BACKGROUND
The heart failure population is ever expanding, with about 23 million people worldwide diagnosed with heart failure. In the United States, acute heart failure (AHF) accounts for over one million hospital admissions.1–3 Despite improvements in morbidity and mortality for patients with chronic HF with reduced EF due to pharmacological and device based therapies, rates of admission, readmission and mortality remain high. Overall, in-hospital mortality is relatively low; it is the early post-discharge period, termed the “vulnerable phase” (VP), where the greatest number of adverse outcomes occurs. (Figure 1).
Figure 1. U-shaped distribution of readmission associated with heart failure management.
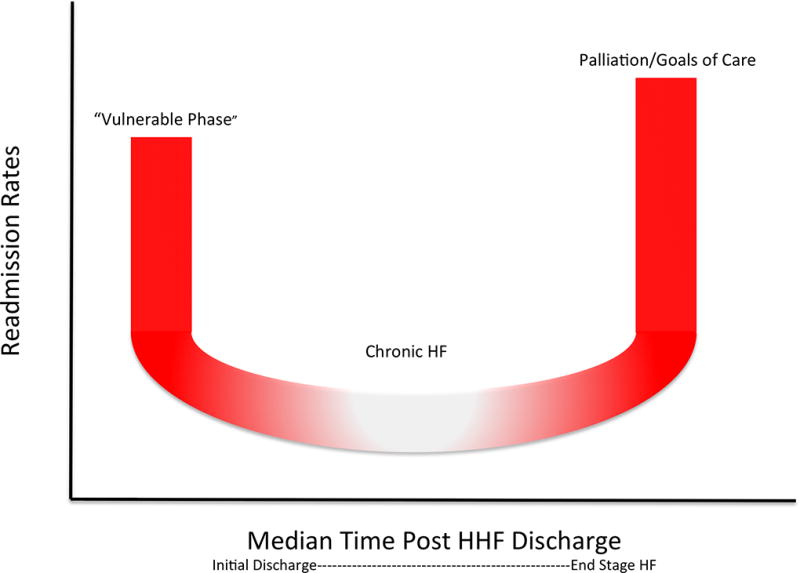
Rehospitalization risk among patients hospitalized for heart failure. Among patients who have repeat hospitalization for heart failure or other cardiovascular-related disease, a three-phase lifetime readmission risk exists. Red indicates period of highest risk for readmission immediately after discharge and just before death. White indicates the lower-risk chronic phase where the rate of readmission levels off prior to end of life.
The VP begins with an AHF exacerbation and lasts up to 6 months post-discharge. Patients who survive this 6-month period following AHF represent a uniform cohort without significant variability amongst clinical profiles or systolic blood pressure classifications at the time of admission, thus suggesting an end point for the VP.4 This VP period is associated with an increased risk of readmission and mortality, with rates of 30% and 10% respectively, within the first few weeks.5 Such poor outcomes may be attributed to cardiac factors (such as myocardial infarctions, atrial fibrillation, and uncontrolled hypertension), non-cardiac comorbidities (such as diabetes, COPD, and infection), patient related factors (medication non-adherence, alcohol and substance abuse, dietary indiscretions), and system-based factors (such as poor access to discharge follow up).6 Additionally, the VP can be further categorized into 3 overlapping subphases: early, middle and late phases. The very early VP includes the acute exacerbation and lasts into the first few days post discharge. This was evident in the European Society of Cardiology Heart Failure Long-Term (ESC-HF-LT) registry where 49% of patients admitted in cardiogenic shock died within the first 24 hours following presentation illustrating the importance of early identification of hypoperfusion as well as appropriate in-hospital triage of these high risk patients.4 The early VP begins at the moment of discharge and readmissions during this time frame have been attributed to both patient and system related factors. The later VP takes into account all precipitating factors and comorbidities within 6 months of discharge.7 (Table 1) As time progresses following a AHF, the readmission and mortality rates gradually decline, as highlighted in the Candesartan in Heart Failure: Assessment of Reduction on Mortality and Morbidity (CHARM) trial. Odds for mortality declined from six-fold during the first month post discharge to two-fold over the time of the trial.8 The susceptibility of patients during the VP presents a potential opportunity to improve patient outcomes by altering the trajectory of an otherwise poor prognosis.9
Table 1.
Summary of Biomarkers and Ability to Risk Stratify
| Biomarkers Predictive of Risk | |||||
|---|---|---|---|---|---|
|
| |||||
| Marker | Group | Study Design | Population | Endpoint | Conclusion |
| BNP/NT-proBNP | Greene et al. | Post-Hoc Analysis of ASTRONAUT cohort | 1351 HHF patients w/EF≤40%, BNP≥ 400pg/mL, or NT-proBNP ≥ 1600 pg/mL | ACM and CVM/HHF at 12 months | Worse CVM/HHF outcomes with two fold increase in NT-proBNP trajectory (HR 1.14, 95% CI 1.02–1.26). No association with ACM (HR 0.95, 95% CI 0.81–1.11) |
| Ambrosy et al. | Post-Hoc Analysis of EVEREST cohort | 2061 HHF patients w/EF≤40% and ≥2 signs/symptoms of fluid overload/CCS | ACM, HHF, and ACM + HHF at 30 days post discharge and overall | Worsening ACM and ACM+HHF outcomes with clinical congestion at 30 days (ACM HR 1.43, 95% CI 1.14–1.58; ACM+HHF HR 1.11, 95% CI 1.06–1.17). No association with HHF (HR 1.06, 95% CI 1.14–1.58) | |
|
| |||||
| Troponin | Greene et al. | Post-Hoc Analysis of ASTRONAUT cohort | 1469 HHF patients w/EF≤40% and troponin measured prior to discharge and at 30 day follow up | ACM and CVM/HHF at 12 months | 1 month troponin elevation predictive of increased ACM (HR 1.59, 95% CI 1.18–2.13) and CVM/HHF (HR 1.28, 95% CI 1.03–1.58) |
|
| |||||
| BUN | Bruocco et al. | Prospective study | 107 patients admitted for AHF and systolic dysfunction | BUN on admission and at discharge | BUN increase of >20% at discharge associated with poorer outcome independent of congestion (univariate HR 2.72, 95% CI 1.03–7.28, multivariate HR 3.00, 95% CI 1.12–8.06) |
|
| |||||
| Serum Osmolality | Vaduganathan et al. | Post-Hoc Analysis of EVEREST cohort | 3744 patients with EF≤40% and ≥2 signs/symptoms of fluid overload/CCS | ACM after 9.9 months | Lower discharge serum osmolality was predictive of worse post-discharge outcomes (HR 1.61, 95%CI 1.19–1.75 |
|
| |||||
| Hematocrit | Greene et al. | Post-Hoc Analysis of EVEREST cohort | 1684 HHF patients w/EF≤40% and hematocrit measured on admission and discharge/hospital day 7 | ACM and CVM/HHF at 100 days post discharge | Every 5% increase of in-hospital hematocrit associated with decreased ACM (HR 0.81, 95% CI 0.70–0.95) and CVM/HHF at ≤100 days (HR 0.73, 95% CI 0.71–0.76) |
Abbreviations: ACM, all cause mortality; ASTRONAUT, Aliskiren Trial in Acute Heart Failure Outcomes; BNP, B-type natriuretic peptide; BUN, blood urea nitrogen; CCS, composite congestion score; CI, confidence interval; CVM, cardiovascular mortality; EF, ejection fraction; EVEREST, Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study with Tolvaptan; HHF, hospitalization for heart failure; HR, hazard ratio; NT-proBNP, N-terminal pro-BNP
AREAS OF UNCERTAINTY
PATHOPHYSIOLOGY OF THE VULNERABLE PHASE
Although there is a significant variability within the AHF population, the pathophysiology of the VP can be attributed to numerous contributing factors including the short-term worsening of hemodynamics attributed to a failure to relieve congestion during the index hospitalization, with progressively increased left ventricular (LV) filling pressures.10, 11 This elevation of LV pressure ultimately leads to persistent hemodynamic congestion and long term persistent multi-organ injury reflected in abnormalities in markers including troponin and creatinine.
Often it is the signs and symptoms associated with congestion that ultimately lead to heart failure admissions and subsequent readmissions.12–14 During AHF, intravenous diuretics are employed with the goal of lowering elevated filling pressures. Over the course of the hospitalization, there is a resolution of symptomatic congestion.5 However, about 20% of patients are discharged despite persistent signs and symptoms of HF4. A negligible decrease or an increase in body weight suggests a possible failure to relieve clinical congestion during index hospitalization, which may potentially contribute to the high post-discharge event rate in the ESC-HF-LT registry.4 In the Romanian Acute Heart Failure Syndrome (RO-AHF) registry, regardless of age, gender and LVEF, 83% of AHF patients reported their status to be improved at discharge.15 However, despite symptomatic improvement, there often remains persistent hemodynamic, subclinical congestion at the time of discharge.5
Given the poor hemodynamic reserve commonly seen following a heart failure exacerbation, the AHF patient population is exquisitely sensitive to changes in LV filling pressures with even the most modest increase potentially leading to worsening of symptoms, worsening multi-organ failure, and readmission.10, 11 This cycle of admission and readmission ultimately predisposes patients to worse outcomes with poor prognosis as well as an increased risk of morbidity and mortality.16
AHF patients often are burdened with numerous comorbidities that contribute to early post discharge event rates and predispose this population to AHF during the VP. Addressing both cardiac and non-cardiac comorbidities is vital in preventing decompensation during the VP; approximately 40% of deaths and readmissions within 60 days of AHF were secondary to non-cardiovascular causes.12
The current culture of medicine, where system based failures predisposes patients to delayed care, contributes to the worsening of outcomes during the VP. The inappropriate triage of critically ill and complex patients during the very early VP, to hospital facilities or care settings poorly equipped to manage them, leaves the patient at risk for worse outcomes compared to those who initiated their care at facilities equipped to handle high acuity patients.4 The lack of a comprehensive discharge plan, either via difficulty arranging immediate outpatient follow up. Lack of resources to pay for medications, follow-up and transportation, or poor medical education leaves this population vulnerable for deterioration and rehospitalization.
DATA SOURCES
IDENTIFICATION OF PATIENTS AT HIGHEST RISK
Although the VP following AHF is marked by high risk for post-discharge events, there are certain subgroups of patients at higher risk than others.17, 18 The identification of these high-risk groups with the use of emerging prognostic biomarkers as well as risk-stratifying tools may allow for targeted treatment of higher risk patients during hospitalization or immediate post-discharge period. (table 2).
Table 2.
Summary of available methods used in the assessment of overall volume status
| Methods of Assessing Volume Status | |||||
|---|---|---|---|---|---|
| Volume Assessment |
Method | Study | Population | End point | Conclusion |
| PAC | Invasive | ESCAPE Trial | 433 patients w/EF <30% admitted for NYHA class IV symptoms | Number of days hospitalized or die during 6 month period | PAC did not improve primary outcomes (HR 1.00 95%CI 0.82–1.21) and was associated with more in-hospital adverse events |
| CardioMEMS | Invasive | CHAMPION Trial | 550 patients with moderate heart failure symptoms (NYHA class III) | Heart-failure-related hospitalizations up to 6 months post implantation | Reduction in HHF during 6 moth period (HR 0.72, 95%CI 0.60–0.85) |
| Ultrasound | Non-invasive | Gargani et al. | 100 patients admitted for acute heart failure | B-lines present on admission and discharge | >15 B-lines present upon discharge were associated with higher risk for HHF within 6 months (HR 24.12 CI 3.15 –184.55 P=0.002). |
Abbreviations: CHAMPION, CadioMEMS Heart Sensor Allows Monitoring of Pressure to Improve Outcomes in New York Heart Association Class III Heart Failure Patients; CI, confidence interval; ESCAPE, Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness; EF, ejection fraction; HHF, hospitalization for heart failure; HR, hazard ratio; NYHA, New York Heart Association; PAC, pulmonary artery catheterization
Natriuretic peptides (NP) levels are amongst the most powerful post-discharge risk stratification markers in HF.19, 20 Serial measurements of NP may help identify patients at high risk for decompensation during the VP, especially during the transition from hospitalization to early outpatient follow up.21 Greene et al. analyzed both baseline and 30 day post discharge levels of NT-proBNP in the Aliskiren Trial in Acute Heart Failure Outcomes (ASTRONAUT) cohort and found that an increased trajectory in NT-proBNP was independently associated with cardiovascular mortality (CVM) and AHF (HR 1.14, 95% CI 1.02–1.26).22 Similarly, when coupled with improvement in clinical congestion, a downward trend in NT-proBNP represented an overall lowering risk for HF readmission.23 However, the Guiding Evidence Based Therapy Using Biomarker Intensified Treatment in Heart Failure (GUIDE-IT) study, which followed 894 patients with HFrEF, found that the use of NT-proBNP was not useful in predicting the time-to-first AHF or CVM in this population (HR 0.98, 95% CI 0.79–1.22; P=0.88).24 The fact that the control arm underwent more frequent outpatient visits per year compared to what typically occurs in standard practice suggests that more frequent outpatient follow up above usual care improves outcomes. This does not imply guided biomarker therapy is better; rather, perhaps more frequent follow up of patients is needed.
During AHF, a significant number of patients have elevations in troponin secondary to myocardial injury outside of an acute coronary syndrome. The elevation in troponin levels has been associated with worsening HF exacerbation, more severe symptoms, greater needs for aggressive supportive measures, and worse overall outcomes.25 Analysis of the ASTRONAUT cohort found that troponin elevations at the 30-day post discharge mark were associated with increased all-cause mortality (ACM) (HR 1.59, 95% CI 1.18–2.13) and cardiovascular mortality/AHF (HR 1.28, 95% CI 1.03–1.58) at 12 months. Assessing troponin levels post-discharge identifies patients with long-term persistence of myocardial injury; such patients are at the highest risk for readmission and may serve as a complement to NP levels in risk-stratification.26
Activation of the RAAS in response to decreased renal blood flow leads to a compensatory mechanism aimed at augmenting the inadequate arterial pressure. Consequently there are often fluctuations in blood urea nitrogen (BUN) levels during AHF, reflecting both congestion and fluid retention.27 Fluctuations in BUN levels during AHF have been investigated by numerous trials. Two retrospective analysis suggested BUN could serve as a marker of neurohormonal activation even in the presence of renal dysfunction.28, 29 In 171 patients admitted with AHF, there was a rise in BUN independent of basal renal dysfunction thus pointing to the activation of RAAS as the inciting event.29 As with the previous markers, decreases in BUN without resolution of clinical congestion were not associated with improved outcomes.
Another potential surrogate marker for assessing the underlying risk of AHF patients is serum osmolality. In a post-hoc analysis of the EVEREST trial, lower discharge serum osmolality was predictive of higher ACM (HR 1.61, 95%CI 1.19–1.75) and those patients at the lower spectrum of osmolality demonstrated many features of advanced HF. This marker may represent a marker of residual congestion beyond currently available parameters, including serum sodium and natriuretic peptides.30
In addition to markers of risk, numerous risk stratification tools have been developed to aid in the identification of patients at highest risk based on their position on the VP timeline. During the earlier portion of the VP, both the Acute Decompensated Heart Failure National Registry (ADHERE) risk tree and the Improving Heart Failure Risk Stratification in the ED (STRATIFY) tool may be used to stratify risk.13, 31 Once patients progress to the early VP, the Pro-BNP Outpatient Tailored Chronic Heart Failure Therapy (PROTECT) risk score and the Center for Outcome Research and Evaluation (CORE) online readmission risk calculator for heart failure can help identify patients at risk for poor outcomes within 7 days of discharge and the 30 day all cause readmission rates, respectively. The Get With the Guidelines–Heart Failure (GWTG-HF) risk score aids in stratifying patients during the late subphase of VP and predicts ACM for future AHF.
Potential Solutions
OPTIMIZING MANAGEMENT
Despite being able to identify markers predictive of worse outcomes, the greatest threat to AHF patients is the risk of readmission secondary to persistent congestion.3, 33, 34 The primary focus during an AHF hospitalization must be the achievement of both clinical and subclinical decongestion. This can only be accomplished by identifying those patients with persistent congestion despite clinical improvement. Thus, the assessment and grading of congestion prior to hospital discharge remains a crucial opportunity to treat patients who have yet to reach optimal euvolemia.34
PHARMACOLOGIC THERAPIES
Once optimal decongestion has been achieved…
Despite the challenge associated with the management of heart failure with preserved ejection fraction due to the lack of life saving therapies, a wide spectrum of medications has been shown to reduce mortality in those patients with heart failure and a reduced ejection fraction (HFrEF). These medications include beta-blockers, angiotensin converting enzyme (ACE) inhibitors, aldosterone receptor blockers (ARBs), aldosterone antagonists, and angiotensin receptor-neprilysin inhibitors (ARNIs). Yet despite their proven clinical efficacy, the initiation and uptitration of these medications remains suboptimal. Analysis of the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF) registry revealed that fewer than 10% of the patients were at target beta-blocker doses upon discharge with the average beta-blocker dose being less than 50% of the target dose.35, 36 Further compounding the problem was the failure to up-titrate medications within 90 days post-discharge. Numerous studies, including the Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training (HF-ACTION), Systolic Heart failure treatment with the If inhibitor ivabradine Trial (SHIFT), and Cardiac Insufficiency Bisoprolol Study in Elderly (CIBIS-ELD) trials, have demonstrated the overall suboptimal dosing of beta-blockers upon discharge.37–39 With regards to ACE inhibitors and ARBs, hyperkalemia and renal dysfunction remain the major concerns leading to underutilization, with nearly 20% of the eligible HFrEF patients not receiving ACE inhibitors or ARBs.40 The utilization of mineralocorticoid receptor antagonists is equally poor, with less than a third of eligible patients receiving prescriptions at discharge.41, 42 Similarly, in the subset of African American population with heart failure intolerant of ACE inhibitors, the usage rate of hydralazine with isosorbide dintirate remains below 25% despite evidence showing a mortality benefit in this population.43, 44
Digoxin is another underutilized medical therapy that may improve outcomes. While there is no mortality benefit associated with its use, digoxin decreases HF hospitalization by 28%.45 Furthermore, in high-risk patients, defined as New York Heart Association class III-IV symptoms, left ventricular ejection fraction <25% or cardiothoracic ratio >55%, digoxin has also been showed to reduce the composite of all-cause mortality or hospitalization, while withdrawing digoxin therapy leads to an increased risk of heart failure.46, 47 Use of digoxin remains controversial however.
The index hospitalization provides an opportune time for both the initiation and appropriate uptitration of guideline directed medical therapy (GDMT) in a monitored setting. Studies have indicated that initiation and aggressive uptitration of medications prior to discharge were associated with a significant reduction in the adjusted risk of death and re-hospitalization. This was seen in the analysis of beta-blocker usage in eligible patients within the OPTIMIZE-HF trial cohort.48 Additionally, an analysis of the metabolic exercise test data combined with cardiac and kidney indexes (MECKI) score database suggests higher dose beta-blockers were associated with an overall better prognosis compared to those on medium and low doses.49 The GWTG-HF registry revealed that patients who were continued or newly started on medication prior to discharge had a significantly improved thirty-day mortality in comparison to those not started on therapy with one-year mortality was 28.2% for patients continued and 29.7% for patients started on ACEi/ARB compared to 41.6% for patients discontinued and 41.7% for patients not started on therapy.50
DEVICE THERAPIES
Patients with severe heart failure must be evaluated for implantable cardiac defibrillators (ICDs) or cardiac resynchronization therapy (CRT) prior to discharge. While the Danish Study to Assess the Efficiency of ICDs in Patients With Non-ischemic Systolic Heart Failure on Mortality (DANISH) study raises some questions regarding the use of ICDs in all HFrEF patients, ICD therapy remains an integral component in the care of HFrEF patients.51, 52 CRT provides the added benefit of reverse remodeling, which in turn leads to reductions in cardiac volumes, improvements in ejection fractions, and subsequently a reduction in heart failure events and mortality.53, 54
NON-PHARMACOLOGIC SYSTEM STRATEGIES
Patient education, home based monitoring (either via the patient or by a visiting nurse service [VNS]), and close follow up in multidisciplinary heart failure clinics are all additional strategies that serve to address system based factors that potentiate readmissions following AHF. Close post-discharge follow up within 1 week of discharge led to fewer readmissions at 30 days (risk-adjusted HR 0.85, 95% CI, 0.78–093).55 Additionally, a meta-analysis of transitional care services such as follow up with home-visiting programs, multidisciplinary heart failure clinics and structured telephone support, revealed that these interventions result in a reduction in all-cause readmission and mortality at 3–6 months post discharge.56 Home visiting programs resulted in a significant reduction in both mortality and readmissions (HR of 0.77 and 0.75, respectively), while multidisciplinary heart failure clinics had even higher reductions in mortality and readmissions at the 6-month mark (HR 0.56 and 0.70, respectively). However, there are some conflicting data in regards to ideal or best transitional care management. Visiting nurse programs and increased surveillance via telemonitoring failed to demonstrate any significant improvement in outcomes.57–60 Observational data failed to consistently support an outcomes benefit associated with dietary modifications towards a salt restricted diet. In fact, in the setting of high diuretic doses, a normal sodium diet was shown to reduce rates of renal dysfunction and readmission when compared to a low sodium diet.61–63 There are inconsistent impacts on outcomes for other interventions such as regular post-discharge telephone calls and the practice of alerting outpatient physicians to patient discharge.64
COMORBIDITY OPTIMIZATION
The AHF population frequently has significant cardiac (e.g. atrial fibrillation, ischemia, valvular heart disease) and non-cardiac (e.g. diabetes mellitus, sleep disordered breathing, chronic lung disease, anemia, depression, chronic kidney disease) comorbidities that contribute substantially to early post-discharge event rates. These comorbid conditions and their management can directly and indirectly contribute to poor outcomes resulting in rehospitalization. Addressing these comorbidities is crucial to improving post-discharge outcomes, especially given that 40% of all deaths and re-hospitalizations within 60 days following a HF hospitalization are due to non-cardiovascular causes.12
POTENTIAL FUTURE OPTIONS: MOVING TO THE LEFT
Rather than waiting one to three months per inclusion criteria of SHIFT, EMPHASIS and PARADIGM-HF trials, earlier initiation of novel therapies during the index hospitalization could potentially medically optimize patients and prevent future AHF. In addition, there are other novel therapies currently under investigation that might further reduce post-discharge risk among heart failure patients. These include ongoing clinical trials with novel agents including a sodium glucose co-transporter 2 inhibitor canagliflozin, a subcutaneous natriuretic peptide omecamtiv mecarbil (GALACTIC-HF, NCT02929329) and a sub-cutaneous guanylate cyclase stimulator vericiguat (VICTORIA, NCT02861534).65–67 The Canagliflozin Cardiovascular Assessment Study (CANVAS) found that in patients with type-2 diabetes mellitus (T2DM) at high risk for cardiovascular events, treatment with canagliflozin lead to an overall reduction in cardiovascular events (HR 0.86, 95%CI 0.75–0.97) and AHF (HR 0.67, 95%CI 0.52–0.87).68 The findings of the CANVAS trial can potentially lead to canagliflozin being included as an adjunct treatment for the large portion of the HF population with T2DM, however these findings are yet to be incorporated into the ACC/AHA/HFSA guidelines for the management of heart failure.69 Recently, the Chronic Oral Study of Myosin Activation to Increase Contractility in Heart Failure (COSMIC-HF) trial demonstrated omecamtiv’s ability to improve cardiac output while simultaneously reducing ventricular diameter in patients with chronic HF.66 These novel therapies may ultimately prove to be useful tools to help reduce readmissions and mortality in heart failure patients during this VP. Appropriate allocation of resources focused on close follow up and preventative measures for those at the highest risk of readmission and mortality may also provide a unique, patient centered opportunity to escort the AHF population through the vulnerable period and into a stable chronic heart failure status.17, 18, 70, 71
CONCLUSION
The VP following AHF is a critical period lasting 6 months post-discharge during which there is heightened risk for adverse outcomes. The risk for readmission begins and continues throughout hospitalization: Failure to adequately decongest patients and maximize proven GDMT prior to discharge or soon after discharge increases risk for adverse events. The identification of patients at risk of poor post-discharge outcomes, and the resolution of both clinical and subclinical congestion remains the most imperative of goal prior to discharge following AHF. Once at decongestive state, proper implementation of heart failure therapies and close outpatient follow up will bridge patients through the VP and into a stable, chronic heart failure status.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.Fonarow GC, Stough WG, Abraham WT, et al. Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE-HF Registry. J Am Coll Cardiol. 2007;50:768–77. doi: 10.1016/j.jacc.2007.04.064. [DOI] [PubMed] [Google Scholar]
- 3.Adams KF, Jr, Fonarow GC, Emerman CL, et al. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE) Am Heart J. 2005;149:209–16. doi: 10.1016/j.ahj.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 4.Chioncel O, Mebazaa A, Harjola VP, et al. Clinical phenotypes and outcome of patients hospitalized for acute heart failure: the ESC Heart Failure Long-Term Registry. Eur J Heart Fail. 2017;19:1242–1254. doi: 10.1002/ejhf.890. [DOI] [PubMed] [Google Scholar]
- 5.Greene SJ, Fonarow GC, Vaduganathan M, et al. The vulnerable phase after hospitalization for heart failure. Nat Rev Cardiol. 2015;12:220–9. doi: 10.1038/nrcardio.2015.14. [DOI] [PubMed] [Google Scholar]
- 6.Fonarow GC, Abraham WT, Albert NM, et al. Factors identified as precipitating hospital admissions for heart failure and clinical outcomes: findings from OPTIMIZE-HF. Arch Intern Med. 2008;168:847–54. doi: 10.1001/archinte.168.8.847. [DOI] [PubMed] [Google Scholar]
- 7.Yilmaz MBMA. Definition and characteristics of the vulnerable phase in heart failure. Medicographia. 2015;37:44–7. [Google Scholar]
- 8.Solomon SD, Dobson J, Pocock S, et al. Influence of nonfatal hospitalization for heart failure on subsequent mortality in patients with chronic heart failure. Circulation. 2007;116:1482–7. doi: 10.1161/CIRCULATIONAHA.107.696906. [DOI] [PubMed] [Google Scholar]
- 9.Marti CN, Fonarow GC, Gheorghiade M, et al. Timing and duration of interventions in clinical trials for patients with hospitalized heart failure. Circ Heart Fail. 2013;6:1095–101. doi: 10.1161/CIRCHEARTFAILURE.113.000518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zile MR, Bennett TD, St John Sutton M, et al. Transition from chronic compensated to acute decompensated heart failure: pathophysiological insights obtained from continuous monitoring of intracardiac pressures. Circulation. 2008;118:1433–41. doi: 10.1161/CIRCULATIONAHA.108.783910. [DOI] [PubMed] [Google Scholar]
- 11.Adamson PB, Magalski A, Braunschweig F, et al. Ongoing right ventricular hemodynamics in heart failure: clinical value of measurements derived from an implantable monitoring system. J Am Coll Cardiol. 2003;41:565–71. doi: 10.1016/s0735-1097(02)02896-6. [DOI] [PubMed] [Google Scholar]
- 12.O'Connor CM, Miller AB, Blair JE, et al. Causes of death and rehospitalization in patients hospitalized with worsening heart failure and reduced left ventricular ejection fraction: results from Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study with Tolvaptan (EVEREST) program. Am Heart J. 2010;159:841–849. e1. doi: 10.1016/j.ahj.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 13.Yancy CW, Lopatin M, Stevenson LW, et al. Clinical presentation, management, and in-hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Heart Failure National Registry (ADHERE) Database. J Am Coll Cardiol. 2006;47:76–84. doi: 10.1016/j.jacc.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 14.Abraham WT, Adams KF, Fonarow GC, et al. In-hospital mortality in patients with acute decompensated heart failure requiring intravenous vasoactive medications: an analysis from the Acute Decompensated Heart Failure National Registry (ADHERE) J Am Coll Cardiol. 2005;46:57–64. doi: 10.1016/j.jacc.2005.03.051. [DOI] [PubMed] [Google Scholar]
- 15.Chioncel O, Vinereanu D, Datcu M, et al. The Romanian Acute Heart Failure Syndromes (RO-AHFS) registry. Am Heart J. 2011;162:142–53. e1. doi: 10.1016/j.ahj.2011.03.033. [DOI] [PubMed] [Google Scholar]
- 16.Setoguchi S, Stevenson LW, Schneeweiss S. Repeated hospitalizations predict mortality in the community population with heart failure. Am Heart J. 2007;154:260–6. doi: 10.1016/j.ahj.2007.01.041. [DOI] [PubMed] [Google Scholar]
- 17.Desai AS, Stevenson LW. Rehospitalization for heart failure: predict or prevent? Circulation. 2012;126:501–6. doi: 10.1161/CIRCULATIONAHA.112.125435. [DOI] [PubMed] [Google Scholar]
- 18.Gheorghiade M, Pang PS, Ambrosy AP, et al. A comprehensive, longitudinal description of the in-hospital and post-discharge clinical, laboratory, and neurohormonal course of patients with heart failure who die or are re-hospitalized within 90 days: analysis from the EVEREST trial. Heart Fail Rev. 2012;17:485–509. doi: 10.1007/s10741-011-9280-0. [DOI] [PubMed] [Google Scholar]
- 19.Iwanaga Y, Nishi I, Furuichi S, et al. B-type natriuretic peptide strongly reflects diastolic wall stress in patients with chronic heart failure: comparison between systolic and diastolic heart failure. J Am Coll Cardiol. 2006;47:742–8. doi: 10.1016/j.jacc.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 20.Konstam MA, Gheorghiade M, Burnett JC, Jr, et al. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial. JAMA. 2007;297:1319–31. doi: 10.1001/jama.297.12.1319. [DOI] [PubMed] [Google Scholar]
- 21.Shah KB, Kop WJ, Christenson RH, et al. Post-discharge changes in NT-proBNP and quality of life after acute dyspnea hospitalization as predictors of one-year outcomes. Clin Biochem. 2010;43:1405–10. doi: 10.1016/j.clinbiochem.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 22.Greene SJ, Maggioni AP, Fonarow GC, et al. Clinical profile and prognostic significance of natriuretic peptide trajectory following hospitalization for worsening chronic heart failure: findings from the ASTRONAUT trial. Eur J Heart Fail. 2015;17:98–108. doi: 10.1002/ejhf.201. [DOI] [PubMed] [Google Scholar]
- 23.Ambrosy AP, Pang PS, Khan S, et al. Clinical course and predictive value of congestion during hospitalization in patients admitted for worsening signs and symptoms of heart failure with reduced ejection fraction: findings from the EVEREST trial. Eur Heart J. 2013;34:835–43. doi: 10.1093/eurheartj/ehs444. [DOI] [PubMed] [Google Scholar]
- 24.Felker GM, Anstrom KJ, Adams KF, et al. Effect of Natriuretic Peptide-Guided Therapy on Hospitalization or Cardiovascular Mortality in High-Risk Patients With Heart Failure and Reduced Ejection Fraction: A Randomized Clinical Trial. JAMA. 2017;318:713–720. doi: 10.1001/jama.2017.10565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Januzzi JL, Jr, Filippatos G, Nieminen M, et al. Troponin elevation in patients with heart failure: on behalf of the third Universal Definition of Myocardial Infarction Global Task Force: Heart Failure Section. Eur Heart J. 2012;33:2265–71. doi: 10.1093/eurheartj/ehs191. [DOI] [PubMed] [Google Scholar]
- 26.Greene SJ, Butler J, Fonarow GC, et al. Pre-discharge and early post-discharge troponin elevation among patients hospitalized for heart failure with reduced ejection fraction: findings from the ASTRONAUT trial. Eur J Heart Fail. 2017 doi: 10.1002/ejhf.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schrier RW. Blood urea nitrogen and serum creatinine: not married in heart failure. Circ Heart Fail. 2008;1:2–5. doi: 10.1161/CIRCHEARTFAILURE.108.770834. [DOI] [PubMed] [Google Scholar]
- 28.Klein L, Massie BM, Leimberger JD, et al. Admission or changes in renal function during hospitalization for worsening heart failure predict postdischarge survival: results from the Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure (OPTIME-CHF) Circ Heart Fail. 2008;1:25–33. doi: 10.1161/CIRCHEARTFAILURE.107.746933. [DOI] [PubMed] [Google Scholar]
- 29.Ruocco G, Pellegrini M, De Gori C, et al. The prognostic combined role of B-type natriuretic peptide, blood urea nitrogen and congestion signs persistence in patients with acute heart failure. J Cardiovasc Med (Hagerstown) 2016;17:818–27. doi: 10.2459/JCM.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 30.Vaduganathan M, Marti CN, Mentz RJ, et al. Serum Osmolality and Postdischarge Outcomes After Hospitalization for Heart Failure. Am J Cardiol. 2016;117:1144–50. doi: 10.1016/j.amjcard.2015.12.059. [DOI] [PubMed] [Google Scholar]
- 31.Collins SP, Jenkins CA, Harrell FE, Jr, et al. Identification of Emergency Department Patients With Acute Heart Failure at Low Risk for 30-Day Adverse Events: The STRATIFY Decision Tool. JACC Heart Fail. 2015;3:737–47. doi: 10.1016/j.jchf.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keenan PS, Normand SL, Lin Z, et al. An administrative claims measure suitable for profiling hospital performance on the basis of 30-day all-cause readmission rates among patients with heart failure. Circ Cardiovasc Qual Outcomes. 2008;1:29–37. doi: 10.1161/CIRCOUTCOMES.108.802686. [DOI] [PubMed] [Google Scholar]
- 33.O'Connor CM, Stough WG, Gallup DS, et al. Demographics, clinical characteristics, and outcomes of patients hospitalized for decompensated heart failure: observations from the IMPACT-HF registry. J Card Fail. 2005;11:200–5. doi: 10.1016/j.cardfail.2004.08.160. [DOI] [PubMed] [Google Scholar]
- 34.Gheorghiade M, Follath F, Ponikowski P, et al. Assessing and grading congestion in acute heart failure: a scientific statement from the acute heart failure committee of the heart failure association of the European Society of Cardiology and endorsed by the European Society of Intensive Care Medicine. Eur J Heart Fail. 2010;12:423–33. doi: 10.1093/eurjhf/hfq045. [DOI] [PubMed] [Google Scholar]
- 35.DeVore AD, Mi X, Mentz RJ, et al. Discharge heart rate and beta-blocker dose in patients hospitalized with heart failure: Findings from the OPTIMIZE-HF registry. Am Heart J. 2016;173:172–8. doi: 10.1016/j.ahj.2015.10.026. [DOI] [PubMed] [Google Scholar]
- 36.Fonarow GC, Abraham WT, Albert NM, et al. Dosing of beta-blocker therapy before, during, and after hospitalization for heart failure (from Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure) Am J Cardiol. 2008;102:1524–9. doi: 10.1016/j.amjcard.2008.07.045. [DOI] [PubMed] [Google Scholar]
- 37.Fiuzat M, Wojdyla D, Kitzman D, et al. Relationship of beta-blocker dose with outcomes in ambulatory heart failure patients with systolic dysfunction: results from the HF-ACTION (Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training) trial. J Am Coll Cardiol. 2012;60:208–15. doi: 10.1016/j.jacc.2012.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Swedberg K, Komajda M, Bohm M, et al. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet. 2010;376:875–85. doi: 10.1016/S0140-6736(10)61198-1. [DOI] [PubMed] [Google Scholar]
- 39.Dungen HD, Apostolovic S, Inkrot S, et al. Titration to target dose of bisoprolol vs. carvedilol in elderly patients with heart failure: the CIBIS-ELD trial. Eur J Heart Fail. 2011;13:670–80. doi: 10.1093/eurjhf/hfr020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fonarow GC, Abraham WT, Albert NM, et al. Association between performance measures and clinical outcomes for patients hospitalized with heart failure. JAMA. 2007;297:61–70. doi: 10.1001/jama.297.1.61. [DOI] [PubMed] [Google Scholar]
- 41.Albert NM, Yancy CW, Liang L, et al. Use of aldosterone antagonists in heart failure. JAMA. 2009;302:1658–65. doi: 10.1001/jama.2009.1493. [DOI] [PubMed] [Google Scholar]
- 42.Epstein M, Reaven NL, Funk SE, et al. Evaluation of the treatment gap between clinical guidelines and the utilization of renin-angiotensin-aldosterone system inhibitors. Am J Manag Care. 2015;21:S212–20. [PubMed] [Google Scholar]
- 43.Golwala HB, Thadani U, Liang L, et al. Use of hydralazine-isosorbide dinitrate combination in African American and other race/ethnic group patients with heart failure and reduced left ventricular ejection fraction. J Am Heart Assoc. 2013;2:e000214. doi: 10.1161/JAHA.113.000214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taylor AL, Ziesche S, Yancy C, et al. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med. 2004;351:2049–57. doi: 10.1056/NEJMoa042934. [DOI] [PubMed] [Google Scholar]
- 45.Digitalis Investigation G. The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med. 1997;336:525–33. doi: 10.1056/NEJM199702203360801. [DOI] [PubMed] [Google Scholar]
- 46.Gheorghiade M, Patel K, Filippatos G, et al. Effect of oral digoxin in high-risk heart failure patients: a pre-specified subgroup analysis of the DIG trial. Eur J Heart Fail. 2013;15:551–9. doi: 10.1093/eurjhf/hft010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Young JB, Gheorghiade M, Uretsky BF, et al. Superiority of "triple" drug therapy in heart failure: insights from the PROVED and RADIANCE trials. Prospective Randomized Study of Ventricular Function and Efficacy of Digoxin. Randomized Assessment of Digoxin and Inhibitors of Angiotensin-Converting Enzyme. J Am Coll Cardiol. 1998;32:686–92. doi: 10.1016/s0735-1097(98)00302-7. [DOI] [PubMed] [Google Scholar]
- 48.Fonarow GC, Abraham WT, Albert NM, et al. Prospective evaluation of beta-blocker use at the time of hospital discharge as a heart failure performance measure: results from OPTIMIZE-HF. J Card Fail. 2007;13:722–31. doi: 10.1016/j.cardfail.2007.06.727. [DOI] [PubMed] [Google Scholar]
- 49.Paolillo S, Mapelli M, Bonomi A, et al. Prognostic role of beta-blocker selectivity and dosage regimens in heart failure patients. Insights from the MECKI score database. Eur J Heart Fail. 2017;19:904–914. doi: 10.1002/ejhf.775. [DOI] [PubMed] [Google Scholar]
- 50.Gilstrap LG, Fonarow GC, Desai AS, et al. Initiation, Continuation, or Withdrawal of Angiotensin-Converting Enzyme Inhibitors/Angiotensin Receptor Blockers and Outcomes in Patients Hospitalized With Heart Failure With Reduced Ejection Fraction. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.116.004675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kober L, Thune JJ, Nielsen JC, et al. Defibrillator Implantation in Patients with Nonischemic Systolic Heart Failure. N Engl J Med. 2016;375:1221–30. doi: 10.1056/NEJMoa1608029. [DOI] [PubMed] [Google Scholar]
- 52.Bilchick KC, Wang Y, Cheng A, et al. Seattle Heart Failure and Proportional Risk Models Predict Benefit From Implantable Cardioverter-Defibrillators. J Am Coll Cardiol. 2017;69:2606–2618. doi: 10.1016/j.jacc.2017.03.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moss AJ, Hall WJ, Cannom DS, et al. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med. 2009;361:1329–38. doi: 10.1056/NEJMoa0906431. [DOI] [PubMed] [Google Scholar]
- 54.Goldenberg I, Kutyifa V, Klein HU, et al. Survival with cardiac-resynchronization therapy in mild heart failure. N Engl J Med. 2014;370:1694–701. doi: 10.1056/NEJMoa1401426. [DOI] [PubMed] [Google Scholar]
- 55.Hernandez AF, Greiner MA, Fonarow GC, et al. Relationship between early physician follow-up and 30-day readmission among Medicare beneficiaries hospitalized for heart failure. JAMA. 2010;303:1716–22. doi: 10.1001/jama.2010.533. [DOI] [PubMed] [Google Scholar]
- 56.Feltner C, Jones CD, Cene CW, et al. Transitional care interventions to prevent readmissions for persons with heart failure: a systematic review and meta-analysis. Ann Intern Med. 2014;160:774–84. doi: 10.7326/M14-0083. [DOI] [PubMed] [Google Scholar]
- 57.Cleland JG, Louis AA, Rigby AS, et al. Noninvasive home telemonitoring for patients with heart failure at high risk of recurrent admission and death: the Trans-European Network-Home-Care Management System (TEN-HMS) study. J Am Coll Cardiol. 2005;45:1654–64. doi: 10.1016/j.jacc.2005.01.050. [DOI] [PubMed] [Google Scholar]
- 58.Jaarsma T, van der Wal MH, Lesman-Leegte I, et al. Effect of moderate or intensive disease management program on outcome in patients with heart failure: Coordinating Study Evaluating Outcomes of Advising and Counseling in Heart Failure (COACH) Arch Intern Med. 2008;168:316–24. doi: 10.1001/archinternmed.2007.83. [DOI] [PubMed] [Google Scholar]
- 59.Chaudhry SI, Mattera JA, Curtis JP, et al. Telemonitoring in patients with heart failure. N Engl J Med. 2010;363:2301–9. doi: 10.1056/NEJMoa1010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Koehler F, Winkler S, Schieber M, et al. Impact of remote telemedical management on mortality and hospitalizations in ambulatory patients with chronic heart failure: the telemedical interventional monitoring in heart failure study. Circulation. 2011;123:1873–80. doi: 10.1161/CIRCULATIONAHA.111.018473. [DOI] [PubMed] [Google Scholar]
- 61.Paterna S, Parrinello G, Cannizzaro S, et al. Medium term effects of different dosage of diuretic, sodium, and fluid administration on neurohormonal and clinical outcome in patients with recently compensated heart failure. Am J Cardiol. 2009;103:93–102. doi: 10.1016/j.amjcard.2008.08.043. [DOI] [PubMed] [Google Scholar]
- 62.Parrinello G, Di Pasquale P, Licata G, et al. Long-term effects of dietary sodium intake on cytokines and neurohormonal activation in patients with recently compensated congestive heart failure. J Card Fail. 2009;15:864–73. doi: 10.1016/j.cardfail.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 63.Licata G, Di Pasquale P, Parrinello G, et al. Effects of high-dose furosemide and small-volume hypertonic saline solution infusion in comparison with a high dose of furosemide as bolus in refractory congestive heart failure: long-term effects. Am Heart J. 2003;145:459–66. doi: 10.1067/mhj.2003.166. [DOI] [PubMed] [Google Scholar]
- 64.Bradley EH, Curry L, Horwitz LI, et al. Hospital strategies associated with 30-day readmission rates for patients with heart failure. Circ Cardiovasc Qual Outcomes. 2013;6:444–50. doi: 10.1161/CIRCOUTCOMES.111.000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen HH, Glockner JF, Schirger JA, et al. Novel protein therapeutics for systolic heart failure: chronic subcutaneous B-type natriuretic peptide. J Am Coll Cardiol. 2012;60:2305–12. doi: 10.1016/j.jacc.2012.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Teerlink JR, Felker GM, McMurray JJ, et al. Chronic Oral Study of Myosin Activation to Increase Contractility in Heart Failure (COSMIC-HF): a phase 2, pharmacokinetic, randomised, placebo-controlled trial. Lancet. 2016;388:2895–2903. doi: 10.1016/S0140-6736(16)32049-9. [DOI] [PubMed] [Google Scholar]
- 67.Selim A, Zolty R, Chatzizisis YS. New heart failure pharmacotherapy in clinical trials: a hope in progress. Expert Rev Cardiovasc Ther. 2017;15:649–651. doi: 10.1080/14779072.2017.1358614. [DOI] [PubMed] [Google Scholar]
- 68.Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med. 2017;377:644–657. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 69.Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation. 2017;136:e137–e161. doi: 10.1161/CIR.0000000000000509. [DOI] [PubMed] [Google Scholar]
- 70.Chun S, Tu JV, Wijeysundera HC, et al. Lifetime analysis of hospitalizations and survival of patients newly admitted with heart failure. Circ Heart Fail. 2012;5:414–21. doi: 10.1161/CIRCHEARTFAILURE.111.964791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bloom MCR, Butler J. EVALUATION AND MANAGEMENT OF ACUTE HEART FAILURE. In: HR Fuster V, Narula J, Eapen ZJ, editors. Hurst's The Heart. 14. New York, NY: McGraw-Hill Education; [Google Scholar]


