Abstract
Background:
The east-to-west spread of carbapenem-resistant Enterobacteriaceae (CRE) represents an opportunity to explore strategies to limit spread in nonendemic areas. We evaluated CRE emergence and regional support for containment strategies.
Methods:
A 17-question cross-sectional survey was administered to infection prevention programs in Orange County, CA (31 hospitals serving 3 million residents), between January and September 2014. Questions addressed newly detected hospital- and community-onset CRE cultures (2008-2013), current CRE control strategies, and support for prevention strategies for a hypothetical regional intervention.
Results:
Among 31 hospitals, 21 (68%, representing 17 infection prevention programs) completed the survey. CRE was scarcely detected between 2009-2010; within 4 years, 90% of hospitals reported CRE, with 2.5-fold higher community-onset than hospital-onset CRE. Between 2011 and 2013, annual CRE incidence increased 4.7-fold (1.4-6.3 cases/10,000 admissions). Support for a regional CRE prevention bundle was unanimous. Although 22% bathed patients positive for CRE with chlorhexidine gluconate and 11% actively screened for CRE, 86% and 57%, respectively, would consider these strategies in a regional intervention.
Conclusions:
CRE epidemiology in Orange County parallels early progression previously seen in now-endemic areas, representing an opportunity to consider interventions to prevent endemic spread. Many facilities would consider proactive strategies, such as chlorhexidine bathing, in the setting of a regional collaborative.
Keywords: Emerging infections, Antibiotic resistance, Infection prevention of multidrug-resistant organisms
The global rise of carbapenem-resistant Enterobactereciae (CRE) over the past decade marks an important opportunity to consider the role of aggressive and proactive infection prevention efforts in the fight against multidrug-resistant organisms (MDROs).1-5 Amongst MDROs, CRE is among the deadliest, with mortality of infection reported to range from 30%-50%.6-12 Mobile genetic elements associated with CRE resistance mechanisms exacerbate the risks of transmission associated with CRE carriage, with epidemiologic evidence of rapid spread in prior outbreaks.7,13 In the context of extremely limited therapeutic options, CRE represents a high-risk MDRO for which primary prevention strategies to limit CRE spread could prove critical.4,5,7,14
When applied across communities, aggressive and proactive infection prevention interventions can mitigate both acquisition and transmission risk and significantly influence CRE spread.5,15,16 Most notably, a national infection prevention intervention in Israel successfully decreased the monthly incidence of hospital-onset CRE by nearly 80% using interventions such as aggressive hand hygiene compliance, contact precautions, patient cohorting, and active surveillance screening.4,5 To date, hospital and regional interventions have only occurred in the setting of CRE outbreaks. However, the greatest potential for influence of regional horizontal strategies in infection prevention is at the earliest opportunity, before widespread dissemination occurs.15,17
In this study, we describe the emergence of CRE in Orange County, CA, the sixth largest county in the United States, and assess the current infection prevention strategies employed by acute care facilities specifically against CRE. Although facility and regional level interventions are most often considered in the setting of an outbreak, it is unclear how such interventions would be supported before MDRO spread reaches endemic proportions within a community. We therefore assessed the level of support for a hypothetical regional CRE collaborative involving primary prevention strategies to limit CRE spread in hospital settings.
METHODS
Data collection and survey design
We administered a survey consisting of 14 questions and 3 data tables (Appendix S1) to the infection prevention and control (IPC) programs of 31 hospitals in Orange County, CA, between January and December 2014. This survey was approved by the University of California Irvine Institutional Review Board. Participation was voluntary and facilities were recruited with the help of the Orange County chapter of the Association for Professionals in Infection Control and Epidemiology. Descriptive data characterizing participating hospital metrics such as hospital admissions, length of stay, patient demographic characteristics, and case mix were obtained from a mandatory state hospitalization data set.18 The survey was completed either on paper or online through SurveyMonkey (www.surveymonkey.com), per hospital preference. Any surveys with incomplete information were followed-up with a telephone call and in-person meeting with hospital IPC programs to encourage completion. Participants were asked to complete data tables reporting aggregated facility-level hospital-onset (HO) and community-onset (CO) CRE cases per year from 2008-2013. Incident cases were defined based on clinical cultures, which represented newly detected (ie, first-known positive CRE for that patient in that hospital) and were designated as HO (>2 days after admission) or CO (≤2 days) by dates. CRE were defined according to each facilities’ laboratory definitions (see Results). Incident CRE cases were confirmed with microbiologic laboratory reports. CRE cases were designated as CO if date of culture positivity was ≤2 days after admission and otherwise as HO.
The survey additionally included a series of questions to assess CRE surveillance definition, strategies in place to address CRE, and support for countywide regional CRE collaborative strategies. The latter 2 were aimed at evaluating current practice and the level of support for IPC strategies to limit intra- and interfacility CRE spread. These strategies included contact precautions for patients with CRE infection, cohort nursing, interfacility communication of patients admitted from or discharged to other facilities, rectal screening for high-risk patients (ie, patients with indwelling devices, from nursing home or long-term acute care facilities, or patients in same or neighboring rooms of patients with known CRE infection), 1-day point prevalence screens, and daily bathing with chlorhexidine gluconate (CHG). For support for a regional response, respondents were asked to rate their willingness to support each strategy above on 5-point Likert scale ranging from “definitely will support” to “definitely will not support.” Survey items are provided in Appendix S1.
Data analysis
Descriptive proportions were calculated using the total number of hospitals responding to each survey item as the denominator. When assessing support for regional strategies for CRE prevention, respondents were designated as “supportive” if they chose either “definitely will support” or “possibly will support” responses. CRE incidence was reported as the number of new hospital or community onset cases per 1,000 annual admissions.
RESULTS
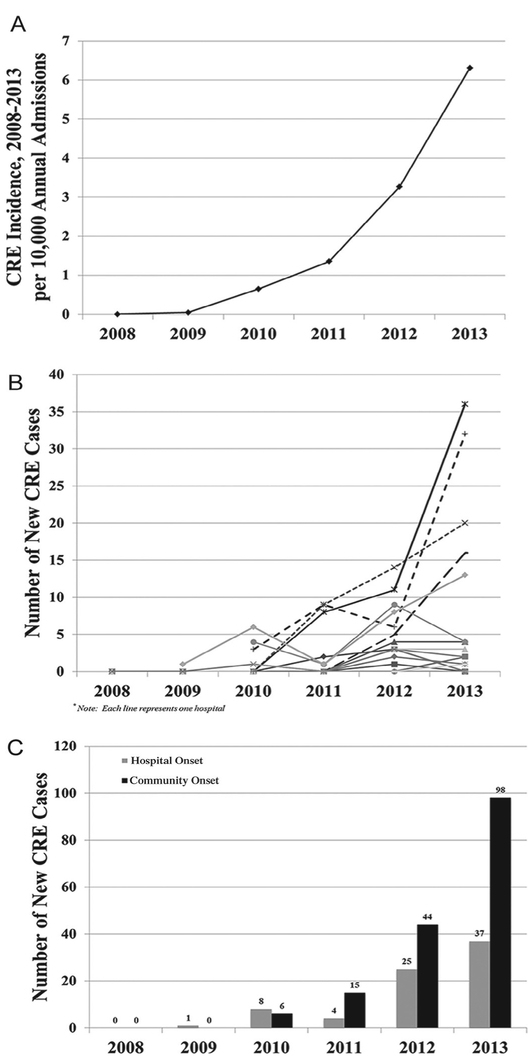
Among 31 hospitals, 17 infection prevention programs responded, representing 21 hospitals (response rate, 68%). Table 1 shows the characteristics and case mix of participating hospitals in 2013. Together, they represented 212,047 annual admissions and 931,216 annual patient days; characteristics were similar to statewide demographic characteristics of hospital admissions.18 Few cases of CRE were seen in Orange County between 2009 and 2010, with only 24% of hospitals reporting CRE cases. By the end of the study period, CRE had been detected in 90% of hospitals. Between 2011 and 2013, the annual incidence of CRE cases rose 4.7-fold, from 1.4-6.3 cases/10,000 admissions representing an absolute number of CRE cases increase from 72-253 within 2 years (Fig 1A). Much of this rise was concentrated within only 5 (24%) facilities reporting 20 or more cases and accounting for 198 (78%) of all cases by 2013 despite representing only 29% of all patient days in participating hospitals that year (Fig 1B). Among these 4 facilities, 1 was an academic tertiary care medical center and the remaining 3 were community hospitals. Aside from 1 hospital, most CRE cases in the county were CO (Fig 1C), although respondents commented that HO cases may have been misclassified because detection was commonly noted in transfers from nursing homes. Among CRE isolates, all were clinical cultures except 2 that were screening cultures only; 69.7% were Klebsiella species (97% of which were Klebsiella pneumoniae), 16.9% were Escherichia coli, and 13.4% were Enterobacter species.
Table 1.
Characteristics of participating hospitals, Orange County, California, 2013
| Hospital characteristic | Acute care hospitals |
|---|---|
| Number of beds | 183 (27-457) |
| Number of admissions | 11,120 (147-29,665) |
| Length of stay | 4.4 (3.4-95.6) |
| No. of admissions by age, y | |
| < 18 | 35,926 (16.9) |
| 19-49 | 65,823 (31.0) |
| 50-65 | 39,532 (18.6) |
| 66-85 | 52,999 (25.0) |
| ≥ 85 | 17,767 (8.4) |
| Male gender | 88,098 (41.6) |
| Number of admissions by race | |
| White | 154,572 (72.9) |
| Black | 4,707 (2.2) |
| Asian | 28,702 (13.5) |
| Other | 24,066 (11.4) |
| Number of admissions by insurance carrier | |
| Medicare | 73,807 (34.8) |
| Medicaid | 42,275 (19.9) |
| Private | 75,698 (35.7) |
| Other | 20,228 (9.6) |
| Mean Elixhauser Comorbidity Index score (Mean SD) | 2.0(0-13) |
NOTE. Values are presented as median (range) or n (%).
Fig 1.
Emergence of carbapenem-resistant Enterobacteriaceae (CRE) in Orange County, CA, 2008-2013. A, CRE incidence, Orange County, California. B, CRE incidence per hospital, with burden concentrated within a few hospitals. C, Hospital- and community-onset CRE incident cases.
At the time of survey, 82% (14 out of 17) of infection prevention programs defined CRE as intermediate or full resistance to any carbapenem; 12% (2 out of 17) defined CRE as intermediate or full resistance to all carbapenems except ertapenem and 1 program did not respond. Infection prevention strategies in place for CRE at the time of survey are shown in Table 2. All facilities were using contact precautions (gowns and gloves) and single-room isolation and had a process in place to track CRE, with 14 (67%) having an electronic medical record flag in place. The majority reported some form of communication to other facilities regarding CRE status. Few hospitals (11%) had instituted active screening for high-risk or CRE-exposed patients. One hospital screened patients in neighboring rooms of patients found to have HO CRE. One facility screens all admitted patients received from nursing homes or other acute care hospitals.
Table 2.
Infection prevention strategies currently used for carbapenem-resistant Enterobacteriaceae (CRE) at participating hospitals
| Infection prevention strategies currently in place for patients with CRE infection |
Adoption among respondents* |
|---|---|
| Contact precautions (gown, gloves, private room, dedicated patient care equipment) |
21 (100) |
| Notify next facility of multidrug-resistant organism detection for patient transferred to another facility |
19 (90) |
| Patient asked to minimize leaving room | 18 (86) |
| Notify prior facility of multidrug-resistant organism detection for patient received by transfer |
14 (67) |
| Patient asked to wear protective gown when leaving room | 12 (57) |
| Cohort patients with same multidrug-resistant organism in a single room |
10 (48) |
| Active CRE screening for exposed patients | |
| Roommate of CRE patient | 5 (28) |
| Neighboring room inhabitants of CRE patient | 1 (6) |
| Roommate/neighboring rooms if CRE cluster | 5 (28) |
| Daily chlorhexidine gluconate bathing† | 4 (22) |
| Active effort to minimize device use | 4 (22) |
| Active effort to minimize antibiotic use ***Active CRE screening for patients admitted |
2 (11) |
| From long-term acute care | 2 (11) |
| Undergoing hemodialysis | 4 (6) |
| From nursing home | 1 (6) |
| From other hospitals | 1 (6) |
| With indwelling urinary catheters | 0 |
| With indwelling central line catheters | 0 |
| One-to-one nursing cohort (nurse only takes care of CRE patients) |
0 |
NOTE. Values are presented as n (%).
Percentage calculated per number of respondents for each question, ranging from 86%-100% (18 to 21) response.
One hospital had adopted housewide chlorhexidine gluconate bathing; the remainder used chlorhexidine gluconate in at least 1 intensive care unit and used chlorhexidine gluconate bathing for patients with drug-resistant organisms, including CRE.
Table 3 shows how many hospitals would support pursuing specific infection prevention strategies in a hypothetical regional CRE collaborative. Support for contact precautions and interfacility communication was unanimous. Although more resource-intensive interventions such as biannual prevalence screening or screening of high-risk incoming patients were less well-supported, more facilities were willing to consider these interventions in the setting of a hypothetical collaborative compared with current practices.
Table 3.
Support for strategies in a regional carbapenem-resistant Enterobacteriaceae (CRE) infection prevention collaborative
| Percentage of respondents |
||||
|---|---|---|---|---|
| Definitely or possibly will support |
Uncertain | Definitely or possibly will not support |
||
| Isolation and precautions of patients with CRE infection | ||||
| Single room isolation | 100 | 0 | 0 | |
| Place in contact precautions | 100 | 0 | 0 | |
| Active observation for hand hygiene and contact precautions adherence | 95 | 5 | 0 | |
| Active intra- and interfacility communication | ||||
| Communicate with treating registered nurse and physician, providing education on CRE and regional collaborative efforts | 100 | 0 | 0 | |
| Communicate with facilities receiving hospital patients about CRE status and regional collaborative to institute bundle | 100 | 0 | 0 | |
| Prevalence screening for CRE | ||||
| Perform annual 1-d prevalence screen for CRE among all patients in 1 intensive care unit and 1 nonintensive care unit | 71 | 10 | 19 | |
| Perform biannual 1-d prevalence screen for CRE among all patients in 1 intensive care unit and 1 nonintensive care unit | 43 | 38 | 19 | |
| Screening nearby patients | ||||
| Screen roommate of patient found to have CRE infection | 81 | 19 | 0 | |
| Screen neighboring rooms of patient found to have CRE infection | 38 | 48 | 14 | |
| CRE screening of patients from other facilities | ||||
| Screen all admitted from nursing home | 48 | 38 | 14 | |
| Screen all admitted from long-term acute care facilities | 48 | 38 | 14 | |
| Screen all transfers from acute care hospitals | 57 | 29 | 14 | |
| Daily chlorhexidine gluconate bathing for hospitalized patients with CRE infection | 86 | 14 | 0 | |
CHG bathing for adult inpatients (regardless of MDRO status) had been implemented in at least 1 intensive care unit at 65% (11 of 17 respondents) of facilities at the time of survey, the remainder did not use CHG bathing as an infection prevention strategy for any inpatient. One facility bathed patients housewide, regardless of MDRO status. CHG bathing of known CRE carriers was widely supported (86% of facilities) for a hypothetical CRE prevention bundle.
CONCLUSIONS
The emergence of CRE in Orange County, CA, during late 2009 to early 2010 has been accompanied by a precipitous rise in prevalence, from 1.4-6.3 cases per 10,000 admissions between 2011 and 2013. This parallels early epidemiology seen in other areas where CRE is now endemic.2,3,19,20 Although CRE has emerged in our county, it is not yet endemic, representing an important opportunity to consider regional approaches to curb further spread.
CRE incidence increased rapidly in our county despite the fact that all facilities surveyed reported instituting prompt contact precautions and providing interfacility communication, suggesting that such efforts to contain CRE within facilities are insufficient to influence regional spread. This may be due to the fact that clinical cultures only identify a small fraction of CRE carriers and because interfacility communication only occurs during direct transfers, which represent only 6% of patient-sharing events among hospitals in this county.21
CRE was detected in 90% of responding hospitals, with CO cases outnumbering HO cases 2.5-fold. It is unclear whether HO cases may represent delayed identification of imported cases either due to delayed culturing or unmasking of CRE due to selective pressure by treatment antibiotic agents. In addition, we were not able to distinguish CO CRE cases from Healthcare Associated Community Onset cases due to lack of information on whether these individuals were recently hospitalized or in nursing homes just before their admission to the reporting hospital. We recognize that CRE has been reported to be more commonly found in long-term care settings compared with hospitals.1,3,22 The disproportionately high number of imported CRE cases highlights the importance of pursuing a regional collaborative approach to minimize CRE spread across hospitals, skilled nursing facilities, and long-term acute care facilities.
The Centers for Disease Control and Prevention (CDC) estimates that infection prevention and antibiotic stewardship interventions occurring at a national level could prevent 619,000 health care-associated infections due to MDROs over 5 years.23 Lee et al24 modeled the differential influence of the CDC CRE prevention toolkit when adopted at the facility level only versus a coordinated regional effort and found that a regional effort would result in an additional 21% reduction in CRE prevalence. Regional strategies to interrupt acquisition and transmission as early as possible among shared patients represents a unique opportunity to prevent endemic MDRO prevalence. In response to international and national outbreaks of CRE, the CDC issued guidance that promoted implementation of a series of core strategies at both facility and regional levels during 2012.2,3,5,16,17 Adoption of such strategies in areas where CRE is not yet endemic is unknown. Although primary prevention strategies are less commonly used in infection prevention when prevalence is rare or low, pre-emptive action taken before spread could serve a critical role in preventing this pathogen from gaining a foothold in a region.
In this study, we sought to contrast infection prevention practices currently in place with support for practices if they were part of a regional collaborative within the context of a newly emerging MDRO. Aside from contact precautions that would be implemented for any MDRO, we found that few facilities had implemented proactive strategies such as active screening protocols for early identification of CRE carriers or exposed patients. This level of response is not surprising in a community where CRE levels are low. Importantly, support for such strategies was higher in the context of a hypothetical regional collaborative, implying that public health initiatives have the potential to influence additional actions. Although this survey did not assess attitudes and beliefs, a possible reason for higher support in the setting of a regional collaboration may include acknowledgment of the importance of such strategies for the communities they serve, or recognition that early proactive efforts may result in future gains for their hospitals.
Infection prevention programs were very willing to perform targeted periodic monitoring of hand hygiene and contact precautions for rooms housing CRE carriers. Broad support for this strategy is not surprising given hand hygiene and contact precautions compliance monitoring is well embedded within activities at most infection prevention programs. In addition, we found that daily CHG bathing of known CRE carriers was among the best supported active interventions among hospitals for a regional collaborative to prevent CRE. Body surface decolonization with daily CHG bathing has been shown to reduce the risk of acquisition and transmission of MDROs and the risk for health care-associated infections, including surgical site infections and central line-associated bloodstream infections.25-31 Daily CHG has also been shown to reduce the risk of CRE acquisition and transmission and several other studies have demonstrated its effectiveness in preventing HAIs from gram negative pathogen.4,26,32-35 Favorability of this intervention may have stemmed from the fact that the majority of hospitals had already introduced CHG bathing into at least 1 hospital intensive care unit. The high willingness to adopt either periodic compliance monitoring or CHG bathing was likely also due to the targeted nature of these strategies, which would currently need to be applied to very few patients.
Among all proposed regional collaborative intervention strategies, active screening received the most mixed support. These findings are not unexpected in light of the high cost and resource-intensive nature of surveillance screening and the unclear role of screening cultures in guiding infection prevention strategies, particularly when prevalence is low. Given the body of literature supporting early identification of CRE and prompt prevention responses resulting in significantly decreased risk of acquisition and transmission where CRE is endemic, further studies are needed to address the role of screening strategies and their cost-effectiveness in early prevention efforts.4,5,16,36
Our study has several limitations. Current strategies and practices for CRE prevention were assessed by facility report without concomitant assessment of compliance. Second, we were unable to identify the exact source or time of CRE acquisition, making any assignment of HO or CO limited due to lack of complete knowledge of recent exposures to a health care facility. Third, this survey period encompassed a time when CRE definitions had been revised twice; thus, responding facilities were likely in varying phases of adopting guidance.37
Contact precautions and interfacility communication remain the most common responses to CRE despite mounting media and literature documenting outbreaks and the rise of CRE to endemic proportions in other areas of the country. The arrival of CRE in Orange County, CA, was anticipated as CRE traversed the United States from east to west. Upon arrival, our study describes a rapid increase in numbers. We found that many facilities are willing to consider more proactive infection prevention strategies, such as hand hygiene and contact precaution monitoring and targeted CHG bathing, in the setting of a regional collaborative to limit CRE spread. A sizeable minority were willing to perform some form of targeted screening of roommates or transfers from high-risk facilities, and this fraction may increase as incidence rises in a region. Investment in public health initiatives may be critically important for motivating hospitals and engendering organized efforts to prevent emerging diseases from gaining a foothold in nonendemic areas.
Supplementary Material
Acknowledgments
This work was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through grant No. KL2 TR000147 to SKG. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
SUPPLEMENTARY DATA
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.ajic.2017.06.004.
Preliminary findings from this work were presented at the Infectious Disease Society of America Annual Meeting, October, 2014, Philadelphia, PA.
Conflicts of interest: SG has served as coinvestigator for studies in which participating facilities may have received contributed product from Clorox. SSH, AG, and RS have conducted clinical studies for which participating hospitals and nursing homes have received contributed product from Sage Products, Molnlycke, 3M, and Clorox.
References
- 1.Munoz-Price LS, Poirel L, Bonomo RA, Schwaber MJ, Daikos GL, Cormican M, et al. Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect Dis 2013;13:785–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bratu S, Landman D, Haag R, Recco R, Eramo A, Alam M, et al. Rapid spread of carbapenem-resistant Klebsiella pneumoniae in New York City: a new threat to our antibiotic armamentarium. Arch Intern Med 2005;165:1430–5. [DOI] [PubMed] [Google Scholar]
- 3.Won SY, Munoz-Price LS, Lolans K, Hota B, Weinstein RA, Hayden MK. Emergence and rapid regional spread of Klebsiella pneumoniae carbapenemase-producing Enterobacteriaceae. Clin Infect Dis 2011;53:532–40. [DOI] [PubMed] [Google Scholar]
- 4.Munoz-Price LS, Hayden MK, Lolans K, Won S, Calvert K, Lin M, et al. Successful control of an outbreak of Klebsiella pneumoniae carbapenemase-producing K. pneumoniae at a long-term acute care hospital. Infect Control Hosp Epidemiol 2010;31:341–7. [DOI] [PubMed] [Google Scholar]
- 5.Schwaber MJ, Lev B, Israeli A, Solter E, Smollan G, Rubinovitch B, et al. Containment of a country-wide outbreak of carbapenem-resistant Klebsiella pneumoniae in Israeli hospitals via a nationally implemented intervention. Clin Infect Dis 2011;52:848–55. [DOI] [PubMed] [Google Scholar]
- 6.Borer A, Saidel-Odes L, Riesenberg K, Eskira S, Peled N, Nativ R, et al. Attributable mortality rate for carbapenem-resistant Klebsiella pneumoniae bacteremia. Infect Control Hosp Epidemiol 2009;30:972–6. [DOI] [PubMed] [Google Scholar]
- 7.Gupta N, Limbago BM, Patel JB, Kallen AJ. Carbapenem-resistant Enterobacteriaceae: epidemiology and prevention. Clin Infect Dis 2011;53:60–7. [DOI] [PubMed] [Google Scholar]
- 8.Schwaber MJ, Klarfeld-Lidji S, Navon-Venezia S, Schwartz D, Leavitt A, Carmeli Y. Predictors of carbapenem-resistant Klebsiella pneumoniae acquisition among hospitalized adults and effect of acquisition on mortality. Antimicrob Agents Chemother 2008;52:1028–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kalpoe JS, Sonnenberg E, Factor SH, del Rio Martin J, Schiano T, Patel G, et al. Mortality associated with carbapenem-resistant Klebsiella pneumoniae infections in liver transplant recipients. Liver Transpl 2012;18:468–74. [DOI] [PubMed] [Google Scholar]
- 10.Bass SN, Bauer SR, Neuner EA, Lam SW. Impact of combination antimicrobial therapy on mortality risk for critically ill patients with carbapenem-resistant bacteremia. Antimicrob Agents Chemother 2015;59:3748–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andria N, Henig O, Kotler O, Domchenko A, Oren I, Zuckerman T, et al. Mortality burden related to infection with carbapenem-resistant Gram-negative bacteria among haematological cancer patients: a retrospective cohort study.J Antimicrob Chemother 2015;70:3146–53. [DOI] [PubMed] [Google Scholar]
- 12.Falagas ME, Tansarli GS, Karageorgopoulos DE, Vardakas KZ. Deaths attributable to carbapenem-resistant Enterobacteriaceae infections. Emerg Infect Dis 2014;20:1170–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nordmann P, Cuzon G, Naas T. The real threat of Klebsiella pneumoniae carbapenemase-producing bacteria. Lancet Infect Dis 2009;9:228–36. [DOI] [PubMed] [Google Scholar]
- 14.Borer A, Eskira S, Nativ R, Saidel-Odes L, Riesenberg K, Livshiz-Riven I, et al. A multifaceted intervention strategy for eradication of a hospital-wide outbreak caused by carbapenem-resistant Klebsiella pneumoniae in Southern Israel. Infect Control Hosp Epidemiol 2011;32:1158–65. [DOI] [PubMed] [Google Scholar]
- 15.Bilavsky E, Schwaber MJ, Carmeli Y. How to stem the tide of carbapenemase-producing Enterobacteriaceae?: proactive versus reactive strategies. Curr Opin Infect Dis 2010;23:327–31. [DOI] [PubMed] [Google Scholar]
- 16.Kochar S, Sheard T, Sharma R, Hui A, Tolentino E, Allen G, et al. Success of an infection control program to reduce the spread of carbapenem-resistant Klebsiella pneumoniae. Infect Control Hosp Epidemiol 2009;30:447–52. [DOI] [PubMed] [Google Scholar]
- 17.Centers for Disease Control National Center for Emerging and Zoonotic Infectious Disease Facility guidance for control of carbapenem-resistant Enterobacteriaceae (CRE). Atlanta, GA: 2015. [Google Scholar]
- 18.Office of Statewide Health Planning and Development. California Inpatient Data Reporting Manual. In: California Department of Health, ed. Vol 7th ed, version 7.6;; September 2011. Available from: http://www.oshpd.ca.gov/HID/MIRCal/Text_pdfs/ManualsGuides/IPManual/TofC.pdf. Accessed August, 2016 [Google Scholar]
- 19.Arnold RS, Thom KA, Sharma S, Phillips M, Kristie Johnson J, Morgan DJ. Emergence of Klebsiella pneumoniae carbapenemase-producing bacteria. South Med J 2011;104:40–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Molton JS, Tambyah PA, Ang BS, Ling ML, Fisher DA. The global spread of healthcare-associated multidrug-resistant bacteria: a perspective from Asia. Clin Infect Dis 2013;56:1310–8. [DOI] [PubMed] [Google Scholar]
- 21.Huang SS, Avery TR, Song Y, Elkins KR, Nguyen CC, Nutter SK, et al. Quantifying interhospital patient sharing as a mechanism for infectious disease spread. Infect Control Hosp Epidemiol 2010;31:1160–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Munoz-Price LS. Carbapenem-resistant Enterobacteriaceae, long-term acute care hospitals, and our distortions of reality. Infect Control Hosp Epidemiol 2013;34:835–7. [DOI] [PubMed] [Google Scholar]
- 23.Slayton RB, Toth D, Lee BY, Tanner W, Bartsch SM, Khader K, et al. Vital signs: estimated effects of a coordinated approach for action to reduce antibiotic-resistant infections in health care facilities—United States. MMWR Morb Mortal Wkly Rep 2015;64:826–31. [PMC free article] [PubMed] [Google Scholar]
- 24.Lee BY, Bartsch SM, Wong KF, McKinnell JA, Slayton RB, Miller LG, et al. The potential trajectory of carbapenem-resistant Enterobacteriaceae, an emerging threat to health-care facilities, and the impact of the centers for disease control and prevention toolkit. Am J Epidemiol 2016;183:471–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Climo MW, Yokoe DS, Warren DK, Perl TM, Bolon M, Herwaldt LA, et al. Effect of daily chlorhexidine bathing on hospital-acquired infection. N Engl J Med 2013;368:533–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang SS, Septimus E, Kleinman K, Moody J, Hickok J, Avery TR, et al. Targeted versus universal decolonization to prevent ICU infection. N Engl J Med 2013;368:2255–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang SS, Septimus E, Hayden MK, Kleinman K, Sturtevant J, Avery TR, et al. Effect of body surface decolonisation on bacteriuria and candiduria in intensive care units: an analysis of a cluster-randomised trial. Lancet Infect Dis 2016;16:70–9. [DOI] [PubMed] [Google Scholar]
- 28.Rao N, Cannella BA, Crossett LS, Yates AJ Jr, McGough RL 3rd, Hamilton CW. Preoperative screening/decolonization for Staphylococcus aureus to prevent orthopedic surgical site infection: prospective cohort study with 2-year follow-up. J Arthroplasty 2011;26:1501–7. [DOI] [PubMed] [Google Scholar]
- 29.Montecalvo MA, McKenna D, Yarrish R, Mack L, Maguire G, Haas J, et al. Chlorhexidine bathing to reduce central venous catheter-associated bloodstream infection: impact and sustainability. Am J Med 2012;125:505–11. [DOI] [PubMed] [Google Scholar]
- 30.Johnson AJ, Daley JA, Zywiel MG, Delanois RE, Mont MA. Preoperative chlorhexidine preparation and the incidence of surgical site infections after hip arthroplasty. J Arthroplasty 2010;25(6 Suppl): 98–102. [DOI] [PubMed] [Google Scholar]
- 31.O’Horo JC, Silva GL, Munoz-Price LS, Safdar N. The efficacy of daily bathing with chlorhexidine for reducing healthcare-associated bloodstream infections: a meta-analysis. Infect Control Hosp Epidemiol 2012;33:257–67. [DOI] [PubMed] [Google Scholar]
- 32.Lin MY, Lolans K, Blom DW, Lyles RD, Weiner S, Poluru KB, et al. The effectiveness of routine daily chlorhexidine gluconate bathing in reducing Klebsiella pneumoniae carbapenemase-producing Enterobacteriaceae skin burden among long-term acute care hospital patients. Infect Control Hosp Epidemiol 2014;35:440–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hayden MK, Lin MY, Lolans K, Weiner S, Blom D, Moore NM, et al. Prevention of colonization and infection by Klebsiella pneumoniae carbapenemase-producing enterobacteriaceae in long-term acute-care hospitals. Clin Infect Dis 2015;60:1153–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cassir N, Thomas G, Hraiech S, Brunet J, Fournier PE, La Scola B, et al. Chlorhexidine daily bathing: impact on health care-associated infections caused by gram-negative bacteria. Am J Infect Control 2015;43:640–3. [DOI] [PubMed] [Google Scholar]
- 35.Chen W, Cao Q, Li S, Li H, Zhang W. Impact of daily bathing with chlorhexidine gluconate on ventilator associated pneumonia in intensive care units: a meta-analysis.J Thorac Dis 2015;7:746–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ben-David D, Maor Y, Keller N, Regev-Yochay G, Tal I, Shachar D, et al. Potential role of active surveillance in the control of a hospital-wide outbreak of carbapenem-resistant Klebsiella pneumoniae infection. Infect Control Hosp Epidemiol 2010;31:620–6. [DOI] [PubMed] [Google Scholar]
- 37.Rennie RP,Jones RN. Effects of breakpoint changes on carbapenem susceptibility rates of Enterobacteriaceae: results from the SENTRY Antimicrobial Surveillance Program, United States, 2008 to 2012. Can J Infect Dis Med Microbiol 2014;25:285–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.