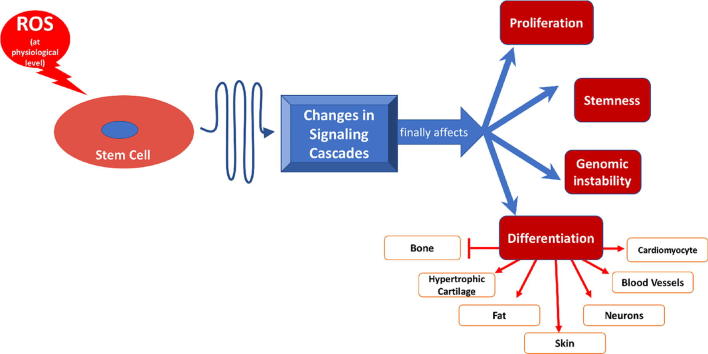
Graphical abstract
Keywords: Stem cells, Reactive oxygen species, Differentiation, Osteogenesis, Potency
Abstract
Reactive oxygen species (ROS) are produced as by-products of several intracellular metabolic pathways and are reduced to more stable molecules by several protective pathways. The presence of high levels of ROS can be associated with disturbance of cell function and could lead to apoptosis. The presence of ROS within the physiological range has many effects on several signalling pathways. In stem cells, this role can range between keeping the potency of the naive stem cells to differentiation towards a certain lineage. In addition, the level of certain ROS would change according to the differentiation stage. For example, the presence of ROS can be associated with increasing the proliferation of mesenchymal stem cells, decreasing the potency of embryonic stem cells and adding to the genomic stability of induced pluripotent stem cells. ROS can enhance the differentiation of stem cells into cardiomyocytes, adipocytes, endothelial cells, keratinocytes and neurons. In the meantime, ROS inhibits osteogenesis and enhances the differentiation of cartilage to the hypertrophic stage, which is associated with chondrocyte death. Thus, ROS may form a link between naïve stem cells in the body and the environment. In addition, monitoring of ROS levels in vitro may help in tissue regeneration studies.
Introduction
Reactive oxygen species (ROS) have been known for a long time for the destructive effect when the oxidative effect exceeds the natural resistance by the antioxidant system. Many years and intensive research was required to convince the scientific community that both oxidant and antioxidant species have physiological roles, especially in metabolism, intracellular signal transmission and regulation of cellular functions [1], [2], [3]. Investigating the cellular roles provided some clues regarding stem cell biology, including the preservation of cell potency and guiding their differentiation, as well as their intense defence against oxidative stress-induced cell death [4]. In 2007, Jang and Sharkis showed that maintaining low levels of ROS corresponded to the quiescent state of stem cells in vivo and was a crucial feature of stem cell precursors [5]. Three years later, Oscar et al. reported a link between specific inflammatory mediators and the regulation of the stem cells’ regenerative capacity; one example was preserving the potency of embryonic stem cells (ESCs) through the inhibition of PLA2, COX, and LOX. These findings were confirmed through the effect on these cells [6]. Despite the significant literature that discusses the interaction between ROS and stem cells, it is difficult to stratify the role of ROS from the potency/differentiation perspective, which is the main aim of this minireview.
An overview on reactive oxygen species
ROS can result from reduction of an electron in oxygen. Among other forms, three forms are found in the intracellular compartment: hydrogen peroxide (H2O2), superoxide anions (O2−) and hydroxyl radicals (OH−). Superoxide dismutase (SOD) is an enzyme, which uses the intracellular antioxidants to reduce these oxidants into H2O and O2 through various steps [7].
Mitochondria represent a major source of ROS through, at least, ten ROS-generating systems. For example, pyruvate dehydrogenase and α-ketoglutarate dehydrogenase are enzymes in the Krebs cycle that produce a significant amount of O2− and H2O2. Also, the inter-mitochondrial membrane protein, p66Shc, and the outer membrane enzyme, monoamine oxidase, are other important mitochondrial ROS sources [5]. The membrane-bound NADPH oxidase (NOX) is considered as another major producer of ROS. This enzyme reduces O2 to O2− by using NADPH as an electron donor. The unstable molecule reacts with nitric oxide (NO) to produce peroxynitrite (NO3–) or converts to hydrogen peroxide (H2O2) by superoxide dismutase. H2O2 may disrupt cell signalling, especially the pathways induced by growth factors, or react with Fe3+ to produce hydroxyl radicals [8]. Acute hypoxia can also influence the generation of ROS through complex III, which is involved in alteration of gene expression [9].
Within the cell, ROS contributes to many normal and abnormal pathways, including cell proliferation, adhesion and survival [10]. ROS can function as secondary messengers through reversible oxidation of the amino acid, cysteine, of certain proteins, which modifies their actions, in particular cyclin D1 and forkhead proteins [11], [12]. ROS-induced oxidative stress can result in injury to various organelles through damaging proteins, lipids or even DNA. This sort of molecular interaction and alteration can lead to cell death. Even worse, sub-lethal levels of ROS can lead to carcinogenesis through activating certain signalling pathways responsible for increasing proliferation. For example, ROS enhances the production of NFκB, signal transducer and activator transcription (STAT) and activator protein-1 (AP1) [13]. ROS can induce prostate cancer through the involvement of Nox5 and inhibition of the JNK signalling pathway, as well as protein kinase C zeta [14]. The mitochondrial DNA is particularly exposed to ROS damage, being in close proximity to the production source of ROS and being deprived of histone and non-histone proteins [15].
Under normal conditions, ROS production is controlled by an efficient ROS scavenging system, which consists of antioxidant molecules that counterbalance ROS through direct reactions. Glutathione (GSH) is an abundant and potent antioxidant that reduces oxidized proteins and H2O2 through the glutaredoxin and thioredoxin system. Cellular redox homeostasis controlled by ROS production versus antioxidant defence is critical for the regulation of both physiological and pathophysiological cellular functions. The natural antioxidant list extends to include superoxide dismutase, catalase, glutathione reductase, glutathione S-transferases, glutathione peroxidases and other low-molecular-weight molecules, such as ascorbic acid and α-tocopherols [16], [17], [18]. Although the antioxidant protection levels have been described in several cell types, it has yet to be fully explored for stem cells.
Reactive oxygen species and keeping the stem cell potency
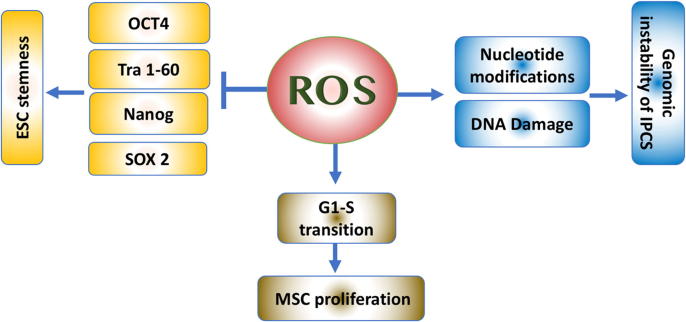
As the term ‘stem cells’ covers cells from different sources at different stages of development, the definition of the role of ROS on stem cells is complex. Stem cells vary in their origin, potential of differentiation, epigenetic markings, and stage of maturity. The presence of ROS balance within the stem cells is not only important for differentiation but also to keep their potency. Multiple studies showed that ROS play different, but vital, roles in various types of stem cells. The interaction between stem cells and ROS in terms of keeping their potency is summarized in Fig. 1.
Fig. 1.
The effect of ROS varies on different types of stem cells. While blocking ESC potency, ROS can increase the likelihood of genomic instability in IPSCs and increase MSC proliferation.
Embryonic stem cells (ESCs)
ESCs are derived from the embryonic inner cell mass, at the blastocyst stage of development [19]. The progeny of the blastocyst are the precursors for all cell types derived from the three embryonic germ layers, when given the sufficient and necessary stimulation [20].
Oxygen level fluctuations and ROS have a very important role in ESC proliferation in addition to differentiation, as the early embryonic developmental stages occur under low oxygen tension. The latter was estimated to be around 2.4% prior to implantation [21].
Furthermore, the ESC markers of pluripotency, OCT4, Tra 1-60, Nanog, and Sox2, are downregulated in response to increasing levels of ROS, which enhances the ESC differentiation along the mesodermal and endodermal lineages. Interestingly, this potency can be restored by the use of antioxidants. Such effects are modulated through different members of the mitogen-activated protein kinase family (MAPK), which influence multiple signalling pathways [22].
Adult stem cells
Adult stem cells (ASCs) are multipotent cells that can be found in adult tissues. These cells are characterized by having the ability of self-renewal, as well as differentiation into most of the cell types in the body. ASCs can be found in almost all tissues in the body, including bone marrow, peripheral blood, skeletal muscle, dental pulp tissue, skin and gastrointestinal tract lining and can be isolated with relative ease from adipose tissue, umbilical cord blood, amniotic fluid, as well as foetal liver and bones [23], [24], [25], [26]. ASCs have a limited proliferation ability and their main function is to support tissue homeostasis by producing cells that replace lost or dead cells [27].
The bone marrow is considered as the reservoir of stem cells in the human body with two distinct populations. Hematopoietic stem cells are a subpopulation of ASCs that differentiate into various types of blood cells, including both the myeloid and lymphoid lineages. While, the former would differentiate into monocytes, macrophages, neutrophils, basophils, eosinophils, erythrocytes and megakaryocytes, the latter would give rise to T-cells, B-cells, NK cells, and some dendritic cells [28], [29]. The other population within the bone marrow is the mesenchymal stem cells (MSCs), which multi-lineage differentiate into the mesodermal lineage by default (such as chondrocytes, osteocytes, and adipocytes), as well as ectodermal and endodermal derived cells [19], [30], [31], [32].
Interestingly, the potency of adult stem cells was correlated to the mitochondrial location within the cytoplasm. The perinuclear arrangement of mitochondria was associated with higher differentiation potential of the cells. These cells had lower ATP content per cell, as well as higher rate of oxygen consumption [33]. ROS plays a role even in MSC proliferation. With the basal level of ROS, MSCs would remain quiescent. The ROS level would increase before the cells enter the S phase of the cell cycle, and antioxidants block the G1-S transition [34]. Urao et al., in 2008, found that deletion of Nox2 causes reduced stem cell mobilization from the bone marrow to peripheral blood [35]. The interaction between ROS and MSCs encouraged many researchers to investigate the potential role of MSCs in severe inflammatory conditions, such as pancreatitis, with inconsistent results [36].
Induced pluripotent stem cells
Induced pluripotent stem cells (IPSCs) combine the advantages of adult and embryonic stem cells. The latter combines the pluripotency with the proliferation potential, which makes them a good model for studying diseases and drug testing without having any ethical concerns. In addition, these cells can be generated to be patient-specific and/or disease-specific, which is not possible with ESCs [37].
One of the methods used to generate tissue-specific pluripotent cells is via transfection with the transcription factors, OCT4, SOX2, KLF4, and c-MYC (collectively known as the four factors or 4F). A key concern with reprogramming adult cells into IPSCs is the increased load of genomic abnormalities that are not originally found in the parent cells [38]. During reprogramming of IPSCs, mitochondria become progressively smaller and less active. The cellular metabolism shifts from oxidative respiration to oxidative glycolysis, which could result in the accumulation of reactive oxygen species and oxidative stress in the cells [39]. Increasing levels of ROS can result in the modification of individual nucleotide bases, single and double-strand breaks, as well as telomere attrition [40]. Checking the integrity of the chromosomes, as well as the genome, is a crucial step for approving the safety of newly generated IPSCs, especially for clinical use [41].
Reactive oxygen species and stem cell differentiation
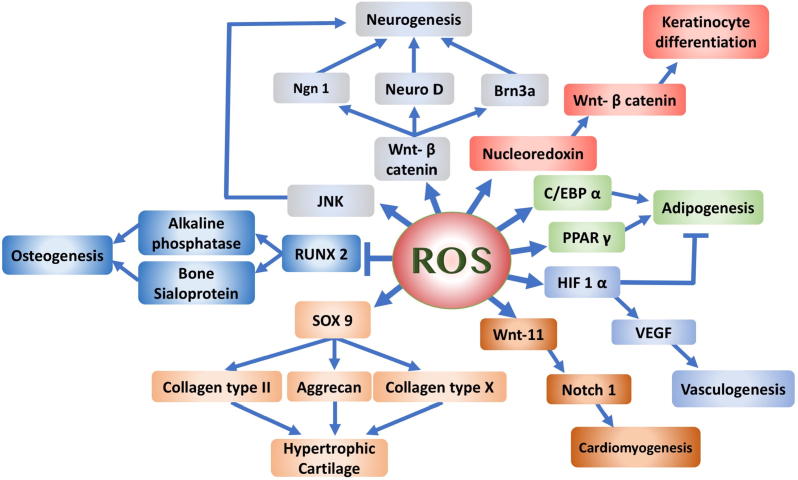
ROS are not only crucial for keeping stem cell potency, but also for their differentiation potential, possibly through a cell signalling effect induced under the effect of Nox4. The effect of ROS on the differentiation of stem cells is illustrated in Fig. 2.
Fig. 2.
Diagrammatic representation of the possible cascade of molecular events induced by ROS affecting various differentiation pathways. ROS were shown to block osteogenic differentiation, enhance terminal differentiation of chondrocytes and induce differentiation of neurons, cardiomyocytes, vasculature and keratinocytes. The role in fat development is controversial.
Bones
Moody et al., showed that oxidative stress caused by ROS was related to a decrease in the skeletal integrity by reducing osteogenic differentiation potential in MSCs [42]. In the meantime, using antioxidants such as vitamin C or E can restore the osteogenic differentiation properties, which highlight the possible role of antioxidants in promoting bone formation [43], [44].
Chen et al. showed that osteogenic differentiation of MSCs was associated with reduction of intracellular ROS levels, based on the upregulation of intracellular antioxidant systems, such as SOD [45]. H2O2 treated MSCs exhibited a reduction in the gene expression of the osteogenic transcription factor, Runx-2, as well as downstream markers, such as alkaline phosphatase and bone sialoprotein [46]. Alkaline phosphatase is an enzyme responsible for the mineralization of bone matrix and is a marker for osteogenic differentiation. The enzymatic activity has been shown to decrease in response to ROS [47]. Furthermore, the addition of ROS to bone marrow-derived stromal cells or osteoblastic precursors inhibited the expression of different osteogenic markers in a dose dependent manner [34], [48]. Thus, there is an inverse correlation between the level of ROS and bone differentiation.
Cartilage
MSCs give rise to two types of cartilage during foetal development: permanent hyaline and transient cartilage. The permanent subtype is located at the ends of the developing bones and is associated with synthesizing the classic extracellular matrix of articular cartilage. The transient form arises prior to skeletal bone formation and passes into three stages: (1) commitment to chondrocyte differentiation by stem cells known as mesenchymal condensation; (2) chondrocyte proliferation in the growth plate; and (3) proliferating chondrocyte differentiation to hypertrophic chondrocytes [49]. The following stage is the formation of the scaffolds where the chondrocytes that are located in the middle of the diaphysis stop proliferating and undergo hypertrophy. Then the cells are either transformed into osteoblasts or proceed to apoptosis and are replaced by the osteoprogenitors [50], [51].
ROS are needed in the early stages of chondrogenesis during in vitro studies. The presence of certain ROS was associated with increased markers of chondrogenesis and the use of antioxidants was inhibitory for differentiation [52], [53].
On the contrary, Morita et al. demonstrated that ROS mediated the inhibition of proliferation in chondrocytes and induced the differentiation into hypertrophic chondrocytes. The same study showed a higher level of intracellular ROS levels in pre-hypertrophic and hypertrophic chondrocytes compared with proliferating chondrocytes and surrounding tissues. In addition, treating the cells with antioxidants blocked the chondrocyte hypertrophy [54]. These findings can be correlated to the presence of cartilage in a hypoxic atmosphere, being an avascular organ, as well as the gradual decrease of catalase during chondrogenic differentiation [52], [53]. Henceforth, ROS may have a counteracting role in cartilage homeostasis and low levels of ROS could be required in the initial stages of chondrogenic differentiation modelling in the lab.
Cardiomyocytes
In ESCs, ROS play an antagonistic role in cardiovascular differentiation. The intermittent exposure to ROS, especially at low levels, increases ESC differentiation into cardiomyocytes and enhances new vessel formation. On the other hand, continuous exposure would inhibit cardiomyogenesis and vasculogenesis [55]. Buggisch et al., in 2007, proposed that glucose-induced production of mitochondrial ROS activates the p38 phosphorylase system via Nox4. Hyperglycaemia has been implicated in increased ROS production, which is involved in the redox state in cardiac differentiation. An examination of cardiac redox status of ES cells during different glucose conditions concluded that in low glucose media, cardiomyogenic potential is impaired [56], [57]. Wnt-11 gene activation is required for cardiomyocyte differentiation. The latter is activated by hypoxia and ROS in order to upregulate Notch1 signalling. Boopathy et al. showed that balancing Notch activation and H2O2 repair and regeneration can be crucial for future implementation of MSC-based cell therapy for the heart [58].
Blood vessels
ROS can induce vascular endothelial growth factor (VEGF), the main angiogenic inducer, through an indirect approach. H2O2 and other ROS can induce the alpha subunit of hypoxia-inducible factor-1 in a dose dependent manner. The latter is a known inducer of VEGF and the link with Nox4 expression was shown through a cascade of molecules, such as ERK1 and 2 as well as JNK activation [59], [60], [61]. In addition, epidermal growth factor and angiopoietin-1 can be directly affected by ROS. The production of these two factors supports the neovascularization through influencing cell migration and proliferation [62].
Adipose tissue
The combination of CCAAT enhancer binding protein α (CEBPα) with peroxisome proliferator-activated receptor γ (PPARγ) would not only involve the commitment of the cells into adipogenic differentiation, but also the terminal differentiation. There is a reciprocal induction between PPARγ and CEBPα as in a positive feedback fashion, which can be stimulated by ROS, especially H2O2. The latter works upstream of CEBPα and PPARγ and regulates their expression [63], [64].
Another theory for ROS effects on adipocytes indicated an anti-adipogenic role, which was introduced by Carriere et al. in 2003 and 2004 [65], [66]. Their observation correlated hypoxia-inducible factor 1-alpha (HIF-1 α) to inhibition of adipogenesis, as the latter inhibits mitochondrial electron transport, producing redox changes in the electron carriers and thereby ROS. These observations were supported by the work of Galinier et al. (2006), who showed that adipose tissue from Zucker obese rats had higher levels of glutathione and vitamin C in a lower redox state than the fat of lean animals. This indicates that obesity is associated with reduced ROS formation [67].
To understand the effects of ROS on preadipocytes and adipocyte differentiation, a dissection of the pathways on a molecular level should take place as it is highly dependent on specific growth factors, which influence the oxidative balance. The prior studies analysed systemic markers involved in energy metabolism (such as leptin and adiponectin) rather than intracellular redox changes, which may be completely different. On the cellular level, pro-inflammatory cytokines such as interferon-γ, transforming growth factor β, tumour necrosis factor-α and interleukin-6 have been shown to inhibit preadipocyte differentiation and lipid accumulation [68].
Skin
Mitochondrial-derived ROS have an important role in skin development and regeneration through influencing Notch and β-catenin signalling pathways. Knockout mice of mitochondrial transcription factor A (TFAM) were associated with loss of mice hair, a defective epidermal barrier and impaired keratinocyte differentiation [69]. Keratinocyte proliferation can be enhanced by irradiation by a 780 nm low power diode laser, which increases the synthesis of ROS within the cells [70].
Furthermore, exposure of the skin to the environmental pollutant, tetrachlorodibenzo-p-dioxin, results in a clinical condition, chloracne. Kennedy et al. (2012) investigated the molecular mechanism and showed upregulation of 40% of the genes responsible for the differentiation of the epidermis, as well as most of the genes responsible for de novo ceramide synthesis. These effects were mediated through increasing the mitochondrial production of ROS by 151% and was reduced by antioxidants [71]. When ROS levels increase in the mitochondria, nucleoredoxin can be targeted. The latter is a regulator of the Wnt/ β catenin pathway with the ultimate result of enhancing epidermal differentiation [69]. Thus, ROS could have a positive role in enhancing stem cell differentiation into the skin multilayers.
Neurons
The role of ROS as an important factor in the regulation of neuronal differentiation is highlighted in many in vitro approaches using cells derived from neuroblastoma, teratocarcinoma and ESC cell lines [72]. Neural stem cells have the ability to differentiate into the three types of cells that can be found in the brain, which are the neurons, astrocytes and oligodendrocytes. In the meantime, these cells keep their self-renewal ability. In addition, ROS scavenging agents can repress neurosphere formation. The surviving cells are significantly reduced in number throughout the culture period [73].
Moliner et al. showed that enhanced differentiation of ESCs to neurons in spheres was associated with increased gene expression of the pathways related to mitochondrial metabolic pathways and ROS production [74]. In clonal cortical cultures, ROS are produced early in the culture environment and lead to cellular differentiation into both the large pyramidal-like and calretinin expressing neurons [75]. Le Belle et al. reported increased oxidative stress that resulted from pharmacological inhibition of Nox enzyme, which promoted neuroepithelial stem cell cellular activity and self-renewal [76]. Furthermore, ROS- mediated neurogenesis is dependent on activation of JNK signalling [77]. Different members of the Wnt signalling pathway play an important role as well, including Wnt-3a and Wnt-7a. The Wnt/ β-catenin pathway is activated in response to ROS, as mentioned earlier [78]. Wnt can induce the expression of the sensory neuron markers, including neuroD, Brn3a and neurogenin 1 (Ngn1) through the activation of Tlx3 [79]. Table 1 summaries the effects of ROS on the pluripotency and differentiation of stem cells.
Table 1.
Summary of ROS effects on the pluripotency and differentiation of stem cells.
| Cells/Process | Oxidant/ anti-oxidant treatment | Outcome | Notes | Reference |
|---|---|---|---|---|
| Embryonic stem cells | Various ROS | Enhance mesodermal and endodermal differentiation | Down regulation of Oct4, Tra 1-60, Nanog, and Sox2 | [22] |
| Adult stem cells | N-acetyl Cystine | Decrease cell proliferation | ROS are essential for G1-S transition | [26] |
| IPSCs | Various ROS | Multiple mutations | Checking DNA integrity is a crucial step before clinical use | [40], [41] |
| Osteogenesis | Vit C and Vit E | Promote osteogenesis | Restore osteogenic differentiation. | [43], [44] |
| SOD Upregulation | Promote osteogenesis | Reduction of ROS levels | [45] | |
| H2O2 | Inhibit osteogenesis | Reduction of Osteogenic genetic markers (Runx-2 and ALP) | [46] | |
| Chondrogenesis | H2O2N-acetyl Cystine | Increases differentiation markersInhibition of chondrogenic markers | ROS are essential for survival and differentiation of chondrocytes | [52] |
| Cardiomyogenesis | Glucose induced ROS production | Induced differentiation to cardiac cells | P38 phosphorylation via Nox4 | [56] |
| H2O2 balance with NOTCH system byproducts | Future target for cell-based therapy | Activate Wnt-11 gene and induce cardiomyocyte differentiation | [58] | |
| Nox4 | Considered a pro-cardiogenesis gene | Activate p38-MAPK pathway | [80] | |
| Blood vasculogenesis | H2O2 and Nox4 | Promote angiogenesis | Induce HIF-1-α and VEGF | [60] |
| Adipogenesis | H2O2 | Induce Adipocyte differentiation | Upregulation of CEBPα and PPARγ expression | [64] |
| Inhibition of mitochondrial derived ROS | Inhibition of Adipogenesis | HIF-1 α mediated | [67] | |
| Keratogenesis | Various ROS | Enhance keratinocyte proliferation and differentiation of epidermis | Upregulation of Notch and β-catenin signalling | [69], [70] |
| Neurogenesis | H2O2 | Promoted neuronal stem cells proliferation | Increase Intracellular Ca+2 , phosphorylation of several mediators | [81] |
Conclusions and futures perspectives
Free radicals or ROS can affect stem cell differentiation through a multitude of factors that include the concentration, duration of exposure, continuous versus intermittent exposure, cellular content of antioxidants, and simultaneous co-exposure to other factors. All these elements are important towards further understanding stem cell biology. Knowing how such molecules may function in such complicated pathways may open the door for the development of regenerative applications based on stem cells for various medical conditions.
The physiological concentration of specific ROS at certain time-points seems to be crucial for keeping the potency of the cells or their differentiation towards a certain lineage. Furthermore, the specific role of certain subsets of stem cells has not been well clarified. The limit between the beneficial and the toxic doses of ROS has yet to be determined. These findings might allow a new potential for adding certain ROS at a sub-toxic concentration for a limited time as an extra component in the differentiation protocols. Further studies are required to compare between different types of ROS and antioxidants and the differentiation efficiency, as well as the ultimate dose and frequency duration of administration for the cells.
Conflict of interest
The authors would like to declare no conflict of interest.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Biographies

Ahmed Nugud graduated from the College of Medicine, University of Sharjah, Sharjah, UAE. Ahmed is currently an Intern House Officer at Dubai Health Authority, Dubai, UAE and a research fellow at Sharjah Institute for Medical Research. He published about 8 articles, including one review and a book chapter. He obtained several undergraduate and faculty research grants from University of Sharjah and Boehringer Ingelheim. He won the prestigious award of His Highness Shk. Hamdan Award for academic excellence, and multiple best poster and oral presentations at national and international meetings.

Divyasree Sandeep was graduated from the University of Kerala and obtained her Master degree in Genetics. Divyasree had her PhD degree in Biochemistry from Mahatma Gandhi University, Kerala, India. The main focus of her thesis was the intracellular effect of reactive oxygen species. Sandeep joined Sharjah Institute for Medical Research in 2014, when she gained her interest in stem cell research. She published about 12 research articles and a book chapter. Divyasree presented her research work in several international symposia and conferences and obtained two prestigious awards.

Ahmed El-Serafi was graduated from the College of Medicine, Suez Canal University, Egypt and obtained his Master degree in Medical Biochemistry. He had his PhD degree in the field of stem cell biology from the Centre for Human Development, Stem Cells and Regeneration, University of Southampton, UK. Ahmed is currently a faculty member in the College of Medicine University of Sharjah, UAE, Suez Canal University, Egypt (on leave) and a visiting professor to Linköping University, Sweden. He published about 30 articles, including two reviews and a book chapter. He obtained several research grants and international awards. Ahmed is leading the stem cell research in Sharjah as well as in the Burn Unit in Linköping.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Tandon R., Sinha M.K., Garg H., Khanna R., Khanna H.D. Oxidative stress in patients with essential hypertension. Natl Med J India. 2005;18(6):297–299. [PubMed] [Google Scholar]
- 2.Chandra J., Samali A., Orrenius S. Triggering and modulation of apoptosis by oxidative stress. Free Radic Biol Med. 2000;29(3–4):323–333. doi: 10.1016/s0891-5849(00)00302-6. [DOI] [PubMed] [Google Scholar]
- 3.Shaban S., El-Husseny M.W.A., Abushouk A.I., Salem A.M.A., Mamdouh M., Abdel-Daim M.M. Effects of antioxidant supplements on the survival and differentiation of stem cells. Oxid Med Cell Longev. 2017;2017:5032102. doi: 10.1155/2017/5032102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Madhavan L., Ourednik V., Ourednik J. Neural stem/progenitor cells initiate the formation of cellular networks that provide neuroprotection by growth factor-modulated antioxidant expression. Stem Cells. 2008;26(1):254–265. doi: 10.1634/stemcells.2007-0221. [DOI] [PubMed] [Google Scholar]
- 5.Jang Y.Y., Sharkis S.J. A low level of reactive oxygen species selects for primitive hematopoietic stem cells that may reside in the low-oxygenic niche. Blood. 2007;110(8):3056–3063. doi: 10.1182/blood-2007-05-087759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yanes O., Clark J., Wong D.M., Patti G.J., Sanchez-Ruiz A., Benton H.P. Metabolic oxidation regulates embryonic stem cell differentiation. Nat Chem Biol. 2010;6(6):411–417. doi: 10.1038/nchembio.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Droge W. Aging-related changes in the thiol/disulfide redox state: implications for the use of thiol antioxidants. Exp Gerontol. 2002;37(12):1333–1345. doi: 10.1016/s0531-5565(02)00175-4. [DOI] [PubMed] [Google Scholar]
- 8.Nathan C., Cunningham-Bussel A. Beyond oxidative stress: an immunologist's guide to reactive oxygen species. Nat Rev Immunol. 2013;13(5):349–361. doi: 10.1038/nri3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klimova T., Chandel N.S. Mitochondrial complex III regulates hypoxic activation of HIF. Cell Death Differ. 2008;15(4):660–666. doi: 10.1038/sj.cdd.4402307. [DOI] [PubMed] [Google Scholar]
- 10.Remacle J., Raes M., Toussaint O., Renard P., Rao G. Low levels of reactive oxygen species as modulators of cell function. Mutat Res. 1995;316(3):103–122. doi: 10.1016/0921-8734(95)90004-7. [DOI] [PubMed] [Google Scholar]
- 11.Ross S.H., Lindsay Y., Safrany S.T., Lorenzo O., Villa F., Toth R. Differential redox regulation within the PTP superfamily. Cell Signal. 2007;19(7):1521–1530. doi: 10.1016/j.cellsig.2007.01.026. [DOI] [PubMed] [Google Scholar]
- 12.Blanchetot C., Boonstra J. The ROS-NOX connection in cancer and angiogenesis. Crit Rev Eukaryot Gene Expr. 2008;18(1):35–45. doi: 10.1615/critreveukargeneexpr.v18.i1.30. [DOI] [PubMed] [Google Scholar]
- 13.Vallee A., Lecarpentier Y. Crosstalk between peroxisome proliferator-activated receptor gamma and the canonical WNT/beta-catenin pathway in chronic inflammation and oxidative stress during carcinogenesis. Front Immunol. 2018;9:745. doi: 10.3389/fimmu.2018.00745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holl M., Koziel R., Schafer G., Pircher H., Pauck A., Hermann M. ROS signaling by NADPH oxidase 5 modulates the proliferation and survival of prostate carcinoma cells. Mol Carcinog. 2016;55(1):27–39. doi: 10.1002/mc.22255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eblin K.E., Jensen T.J., Wnek S.M., Buffington S.E., Futscher B.W., Gandolfi A.J. Reactive oxygen species regulate properties of transformation in UROtsa cells exposed to monomethylarsonous acid by modulating MAPK signaling. Toxicology. 2009;255(1–2):107–114. doi: 10.1016/j.tox.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi H., Hudson L.G., Liu K.J. Oxidative stress and apoptosis in metal ion-induced carcinogenesis. Free Radic Biol Med. 2004;37(5):582–593. doi: 10.1016/j.freeradbiomed.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 17.Leonard S.S., Harris G.K., Shi X. Metal-induced oxidative stress and signal transduction. Free Radic Biol Med. 2004;37(12):1921–1942. doi: 10.1016/j.freeradbiomed.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 18.Osburn W.O., Kensler T.W. Nrf2 signaling: an adaptive response pathway for protection against environmental toxic insults. Mutat Res. 2008;659(1–2):31–39. doi: 10.1016/j.mrrev.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salem H.K., Thiemermann C. Mesenchymal stromal cells: current understanding and clinical status. Stem Cells. 2010;28(3):585–596. doi: 10.1002/stem.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomson J.A., Odorico J.S. Human embryonic stem cell and embryonic germ cell lines. Trends Biotechnol. 2000;18(2):53–57. doi: 10.1016/s0167-7799(99)01410-9. [DOI] [PubMed] [Google Scholar]
- 21.Ottosen L.D.M., Hindkjær J., Husth M., Petersen D.E., Kirk J., Ingerslev H.J. Observations on intrauterine oxygen tension measured by fibre-optic microsensors. Reproductive BioMed Online. 2006;13(3):380–385. doi: 10.1016/s1472-6483(10)61443-5. [DOI] [PubMed] [Google Scholar]
- 22.Ji A.-R., Ku S.-Y., Cho M.S., Kim Y.Y., Kim Y.J., Oh S.K. Reactive oxygen species enhance differentiation of human embryonic stem cells into mesendodermal lineage. Exp Mol Med. 2010;42(3):175–186. doi: 10.3858/emm.2010.42.3.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Serakinci N., Keith W.N. Therapeutic potential of adult stem cells. Eur J Cancer. 2006;42(9):1243–1246. doi: 10.1016/j.ejca.2006.01.036. [DOI] [PubMed] [Google Scholar]
- 24.Bianco P., Robey P.G., Simmons P.J. Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell. 2008;2(4):313–319. doi: 10.1016/j.stem.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.El-Serafi A.T., Wilson D.I., Roach H.I., Oreffo R.O. Developmental plasticity of human foetal femur-derived cells in pellet culture: self assembly of an osteoid shell around a cartilaginous core. Eur Cell Mater. 2011;21:558–567. doi: 10.22203/ecm.v021a42. [DOI] [PubMed] [Google Scholar]
- 26.Maraldi T., Guida M., Zavatti M., Resca E., Bertoni L., La Sala G.B. Nuclear Nox4 role in stemness power of human amniotic fluid stem cells. Oxid Med Cell Longev. 2015;2015 doi: 10.1155/2015/101304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Campisi J., d'Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8(9):729–740. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- 28.Verfaillie C.M. Hematopoietic stem cells for transplantation. Nat Immunol. 2002;3(4):314–317. doi: 10.1038/ni0402-314. [DOI] [PubMed] [Google Scholar]
- 29.Orkin S.H., Morrison S.J. Stem-cell competition. Nature. 2002;418(6893):25–27. doi: 10.1038/418025a. [DOI] [PubMed] [Google Scholar]
- 30.Bianchi G., Borgonovo G., Pistoia V., Raffaghello L. Immunosuppressive cells and tumour microenvironment: focus on mesenchymal stem cells and myeloid derived suppressor cells. Histol Histopathol. 2011;26(7):941–951. doi: 10.14670/HH-26.941. [DOI] [PubMed] [Google Scholar]
- 31.Prockop D.J. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276(5309):71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 32.Granero-Molto F., Weis J.A., Longobardi L., Spagnoli A. Role of mesenchymal stem cells in regenerative medicine: application to bone and cartilage repair. Expert Opin Biol Ther. 2008;8(3):255–268. doi: 10.1517/14712598.8.3.255. [DOI] [PubMed] [Google Scholar]
- 33.Lonergan T., Brenner C., Bavister B. Differentiation-related changes in mitochondrial properties as indicators of stem cell competence. J Cell Physiol. 2006;208(1):149–153. doi: 10.1002/jcp.20641. [DOI] [PubMed] [Google Scholar]
- 34.Lyublinskaya O.G., Borisov Y.G., Pugovkina N.A., Smirnova I.S., Obidina J.V., Ivanova J.S. Reactive oxygen species are required for human mesenchymal stem cells to initiate proliferation after the quiescence exit. Oxid Med Cell Longev. 2015;2015 doi: 10.1155/2015/502105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Urao N., Inomata H., Razvi M., Kim H.W., Wary K., McKinney R. Role of nox2-based NADPH oxidase in bone marrow and progenitor cell function involved in neovascularization induced by hindlimb ischemia. Circ Res. 2008;103(2):212–220. doi: 10.1161/CIRCRESAHA.108.176230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ahmed S.M., Morsi M., Ghoneim N.I., Abdel-Daim M.M., El-Badri N. Mesenchymal stromal cell therapy for pancreatitis: a systematic review. Oxid Med Cell Longev. 2018;2018:3250864. doi: 10.1155/2018/3250864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 38.Hussein S.M., Batada N.N., Vuoristo S., Ching R.W., Autio R., Narva E. Copy number variation and selection during reprogramming to pluripotency. Nature. 2011;471(7336):58–62. doi: 10.1038/nature09871. [DOI] [PubMed] [Google Scholar]
- 39.Ji J., Ng S.H., Sharma V., Neculai D., Hussein S., Sam M. Elevated coding mutation rate during the reprogramming of human somatic cells into induced pluripotent stem cells. Stem Cells. 2012;30(3):435–440. doi: 10.1002/stem.1011. [DOI] [PubMed] [Google Scholar]
- 40.Ji J., Sharma V., Qi S., Guarch M.E., Zhao P., Luo Z. Antioxidant supplementation reduces genomic aberrations in human induced pluripotent stem cells. Stem Cell Rep. 2014;2(1):44–51. doi: 10.1016/j.stemcr.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mayshar Y., Ben-David U., Lavon N., Biancotti J.C., Yakir B., Clark A.T. Identification and classification of chromosomal aberrations in human induced pluripotent stem cells. Cell Stem Cell. 2010;7(4):521–531. doi: 10.1016/j.stem.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 42.Mody N., Parhami F., Sarafian T.A., Demer L.L. Oxidative stress modulates osteoblastic differentiation of vascular and bone cells. Free Radic Biol Med. 2001;31(4):509–519. doi: 10.1016/s0891-5849(01)00610-4. [DOI] [PubMed] [Google Scholar]
- 43.Basu S., Michaelsson K., Olofsson H., Johansson S., Melhus H. Association between oxidative stress and bone mineral density. Biochem Biophys Res Commun. 2001;288(1):275–279. doi: 10.1006/bbrc.2001.5747. [DOI] [PubMed] [Google Scholar]
- 44.Shouhed D., Kha H.T., Richardson J.A., Amantea C.M., Hahn T.J., Parhami F. Osteogenic oxysterols inhibit the adverse effects of oxidative stress on osteogenic differentiation of marrow stromal cells. J Cell Biochem. 2005;95(6):1276–1283. doi: 10.1002/jcb.20497. [DOI] [PubMed] [Google Scholar]
- 45.Chen C.T., Shih Y.R., Kuo T.K., Lee O.K., Wei Y.H. Coordinated changes of mitochondrial biogenesis and antioxidant enzymes during osteogenic differentiation of human mesenchymal stem cells. Stem Cells. 2008;26(4):960–968. doi: 10.1634/stemcells.2007-0509. [DOI] [PubMed] [Google Scholar]
- 46.Arai M., Shibata Y., Pugdee K., Abiko Y., Ogata Y. Effects of reactive oxygen species (ROS) on antioxidant system and osteoblastic differentiation in MC3T3-E1 cells. IUBMB Life. 2007;59(1):27–33. doi: 10.1080/15216540601156188. [DOI] [PubMed] [Google Scholar]
- 47.Krampera M., Pasini A., Rigo A., Scupoli M.T., Tecchio C., Malpeli G. HB-EGF/HER-1 signaling in bone marrow mesenchymal stem cells: inducing cell expansion and reversibly preventing multilineage differentiation. Blood. 2005;106(1):59–66. doi: 10.1182/blood-2004-09-3645. [DOI] [PubMed] [Google Scholar]
- 48.Almeida M., Han L., Martin-Millan M., Plotkin L.I., Stewart S.A., Roberson P.K. Skeletal involution by age-associated oxidative stress and its acceleration by loss of sex steroids. J Biol Chem. 2007;282(37):27285–27297. doi: 10.1074/jbc.M702810200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kronenberg H.M. Developmental regulation of the growth plate. Nature. 2003;423(6937):332–336. doi: 10.1038/nature01657. [DOI] [PubMed] [Google Scholar]
- 50.Adams C.S., Shapiro I.M. The fate of the terminally differentiated chondrocyte: evidence for microenvironmental regulation of chondrocyte apoptosis. Crit Rev Oral Biol Med. 2002;13(6):465–473. doi: 10.1177/154411130201300604. [DOI] [PubMed] [Google Scholar]
- 51.Pelttari K., Steck E., Richter W. The use of mesenchymal stem cells for chondrogenesis. Injury. 2008;39(1 SUPPL.):58–65. doi: 10.1016/j.injury.2008.01.038. [DOI] [PubMed] [Google Scholar]
- 52.Li Q., Gao Z., Chen Y., Guan M.X. The role of mitochondria in osteogenic, adipogenic and chondrogenic differentiation of mesenchymal stem cells. Protein Cell. 2017;8(6):439–445. doi: 10.1007/s13238-017-0385-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim K.S., Choi H.W., Yoon H.E., Kim I.Y. Reactive oxygen species generated by NADPH oxidase 2 and 4 are required for chondrogenic differentiation. J Biol Chem. 2010;285(51):40294–40302. doi: 10.1074/jbc.M110.126821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morita K., Miyamoto T., Fujita N., Kubota Y., Ito K., Takubo K. Reactive oxygen species induce chondrocyte hypertrophy in endochondral ossification. J Exp Med. 2007;204(7):1613–1623. doi: 10.1084/jem.20062525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sauer H., Bekhite M.M., Hescheler J., Wartenberg M. Redox control of angiogenic factors and CD31-positive vessel-like structures in mouse embryonic stem cells after direct current electrical field stimulation. Exp Cell Res. 2005;304(2):380–390. doi: 10.1016/j.yexcr.2004.11.026. [DOI] [PubMed] [Google Scholar]
- 56.Buggisch M., Ateghang B., Ruhe C., Strobel C., Lange S., Wartenberg M. Stimulation of ES-cell-derived cardiomyogenesis and neonatal cardiac cell proliferation by reactive oxygen species and NADPH oxidase. J Cell Sci. 2007;120(Pt 5):885–894. doi: 10.1242/jcs.03386. [DOI] [PubMed] [Google Scholar]
- 57.Li J., Stouffs M., Serrander L., Banfi B., Bettiol E., Charnay Y. The NADPH oxidase NOX4 drives cardiac differentiation: role in regulating cardiac transcription factors and MAP kinase activation. Mol Biol Cell. 2006;17(9):3978–3988. doi: 10.1091/mbc.E05-06-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boopathy A.V., Pendergrass K.D., Che P.L., Yoon Y.S., Davis M.E. Oxidative stress-induced Notch1 signaling promotes cardiogenic gene expression in mesenchymal stem cells. Stem Cell Res Ther. 2013;4(2):43. doi: 10.1186/scrt190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jiang B.H., Rue E., Wang G.L., Roe R., Semenza G.L. Dimerization, DNA binding, and transactivation properties of hypoxia-inducible factor 1. J Biol Chem. 1996;271(30):17771–17778. doi: 10.1074/jbc.271.30.17771. [DOI] [PubMed] [Google Scholar]
- 60.Xia C., Meng Q., Liu L.Z., Rojanasakul Y., Wang X.R., Jiang B.H. Reactive oxygen species regulate angiogenesis and tumor growth through vascular endothelial growth factor. Cancer Res. 2007;67(22):10823–10830. doi: 10.1158/0008-5472.CAN-07-0783. [DOI] [PubMed] [Google Scholar]
- 61.Sauer H., Wartenberg M. Reactive oxygen species as signaling molecules in cardiovascular differentiation of embryonic stem cells and tumor-induced angiogenesis. Antioxid Redox Signal. 2005;7(11–12):1423–1434. doi: 10.1089/ars.2005.7.1423. [DOI] [PubMed] [Google Scholar]
- 62.Zhou Q., Liu L.Z., Fu B., Hu X., Shi X., Fang J. Reactive oxygen species regulate insulin-induced VEGF and HIF-1alpha expression through the activation of p70S6K1 in human prostate cancer cells. Carcinogenesis. 2007;28(1):28–37. doi: 10.1093/carcin/bgl085. [DOI] [PubMed] [Google Scholar]
- 63.Kanda Y., Hinata T., Kang S.W., Watanabe Y. Reactive oxygen species mediate adipocyte differentiation in mesenchymal stem cells. Life Sci. 2011;89(7–8):250–258. doi: 10.1016/j.lfs.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 64.Lee H., Lee Y.J., Choi H., Ko E.H., Kim J.W. Reactive oxygen species facilitate adipocyte differentiation by accelerating mitotic clonal expansion. J Biol Chem. 2009;284(16):10601–10609. doi: 10.1074/jbc.M808742200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Carrière A., Fernandez Y., Rigoulet M., Pénicaud L., Casteilla L. Inhibition of preadipocyte proliferation by mitochondrial reactive oxygen species. FEBS Lett. 2003;550(1–3):163–167. doi: 10.1016/s0014-5793(03)00862-7. [DOI] [PubMed] [Google Scholar]
- 66.Carriere A., Carmona M.C., Fernandez Y., Rigoulet M., Wenger R.H., Penicaud L. Mitochondrial reactive oxygen species control the transcription factor CHOP-10/GADD153 and adipocyte differentiation: a mechanism for hypoxia-dependent effect. J Biol Chem. 2004;279(39):40462–40469. doi: 10.1074/jbc.M407258200. [DOI] [PubMed] [Google Scholar]
- 67.Galinier A., Carriere A., Fernandez Y., Caspar-Bauguil S., Periquet B., Periquet A. Site specific changes of redox metabolism in adipose tissue of obese Zucker rats. FEBS Lett. 2006;580(27):6391–6398. doi: 10.1016/j.febslet.2006.10.052. [DOI] [PubMed] [Google Scholar]
- 68.Gustafson B., Smith U. Cytokines promote Wnt signaling and inflammation and impair the normal differentiation and lipid accumulation in 3T3-L1 preadipocytes. J Biol Chem. 2006;281(14):9507–9516. doi: 10.1074/jbc.M512077200. [DOI] [PubMed] [Google Scholar]
- 69.Hamanaka R.B., Chandel N.S. Mitochondrial metabolism as a regulator of keratinocyte differentiation. Cell Logist. 2013;3(1) doi: 10.4161/cl.25456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Grossman N., Schneid N., Reuveni H., Halevy S., Lubart R. 780 nm low power diode laser irradiation stimulates proliferation of keratinocyte cultures: involvement of reactive oxygen species. Lasers Surg Med. 1998;22(4):212–218. doi: 10.1002/(sici)1096-9101(1998)22:4<212::aid-lsm5>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 71.Kennedy L.H., Sutter C.H., Leon Carrion S., Tran Q.T., Bodreddigari S., Kensicki E. 2,3,7,8-Tetrachlorodibenzo-p-dioxin-mediated production of reactive oxygen species is an essential step in the mechanism of action to accelerate human keratinocyte differentiation. Toxicol Sci. 2013;132(1):235–249. doi: 10.1093/toxsci/kfs325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vieira H.L.A., Alves P.M., Vercelli A. Modulation of neuronal stem cell differentiation by hypoxia and reactive oxygen species. Prog Neurobiol. 2011;93(3):444–455. doi: 10.1016/j.pneurobio.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 73.Yoneyama M., Kawada K., Gotoh Y., Shiba T., Ogita K. Endogenous reactive oxygen species are essential for proliferation of neural stem/progenitor cells. Neurochem Int. 2010;56(6–7):740–746. doi: 10.1016/j.neuint.2009.11.018. [DOI] [PubMed] [Google Scholar]
- 74.Moliner A., Enfors P., Ibanez C.F., Andang M. Mouse embryonic stem cell-derived spheres with distinct neurogenic potentials. Stem Cells Dev. 2008;17(2):233–243. doi: 10.1089/scd.2007.0211. [DOI] [PubMed] [Google Scholar]
- 75.Tsatmali M., Walcott E.C., Makarenkova H., Crossin K.L. Reactive oxygen species modulate the differentiation of neurons in clonal cortical cultures. Mol Cell Neurosci. 2006;33(4):345–357. doi: 10.1016/j.mcn.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Le Belle J.E., Orozco N.M., Paucar A.A., Saxe J.P., Mottahedeh J., Pyle A.D. Proliferative neural stem cells have high endogenous ROS levels that regulate self-renewal and neurogenesis in a PI3K/Akt-dependant manner. Cell Stem Cell. 2011;8(1):59–71. doi: 10.1016/j.stem.2010.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sart S., Song L., Li Y. Controlling redox status for stem cell survival, expansion, and differentiation. Oxid Med Cell Longev. 2015;2015 doi: 10.1155/2015/105135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Visweswaran M., Pohl S., Arfuso F., Newsholme P., Dilley R., Pervaiz S. Multi-lineage differentiation of mesenchymal stem cells – to Wnt, or not Wnt. Int J Biochem Cell Biol. 2015;68:139–147. doi: 10.1016/j.biocel.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 79.Kondo T., Matsuoka A.J., Shimomura A., Koehler K.R., Chan R.J., Miller J.M. Wnt signaling promotes neuronal differentiation from mesenchymal stem cells through activation of Tlx3. Stem Cells. 2011;29(5):836–846. doi: 10.1002/stem.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jaulmes A., Sansilvestri-Morel P., Rolland-Valognes G., Bernhardt F., Gaertner R., Lockhart B.P. Nox4 mediates the expression of plasminogen activator inhibitor-1 via p38 MAPK pathway in cultured human endothelial cells. Thromb Res. 2009;124(4):439–446. doi: 10.1016/j.thromres.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 81.Lee S.H., Na S.I., Heo J.S., Kim M.H., Kim Y.H., Lee M.Y. Arachidonic acid release by H2O2 mediated proliferation of mouse embryonic stem cells: involvement of Ca2+/PKC and MAPKs-induced EGFR transactivation. J Cell Biochem. 2009;106(5):787–797. doi: 10.1002/jcb.22013. [DOI] [PubMed] [Google Scholar]