | ||
| 2-dose (Days 1, 180) | 10–14 | 131 | 145 | (122–172) | 0.92 | (0.69–1.22) | ||
| 15–18 | 112 | 92 | (75–112) | 0.57 | (0.43–0.77) | 0.94 | (0.67–1.31) | |
| HPV 6 L1 | ||||||||
| 3-dose (Days 1, 60, 180) | 10–14 | 127 | 488 | (401–593) | 1.00 | |||
| 15–18 | 112 | 367 | (304–443) | 0.71 | (0.54–0.95) | 1.00 | ||
| 2-dose (Days 1, 180) | 10–14 | 131 | 509 | (431–600) | 1.03 | (0.78–1.35) | ||
| 15–18 | 112 | 380 | (310–466) | 0.77 | (0.58–1.03) | 0.95 | (0.68–1.34) | |
| HPV 11 L1 | ||||||||
| 3-dose (Days 1, 60, 180) | 10–14 | 127 | 557 | (452–686) | 1.00 | |||
| 15–18 | 112 | 395 | (322–483) | 0.68 | (0.51–0.92) | 1.00 | ||
| 2-dose (Days 1, 180) | 10–14 | 131 | 655 | (556–772) | 1.18 | (0.88–1.57) | ||
| 15–18 | 112 | 576 | (478–695) | 1.02 | (0.75–1.37) | 1.40 | (1.01–1.94) | |
MFI: median fluorescence intensity; CI: confidence interval; HPV: human papilloma virus.
The other specified dose schedule and age stratum were non-inferior to the 3-dose schedule (Days 1, 60, 180+) in girls aged 10–14 years if the lower limit of the 95% CI for the MFI ratio was above 0.5 (2-fold difference).
The 2-dose (Days 1 and 180+), 15–18 age group was non-inferior to the 3-dose (Days 1, 60 and 180+), 15–18 age group if the lower limit of the 95% CI for the MFI ratio was above 0.5 (2-fold difference).
Data shown for day 1 refers to the samples collected from the participants randomized to the original two-dose and three-dose groups.
The actual range of MFI values with median and interquartile ranges are shown in Supplemental Table 1.
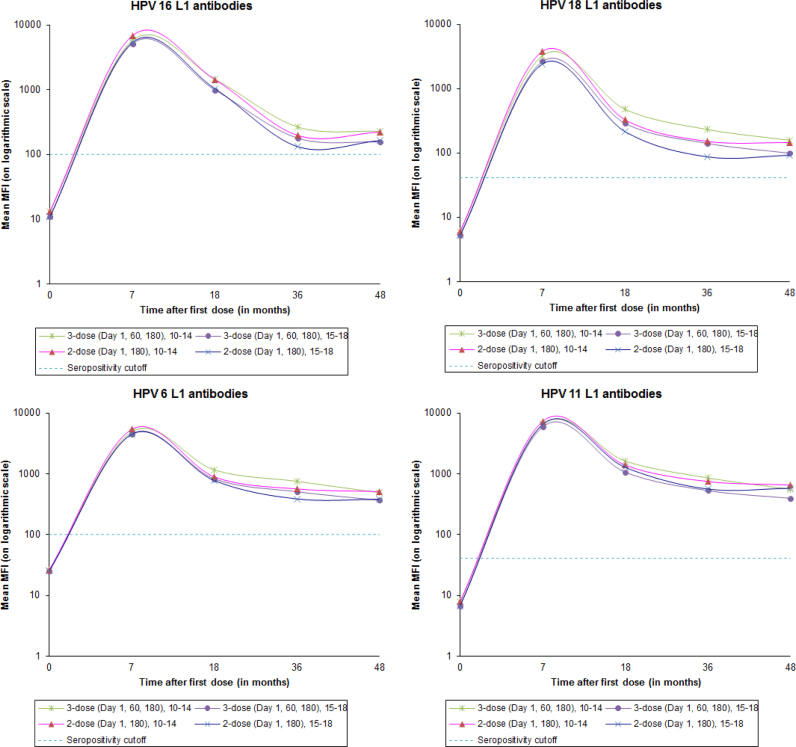
Fig. 1 shows the mean values for HPV 16, 18, 6 and 11 L1 antibodies, over different time points and stratified by age and dose. The kinetics of antibody responses in the older age cohort is similar to that in the younger age cohort irrespective of whether they received two or three doses of the vaccine.
Fig. 1.
Mean MFI values for HPV 16, 18, 6 and 11 L1 antibodies stratified by age.
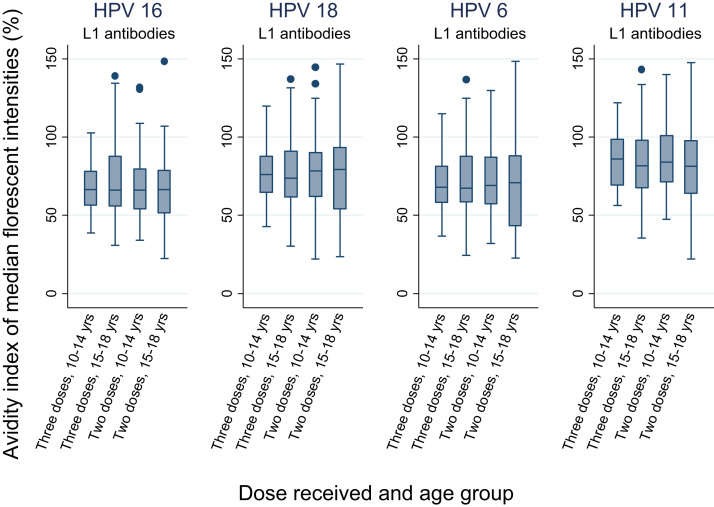
Fig. 2 shows the box plots of the avidity index of MFI for all four targeted HPV types L1 antibodies at 18 months after the first dose by the age groups and doses. The geometric mean indices for all dose-age combinations were non-inferior to three-dose, 10–14 year age group against all four HPV types.
Fig. 2.
Box plots of the avidity index of MFI for HPV types 16, 18, 6 and 11 L1 antibodies at month 18 after the first dose by age group.
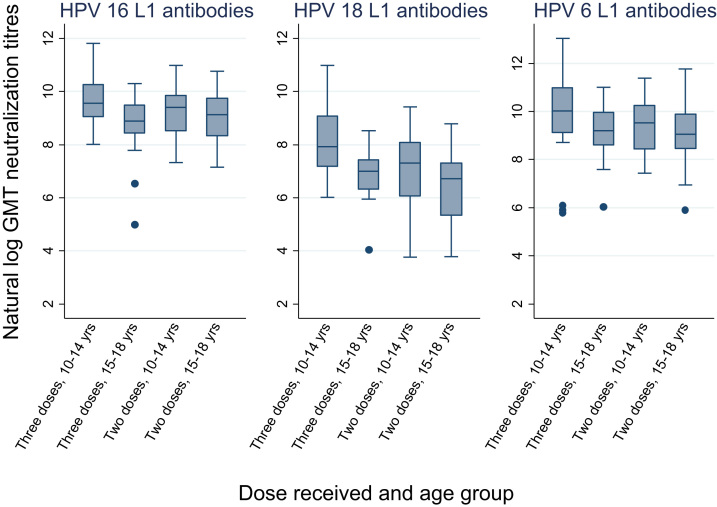
Geometric mean neutralization EC50 titres of HPV 16, 18 and 6 antibodies were measured at 18 months after the first dose (Table 3). The neutralizing antibody titres against all three HPV types in the 15–18 year old two-dose group were inferior to that in the 10–14 year old girls receiving three doses. (Fig. 3) However, compared to the 15–18 year old three-dose cohort the antibody titre in the 15–18 year old two-dose cohort was inferior against HPV 18 only. All the participants receiving two doses at 15–18 years had detectable neutralization antibodies at 18 months. The neutralization assay against HPV 11 was not done essentially to reduce cost and save time
Table 3.
Geometric mean neutralization titres of HPV 16, 18 and 6 L1 antibodies at 18 months after first dose among girls who received three doses (at 1, 60 and 180+ days) and two doses (at 1 and 180+ days) by age.
| HPV type | Age group (years) | No. of samples tested | No. of samples with Detectable neutralization antibodies (%) | Geometric mean neutralization titres (95% CI) | Geometric mean ratio (95% CI)a[2-dose/3-dose, 10–14 age group] | Geometric mean ratio (95% CI)b[2-dose, 15–18 age group/3-dose, 15–18 age group] | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| HPV 16 L1 | ||||||||||
| 3-dose (Days 1, 60, 180) | 10–14 | 33 | 33 | (100.0) | 14,417 | (10,550–19,702) | 1.00 | |||
| 15–18 | 27 | 27 | (100.0) | 6262 | (4095–9577) | 0.43 | (0.36–0.52) | 1.00 | ||
| 2-dose (Days 1, 180) | 10–14 | 27 | 27 | (100.0) | 10,845 | (7556–15,567) | 0.75 | (0.50–1.12) | ||
| 15–18 | 32 | 32 | (100.0) | 9154 | (6476–12,940) | 0.63 | (0.48–0.84) | 1.46 | (1.08–1.98) | |
| HPV 18 L1 | ||||||||||
| 3-dose (Days 1, 60, 180) | 10–14 | 33 | 33 | (100.0) | 3296 | (2109–5150) | 1.00 | |||
| 15–18 | 27 | 26 | (96.3) | 1003 | (690–1459) | 0.30 | (0.21–0.45) | 1.00 | ||
| 2-dose (Days 1, 180) | 10–14 | 27 | 27 | (100.0) | 1112 | (654–1891) | 0.34 | (0.20–0.56) | ||
| 15–18 | 32 | 32 | (100.0) | 632 | (387–1033) | 0.19 | (0.13–0.28) | 0.63 | (0.44–0.91) | |
| HPV 6 L1 | ||||||||||
| 3-dose (Days 1, 60, 180) | 10–14 | 33 | 33 | (100.0) | 19,728 | (11,167–34,852) | 1.00 | |||
| 15–18 | 27 | 27 | (100.0) | 9113 | (5960–13,932) | 0.46 | (0.28–0.77) | 1.00 | ||
| 2-dose (Days 1, 180) | 10–14 | 27 | 27 | (100.0) | 13,369 | (8509–21,006) | 0.68 | (0.52–0.89) | ||
| 15–18 | 32 | 32 | (100.0) | 9124 | (5765–14,439) | 0.46 | (0.31–0.68) | 1.00 | (0.57–1.75) | |
CI: confidence interval; HPV: human papilloma virus.
The other specified dose schedule and age stratum were non-inferior to the standard 2-dose schedule (Days 1, 180) in girls aged 10–14 years if the lower limit of the 95% CI for the geometric mean ratio was above 0.5 (2-fold difference).
The 2-dose, 15–18 age group was non-inferior to the 3-dose, 15–18 age group if the lower limit of the 95% CI for the geometric mean ratio was above 0.5 (2-fold difference).
Fig. 3.
Box plots of neutralisation titres of HPV types 16, 18 and 6 L1 antibodies at month 18 after the first dose by age group.
The frequencies of incident HPV infections in the vaccinated women in different age groups and dose categories are listed in Table 4. The frequency of incident infection from vaccine targeted types (types 16, 18, 6 and 11), the non-vaccine targeted high risk types 31, 33 and 45 and all HPV types in the 15–18 year age group receiving two doses were comparable to the frequencies observed in the younger as well as older age groups receiving three doses, as evident by the overlapping confidence intervals.
Table 4.
Analysis of one-time incident HPV infections by dose cohorts and age.
| Type of HPV infection/Dose received | HPV incidence | ||||
|---|---|---|---|---|---|
|
Participants with ≥ one samples tested | |||||
| Age group | Women assessed | Women with incident infections | Proportion of incidence (%, 95% CI) | ||
| HPV 16/18 infections | |||||
| 3-dose | 10–14 | 412 | 4 | 1.0 | (0.3–2.5) |
| 15–18 | 701 | 7 | 1.0 | (0.4–2.0) | |
| 2-dose | 10–14 | 262 | 2 | 0.8 | (0.1–2.7) |
| 15–18 | 661 | 4 | 0.6 | (0.2–1.5) | |
| HPV 6/11 infections | |||||
| 3-dose | 10–14 | 412 | 4 | 1.0 | (0.3–2.5) |
| 15–18 | 701 | 3 | 0.4 | (0.1–1.2) | |
| 2-dose | 10–14 | 262 | 0 | 0.0 | |
| 15–18 | 661 | 3 | 0.5 | (0.1–1.3) | |
| Non-vaccine-targeted HPV 31, 33 and 45 infections | |||||
| 3-dose | 10–14 | 412 | 14 | 3.4 | (1.9–5.6) |
| 15–18 | 701 | 40 | 5.7 | (4.1–7.7) | |
| 2-dose | 10–14 | 262 | 10 | 3.8 | (1.8–6.9) |
| 15–18 | 661 | 36 | 5.4 | (3.8–7.5) | |
| Non-vaccine-targeted high-risk HPV (31/33/35/39/45/51/52/56/58/59/68) infections | |||||
| 3-dose | 10–14 | 412 | 45 | 10.9 | (8.1–14.3) |
| 15–18 | 701 | 89 | 12.7 | (10.3–15.4) | |
| 2-dose | 10–14 | 262 | 42 | 16.0 | (11.8–21.0) |
| 15–18 | 661 | 101 | 15.3 | (12.6–18.3) | |
| Any HPV (16/18/6/11/26/31/33/35/39/45/51/52/53/56/58/59/66/68/70/73/82) infection | |||||
| 3-dose | 10–14 | 412 | 74 | 18.0 | (14.4–22.0) |
| 15–18 | 701 | 111 | 15.8 | (13.2–18.8) | |
| 2-dose | 10–14 | 262 | 50 | 19.1 | (14.5–24.4) |
| 15–18 | 661 | 120 | 18.2 | (15.3–21.3) | |
HPV: human papilloma virus; CI: confidence interval.
Persistent infection could be assessed in 477 women receiving 3 doses and 512 women receiving 2 doses and none of them had persistent infection from any of the vaccine targeted HPV types.
4. Discussion
The pivotal phase III trials on efficacy of the quadrivalent and the bivalent HPV vaccines in sexually active women aged 16–26 years had as their end-points prevention of high grade cervical intraepithelial neoplasia or adenocarcinoma in situ attributable to the HPV types included in the vaccines [17], [18]. Extension of the license to the adolescent girls (9–15 years) was based on the immunobridging studies [19], [20], which demonstrated that the immune responses in the younger girls were comparable to those in adult women in whom the vaccine efficacies were proven. Licensure in different countries was also possible based on immunobridging studies [21].
WHO recommended two doses of the HPV vaccine (administered at six months interval) for healthy girls below 15 years of age on the basis of immunobridging studies (including preliminary data from the present study) that demonstrated non-inferior immune response of two doses in adolescent girls compared to three doses in young adult women in whom the efficacy against disease was established [9]. Many countries around the globe have adopted this two-dose regime for adolescent girls (the primary target population). The two-dose regime has simplified the logistics, reduced the costs of vaccines and delivery systems and improved compliance.
Our study previously reported that two doses of the quadrivalent vaccine were non-inferior to three doses when administered at least 6 months apart in the 10–18 years age group [13]. In the present analysis we demonstrate that the 15–18 year old adolescents receiving two doses not only have non-inferior immune responses (total antibody titre, neutralizing antibody level and antibody avidity) compared to individuals of the same age receiving 3 doses, but also have comparable rates of incident and persistent infection with the vaccine targeted HPV types. Romanowski et al. also reported that the two-dose schedule of the bivalent vaccine (Cervarix™) was non-inferior to the three-dose schedule in the adolescents aged 15–19 years [19]. However, they only assessed the geometric mean antibody titre at month 7 and did not have any efficacy assessment against the disease end points.
The observation that the two-dose schedule in 15–18 year old adolescents can be as efficacious as three doses has tremendous practical implications. In the recently published position paper WHO has recommended vaccination of multiple age cohorts of girls between 9 and 18 years of age at the time of the vaccine introduction, since such a strategy would result in faster and greater population impact than vaccination of single age cohorts [20]. Extending the upper age limit for the two-dose recommendation to 18 years of age will simplify the logistics of vaccination and greatly benefit many countries introducing the vaccine. Selecting an age cohort within 15–18 years as the primary target age for vaccination will allow many countries to link the HPV vaccination program to adolescent health programs more effectively.
The HPV vaccine has certain unique features that permit the vaccine to be effective with less than the conventional three doses [22]. The virus like particles (VLPs) used as the antigen for the vaccine have repetitive, closely spaced epitopes of the antigen protein on the surface that stimulates a robust and sustained immune response, similar to the live attenuated vaccines. Our present analysis has demonstrated that the antibody titre remains persistently high (more than 10 times the baseline value) even at 4 years after vaccination in all the vaccinated individuals, including those receiving only two doses of the vaccine at 15–18 years. Even though the immune correlate of protection for the HPV vaccine is still unknown, low concentrations of neutralizing antibodies can effectively bind the virus and are able to prevent infection [23]. A hypothetical model based on mouse data demonstrates that the antibodies bind to the virions in the cervical fluid and the exuded serum (due to micro-traumas) to prevent their attachment to the basement membrane [24], [25]. The antibodies can also bind to the virions after they get attached to the basement membrane and block their transfer to the epithelial cells. This dual mode of action of the neutralizing antibodies and the presence of the virions on the basement membrane for several hours, allowing ample time for the antibodies to neutralize them, are the reasons why even a low level of antibody is effective in preventing the infection [22]. We have also demonstrated that the avidity of the antibodies remains persistently high, despite a drop in the antibody titre, a finding corroborated by Brady et al. [26]. The persistence of quality HPV type-specific antibodies (indicated by avidity) could reflect an effective affinity maturation of B-cells that can aid in long-term protection even as antibody titres drop and this can explain the equal protection observed in the two-dose and three-dose group irrespective of age at vaccination in our study.
The primary limitation of our study is that it is an observational study with potential for selection biases among the groups affecting the comparisons. In the context of the current manuscript, the immunogenicity and the HPV genotyping results presented were restricted to the girls/women who received their vaccine doses as per the original randomization protocol (three doses at 1–60–180 days or two doses at 1–180 days). Moreover, we have also demonstrated that the age and the dose groups are comparable by the different socio-demographic parameters and also the duration of follow ups.
The strength of the study lies in the systematic follow up of the large number of recipients of different doses of the vaccine, thus generating convincing evidence that adolescents between 15 and 18 years of age receiving two doses at six months interval have the same high protection against persistent infection from the vaccine-targeted HPV types as is seen in the recipients of three doses either at younger age (10–14 years) or older age (15–18 years). The IARC working group on primary end-points for the prophylactic HPV vaccine trials unanimously agreed that to extend vaccination to additional age groups immuno-bridging based on non-inferiority of the immune response (using a standardized validated immunological test) would be a useful approach for fewer than three doses, and it could apply to groups older than 9–15 years as well [27]. In the present study, in addition to clearly demonstrating the immunological non-inferiority of the 15–18 year old two-dose recipients compared to 15–18 year old three-dose recipients (in whom efficacy is proven), we have also proved the efficacy of the alternate schedule against incident and persistent infections as the viral efficacy end-points.
5. Conclusions
Adolescent girls vaccinated between 15 and 18 years of age with two doses of the quadrivalent HPV vaccine have similar antibody profiles as seen in girls vaccinated at the same age with three doses of the vaccine. The efficacy results in terms of protection against incident and persistent infections from the vaccine targeted HPV types in the girls getting two doses are also comparable to the girls receiving three doses at 15–18 years or even at younger age. The results justify extending the two-dose recommendation to the 15–18 year old healthy adolescents as well.
Acknowledgements
The vaccines used in the study were provided by Merck through a memorandum of understanding (MOU) with WHO. IARC is the autonomous cancer research agency of the WHO. Merck did not have any other role in design, protocol development, conduct, monitoring and evaluation, analysis of biological samples or publication of the study results. Thus the study is independent of the pharmaceutical industry.
The study is generously supported by a grant from the Bill & Melinda Gates Foundation. The funding agency had no role in design, conduct, monitoring and evaluation of the study, analysis of data or reporting of results.
Acknowledgments
Conflicts of interest
Neerja Bhatla has received research funding through her Institute from GlaxoSmithKline and Merck. Smita Joshi has received funds from GlaxoSmithKline through the Jehangir Clinical Development Center to do a HPV vaccine study. Partha Basu has received research funding from GlaxoSmithKline through Chittaranjan National Cancer Institute, India during his previous position at the Institute. The other authors declare no competing interests. MP is a inventor on a patent application by DKFZ on the high-throughput pseudovirion-based neutralisation assay. PS is co-inventor on a patent application by DKFZ on the high-throughput pseudovirion-based neutralisation assay.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.pvr.2018.03.008.
Appendix A. Supplementary material
Supplementary material
References
- 1.International Agency for Research on Cancer . IARC; Lyon: 2007. (IARC Monographs on the Evaluation of Carcinogenic Risks to Humans Volume 90. Human Papillomaviruses). [Google Scholar]
- 2.zur Hausen H. Papillomaviruses in the causation of human cancers – a brief historical account. Virology. 2009;384:260–265. doi: 10.1016/j.virol.2008.11.046. [DOI] [PubMed] [Google Scholar]
- 3.Li N., Franceschi S., Howell-Jones R., Snijders P.J., Clifford G.M. Human papillomavirus type distribution in 30,848 invasive cervical cancers worldwide: variation by geographical region, histological type and year of publication. Int. J. Cancer. 2011;128:927–935. doi: 10.1002/ijc.25396. [DOI] [PubMed] [Google Scholar]
- 4.Smith J.S., Lindsay L., Hoots B., Keys J., Franceschi S., Winer R. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: a meta-analysis update. Int. J. Cancer. 2007;121:621–632. doi: 10.1002/ijc.22527. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization . Vaccine in National Immunization Programme Update. 2017. (Available from: 〈 http://www.who.int/entity/immunization/monitoring_surveillance/VaccineIntroStatus.pptx〉, Accessed June 12, 2017) [Google Scholar]
- 6.Schiller J.T., Castellsague X., Garland S.M. A review of clinical trials of human papillomavirus prophylactic vaccines. Vaccine. 2012;30(Suppl. 5):SF123–SF138. doi: 10.1016/j.vaccine.2012.04.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bosch F.X., Broker T.R., Forman D., Moscicki A.B., Gillison M.L., Doorbar J. Comprehensive control of human papillomavirus infections and related diseases. Vaccine. 2013;31(Suppl. 5):SF1–SF31. doi: 10.1016/j.vaccine.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 8.Erickson B.K., Landers E.E., Huh W.K. Update on vaccination clinical trials for HPV-related disease. Clin. Ther. 2014;36:8–16. doi: 10.1016/j.clinthera.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization, Immunization, Vaccines and Biologicals: Summary of the SAGE April 2014 Meeting. Available from: 〈http://www.who.int/immunization/sage/meetings/2014/april/report_summary_april_2014/en/〉, (Accessed 12 June 2017).
- 10.World Health Organization, Evidence based recommendations on Human Papilloma Virus (HPV) Vaccines Schedules. Background paper for SAGE discussions. Available from: 〈http://www.who.int/immunization/sage/meetings/2014/april/1_HPV_Evidence_based_recommendationsWHO_with_Appendices2_3.pdf〉, (Accessed 12 June 2017).
- 11.Block S.L., Nolan T., Sattler C., Barr E., Giacoletti K.E., Marchant C.D. Comparison of the immunogenicity and reactogenicity of a prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in male and female adolescents and young adult women. Pediatrics. 2006;118:2135–2145. doi: 10.1542/peds.2006-0461. [DOI] [PubMed] [Google Scholar]
- 12.Pedersen C., Petaja T., Strauss G., Rumke H.C., Poder A., Richardus J.H. Immunization of early adolescent females with human papillomavirus type 16 and 18 L1 virus-like particle vaccine containing AS04 adjuvant. J. Adolesc. Health. 2007;40:564–571. doi: 10.1016/j.jadohealth.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 13.Sankaranarayanan R., Prabhu P.R., Pawlita M., Gheit T., Bhatla N., Muwonge R. Immunogenicity and HPV infection after one, two, and three doses of quadrivalent HPV vaccine in girls in India: a multicentre prospective cohort study. Lancet Oncol. 2016;17:67–77. doi: 10.1016/S1470-2045(15)00414-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waterboer T., Sehr P., Michael K.M., Franceschi S., Nieland J.D., Joos T.O. Multiplex human papillomavirus serology based on in situ-purified glutathione s-transferase fusion proteins. Clin. Chem. 2005;51:1845–1853. doi: 10.1373/clinchem.2005.052381. [DOI] [PubMed] [Google Scholar]
- 15.Michael K.M., Waterboer T., Sehr P., Rother A., Reidel U., Boeing H. Seroprevalence of 34 human papillomavirus types in the German general population. PLoS Pathog. 2008;4:e1000091. doi: 10.1371/journal.ppat.1000091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sehr P., Rubio I., Seitz H., Putzker K., Ribeiro-Muller L., Pawlita M. High-throughput pseudovirion-based neutralization assay for analysis of natural and vaccine-induced antibodies against human papillomaviruses. PLoS One. 2013;8:e75677. doi: 10.1371/journal.pone.0075677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dillner J., Kjaer S.K., Wheeler C.M., Sigurdsson K., Iversen O.E., Hernandez-Avila M. Four year efficacy of prophylactic human papillomavirus quadrivalent vaccine against low grade cervical, vulvar, and vaginal intraepithelial neoplasia and anogenital warts: randomised controlled trial. BMJ. 2010;341:c3493. doi: 10.1136/bmj.c3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lehtinen M., Paavonen J., Wheeler C.M., Jaisamrarn U., Garland S.M., Castellsague X. Overall efficacy of HPV-16/18 AS04-adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol. 2012;13:89–99. doi: 10.1016/S1470-2045(11)70286-8. [DOI] [PubMed] [Google Scholar]
- 19.Romanowski B., Schwarz T.F., Ferguson L.M., Peters K., Dionne M., Schulze K. Immunogenicity and safety of the HPV-16/18 AS04-adjuvanted vaccine administered as a 2-dose schedule compared with the licensed 3-dose schedule: results from a randomized study. Hum. Vaccin. 2011;7:1374–1386. doi: 10.4161/hv.7.12.18322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization Human papillomavirus vaccine: WHO position paper, may 2017. Wkly. Epidemiol. Rec. 2017;92:241–298. [Google Scholar]
- 21.Bhatla N., Suri V., Basu P., Shastri S., Datta S.K., Bi D. Immunogenicity and safety of human papillomavirus-16/18 AS04-adjuvanted cervical cancer vaccine in healthy Indian women. J. Obstet. Gynaecol. Res. 2010;36:123–132. doi: 10.1111/j.1447-0756.2009.01167.x. [DOI] [PubMed] [Google Scholar]
- 22.Lowy D.R. HPV vaccination to prevent cervical cancer and other HPV-associated disease: from basic science to effective interventions. J. Clin. Invest. 2016;126:5–11. doi: 10.1172/JCI85446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schiller J.T., Lowy D.R. Understanding and learning from the success of prophylactic human papillomavirus vaccines. Nat. Rev. Microbiol. 2012;10:681–692. doi: 10.1038/nrmicro2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Day P.M., Kines R.C., Thompson C.D., Jagu S., Roden R.B., Lowy D.R. In vivo mechanisms of vaccine-induced protection against HPV infection. Cell Host Microbe. 2010;8:260–270. doi: 10.1016/j.chom.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kines R.C., Thompson C.D., Lowy D.R., Schiller J.T., Day P.M. The initial steps leading to papillomavirus infection occur on the basement membrane prior to cell surface binding. Proc. Natl. Acad. Sci. USA. 2009;106:20458–20463. doi: 10.1073/pnas.0908502106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brady A.M., Panicker G., Meites E., Bulkow L., Hurlburt D., Markowitz L. Maintenance of antibody avidity in Alaska native adolescents receiving quadrivalent human papillomavirus (HPV) vaccine. FASEB J. 2017;31(lb):191. [Google Scholar]
- 27.World Health Organization . Primary End-Points for Prophylactic HPV Vaccine Trials. IARC Working Group Report. Vol. 7. IARC; Lyon: 2014. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material