Integrating physiological and behavioral plasticity into climate change models markedly reduces predicted extinction risk.
Abstract
Extinction rates are predicted to rise exponentially under climate warming, but many of these predictions ignore physiological and behavioral plasticity that might buffer species from extinction. We evaluated the potential for physiological acclimatization and behavioral avoidance of poor climatic conditions to lower extinction risk under climate change in the global hotspot of salamander diversity, a region currently predicted to lose most of the salamander habitat due to warming. Our approach integrated experimental physiology and behavior into a mechanistic species distribution model to predict extinction risk based on an individual’s capacity to maintain energy balance with and without plasticity. We assessed the sensitivity of extinction risk to body size, behavioral strategies, limitations on energy intake, and physiological acclimatization of water loss and metabolic rate. The field and laboratory experiments indicated that salamanders readily acclimatize water loss rates and metabolic rates in ways that could maintain positive energy balance. Projections with plasticity reduced extinction risk by 72% under climate warming, especially in the core of their range. Further analyses revealed that juveniles might experience the greatest physiological stress under climate warming, but we identified specific physiological adaptations or plastic responses that could minimize the lethal physiological stress imposed on juveniles. We conclude that incorporating plasticity fundamentally alters ecological predictions under climate change by reducing extinction risk in the hotspot of salamander diversity.
INTRODUCTION
Extinction rates are predicted to accelerate with every degree that global temperatures warm (1), but most of these predictions likely overestimate extinction risk by ignoring physiological and behavioral plasticity (2). Physiological plasticity has the potential to buffer organisms from warming by reducing the thermal sensitivity of life-sustaining processes and increasing physiological tolerances (3, 4). Behavioral avoidance can also buffer organisms from climate change by minimizing exposure to costly or lethal temperatures (5, 6). However, the potential for plasticity to buffer species from extinction is contentious. Climate warming might exceed plastic capacities (7) or increase costs of behavioral strategies (8). Predictions that incorporate plasticity might help to resolve whether physiological and behavioral responses can reduce extinction risk.
The absence of plasticity represents a major impediment to improving predictions of extinction risk under climate change (9). The most common approach used to predict extinction risk under warming cannot explicitly incorporate plasticity because their predictions are generated using statistical relationships between species occurrence data and climatic data (2). These correlative models operate under the assumption that occurrence data implicitly reflect underlying mechanisms, such as phenotypic plasticity. Alternatively, mechanistic species distribution models (SDMs) are well suited to explore the ecological consequences of plasticity (10). These models estimate habitat suitability by linking functional traits with the biophysical environment to determine whether organisms can acquire the necessary energy for survival and reproduction, that is, the fundamental niche (11). To date, studies have yet to link both physiological and behavioral plasticity to an individual’s capacity to acquire sufficient energy to survive and reproduce. Current evidence suggests that mechanistic and statistical SDMs predict similar responses to climate (12, 13), but the incorporation of plasticity might disrupt the similarities between mechanistic and correlative predictions.
Mechanistic SDMs predict the distribution of habitat suitability through space and time by assessing energy balance. Daily and seasonal fluctuations in the thermal and hydric environment jeopardize energy balance by reducing the rates and durations of activities that supply energy and resources (14, 15). Temperature constrains energy intake in plants and animals by reducing the rates of photosynthesis and the capacity to forage and assimilate energy, respectively (16, 17). Similarly, the vapor content of the air also influences habitat suitability by limiting the duration of foraging activity and increasing lethal dehydration stress (15, 16, 18). Climate change might further exacerbate hydric stress as warming increases the drying power of the air. Physical expectations indicate that warming will increase evaporation rates by increasing the vapor pressure deficit (VPD), one of the primary forces driving evaporation rates (16). High VPDs are known to threaten plants as a major driver of mortality events (16), and the rise in evaporation rates under climate change increases exposure to lethal dehydration events by fourfold in birds (19). Therefore, ecological predictions require knowledge on the physiological and behavioral strategies that organisms use to minimize thermal and hydric stress.
Amphibians are among the most vulnerable organisms on the planet (20), yet they exhibit a high degree of physiological plasticity (7, 21). The empirical evidence of plasticity in amphibians consists primarily of thermal tolerances and timing of metamorphosis, but we lack a rigorous test of plasticity in traits related directly to balancing energy budgets, a key component of the fundamental niche. To address this gap in our knowledge, we evaluated the potential for plasticity in metabolic rates, water loss rates, and behavior to reduce extinction risk for seven species within the Plethodon jordani species complex under climate warming, assuming that carbon emissions continue to rise at current levels [Representative Concentration Pathway 8.5 (RCP8.5)] (22). We estimated extinction risk for the entire species complex because these species can exhibit the greatest biomass among salamanders across the region (23), and they have a wide geographic distribution within the global hotspot of salamander diversity (Fig. 1). As high elevation specialists, these species also share similar climatic requirements reflecting underlying physiological similarities (24). Moreover, recent evidence suggests that populations of these species have recently declined (25), exhibited reductions in body size due to climate (26), and face a widespread loss of suitable habitat under future climate warming (27). Because of their shared physiological requirements, high abundance, and high extinction risk, these species are well suited to assess the role of plasticity in minimizing the loss of biodiversity.
Fig. 1. Acclimatization of physiological traits in the global hotspot of salamander diversity.
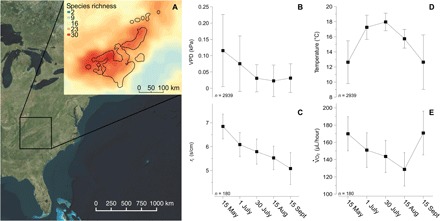
The species richness of the salamander diversity hotspot (60) inset on top of the eastern United States with the geographic range of the P. jordani species complex outlined in black (A). Mean values with SDs of environmental and physiological measurements (adjusted for mass) showing that high VPDs (B) coincide with ri (C), and high temperatures (D) were associated with low volume of oxygen consumption (E) across the seasonal time points.
RESULTS
Salamanders show a high capacity for physiological acclimatization
We determined the capacity of an abundant, widely distributed salamander (Plethodon metcalfi) to acclimatize by adjusting metabolic rates [volume of oxygen consumption (V . O2)] and water loss rates [skin resistance to water loss (ri)] in response to seasonal changes in climate in field-based experiments and under controlled exposure to temperatures and humidities in laboratory. Both laboratory-based experiments and field-based experiments relied upon using a flow-through respirometry system. We collected individuals (n = 180 for field study and n = 120 for laboratory study) along elevational gradients for both experiments in a stratified design. We collected along the elevational gradient due to recent evidence of greater physiological plasticity in salamanders collected from low elevations potentially indicative of local adaptation (28).
In the field and laboratory, salamanders adjusted physiological traits in ways that would maximize time available to forage and minimize energetic costs. In the field, we assessed the effect of body size, elevation, and sampling time period of measurements on ri and V . O2. We also demonstrated that these physiological traits are correlated with either temperature or VPD. The field-based experiments revealed that individuals increased ri by 35% in response to VPDs with the greatest drying potential (Fig. 1), and nearly 20% of the variation of ri was explained by the sampling time period (table S1). We also found that ri was positively correlated to VPDs [r2 = 0.702; slope (β) = 11.1; slope (SE) = 4.19]. Individuals acclimatized by lowering metabolic rates by 24.7% during the warmest part of the year (Fig. 1). Metabolic rates were also negatively correlated with our measurements of air temperature (r2 = 0.53; β = −5.22; SE = 2.81). Sampling time period explained less than 5% of the variation in metabolic rate (table S2), indicating a high degree of variation in acclimatization of V . O2. In the laboratory, individuals maintained high ri or increased ri by as much as 68% in response to controlled exposure to the experimental conditions (fig. S1). Individuals across the elevational gradient exhibited the same capacity to acclimatize (that is, no signal of local adaptation). The magnitude and consistent pattern of acclimatization in the field and laboratory suggest a high potential for ri to minimize the ecological impact of rising VPDs. We combined these responses with validated estimates of air temperature and VPD to predict extinction under climate warming.
Environmental predictions appear valid
Relevant estimates of temperature and VPD are critical to predict energy balance using mechanistic SDMs. We estimated air temperatures and VPDs experienced by salamanders on the basis of the microclim data set, which estimates relevant microhabitat conditions for many organisms (29). We increased the spatial resolution using adiabatic lapse rates to capture the changes in air temperature that occur due to altitude, and we estimated VPDs based on the local dew point temperature on an average night (see Materials and Methods and the Supplementary Materials for calculations). We validated our estimates of temperature and VPD with direct measurements of near-surface conditions (n = 2939) from the field using temperature and humidity data loggers. Our predicted values of current temperatures and VPDs overlapped with the observed values from the field with the exception of 2 months that were warmer than we predicted (fig. S2). We predicted VPDs under future climate warming based on the typical pattern of constant relative humidities during climate change in global circulation models (22). Under climate change, we predicted VPDs to rise by 29.5% by the end of this century across the hotspot of salamander diversity (fig. S3), which is consistent with ~3% rise per decade predicted by general circulation models (16). The spatial distribution of VPDs suggests that high elevations will continue to contain the most suitable habitat by 2100 (fig. S3). Persistence of salamander populations in these areas depends upon whether the degree of climate change challenges their capacity to balance their energy budget.
Plasticity improves performance of the SDM
We integrated physiological acclimatization and avoidance behavior into a simulation-based SDM to predict extinction risk across the global hotspot of salamander diversity. The model predicts annual energy balance, activity, and local extirpation of a site by integrating across an average day for each month of the year. We integrated physiological acclimatization into our predictions based on the consistent upper limits of ri (7 s/cm) recorded in our laboratory and field-based experiments. We compared these estimates with an average ri (4 s/cm) to identify the maximum potential for physiological acclimatization to reduce extinction risk. We also incorporated behavioral avoidance into our SDM because these species are well known for their behavioral avoidance of harsh climatic conditions. In nature, terrestrial salamanders assess climatic conditions at the opening of their refuge and avoid unfavorable thermal and hydric conditions (30). We incorporated behavioral avoidance based on the relative energetic costs associated with activity. In the simulations with avoidance behavior, individuals either foraged on the surface or remained inactive underground depending upon the state with the lowest net energetic costs. Salamanders always abandoned surface activity upon reaching a specific dehydration threshold, a relative amount of mass lost due to water loss during activity (31). We predicted annual energy balance under all possible combinations of body size, dehydration thresholds for activity, physiological acclimatization, and behavioral strategies every 10 years over the next century for a total of 480 simulations across 288,000 sites. With these iterations, we could address the sensitivity of our predictions to ecologically important factors.
Incorporating physiological acclimatization and behavioral avoidance improved our ability to explain the current geographic range of the species complex. Without acclimatization and avoidance, our simulation indicated that 63% of the current geographic range could not support salamanders (that is, annual energy reserves were depleted). With acclimatization and avoidance, more than 90% of their species range was predicted to be suitable (that is, positive energy balance), assuming that individuals remained active until losing 10% of their body mass to water loss, a realistic value based on empirical evidence (31). Including behavioral avoidance improved our SDM by predicting most of their geographic range to support the energetic demands of reproduction (at least 1.7 kJ/year) (32). The SDM overestimated the species range at higher latitudes (Fig. 2), but mechanistic SDMs typically overestimate species ranges to regions that are inhabited by congenerics with similar physiological traits (33, 34). The importance of acclimatization and avoidance under current climates suggests that these responses could be important during climate warming.
Fig. 2. Acclimatization and avoidance behavior reduces extinction risk in the core of salamander diversity hotspot.
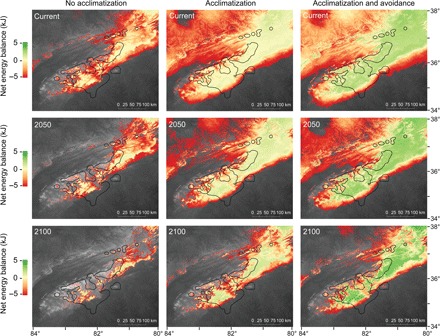
Estimates of energy balance (ranging from green to red) and extinction (grayscale) as warming progresses through time (top to bottom) demonstrate that acclimatization and avoidance behavior reverse extinction in the core of the global hotspot of salamander diversity. Green regions are predicted to have sufficient energy for reproduction, orange regions are in negative energy balance, and grayscale regions are extinct. The grayscale also indicates the elevation ranging from black (80 m) to white (2300 m). The left panels do not include acclimatization, the middle panels include acclimatization, and the right panels include acclimatization and avoidance behavior. The black outline represents the range limits for the P. jordani species complex. The maps have a spatial resolution of 1 km2 over an area of 430 km × 530 km.
Plasticity reduces extinction risk under future warming
Physiological and behavioral plasticity maintained energy balance in the core of the hotspot of salamander diversity under climate warming. Without plasticity, 71% of the sites within the species range were predicted to experience local extirpation by the end of the century. With plasticity, our SDM predicted that only 20% of the sites within the species’ range would experience local extirpation by 2100 for an average-sized salamander (~3 g for our study) (Figs. 2 and 3). Thus, plasticity reduced the number of sites expected to experience local extirpation by 72% by the end of the century. The SDM also predicted that the geographic range would contract by 30% by the end of the century with physiological acclimatization and behavioral avoidance (Fig. 2). Populations that persisted through the next century appear to be limited to high elevations in the core of their range (see Fig. 2 for digital elevation map underlying energy balance). The incorporation of plasticity led to an increase in positive energy balance in the core of their range by as much as twofold (Fig. 2). Therefore, including plasticity reversed the pattern of energy balance over the next century in the core of their range. Warming still appears to negatively affect populations at the range limits as energetic costs exceed energy intake (35), whereas warming positively affects the core of their range by increasing energy intake. We also discovered that local extirpation occurred because of a failure to balance energy budgets, and salamanders never experienced physiological death because of reaching their maximum thermal tolerance.
Fig. 3. Extinction risk is highest for juvenile salamanders.
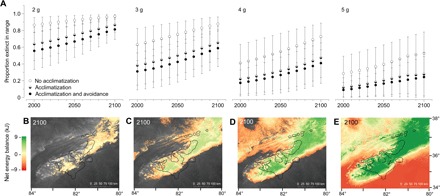
(A) The effects of acclimatization and avoidance behavior are illustrated using empty circles (no acclimatization or avoidance behavior), half-filled circles (acclimatization only), and full circles (acclimatization and avoidance behavior). Each panel illustrates how warming influences extinction risk within the geographic range for each body size (2 to 5 g) over the next century. Average proportions of extinct regions with SDs of four body size classes are shown. (B to E) The spatial distribution of energy balance and extinction for each corresponding body size (2 to 5 g) reveals that juvenile salamanders are disproportionately affected by warming.
Juveniles might experience the greatest physiological stress as the climate warms. In our predictions, large individuals (5 g) experienced little risk of extinction over the next century, whereas small individuals experienced the greatest threat to their physiological capacity to survive (Fig. 3). These differences in body size explained up to 53% of the variation in the number of areas predicted to become locally extirpated within the species range during climate change (table S3). Thus, juveniles or small-bodied terrestrial salamanders might experience the greatest threat to their capacity to forage, defend territories, and find mates in the future. However, we identified three possible responses capable of relieving physiological stress of juveniles. Our additional sensitivity analysis on small individuals indicated that evolving a high ri, maximizing energetic intake, or acclimating metabolic rates can maintain their capacity to find sufficient energy for survival and reproduction (Fig. 4).
Fig. 4. Adaptations or plastic responses required to reverse extinction in the most sensitive life stage.
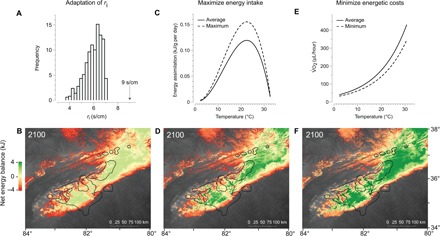
The required phenotypic changes required to maintain habitat suitability under climate warming by (A) changing ri relative to the observed distribution of ri in our experiments, (C) maximizing energy intake, and (E) minimizing energetic costs. Below the phenotypic responses, we demonstrate the reductions of extinction risk and increased energy balance throughout the geographic range of the species complex (B, D, and F). Green regions are predicted to have sufficient energy for growth and survival, orange regions are in negative energy balance, and grayscale regions are extinct. The maps have a spatial resolution of 1 km2 over an area of 430 km × 530 km.
DISCUSSION
Our study suggests that physiological acclimatization and behavioral avoidance have the potential to markedly reduce extinction risk under climate change. Recent meta-analyses have suggested that climate change will exceed the physiological capacities of ectotherms to tolerate warming (7). In contrast, other studies have suggested that plasticity will buffer species from climate change (3), but to date, studies have yet to link plasticity in multiple traits to an individual’s capacity to balance their energy budget. Our results indicate that predictions without explicitly incorporating plasticity should be interpreted cautiously. Our study also demonstrates that the incorporation of physiological and behavioral plasticity can improve the capacity of mechanistic SDMs to explain the geographic range of a species. By incorporating complex traits, mechanistic SDMs might identify the physiological and behavioral mechanisms that support the fundamental niche. We also predicted that warming might disproportionately threaten small individuals and juveniles, a common trend among many taxa under climate change (36).
Reductions in body size represent a universal response to climate warming due to an increase in energetic costs that reduce energy available for growth (36). In our analysis, the higher rates of physiological death in small individuals suggest that studies on salamanders have missed critical processes underlying ecological responses to climate by focusing on an average body size (26, 32). For instance, warming has been linked to reductions in body size of salamander populations by reducing the amount of energy available for growth (26). Our study reveals that large individuals exhibit a substantial advantage under warming in terms of energy balance and activity budgets, likely due to their low surface area–to–volume ratios, higher energy intake, and greater lipid reserves that minimize the risk of starvation. Our analysis is consistent with reductions in energy available for growth, resulting in reductions in body size; however, the reductions in body size might also reflect changes in the age distribution of a population or statistical artifacts due to nonrandom sampling (37). If these alternative explanations are true, the survival of small individuals would rely upon adaptation, plasticity, or greater behavioral avoidance.
We identified three possibilities that could greatly reduce physiological stress of juveniles. These responses could ameliorate physiological stress imposed upon juveniles, but the possibility of one of these scenarios reducing extinction risk appears low, at least in isolation. For instance, we lack evidence that ri is a heritable trait or that the required genetic variation exists for ri to evolve. We also assumed that salamanders had immediate access to prey, and thus, the capacity to maximize energy intake will depend upon prey densities. Any reductions in prey abundances would inhibit their capacity to maximize energy intake. The analysis also indicated that a 20% reduction in metabolic rates could lower extinction risk for juveniles, and our experimental results suggest that salamanders can meet that demand (Fig. 1). However, our simulations required that juveniles reduce metabolic rates by 20% during the entire year, whereas our empirical results indicated that salamanders only acclimatize during the warmest period of the summer. In isolation, these scenarios seem unlikely, but they are not mutually exclusive. Combinations of these responses might interact over time to lower physiological stress. In addition, juveniles might avoid lethal stress by restricting activity to saturated microhabitat, such as under leaf litter. These unexplored strategies might be critical for lowering physiological stress in the most vulnerable stage of life.
Our results suggest that correlative SDMs overestimate extinction risk because they do not capture plasticity. Comparisons between correlative and mechanistic SDMs indicate that these approaches can produce similar predictions under climate warming (12). In our focal system, correlative SDMs have predicted suitable habitat for the global hotspot of salamander diversity to disappear under climate change (27), a result that is similar to our simulations without plasticity. However, incorporating plasticity fundamentally altered our predictions of extinction by maintaining habitat suitability and reversing the change in energy balance during warming in the core of their range. Our analysis suggests that correlative models struggle to capture complex traits, such as phenotypic plasticity. More precisely, correlative models can predict whether populations will experience nonanalogous climates under climatic change (38), but they cannot evaluate whether an individual has the potential to survive and reproduce in nonanalogous climates. Correlative models are likely more appropriate for studies on current distributions, such as the discovery of unknown populations (39) or factors beyond physiology, such as species interactions, that structure species ranges (32). However, spatial layers derived from physiology can be incorporated into a correlative framework, and previous work indicates that incorporating mechanism can reduce the severity of extinction risk under climate change (40). These findings bolster our claim that mechanism promotes a more flexible, and possibly realistic, perspective on the impact of climate warming, particularly if the model addresses uncertainty (41). Studies like these can inform conservation strategies by providing perspective on the processes that threaten biodiversity.
Conservation plans can improve their effectiveness by considering the physiological mechanisms that contribute to population decline (42). For amphibians, enigmatic population declines have hindered the development of effective conservation strategies (43). Understanding the cause of amphibian declines has been complicated by the multitude of factors that contribute to their decline, such as habitat conversion, overexploitation, disease, and climatic factors (44). Salamanders are no exception. Population declines in North and South America have occurred in the absence of a clear cause (25, 43, 45). Here, we contributed to the broader effort of identifying the geographic regions with the highest physiological stress under climate warming. These predictions can be combined with alternative stressors across a landscape (for example, disease risk and habitat destruction) to identify high-priority regions for conservation action. Because of additional threats like novel fungal pathogens (46), an integrative approach provides the best chance to conserve the global hotspot of salamander diversity.
Physiological and behavioral plasticity have been proposed as predictors of a species’ susceptibility to climate change (47). In accordance with this hypothesis, we discovered that physiological and behavioral plasticity reduced estimates of extinction risk under the most extreme warming scenario. We acknowledge that interpreting plasticity can be complicated by an incomplete understanding of the relative costs and benefits of plasticity. Physiological acclimation in lungless salamanders has been shown to reflect a trade-off between water loss rates and metabolic rates due to underlying physical and biochemical linkages (48). The trade-off has the potential to undermine the benefits of acclimation, but in our study, salamanders in the field-based experiments appeared to decouple physiological responses to thermal and hydric stressors without exhibiting an obvious trade-off. We also suggest further studies on the consequences of physiological acclimatization for behavior will improve our understanding of the costs and benefits of plasticity. Even with these caveats, our results underscore the need to include complex biological responses, such as plasticity and adaptation, into ecological predictions of climate change. These considerations will improve our capacity to understand whether physiological and behavioral plasticity can effectively meet the biophysical demand of climate change.
MATERIALS AND METHODS
Animal collection and care
Our study conducted several laboratory-based experiments to confirm that individuals exhibit physiological plasticity in response to temperature and humidity. We collected P. metcalfi from the Nantahala National Forest near Cullowhee, NC (35°20′N, 83°4′W) for all experiments. We collected no more than four individuals from random coordinates that we generated using QGIS (Quantum Geographic Information System Developmental Team), and we sampled more than 100 m from the road to reduce the risk of road effects. We collected individuals equally across the extent of our species’ elevational range at low (1000 m), mid (1300 m), and high (1600 m) sites. After collection, we placed each salamander in a Ziploc bag with moist leaf litter and transported them back to the laboratory. Individuals were maintained in containers (17 cm × 17 cm × 12 cm) throughout their time in the laboratory and provided with moist paper towels to avoid dehydration. All experiments were approved by the Institute for Animal Care and Use Committee at Clemson University (#2014-024), and approval for collections and experimentation were granted by the Nantahala National Forest, the U.S. Fish and Wildlife Service (#MA90761B-0), and the North Carolina Wildlife Resource Commission (#16-SC00746).
Seasonal acclimatization of physiology
Seasonal variation in physiological traits could identify the sensitivity of organisms to environmental cues. We collected 36 individuals at five time points (n = 180) that occurred over the spring, summer, and fall of 2015 to measure variation in physiological traits. After collection, salamanders were maintained at 15°C for 1 week to ensure that salamanders were in a postabsorptive state. Waiting for 1 week did not appear to influence our ability to detect geographic variation of ri in the past, and an acclimation period was critical to control for potential differences in digestion. We then used the flow-through system (described in the Supplementary Materials) to measure physiological traits. Each salamander was measured three times over a period of 2.5 hours at 18°C and a VPD of 0.5 kPa (76% relative humidity). We only analyzed physiological traits during rest, which occurred at least 1 hour after being placed in the chambers, and we excluded any measurements with spikes or irregularities indicative of activity. The values of ri and V . O2 were calculated using a series of equations that convert partial pressures of gases into meaningful physiological values (see eqs. S1 to S4). Resistance of the respiratory system could be ignored because these species lacked lungs and relied upon cutaneous gas exchange to breathe. We included an additional laboratory-based experiment that tested the extremes of their capacity to acclimatize (described in the Supplementary Materials) to demonstrate that salamanders in this population exhibited physiological plasticity.
Environmental data
We measured temperature and humidity in the spring, summer, and fall of 2015 to identify the relationship between physiological plasticity and environmental cues. We distributed 15 iButton data loggers (Hygrochron, Maxim Integrated) to randomly generated coordinates at the collection sites. The local microhabitat associated with each iButton typically consisted of low-lying herbaceous undergrowth in a hardwood, deciduous forest. We programmed the iButtons using Thermodata Viewer (v. 3.1.24; Thermodata Ltd.) to record temperature and relative humidity every 20 min from May to September 2015. We only included nighttime temperatures and humidities in our analyses (n = 2939). The iButtons were placed on the ground in hardwire mesh cages (1-cm gauge) and measured conditions at ~1 cm above the forest floor. We then converted relative humidity to VPDs using the equations described in the Supplementary Materials. We averaged the nightly temperatures and VPDs over the 10 days before collection. We selected 10 days due to the rate at which congenerics adjust physiological traits to temperature in the laboratory (49).
Estimation and validation of environmental data for SDM
We used estimates of air temperature near the surface from the microclim data set (29), which generates estimates of surface temperature across the globe under various types of microclimatic environments. For our model, we assumed that the environmental temperatures were similar to the surface soil temperatures (depth, 0 cm) in 90% shade to reflect the conditions of a typical deciduous forest. We used an adiabatic correction on the temperature layers from the microclim data set that accounts for the effect of altitude on temperature to improve the resolution to ~0.9 km2 (see eq. S5). We estimated the vapor content of the air by treating the daily minimum temperature as the dew point temperature (see eq. S6) because minimum temperatures frequently approach the dew point temperature due to radiative cooling near the surface (50). To estimate VPDs under climate change, we assumed that relative humidities remained constant during climate change, a typical characteristic of general circulation models (22). We validated the estimates of temperature and humidity using the iButton data loggers deployed from March to October in 2015 and 2016 using the same methods described above. We then assessed the relationship between observed and predicted estimates of surface temperature and VPDs. The microclim values were then used to estimate activity and energetics in our foraging-energetic SDM.
Foraging-energetic SDM
We developed a physiologically structured SDM to estimate the change in durations of activity and net energy balance under climate change over the geographic range of the P. jordani species complex. The model was built from a custom code implemented in Python (v. 2.7) that iterated across each pixel (n = 288,000) of the environmental data to calculate activity and energetics. We validated our physiologically structured approach in a recent study by demonstrating the necessity of accurate estimates of resistance to water loss to predict the current distribution of the study species (51). Our modeling approach predicted 83% of the recorded captures between 1940 and the current day to be in positive energy balance. We provided a flow chart that describes how the model iterates through space, time, and phenotypes to generate estimates of activity, energy budgets, and extinction risk (fig. S4).
In our model, individuals need to acquire sufficient energy (that is, prey) while foraging to reproduce and maintain their physiological capacity to survive. We limited the potential for activity to nighttime hours (2100 to 0600) to reflect their nocturnal behavior. We then used durations of activity to calculate energy budgets based on thermally sensitive traits involving energy intake and costs. Body temperatures were estimated using humid operative temperature (Teh) to account for radiative balance at night and the loss of heat associated with evaporative cooling (see eq. S10) (52). Salamanders were active when body temperatures were between 5° and 25°C because Plethodon cease activity outside of this range, and this interval represented the range of values that salamanders selected in the laboratory (30). A salamander could be active until the salamanders lost 10% of their fully hydrated mass, the threshold at which motor function begins to decline (31). We also ran simulations with a 3.5 and 7% dehydration threshold to assess the sensitivity of the output to the dehydration threshold. Upon losing the specified body mass to water loss, salamanders retreated underground (to 2.5- or 30-cm depth, depending on the behavioral strategy) to rehydrate during the day. Our assumption that salamanders rehydrate during the day was supported by the maintenance of high water potential of soil in the southern Appalachian Mountains (53) and the rapid rehydration rates of Plethodon in soil (54).
A key component to foraging-energetic models is the balance between energetic intake and costs. We estimated energy intake while foraging based on the thermal sensitivity of prey consumption (in joules) and energy assimilation (see eq. S11) (55). In our simulations, individuals always have access to prey during activity, and they immediately consume and digest their prey. Water intake from prey was incorporated by assuming that insects had an average composition of water content of ~70% and yielded 22 kJ/g of dry mass (56). Energetic costs were estimated from the variation in resting rates of oxygen consumption (V . O2) across temperature measured in the laboratory in this study (see eq. S12). Rates of V . O2 during activity were estimated to be 1.5-fold higher than resting rates (57), and salamanders were assumed to exhibit resting rates of V . O2 when underground. Reproductive potential was determined by whether an individual could acquire sufficient energetic resources (1.7 kJ) over a year to support their biennial strategy of reproduction (32). While inactive underground, a salamander’s body temperature was calculated on the basis of the soil temperature fluctuations that occur throughout the day at a given depth (see eq. S13). We assumed that salamanders could find a suitable microhabitat during brumation (winter dormancy) throughout the cold months. Energetic costs associated with brumation are less than 1% of their total energy reserves (58); therefore, we assumed no energetic costs associated with subzero temperatures. Because of the difficulty in estimating energy budgets, our model required several assumptions. However, we believed these assumptions to be supported by existing data or result in conservative estimates of extinction. These assumptions and explanations can be found in the Supplementary Materials.
Incorporation of physiological and behavioral plasticity
We ran multiple simulations of the model with and without acclimatization of ri. The values of ri were empirically derived estimates based on the seasonal variation of ri that coincided with changes in VPD (Fig. 1) and plasticity of ri in the laboratory (fig. S3). We modeled physiological plasticity using changes in the mean value of ri between simulations. By using the mean, our simulations estimated the full potential for physiological plasticity to minimize the effect of warming. Simulations with acclimatization incorporated the maximum value of ri (7 s/cm), and simulations without acclimatization incorporated the average value of ri (4 s/cm). We also tested the extent to which behavioral avoidance buffers extinction risk. In simulations with avoidance, salamanders retreated underground to a depth of 30 cm when net energetic costs were higher while foraging relative to remaining inactive underground. This avoidance behavior was used to simulate the potential for individuals to limit exposure to high energetic costs. We selected a depth of 30 cm in simulations with avoidance due to the negligible dampening of temperature beyond this depth. Therefore, this depth represents the maximum depth that behavioral strategies might minimize energetic costs. We used a depth of 2.5 cm in simulations without avoidance to identify the full potential of avoidance to minimize energetic costs. These depths also represented the range of depths that terrestrial salamanders behaviorally selected while underground (59). Comparisons between these depths indicated the greatest extent to which behavioral choices of depth might influence energy balance and extinction.
Predicting extinction
We estimated extinction based on the net energy balance of salamanders and temperatures that they experienced throughout the year. We considered a population at a site to be locally extirpated if body temperature exceeded the critical thermal maximum of 34.9°C while inactive underground (30). From an energetic perspective, populations at a certain geographic location were considered extinct if net energy budgets fell below the lipid reserves for a given body size (see eq. S14). The model assessed energy balance throughout the year (as opposed to the annual net energy balance) to determine whether the climatic conditions depleted energy reserves during any month of the year. Our analysis addresses sensitivity of extinction by adjusting body size, ri, dehydration thresholds, and behavioral strategies.
Plasticity or adaptation required to lower extinction
We ran additional simulations that varied ri, V . O2, and energy assimilation based on empirical evidence to identify the phenotypic changes required for individuals with a small body size (2 g) to avoid extinction. Specifically, we tested whether extreme trait variation in ri due to developmental plasticity or adaptation could reverse estimates of extinction. We tested ri from 8 to 15 s/cm, which represents trait values above the current distribution of ri phenotypes. We also explored the capacity for acclimatization of V . O2 to lower extinction risk, and we ran simulations in which individuals reduced metabolic costs from 10 to 50%. We also increased energy intake based on the upper bound of an SD of energy assimilation (55). Maximizing energy assimilation adjusted energy intake by 22 to 122% depending on body temperature. We determined the phenotypic changes required to maintain habitat suitability by comparing the average energy balance within the species range from current time periods to energy balance by the end of the century with the extreme trait variation.
Supplementary Material
Acknowledgments
We would like to thank D. Wake, K. Zamudio, and the Clemson Writing Group for their comments on this manuscript. Funding: We would like to thank the Highlands Biological Station in Highlands, NC for the opportunity to collect these data through their Grant-in-Aid Program. Funding for the experiments was provided by the NSF Doctoral Dissertation Improvement Grant Program (#1601485) and the Clemson Creative Inquiry program. We thank the Clemson Creative Inquiry program, the Open Access Publishing Fund through the Clemson Library, and the Department of Biological Sciences at Clemson University for funding to cover publication costs. Author contributions: E.A.R. conceptualized, designed, implemented the experiments, conducted the analyses, developed the mechanistic SDM, and wrote the manuscript. J.P.O. and J.D.D. assisted with the experiments. M.W.S. conceptualized and designed the project, as well as provided helpful comments to the manuscript. Competing interests: The authors declare that they have no competing interests. Data and materials availability: Data are available on the Open Science Framework (https://osf.io/e4sp6/), and Python Script with supporting materials is available on GitHub (https://github.com/ecophysiology/salamander_simulation). All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/4/7/eaar5471/DC1
Supplementary Materials and Methods
Table S1. Analysis of covariance and effect sizes for seasonal acclimatization demonstrates that surface area and month have the greatest influence on skin resistance to water loss (ri).
Table S2. Analysis of covariance and effect size for the seasonal acclimatization experiment demonstrates that surface area and month had the greatest effect on metabolic rate (V . O2) during the summer.
Table S3. Analysis of covariance and effect size on the simulated data from the mechanistic SDM illustrates that mass had the largest influence on the number of extinct regions in the species range model.
Fig. S1. Experimental evidence for physiological plasticity of skin resistance to water loss.
Fig. S2. Predicted and observed values of nighttime temperatures and VPDs at our field site near Cullowhee, NC.
Fig. S3. Estimated rise in VPDs through time under climate change and the spatial distribution of VPDs in 2100.
Fig. S4. Flow chart of foraging-energetic model used to estimate extinction.
REFERENCES AND NOTES
- 1.Urban M. C., Accelerating extinction risk from climate change. Science 348, 571–573 (2015). [DOI] [PubMed] [Google Scholar]
- 2.Urban M. C., Bocedi G., Hendry A. P., Mihoub J.-B., Pe’er G., Singer A., Bridle J. R., Crozier L. G., De Meester L., Godsoe W., Gonzalez A., Hellman J. J., Holt R. D., Huth A., Johst K., Krug C. B., Leadley P. W., Palmer S. C. F., Pantel J. H., Schmitz A., Zollner P. A., Travis J. M. J., Improving the forecast for biodiversity under climate change. Science 353, aad8466 (2016). [DOI] [PubMed] [Google Scholar]
- 3.Seebacher F., White C. R., Franklin C. E., Physiological plasticity increases resilience of ectothermic animals to climate change. Nat. Clim. Change 5, 61–66 (2015). [Google Scholar]
- 4.Stillman J. H., Acclimation capacity underlies susceptibility to climate change. Science 301, 65 (2003). [DOI] [PubMed] [Google Scholar]
- 5.Huey R. B., Kearney M. R., Krockenberger A. K., Holtum J. A. M., Jess M., Williams S. E., Predicting organismal vulnerability to climate warming: Roles of behaviour, physiology and adaptation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 367, 1665–1679 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sunday J. M., Bates A. E., Kearney M. R., Colwell R. K., Dulvy N. K., Longino J. T., Huey R. B., Thermal-safety margins and the necessity of thermoregulatory behavior across latitude and elevation. Proc. Natl. Acad. Sci. U.S.A. 111, 5610–5615 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gunderson A. R., Stillman J. H., Plasticity in thermal tolerance has limited potential to buffer ectotherms from global warming. Proc. Biol. Sci. 282, 20150401 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sears M. W., Angilletta M. J. Jr, Schuler M. S., Borchert J., Dillipane K. F., Stegman M., Rusch T. W., Mitchell W. A., Configuration of the thermal landscape determines thermoregulatory performance of ectotherms. Proc. Natl. Acad. Sci. U.S.A. 113, 10595–10600 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sinclair B. J., Marshall K. E., Sewell M. A., Levesque D. L., Willett C. S., Slotsbo S., Dong Y., Harley C. D. G., Marshall D. J., Helmuth B. S., Huey R. B., Can we predict ectotherm responses to climate change using thermal performance curves and body temperatures?. Ecol. Lett. 19, 1372–1385 (2016). [DOI] [PubMed] [Google Scholar]
- 10.Kolbe J. J., Kearney M., Shine R., Modeling the consequences of thermal trait variation for the cane toad invasion of Australia. Ecol. Appl. 20, 2273–2285 (2010). [DOI] [PubMed] [Google Scholar]
- 11.Kearney M. R., Porter W. P., Mapping the fundamental niche: Physiology, climate, and the distribution of a nocturnal lizard. Ecology 85, 3119–3131 (2004). [Google Scholar]
- 12.Kearney M. R., Wintle B. A., Porter W. P., Correlative and mechanistic models of species distribution provide congruent forecasts under climate change. Conserv. Lett. 3, 203–213 (2010). [Google Scholar]
- 13.Buckley L. B., Linking traits to energetics and population dynamics to predict lizard ranges in changing environments. Am. Nat. 171, E1–E19 (2008). [DOI] [PubMed] [Google Scholar]
- 14.Grant B. W., Dunham A. E., Thermally imposed time constraints on the activity of the desert lizard Sceloporus merriami. Ecology 69, 167–176 (1988). [Google Scholar]
- 15.Levy O., Dayan T., Porter W. P., Kronfeld-Schor N., Foraging activity pattern is shaped by water loss rates in a diurnal desert rodent. Am. Nat. 188, 205–218 (2016). [DOI] [PubMed] [Google Scholar]
- 16.Williams A. P., Allen C. D., Macalady A. K., Griffin D., Woodhouse C. A., Meko D. M., Swetnam T. W., Rauscher S. A., Seager R., Grissino-Mayer H. D., Dean J. S., Cook E. R., Gangodagamage C., Cai M., McDowell N. G., Temperature as a potent driver of regional forest drought stress and tree mortality. Nat. Clim. Change 3, 292–297 (2012). [Google Scholar]
- 17.Adolph S. C., Porter W. P., Temperature, activity, and lizard life histories. Am. Nat. 142, 273–295 (1993). [DOI] [PubMed] [Google Scholar]
- 18.McKechnie A. E., Wolf B. O., Climate change increases the likelihood of catastrophic avian mortality events during extreme heat waves. Biol. Lett. 6, 253–256 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Albright T. P., Mutiibwa D., Gerson A. R., Smithh E. K., Talbot W. A., O’Neill J. J., McKechnie A. E., Wolf B. O., Mapping evaporative water loss in desert passerines reveals an expanding threat of lethal dehydration. Proc. Natl. Acad. Sci. U.S.A. 114, 2283–2288 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hof C., Araújo M. B., Jetz W., Rahbek C., Additive threats from pathogens, climate and land-use change for global amphibian diversity. Nature 480, 516–519 (2011). [DOI] [PubMed] [Google Scholar]
- 21.Urban M. C., Richardson J. L., Freidenfelds N. A., Plasticity and genetic adaptation mediate amphibian and reptile responses to climate change. Evol. Appl. 7, 88–103 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Intergovernmental Panel on Climate Change (IPCC), Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, Core Writing Team, R. K. Pachauri, L. A. Meyer, Eds. (IPCC, Geneva, Switzerland, 2014), 151 pp. [Google Scholar]
- 23.Hairston N. G., The experimental test of an analysis of field distributions: Competition in terrestrial salamanders. Ecology 61, 817–826 (1980). [Google Scholar]
- 24.Wiens J. J., Ackerly D. D., Allen A. P., Anacker B. L., Buckley L. B., Cornell H. V., Damschen E. I., Davies T. J., Grytnes J.-A., Harrison S. P., Hawkins B. A., Holt R. D., McCain C. M., Stephens P. R., Niche conservatism as an emerging principle in ecology and conservation biology. Ecol. Lett. 13, 1310–1324 (2010). [DOI] [PubMed] [Google Scholar]
- 25.Caruso N. M., Lips K. R., Truly enigmatic declines in terrestrial salamander populations in Great Smoky Mountains National Park. Divers. Distrib. 19, 38–48 (2012). [Google Scholar]
- 26.Caruso N. M., Sears M. W., Adams D. C., Lips K. R., Widespread rapid reductions in body size of adult salamanders in response to climate change. Glob. Chang. Biol. 20, 1751–1759 (2014). [DOI] [PubMed] [Google Scholar]
- 27.Milanovich J. R., Peterman W. E., Nibbelink N. P., Maerz J. C., Projected loss of a salamander diversity hotspot as a consequence of projected global climate change. PLOS ONE 5, e12189 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Riddell E. A., Sears M. W., Geographic variation of resistance to water loss within two species of lungless salamanders: Implications for activity. Ecosphere 6, 86 (2015). [Google Scholar]
- 29.Kearney M. R., Isaac A. P., Porter W. P., microclim: Global estimates of hourly microclimate based on long-term monthly climate averages. Sci. Data 1, 140006 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spotila J. R., Role of temperature and water in the ecology of lungless salamanders. Ecol. Monogr. 42, 95–125 (1972). [Google Scholar]
- 31.Feder M. E., Londos P. L., Hydric constraints upon foraging in a terrestrial salamander, Desmognathus ochrophaeus (Amphibia: Plethodontidae). Oecologia 64, 413–418 (1984). [DOI] [PubMed] [Google Scholar]
- 32.Gifford M. E., Kozak K. H., Islands in the sky or squeezed at the top? Ecological causes of elevational range limits in montane salamanders. Ecography 35, 193–203 (2012). [Google Scholar]
- 33.Buckley L. B., Urban M. C., Angilletta M. J., Crozier L. G., Rissler L. J., Sears M. W., Can mechanism inform species’ distribution models?. Ecol. Lett. 13, 1041–1054 (2010). [DOI] [PubMed] [Google Scholar]
- 34.Sinervo B., Méndez-de-la-Cruz F. R., Miles D. B., Heulin B., Bastiaans E., Villagrán-Santa Cruz M., Lara-Resendiz R. A., Martínez-Méndez N., Calderón-Espinosa M. L., Meza-Lázaro R. N., Gadsden H. E., Avila L. J., Morando M., De la Riva I. J., Sepúlveda P. V., Rocha C. F. D., Ibargüengoytía N. R., Puntriano C. A., Massot M., Lepetz V., Oksanen T. A., Chapple D. G., Bauer A. M., Branch W. R., Clobert J., Sites J. W. Jr, Erosion of lizard diversity by climate change and altered thermal niches. Science 328, 894–899 (2010). [DOI] [PubMed] [Google Scholar]
- 35.Levy O., Borchert J. D., Rusch T. W., Buckley L. B., Angilletta M. J. Jr, Diminishing returns limit energetic costs of climate change. Ecology 98, 1217–1228 (2017). [DOI] [PubMed] [Google Scholar]
- 36.Sheridan J. A., Bickford D., Shrinking body size as an ecological response to climate change. Nat. Clim. Change 1, 401–406 (2011). [Google Scholar]
- 37.Connette G. M., Crawford J. A., Peterman W. E., Climate change and shrinking salamanders: Alternative mechanisms for changes in plethodontid salamander body size. Glob. Chang. Biol. 8, 2834–2843 (2015). [DOI] [PubMed] [Google Scholar]
- 38.Fitzpatrick M. C., Hargrove W. W., The projection of species distribution models and the problem of non-analog climate. Biodivers. Conserv. 18, 2255–2261 (2009). [Google Scholar]
- 39.Rhoden C. M., Peterman W. E., Taylor C. A., Maxent-directed field surveys identify new populations of narrowly endemic habitat specialists. PeerJ 5, e3632 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mathewson P. D., Moyer-Horner L., Beever E. A., Briscoe N. J., Kearney M., Yahn J. M., Porter W. P., Mechanistic variables can enhance predictive models of endotherm distributions: The American pika under current, past, and future climates. Glob. Chang. Biol. 23, 1048–1064 (2017). [DOI] [PubMed] [Google Scholar]
- 41.Peterman W. E., Gade M., The importance of assessing parameter sensitivity when using biophysical models: A case study using plethodontid salamanders. Popul. Ecol. 59, 275–286 (2017). [Google Scholar]
- 42.Cooke S. J., O’Connor C. M., Making conservation physiology relevant to policy makers and conservation practitioners. Conserv. Lett. 3, 159–166 (2010). [Google Scholar]
- 43.Stuart S. N., Chanson J. S., Cox N. A., Young B. E., Rodrigues A. S. L., Fischman D. L., Waller R. W., Status and trends of amphibian declines and extinctions worldwide. Science 306, 1783–1786 (2004). [DOI] [PubMed] [Google Scholar]
- 44.Grant E. H. C., Miller D. A. W., Schmidt B. R., Adams M. J., Amburgey S. M., Chambert T., Cruickshank S. S., Fisher R. N., Green D. M., Hossack B. R., Johnson P. T. J., Joseph M. B., Rittenhouse T. A. G., Ryan M. E., Waddle J. H., Walls S. C., Bailey L L., Fellers G. M., Gorman T. A., Ray A. M., Pilloid D. S., Price S. J., Saenz D., Sadinski W., Muths E., Quantitative evidence for the effects of multiple drivers on continental-scale amphibian declines. Sci. Rep. 6, 25625 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rovito S. M., Parra-Olea G., Vásquez-Almazán C. R., Papenfuss T. J., Wake D. B., Dramatic declines in neotropical salamander populations are an important part of the global amphibian crisis. Proc. Natl. Acad. Sci. U.S.A. 106, 3231–3236 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yap T. A., Koo M. S., Ambrose R. F., Wake D. B., Vredenburg V. T., Averting a North American biodiversity crisis. Science 349, 481–482 (2015). [DOI] [PubMed] [Google Scholar]
- 47.Somero G. N., The physiology of climate change: How potentials for acclimatization and genetic adaptation will determine ‘winners’ and ‘losers’. J. Exp. Biol. 213, 912–920 (2010). [DOI] [PubMed] [Google Scholar]
- 48.Riddell E. A., McPhail J., Damm J. D., Sears M. W., Trade-offs between water loss and gas exchange influence habitat suitability of a woodland salamander. Funct. Ecol. 32, 916–925 (2018). [Google Scholar]
- 49.Feder M. E., Gibbs A. G., Griffith G. A., Tsuji J., Thermal acclimation of metabolism in salamanders: Fact or artefact?. J. Therm. Biol. 9, 255–260 (1984). [Google Scholar]
- 50.Kimball J. S., Running S. W., Nemani R. R., An improved method for estimating surface humidity from daily minimum temperature. Agric. For. Meteorol. 85, 87–98 (1997). [Google Scholar]
- 51.Riddell E. A., Apanovitch E. K., Odom J. P., Sears M. W., Physical calculations of resistance to water loss improve predictions of species range models. Ecol. Monogr. 87, 21–33 (2017). [DOI] [PubMed] [Google Scholar]
- 52.G. S. Campbell, J. M. Norman, An Introduction to Environmental Biophysics (Springer-Verlag, 1998). [Google Scholar]
- 53.Yeakley J. A., Swank W. T., Swift L. W., Hornberger G. M., Shugart H. H., Soil moisture gradients and controls on a southern Appalachian hillslope from drought through recharge. Hydrol. Earth Syst. Sci. 2, 41–49 (1998). [Google Scholar]
- 54.Spight T. M., The water economy of salamanders: Water uptake after dehydration. Comp. Biochem. Physiol. 20, 767–771 (1967). [Google Scholar]
- 55.Clay T. A., Gifford M. E., Population level differences in thermal sensitivity of energy assimilation in terrestrial salamanders. J. Therm. Biol. 64, 1–6 (2017). [DOI] [PubMed] [Google Scholar]
- 56.Bell G. P., Birds and mammals on an insect diet: A primer on diet composition analysis in relation to ecological energetics. Stud. Avian Biol. 13, 416–422 (1990). [Google Scholar]
- 57.Bennett A. F., Houck L. D., The energetic cost of courtship and aggression in a plethodontid salamander. Ecology 64, 979–983 (1983). [Google Scholar]
- 58.Fitzpatrick L. C., Energy allocation in the Allegheny Mountain salamander, Desmognathus ochrophaeus. Ecol. Monogr. 43, 43–58 (1973). [Google Scholar]
- 59.Taub F. B., The distribution of the red-backed salamander, Plethodon c. cinereus, within the soil. Ecology 42, 681–698 (1961). [Google Scholar]
- 60.Jenkins C. N., Pimm S. L., Joppa L. N., Global patterns of terrestrial vertebrate diversity and conservation. Proc. Natl. Acad. Sci. U.S.A. 110, E2602–E2610 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Whitford W. G., Hutchison V. H., Body size and metabolic rate in salamanders. Physiol. Zool. 40, 127–133 (1967). [Google Scholar]
- 62.J. R. B. Lighton, Measuring Metabolic Rates: A Manual for Scientists (Oxford Univ. Press, 2008). [Google Scholar]
- 63.R. B. Stull, Meteorology for Scientists and Engineers (Brooks/Cole, ed. 2, 2000). [Google Scholar]
- 64.Fridley J. D., Downscaling climate over complex terrain: High finescale (<1000 m) spatial variation of near-ground temperatures in a montane forested landscape (Great Smoky Mountains). J. Appl. Meteorol. Climatol. 48, 1033–1049 (2009). [Google Scholar]
- 65.de V. Weir J. B., New methods for calculating metabolic rate with special reference to protein metabolism. J. Physiol. 109, 1–9 (1949). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.De Vries D. A., Thermal conductivity of soil. Nature 178, 1074 (1956). [Google Scholar]
- 67.Fitzpatrick L. C., Life history patterns of storage and utilization of lipids for energy in amphibians. Am. Zool. 16, 725–732 (1976). [Google Scholar]
- 68.Olejnik S., Algina J., Generalized eta and omega squared statistics: Measures of effect size for some common research designs. Psychol. Methods 8, 434–447 (2003). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/4/7/eaar5471/DC1
Supplementary Materials and Methods
Table S1. Analysis of covariance and effect sizes for seasonal acclimatization demonstrates that surface area and month have the greatest influence on skin resistance to water loss (ri).
Table S2. Analysis of covariance and effect size for the seasonal acclimatization experiment demonstrates that surface area and month had the greatest effect on metabolic rate (V . O2) during the summer.
Table S3. Analysis of covariance and effect size on the simulated data from the mechanistic SDM illustrates that mass had the largest influence on the number of extinct regions in the species range model.
Fig. S1. Experimental evidence for physiological plasticity of skin resistance to water loss.
Fig. S2. Predicted and observed values of nighttime temperatures and VPDs at our field site near Cullowhee, NC.
Fig. S3. Estimated rise in VPDs through time under climate change and the spatial distribution of VPDs in 2100.
Fig. S4. Flow chart of foraging-energetic model used to estimate extinction.


