Abstract
The shift in ocular dominance (OD) of binocular neurons induced by monocular deprivation is the canonical model of synaptic plasticity confined to a postnatal critical period. Developmental constraints on this plasticity not only lend stability to the mature visual cortical circuitry but also impede the ability to recover from amblyopia beyond an early window. Advances with mouse models utilizing the power of molecular, genetic, and imaging tools are beginning to unravel the circuit, cellular, and molecular mechanisms controlling the onset and closure of the critical periods of plasticity in the primary visual cortex (V1). Emerging evidence suggests that mechanisms enabling plasticity in juveniles are not simply lost with age but rather that plasticity is actively constrained by the developmental up-regulation of molecular ‘brakes’. Lifting these brakes enhances plasticity in the adult visual cortex, and can be harnessed to promote recovery from amblyopia. The reactivation of plasticity by experimental manipulations has revised the idea that robust OD plasticity is limited to early postnatal development. Here, we discuss recent insights into the neurobiology of the initiation and termination of critical periods and how our increasingly mechanistic understanding of these processes can be leveraged toward improved clinical treatment of adult amblyopia.
Keywords: GABA, Parvalbumin, Perineuronal net, Dark exposure, Acetylcholine, PSD-95, HDAC
It is well appreciated that there are defined windows in early life when neural circuitry can be robustly restructured in response to experience. These time-limited critical periods have been demonstrated for many brain functions across many brain regions and are thought to allow developing neural circuits to establish an individualized, optimal neural representation of a highly variable environment. The enhanced plasticity corresponds to the peak phases of physical growth and may, therefore, allow for constant perception during expansion of the body surface. For example, visual receptive fields must repeatedly remap as the distance between the two eyes increases. Indeed, experience-dependent matching of stimulus selectivity of the visual input from the two eyes occurs during the critical period (Wang et al., 2010).
The relative stability that follows the critical period may be advantageous in adult circuits, and also allow for conservation of energy/resources. However, the enhanced stability with age also inhibits large-scale adaptations to changes in the input during adulthood. Nonetheless the adult cortex retains the ability to express some forms of synaptic plasticity, but the mechanisms for the induction and expression of plasticity differ from those utilized during the critical period. In this context, it is important to bear in mind that many measures are in current use to study ‘ocular dominance (OD) plasticity.’ Originally defined as a change in the eye preference of the spiking output of V1 neurons (Wiesel & Hubel, 1963), current methods include visually evoked synaptic potentials, intrinsic hemodynamic signals, immediate early gene activation, thalamocortical axon or dendritic spine morphology and motility, and calcium responses in genetically identified cell types. Each of these methods yields different resolutions and may be variably sensitive to subthreshold inputs (Morishita & Hensch, 2008), which are important considerations when informing therapies for recovery of the visual function in both amblyopic children and adults.
During the critical period, an asymmetry in the quality of the visual input across the two eyes leads to reduced visual acuity and a visually evoked spiking response through the affected eye with no obvious pathology in the eye, thalamus, or cortex. The severity of amblyopia depends on the age at initiation and the type of asymmetry, which can be caused by unequal alignment (strabismus), unequal refractive error (anisometropia), or form deprivation (e.g., cataract). The critical period for developing amblyopia in children extends to 8 years and is relatively easy to correct until that age by improving the quality of visual input in the affected eye (reviewed in Daw, 1998; Mitchell & MacKinnon, 2002; Simons, 2005) but becomes increasingly resistant to reversal with age.
In animal models, amblyopia is most often induced by monocular deprivation (MD)—an eyelid suture which significantly occludes the patterned visual input to one eye. Across various species, MD unleashes a sequence of functional and structural changes in V1 that shifts the OD of binocular neurons away from the deprived eye and toward the open eye, resulting in a reduction in deprived-eye acuity (Wiesel & Hubel, 1963, 1970; Olson & Freeman, 1975; Hubel et al., 1977; Movshon & Dürsteler, 1977; Blakemore et al., 1978; LeVay et al., 1978; Shatz & Stryker, 1978; Antonini & Stryker, 1993; Fagiolini et al., 1994; Gordon & Stryker, 1996; Hensch et al., 1998; Trachtenberg & Stryker, 2001; Mataga et al., 2002; Taha & Stryker, 2002; Prusky & Douglas, 2003; Frenkel & Bear, 2004; Sato & Stryker, 2008).
While OD plasticity peaks during the postnatal critical period, it (Lehmann and Lowel, 2008) persists beyond sexual maturity at some level in many species, including rats, mice and cats. The age at initiation and duration of MD then strongly impacts the severity of the subsequent amblyopia, as well as the potential for recovery (Liao et al., 2004; Eaton et al., 2016). Accordingly, short durations of MD within the critical period induce a shift in OD and a reduction in the deprived eye visual acuity that are rapidly corrected by restoring normal vision (Schwarzkopf et al., 2007). In contrast, long-term MD initiated early and persisting through the end of the critical period induces a shift in OD and a loss of visual acuity that is highly resistant to reversal.
The initial response to MD during the critical period is a reduction in functional strength and selectivity of the deprived eye visual responses (Gordon & Stryker, 1996; Hensch et al., 1998; Trachtenberg et al., 2000; Frenkel & Bear, 2004) and disrupts the balance of excitatory ad inhibitory inputs to individual binocular neurons (Saiepour et al., 2015). Depression of deprived-eye responses may occur by synaptic depression at both thalamocortical and intracortical connections. Notably, rapid shifts in the visual response of parvalbumin (PV)-expressing inhibitory interneurons may enable these first functional changes within V1 (Yazaki-Sugiyama et al., 2009; Aton, Broussard et al., 2013; Kuhlman et al., 2013). Depression is then followed by a relatively slow, strengthening of open eye responses (Sawtell et al., 2003; Frenkel & Bear, 2004; Kaneko et al., 2008).
Robust morphological plasticity is also induced by MD during the critical period. An initial degradation of the extracellular matrix by the upregulation of proteases occurs within the first 2 days after MD in the mouse and may elevate spine motility (Mataga et al., 2004; Oray et al., 2004). Studies in cats, monkeys, and humans suggest that structural plasticity is facilitated by a reduction in the neurofilament-light protein within V1, which may de-stabilize the cytoskeleton and promote plasticity (Duffy & Livingstone, 2005; Duffy et al., 2007; Duffy & Mitchell, 2013). Brief MD during the critical period alters the spine density on pyramidal neurons (Mataga et al., 2004; Tropea et al., 2010; Yu et al., 2011; Djurisic et al., 2013) and induces a transient decrease in the density of synapses formed by thalamocortical axons originating from the lateral geniculate nucleus (LGN) (Coleman et al., 2010). Long-term MD yields enduring alterations in the length and extent of thalamocortical arbors serving the two eyes (Hubel et al., 1977; Shatz & Stryker, 1978; Antonini et al., 1999) and a significant reduction in dendritic spine density (Montey & Quinlan, 2011).
Studies from humans and nonhuman primates suggest a protracted decline in visual plasticity that extends into adulthood rather than an abrupt closure of the critical period. However, the residual plasticity that persists in the adult visual cortex appears to differ from the plasticity during the critical period in several important ways: (1) the shift in OD in adults is slower and smaller and may require a longer duration of deprivation to engage; (2) it may not require depression of deprived eye responses for the subsequent strengthening of responses to the nondeprived eye; (3) it may be restricted to synapses in the supragranular and infragranular lamina, as plasticity in layer 4 has been shown to be constrained early in postnatal development; (4) it may be restricted by saturated synapses, setting limits on the amount of recovery of visual function that can be accomplished using this pathway. Additionally, MD in adults does not elicit the robust structural alterations that accompany OD plasticity during the critical period such as increased spine motility and pruning (Mataga et al., 2004; Oray et al., 2004; Lee et al., 2006). Indeed, a general decline in structural plasticity is one of the hallmarks of the termination of the critical period. However, residual increases in the rate of formation and stability of dendritic spines may persist in the adult layer I after MD (Hofer et al., 2009).
Inhibition and critical period induction
Powerful new tools in neuroscience, especially the molecular genetic control available in mice, are beginning to elucidate the cellular and molecular mechanisms that initiate and terminate critical periods. Ocular dominance plasticity peaks during the third postnatal week in rodents, demonstrating that elevated plasticity is not the initial state of immature circuits. Indeed, the maturation of specific inhibitory circuitry is necessary to initiate the critical period, which can be accelerated by activating inhibitory GABAA receptors with allosteric modulators such as benzodiazepines (Hensch et al., 1998; Fagiolini & Hensch, 2000; Iwai et al., 2003; Fagiolini et al., 2004). Promoting early maturation of a specific class of inhibitory interneurons that express the calcium binding protein parvalbumin (PV) by increasing the levels of growth factors (Huang et al., 1999; Hanover et al., 1999; Otx2: Sugiyama et al., 2008; Spatazza et al., 2013) or by removing cell adhesion (PSA: Di Cristo et al., 2007) or DNA binding proteins (MeCP2: Durand et al., 2012; Krishnan et al., 2015) can induce premature initiation of the critical period.
The perisomatic inhibition mediated by these fast-spiking PV interneurons exerts powerful control over the excitability and plasticity of downstream pyramidal neurons, regulating many forms of downstream synaptic plasticity (Katagiri et al., 2007; Kuhlman et al., 2013; Toyoizumi et al., 2013). Several proteins that regulate synaptic strength and/or number are highly enriched at excitatory synapses onto PV interneurons and impact the timing of the critical period and NRG1 (NARP: Chang et al., 2010; Gu et al., 2013, Pelkey et al., 2015; Gu et al., 2016; kaplan et al., 2016; Sun et al., 2016). Accordingly, NARP-deficient mice fail to initiate a critical period unless rescued by enhancing the strength of the inhibitory output or excitatory drive onto PV interneurons (Gu et al., 2013; Gu et al., 2016).
A further increase in perisomatic inhibition is thought to terminate the critical period. Hence, the critical period can be reopened in adulthood by pharmacological reduction of inhibition (Harazouv et al., 2010) or by the knockdown of Otx2 (Beurdeley et al., 2012; Spatazza et al., 2013). Treatment with an NRG1 peptide induces a precocious termination of the critical period, while inhibition of the activity of the NRG receptor (ErbB) reactivates the critical period in adults (Gu et al., 2016). Indeed, a developmental reduction of plasticity at excitatory synapses onto FS interneurons may explain the requirement for longer durations of MD with age (Kameyama et al., 2010). Together, these studies indicate that PV inhibitory neurons exert bidirectional control over OD plasticity (van Versendaal & Levelt, 2016).
Other classes of inhibitory neurons may influence the expression of plasticity, either independently or through the regulation of PV neurons. Interestingly, inhibitory neurons in layer 1 (L1) of the visual cortex and those expressing vasoactive intestinal peptide (VIP) are strongly activated during certain behavioral states and exert cortical effects by disinhibition of pyramidal neurons (Letzkus et al., 2011; Donato et al., 2013; Pfeffer et al., 2013; Pi et al., 2013; Fu et al., 2015). Locomotion activates VIP interneurons, which enhances neural activity in V1 (Niell & Stryker, 2010) and promotes adult plasticity by increasing inhibition onto other interneuron subtypes that target pyramidal neurons (Fu et al., 2014; Fu et al., 2015). Similarly, reinforcement signals (reward and punishment) during the performance of an auditory discrimination task activate VIP neurons in the auditory cortex, which increase the gain of a functional subpopulation of pyramidal neurons by disinhibition (Pi et al., 2013). Thus, disinhibitory circuits which transiently suppress other inhibitory interneurons may be a general mechanism for enabling plasticity in the adult cortex.
Molecular reactivation of critical period in adulthood
Increasing evidence demonstrates that removing molecular ‘brakes’ in adulthood can enhance plasticity and promote recovery from amblyopia. For example, epigenetic mechanisms, such as histone deacetylase (HDAC) activity, may down regulate the expression of genes that promote plasticity over development. Accordingly, HDAC inhibition enhances plasticity in adult V1, allowing for recovery from amblyopia (Putignano et al., 2007; Silingardi et al., 2010; Baroncelli et al., 2016). However, the downstream targets of histone acetylation at specific stages of development remain to be identified.
Alternatively, increased expression of specific genes over development can actively limit rewiring. The expression of Lynx1, an endogenous inhibitor of nicotinic acetylcholine receptors, emerges in V1 coincident with critical period closure, which would dampen the neuromodulatory actions of acetylcholine (Miwa et al., 1999; Morishita et al., 2010). Both genetic deletion of lynx1 and administration of acetylcholinesterase inhibitors enhance spine motility and the morphological plasticity induced by MD (Sajo et al., 2016) and enable recovery of visual acuity following MD throughout life (Morishita et al., 2010). The major histocompatibility complex class I (MHCI) receptor, PirB, is another molecular brake. Disruption of PirB signaling enhances OD plasticity throughout life and facilitates recovery from amblyopia in adults (Syken et al., 2006; Bochner et al., 2014). Another immune system molecule, Stat1, restricts the increase of open eye responses following monocular deprivation, while genetic deletion enhances this component of plasticity (Nagakura et al., 2014). The identification of specific molecules that actively suppress plasticity in the adult visual cortex may inform strategies for pharmacological interventions to reopen the critical period.
Molecular brakes can also present physical barriers to morphological plasticity. Perineuronal nets are highly enriched around PV neurons and reach maturity at the end of the critical period. Disrupting the molecular latticework of this extracellular matrix (Pizzorusso et al., 2002; Pizzorusso et al., 2006; Carulli et al., 2010) or the molecules which bind to it (i.e., Otx2: Beurdeley et al., 2012) enables OD plasticity and recovery from amblyopia in adults. Consistent with this, mice lacking the Nogo receptor (Ngr1), a bimodal receptor for chondroitin sulfate proteoglycans and myelin-derived inhibitory factors (Dickendesher et al., 2012), also retain critical period plasticity into adulthood and spontaneously recover visual acuity following long-term MD (McGee et al., 2005; Stephany et al., 2014). Interestingly, PirB may act in concert with Ngr1 (Atwal et al., 2008) to dampen the morphological plasticity of dendritic spines on layer 5 pyramidal neurons in adults (Bochner et al., 2014) (Fig. 1).
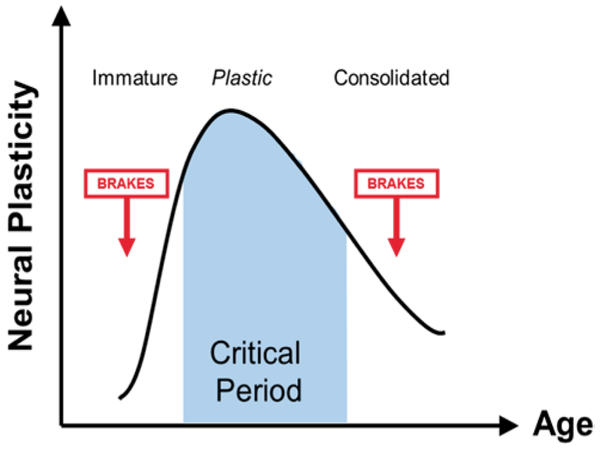
FIG. 1.
Critical period plasticity as a function of age. Initially, immature brain circuits are dominated by excitatory inputs and fail to express plasticity. As inhibitory circuits mature, a highly plastic critical period is induced. Plasticity then declines with age as inhibitory circuits and brake-like factors dominate, harboring the potential for plasticity throughout life. Dynamic changes in the excitatory/inhibitory balance across age are shown below the graph. Figure courtesy of Takao Hensch (Harvard University).
Interestingly, one recently discovered molecular brake may lie within the dendritic spine itself. Postsynaptic density protein 95 (PSD-95), an intracellular scaffold highly enriched at excitatory synapses, is thought to accelerate maturation of excitatory synapses. PSD-95 promotes the incorporation of AMPA-type glutamate receptors into synapses containing only NMDA receptors, which are normally functionally “silent” at the resting membrane potential. In contrast, the immediate early gene Arc promotes removal of AMPA receptors from cortical synapses and deletion precludes visual plasticity (McCurry et al., 2010). Genetic reduction of PSD-95 in adulthood increases the number of silent synapses and reactivates the juvenile form of OD plasticity, characterized by a rapid and robust deprived-eye depression (Huang et al., 2015). Notably, no changes in GABAergic or NMDA receptor currents are observed, suggesting that the reactivation of plasticity by PSD-95 deletion lies downstream of the regulation of inhibitory circuitry. A conversion of ‘silent’ to functional synapses has been proposed as a general mechanism to constrain plasticity across brain regions (Greifzu et al., 2014; Huang et al., 2015).
Environmental reactivation of critical period in adulthood
Characteristics of the physical or sensory environment strongly impact the function and plasticity of cortical circuits. Remarkably, adding social, sensory, or motor enrichment to the typically impoverished environment of the laboratory rodent influences the expression and time course of OD plasticity. Robust OD plasticity persists into adulthood when mice are raised in large complex cages with multisensory and motor enrichment (Sale et al., 2007; Scali et al., 2012; Greifzu et al., 2014). In fact, enriched rearing may better reflect the sensorimotor environment of primates including humans. At a molecular level, exposure to enriched environments in adulthood increases H3 acetylation (Baroncelli et al., 2016), reduces the expression of PV and GAD67 within inhibitory neurons of the visual cortex, weakens GABA signaling, and fosters plasticity in both the cortex and hippocampus (Sale et al., 2007; Donato et al., 2013; Greifzu et al., 2014).
In this regard, it is intriguing that total visual deprivation also reactivates robust plasticity in adult V1 and promotes recovery from chronic MD (He et al., 2007; Montey & Quinlan, 2011; Duffy & Mitchell, 2013; Stodieck et al., 2014; Eaton et al., 2016; Mitchell et al., 2016). The BCM theory of a sliding synaptic modification threshold forecasted that dark exposure would enhance plasticity and indeed several mechanisms are engaged by the dark exposure that are predicted to lower the threshold for synaptic plasticity in pyramidal neurons (Cooper & Bear, 2012). For example, the composition of the NMDA type glutamate receptors is reset to a “juvenile” form (containing the NR2B subunit; Quinlan et al., 1999) which exhibit enhanced temporal summation (Yashiro et al., 2005; He et al., 2006). In addition, forms of synaptic plasticity typically limited to juveniles are re-expressed (Huang et al., 2010; Montey & Quinlan, 2011), spines on pyramidal neurons are shifted toward an immature structure and dynamics (Tropea et al., 2010), and immature excitatory synapses on pyramidal neurons are strengthened, thereby increasing excitability and expanding the integration window for spike-timing dependent plasticity (Goel & Lee, 2007; He et al., 2007; Guo et al., 2012).
In contrast, dark exposure decreases the excitability of PV interneurons, and the reactivated plasticity can be reversed by increasing the strength of their excitatory synaptic inputs (Gu et al., 2016). A loss of specific neurofilament protein associated with cytoskeletal stability is observed in the LGN following dark exposure, which may further contribute to the reactivation of structural OD plasticity (O’Leary et al., 2012; Duffy et al., 2016). Importantly, dark exposure restores a period of susceptibility to MD and also promotes the recovery of visual function in adults with amblyopia, as has been demonstrated in rats, mice, and cats (He et al., 2006; He et al., 2007; Duffy & Mitchell, 2013; Stodieck et al., 2014; Duffy et al., 2016). The reactivation of plasticity by dark exposure has also been shown to strengthen the thalamic input to the cortex (Montey & Quinlan, 2011). Thus, the seemingly opposite interventions of environmental enrichment and dark exposure may both enhance cellular plasticity by the removal of functional and structural constraints that normally accumulate over development to stabilize the V1 circuitry.
It is important to note that dark exposure alone does not impact visual acuity or neuronal stimulus selectivity, which is regained only after repetitive visual experience (Montey et al., 2013; Eaton et al., 2016). Likewise, enrichment or locomotion alone does not strengthen visual performance (Kaneko & Stryker, 2014; Greifzu et al., 2016). This suggests that the recovery from amblyopia in adulthood is a two-stage process that requires (1) the reactivation of plasticity in the adult amblyopic cortex (permissive step) and (2) focused visual experience to stimulate perceptual learning (instructive step). One of the challenges, therefore, is to identify the optimal visual stimulation to drive recovery of the function, which may differ based on age and depth of amblyopia (Montey & Quinlan, 2011; Eaton et al., 2016). However, prolonged plasticity by environmental enrichment makes it unclear if complex environments better mimic those of primates including humans. At a minimum, these experiments provides a valuable condition with which to better understand the biological basis of critical period closure.
Reactivating plasticity to enhance recovery
The reactivation of plasticity in the primary visual cortex has revised the idea that critical periods are strictly limited to early postnatal development (Bavelier et al., 2010; Takesian & Hensch, 2013; Sengpiel, 2014). However, the mechanisms that engage the cortical plasticity necessary to treat amblyopia may be very different from the plasticity that enables the cortex to regain sensitivity to MD. Indeed, it has long been known that the critical period for plasticity in response to MD differs from the critical period for recovery of the binocularity and orientation selectivity by removing the MD (Liao et al., 2004). Although initially assumed to overlap with the critical period for susceptibility to amblyopia, it is now clear that the treatment window for the reversal of amblyopia in humans may extend beyond early life (reviewed in Daw, 1998). It is, therefore, important that key molecular effectors be tested in their ability to recover (not induce) amblyopia in adults.
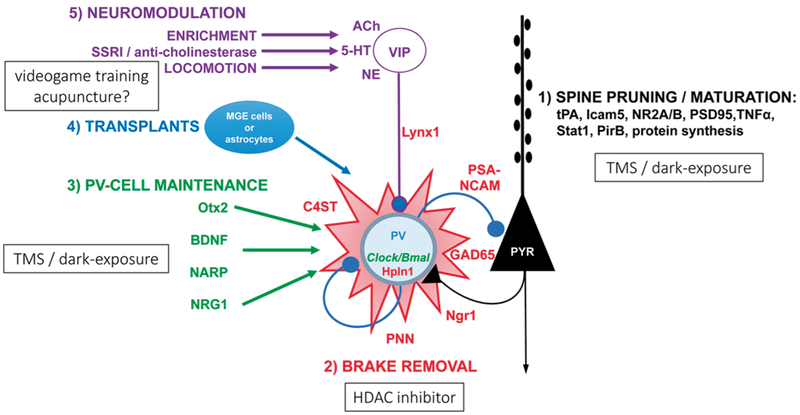
Mechanistic studies performed primarily in mice, have identified novel therapies with translational potential to reverse the developmental constraints on recovery from amblyopia. Several commonly prescribed drugs, such as cholinesterase inhibitors (Morishita et al., 2010), valproate (Gervain et al., 2013; Lennartsson et al., 2015), or selective serotonin reuptake inhibitors (Maya Vetencourt et al., 2008), could be repurposed to reactivate plasticity in adult amblyopic patients. Interestingly, a reduction in PV interneuron function may be a mechanism common to several of these interventions. The SSRI antidepressant fluoxetine reduces the basal levels of extracellular GABA (Maya Vetencourt et al., 2008) and the number of PV interneurons surrounded by dense perineuronal nets (Guirado et al., 2014). Similarly, dark exposure may rejuvenate intracortical inhibition by reducing the excitatory drive onto PV neurons (Gu et al., 2016) (Fig. 2).
Fig. 2.
Pathways known to enhance plasticity in the adult visual cortex.
Future work that explores noninvasive ways to tap into these mechanisms to trigger plasticity may generate novel amblyopia treatments for adults. It will also be important to learn if the success of behavioral manipulations, such as dark rearing, environmental enrichment and exercise, promotes the recovery from amblyopia in rodents by reversing molecular brakes or engaging residual plasticity mechanisms that are normally expressed in the adult cortex (Sale et al., 2007; He et al., 2007; Duffy & Mitchell, 2013; Eaton et al., 2016). Such biological insights gleaned from animal model systems have provided the foundation for a number of promising ongoing clinical trials aimed at improving vision in amblyopic patients (Stryker & Löwel, this volume).
In addition, it is important to keep in mind that while robust OD plasticity is lost with age, the adult visual cortex does retain the ability to learn. This is reflected in the success of visual training (repetitive visual task performance, visual perceptual learning) to promote enhancement of acuity and recovery of stereoscopic vision in both amblyopic humans and experimental animals (Levi & Li, 2009; Bonaccorsi et al., 2014; Kawato et al., 2014; Sengpiel, 2014). Recent approaches to visual training include dichoptic visual stimulation (in humans and cats) to normalize the quality of visual input across the strong and weak eye, and the use of action video games, to recruit neuromodulatory pathways that engage attention and motivation (Stryker & Löwel, this volume; Mitchell & Duffy, 2014; Hess & Thompson, 2015; Murphy et al., 2015; Levi et al., 2015). However, the improvements in visual acuity achieved with these methods in humans have been relatively modest to date (Tsirlin et al., 2015, but see; Hess & Thompson, 2015).
Expanding the focus beyond ocular dominance
The primary aspects of visual system function assessed in animal studies of amblyopia are OD and spatial acuity. However, amblyopia is associated with a range of visual deficits, including loss of stereoscopic depth perception, crowding, impairments in shape discrimination, deficits in motion and direction perception, and object tracking (reviewed in Daw, 2013). Some of these impairments, such as the loss of stereoscopic depth perception and visual crowding, may greatly impact the quality of the life of the amblyopic patient (Levi et al., 2015). Expanding the focus of animal studies of amblyopia beyond the recovery of OD will broaden the ability of this work to inform clinical strategies.
Furthermore, separable neuronal response properties of individual V1 neurons have distinct, overlapping critical periods (reviewed in Kiorpes, 2015). For example, it has long been known that the critical period for direction selectivity in kittens precedes the critical period for OD (Daw & Wyatt, 1976). In the primate visual system, critical periods for basic spectral sensitivities end relatively early (6 months), whereas those for complex representations such as contrast sensitivity and binocularity extend much later (25 months; Harwerth et al., 1986). There is also evidence that some manipulations may globally reinstate V1 plasticity across these distinct visual functions. For example, dark exposure in adulthood, which reactivates plasticity for the recovery of normal OD in amblyopic rats, mice, and kittens (He et al., 2007; Montey & Quinlan, 2011; Duffy & Mitchell, 2013; Stodieck et al., 2014; Eaton et al., 2016; Mitchell et al., 2016) promotes the recovery of stimulus selectivity and visual response strength (Montey et al., 2013). As critical periods for different visual functions may depend on separate underlying mechanisms, some manipulations may restore only selective features of V1 responses. For example, a genetic deletion of PSD-95 disrupts the development of orientation preference in the mouse visual cortex, without impacting the development or plasticity of OD in juveniles (Fagiolini et al., 2003).
It is particularly important to ask if the interventions that promote the recovery of OD and/or visual acuity also promote the visual functions that underlie stereopsis, such as retinal disparity tuning and/or binocular matching of stimulus preference. Binocular integration in the primary visual cortex is an important first step in the perception of depth from retinal disparity (Scholl et al., 2013). It has been demonstrated that shortly after eye opening, V1 neurons exhibit orientation tuning and respond to visual stimulation of either eye; however, the orientation preference through each eye, which is initially mismatched, becomes tuned to similar orientations during the critical period (Wang et al., 2010). Indeed, manipulations that prolong V1 plasticity, such as environmental enrichment, accelerate binocular matching of the stimulus selectivity in the developing mouse primary visual cortex (Gu et al., 2016). In contrast, manipulations that accelerate plasticity in the cortex, such as heterozygous loss of Mecp2, prevent the acquisition of matched stimulus selectivity (Krishnan et al., 2015). Recovery of stereopsis in rodents can be assessed through behavioral measures such as visual cliff or SLAG performance (Gil-Pagés et al., 2013). Incorporation of physiological and psychophysical assessments that examine contrast sensitivity, direction selectivity, and stereoscopy would greatly improve assessement of the treatment efficacy across multiple aspects of vision in patients with amblyopia.
Expanding the focus beyond primary visual cortex
The majority of animal work on amblyopia has focused on regions early in the visual pathway, as MD induces significant structural re-arrangements in V1, including pruning of thalamocortical inputs that serve the deprived eye (Wiesel & Hubel, 1963; Hubel et al., 1977; Shatz & Stryker, 1978). Long-term MD induces a near complete loss of stimulus selectivity for input coming in through the chronically deprived eye (Montey & Quinlan, 2011). Given these severe structural and functional deficits in V1, it is even more remarkable that full recovery of visual acuity has been demonstrated with some interventions.
However, the magnitude of compromised vision observed in psychophysical experiments is often not mirrored by changes in the function of V1 neurons, suggesting that physiological changes may be propagated and amplified in higher cortical areas (Shooner et al., 2015). Indeed, psychophysical and neural recording data suggest that amblyopia is also associated with abnormalities in extrastriate regions (reviewed in Kiorpes, 2015). For example, deficits in higher order visual functions, such as motion perception, which have been described in amblyopic monkeys (Kiorpes et al., 2006) may be partly explained by aberrant development of the extrastriate area MT/V5. Here neurons driven by the amblyopic eye exhibit reduced sensitivity to coherent motion and reduced ability to integrate motion information over time (El-Shamayleh et al., 2010).
Higher brain areas and neuromodulatory pathways are also potential targets to facilitate visual responses and plasticity within V1 of amblyopic adults. For example, children with macular degeneration show large regions of V1 that are unresponsive during passive viewing of the visual stimuli, but can be activated by engaging the subjects in a stimulus-related task, suggesting a powerful role of top-down influences. Remarkably, the same visual task-related responses are not observed in simulated lesion zones in normal binocular subjects, suggesting that macular degeneration may potentiate or unmask feedback signals (Masuda et al., 2008).
Regions outside of the primary sensory cortices are thought to express late, prolonged windows of plasticity that extend well beyond that of V1. Thus, devising treatments to target these regions may be an effective strategy for recovery of visual function in adulthood that does not require the reactivation of plasticity in V1. Advanced tools enabling the monitoring, activation, or silencing of specific neural circuits in mice or higher species will contribute to our understanding of the top-down influences on plasticity within V1. Future primate studies will also be essential to examine plasticity within higher order visual regions.
Path forward
Rapidly evolving genetic, imaging, and physiological tools have allowed mechanistic insights into how critical period plasticity is regulated, including the identification of molecular ‘triggers’ and ‘brakes’ that control the initiation and termination in V1. Much of this knowledge has been gleaned recently from mouse models, which offer unprecedented experimental control of specific neuronal and synaptic populations, including optogenetic, chemogenetic, and magnetogenetic approaches. However, to better inform amblyopia treatment, mechanistic work should be expanded to additional species, especially those with a columnar organization of ocular preference and neurons tuned for small retinal disparities. The increasing availability of molecular genetic techniques such as CRISPR makes this a likely goal.
In addition, examination of the incidence and expression of amblyopia across human populations may elucidate the impact of environmental and genetic factors on individual differences in visual plasticity. An assessment of OD plasticity across a large number of recombinant inbred mouse strains revealed striking variability in response to MD. Interestingly, there was no correlation between the weakening of deprived eye responses and the strengthening of nondeprived eye responses, suggesting that these two pathways may be regulated by separate genetic factors (Heimel et al., 2008). In addition, several molecules implicated in regulating the timing of the critical period, including the constraints on adult plasticity, are the known risk factors for neurodevelopmental disorders such as schizophrenia. These include HDAC and NRG1 (Rico & Marín, 2011; Penzes et al., 2013). Interestingly, male schizophrenics are two times less likely to have refractive errors (Caspi et al., 2009), raising the possibility that common genetic risk factors contribute generally to the maturation of neuronal circuitry, including the normal development of binocular vision. Further work is necessary to identify human populations that may be at a greater or at a lesser risk for the development of amblyopia.
Capitalizing on these biological insights, one goal is to develop targeted strategies to guide clinical trials by enhancing plasticity in the postcritical period visual cortex in humans. In addition, such a critical period regulation could also be extended to strabismus, eye movement control disorders, and the restoration of the optimal neural function after damage from a stroke or other traumatic brain injuries.
Recommendations.
During developmental ‘critical periods,’ neural circuitry can be potently shaped by experience. Although the brain retains the capacity to re-wire beyond early life, adult forms of plasticity may utilize mechanisms distinct from those available to juveniles. Understanding the differences between developmental and adult plasticity, including differences in how they are typically measured, will provide key insights into novel therapies for recovery of the visual function from amblyopia in both children and adults.
Evolving tools in neuroscience have shed new light on the ‘triggers’ and ‘brakes’ that determine the onset and offset of critical periods. Strikingly, the brain’s intrinsic potential for plasticity is not lost with age but instead is actively constrained beyond early critical periods. Indeed, lifting molecular ‘brakes’ unmasks potent plasticity in adulthood. Ongoing work to determine how the various ‘brakes’ act within common cellular and circuit networks will lead to targeted therapeutic strategies to promote plasticity and biologically-inspired clinical studies for amblyopia recovery.
Most animal studies have focused on reinstating a period of susceptibility to MD in adulthood. Yet, the plasticity necessary to recover from amblyopia may be different from that required to recover sensitivity to MD. Thus, future work should emphasize animal studies that specifically examine recovery of the visual function in amblyopic brains.
Amblyopia is associated with a range of visual impairments beyond acuity, but the majority of studies in mouse models of amblyopia have focused exclusively on OD shifts. Future work should identify other physiological measures and behavioral paradigms to examine widespread visual functions beyond OD, such as contrast sensitivity and stereopsis that can be applied across species.
Some of the deficits associated with amblyopia may result from abnormalities in regions beyond the primary visual cortex (V1). Moreover, signals from higher brain areas may facilitate visual responses and plasticity within V1. Thus, understanding developmental trajectories and critical period mechanisms throughout the visual system may identify new treatments for the recovery from amblyopia in adulthood.
Future work should include the development of better models for amblyopia across animal species and humans. Identifying biochemical correlates of plasticity will allow us to compare developmental trajectories more readily across species. Capitalizing on genetic diversity in mice and humans will provide insights into the individual variability that influences the etiology or recovery from amblyopia.
Acknowledgments
The authors would like to thank Anne Takesian,* who served as the scribe for this discussion section. We would also like to thank all discussion group participants: Mark Bear, Dennis Dacey, Kevin Duffy, Elizabeth Engle, Donald Mitchell, Hirofumi Morishita, Kathryn Murphy, Siegrid Löwel, Anu Sharma, Michael Steinmetz, and Jan Wensveen.
Footnotes
FM Kirby Neurobiology Center, Boston Children’s Hospital, Harvard Medical School, Boston, MA, USA.
References
- Antonini A, Fagiolini M & Stryker MP (1999). Anatomical correlates of functional plasticity in mouse visual cortex. Journal of Neuroscience 19, 4388–4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonini A & Stryker MP (1993). Rapid remodeling of axonal arbors in the visual cortex. Science 260, 1819–1821. [DOI] [PubMed] [Google Scholar]
- Aton SJ, Broussard C, Dumoulin M, Seibt J, Watson A, Coleman T & Frank MG (2013). Visual experience and subsequent sleep induce sequential plastic changes in putative inhibitory and excitatory cortical neurons. Proceedings of the National Academy of Sciences of the United States of America 110, 3101–3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwal JK, Pinkston-Gosse J, Syken J, Stawicki S, Wu Y, Shatz C & Tessier-Lavigne M (2008). PirB is a functional receptor for myelin inhibitors of axonal regeneration. Science 322, 967–970. [DOI] [PubMed] [Google Scholar]
- Baroncelli L, Sale A, Viegi A, Maya Vetencourt JF, De Pasquale R, Baldini S & Maffei L (2010). Experience-dependent reactivation of ocular dominance plasticity in the adult visual cortex. Experimental Neurology 226, 100–109. [DOI] [PubMed] [Google Scholar]
- Baroncelli L, Scali M, Sansevero G, Olimpico F, Manno I, Costa M & Sale A (2016). Experience affects critical period plasticity in the visual cortex through an epigenetic regulation of histone post-translational modifications. Journal of Neuroscience 36, 3430–3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavelier D, Levi DM, Li RW, Dan Y & Hensch TK (2010). Removing brakes on adult brain plasticity: From molecular to behavioral interventions. Journal of Neuroscience 30, 14964–14971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurdeley M, Spatazza J, Lee HH, Sugiyama S, Bernard C, Di Nardo AA, Hensch TK & Prochiantz A (2012). Otx2 binding to perineuronal nets persistently regulates plasticity in the mature visual cortex. Journal of Neuroscience 32, 9429–9437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore C, Garey LJ & Vital-Durand F (1978). The physiological effects of monocular deprivation and their reversal in the monkey’s visual cortex. Journal de Physiologie 283, 223–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochner DN, Sapp RW, Adelson JD, Zhang S, Lee H, Djurisi M, Syken J, Dan Y & Shatz CJ (2014). Blocking PirB up-regulates spines and functional synapses to unlock visual cortical plasticity and facilitate recovery from amblyopia. Science Translational Medicine 6, 258ra140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaccorsi J, Berardi N & Sale A (2014). Treatment of amblyopia in the adult: Insights from a new rodent model of visual perceptual learning. Frontiers in Neural Circuits 8, 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carulli D, Pizzorusso T, Kwok JC, Putignano E, Poli A, Forostyak S, Andrews MR, Deepa SS, Glant TT & Fawcett JW (2010). Animals lacking link protein have attenuated perineuronal nets and persistent plasticity. Brain 133, 2331–2347. [DOI] [PubMed] [Google Scholar]
- Caspi A, Vishne T, Reichenberg A, Weiser M, Dishon A, Lubin G, Shmushkevitz M, Mandel Y, Noy S & Davidson M (2009). Refractive errors and schizophrenia. Schizophrenia Research 107, 238–241. [DOI] [PubMed] [Google Scholar]
- Chang MC, Park JM, Pelkey KA, Grabenstatter HL, Xu D, Linden DJ, Sutula TP, McBain CJ & Worley PF (2010). Narp regulates homeostatic scaling of excitatory synapses on parvalbumin-expressing interneurons. Nature Neuroscience 13, 1090–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman JE, Nahmani M, Gavornik JP, Haslinger R, Heynen AJ, Erisir A & Bear MF (2010). Rapid structural remodeling of synapses parallels experience-dependent functional plasticity in mouse primary visual cortex. Journal of Neuroscience 30, 9670–9682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper LN & Bear MF (2012). The BCM theory of synapse modification at 30: Interaction of theory with experiment. Nature Reviews Neuroscience 13, 798–810. [DOI] [PubMed] [Google Scholar]
- Daw NW (1998). Critical periods and amblyopia. Archives of Ophthalmology 116, 502–505. [DOI] [PubMed] [Google Scholar]
- Daw NW (2013). Visual Development (2nd ed.). New York: Springer. [Google Scholar]
- Daw NW & Wyatt HJ (1976). Kittens reared in a unidirectional environment: Evidence for a critical period. Journal of Physiology 257, 155–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Cristo G, Chattopadhyaya B, Kuhlman SJ, Fu Y, Bélanger MC, Wu CZ, Rutishauser U, Maffei L & Huang ZJ (2007). Activity-dependent PSA expression regulates inhibitory maturation and onset of critical period plasticity. Nature Neuroscience 10, 1569–1577. [DOI] [PubMed] [Google Scholar]
- Dickendesher TL, Baldwin KT, Mironova YA, Koriyama Y, Raiker SJ, Askew KL, Wood A, Geoffroy CG, Zheng B, Liepmann CD, Katagiri Y, Benowitz LI, Geller HM & Giger RJ (2012). NgR1 and NgR3 are receptors for chondroitin sulfate proteoglycans. Nature Neuroscience 15, 703–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djurisic M, Vidal GS, Mann M, Aharon A, Kim T, Ferrao Santos A, Zuo Y, Hubener M & Shatz CJ (2013). PirB regulates a structural substrate for cortical plasticity. Proceedings of the National Academy of Sciences of the United States of America 110, 20771–20776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato F, Rompani SB & Caroni P (2013). Parvalbumin-expressing basket-cell network plasticity induced by experience regulates adult learning. Nature 504, 272–276. [DOI] [PubMed] [Google Scholar]
- Duffy KR, Lingley AJ, Holman KD & Mitchell DE (2016). Susceptibility to monocular deprivation following immersion in darkness either late into or beyond the critical period. Journal of Comparative Neurology 524, 2643–2653. [DOI] [PubMed] [Google Scholar]
- Duffy KR & Livingstone MS (2005). Loss of neurofilament labeling in the primary visual cortex of monocularly deprived monkeys. Cerebral Cortex 15, 1146–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy KR & Mitchell DE (2013). Darkness alters maturation of visual cortex and promotes fast recovery from monocular deprivation. Current Biology 23, 382–386. [DOI] [PubMed] [Google Scholar]
- Duffy KR, Murphy KM, Frosch MP & Livingstone MS (2007). Cytochrome oxidase and neurofilament reactivity in monocularly deprived human primary visual cortex. Cerebral Cortex 17, 1283–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand S, Patrizi A, Quast KB, Hachigian L, Pavlyuk R, Saxena A, Carninci P, Hensch TK & Fagiolini M (2012). NMDA receptor regulation prevents regression of visual cortical function in the absence of Mecp2. Neuron 76, 1078–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton NC, Sheehan HM & Quinlan EM (2016). Optimization of visual training for full recovery from severe amblyopia in adults. Learning & Memory 23, 99–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Shamayleh Y, Kiorpes L, Kohn A & Movshon JA (2010). Visual motion processing by neurons in area MT of macque monkeys with experimental amblyopia. Journal of Neuroscience 30, 12198–12209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagiolini M, Fritschy JM, Löw K, Möhler H, Rudolph U & Hensch TK (2004). Specific GABA-A circuits for visual cortical plasticity. Science 303, 1681–1683. [DOI] [PubMed] [Google Scholar]
- Fagiolini M & Hensch TK (2000). Inhibitory threshold for critical period activation in primary visual cortex. Nature 404, 183–186. [DOI] [PubMed] [Google Scholar]
- Fagiolini M, Katagiri H, Miyamoto H, Mori H, Grant SG, Mishina M & Hensch TK (2003). Separable features of visual cortical plasticity revealed by N-methyl-D-aspartate receptor 2A signaling. Proceedings of the National Academy of Sciences of the United States of America 100, 2854–2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagiolini M, Pizzorusso T, Berardi N, Domenici L & Maffei L (1994). Functional postnatal development of the rat primary visual cortex and the role of visual experience: Dark rearing and monocular lid suture. Vision Research 34, 709–720. [DOI] [PubMed] [Google Scholar]
- Frenkel MY & Bear MF (2004). How monocular deprivation shifts ocular dominance in visual cortex of young mice. Neuron 44, 917–923. [DOI] [PubMed] [Google Scholar]
- Fu Y, Kaneko M, Tang Y, Alvarez-Buylla A & Stryker MP (2015). A cortical disinhibitory circuit for enhancing adult plasticity. Elife 2015, e05558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Tucciarone JM, Espinosa JS, Sheng N, Daracy DP, Nicoll RA, Huang ZK & Stryker MP (2014). A cortical circuit for gain control by behavioral state. Cell 2014, 1139–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervain J, Vines BW, Chen LM, Seo RJ, Hensch TK, Werker JF & Young AH (2013). Valproate reopens critical-period learning of absolute pitch. Frontiers in Systems Neuroscience 7, 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil-Pagés M, Stiles RJ, Parks CA, Neier SC, Radulovic M, Oliveros A, Ferrer A, Reed BK, Wilton KM & Schrum AG (2013). Slow angled-descent forepaw grasping (SLAG) could be incorporated into psy: An innate behavioral task for identification of individual experimental mice possessing functional vision. Behavioral and Brain Functions 9, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel A & Lee HK (2007). Persistence of experience-induced homeostatic synaptic plasticity through adulthood in superficial layers of mouse visual cortex. Journal of Neuroscience 27, 6692–6700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon JA & Stryker MP (1996). Experience-dependent plasticity of binocular responses in the primary visual cortex of the mouse. Journal of Neuroscience 16, 3274–3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greifzu F, Kalogeraki E & Löwel S (2016). Environmental enrichment preserved lifelong ocular dominance plasticity, but did not improve visual abilities. Neurobiology of Aging 41, 130–137. [DOI] [PubMed] [Google Scholar]
- Greifzu J, Pielecka-Fortuna F, Kalogeraki E, Kremplar K, Favaro PD, Schlüter OM & Löwel S (2014). Environmental enrichment extends ocular dominance plasticity into adulthood and protects from stroke-induced impairments of plasticity. Proceedings of the National Academy of Sciences of the United States of America 111, 1150–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Huang S, Chang MC, Worley P, Kirkwood A & Quinlan EM (2013). Obligatory role for the immediate early gene NARP in critical period plasticity. Neuron 79, 335–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Tran T, Murase S, Borrell A, Kirkwood A & Quinlan EM (2016). Neuregulin-dependent regulation of fast-spiking interneuron excitability controls the timing of the critical period. Journal of Neuroscience 36, 10285–10295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guirado R, Perez-Rando M, Sanchez-Matarredona D, Castrén E & Nacher J (2014). Chronic fluoxetine treatment alters the structure, connectivity and plasticity of cortical interneurons. International Journal of Neuropsychopharmacology 17, 1635–1646. [DOI] [PubMed] [Google Scholar]
- Guo Y, Huang S, de Pasquale R, McGehrin K, Lee HK, Zhao K & Kirkwood A (2012). Dark exposure extends the integration window for spike-timing-dependent plasticity. Journal of Neuroscience 32, 15027–15035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanover JL, Huang ZJ, Tonegawa S & Stryker MP (1999). Brain-derived neurotrophic factor overexpression induces precocious critical period in mouse visual cortex. Journal of Neuroscience 19, RC40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harauzov A, Spolidoro M, Dicristo G, Pasquale RD, Cancedda L, Pizzorusso T, Viegi A, Berardi N & Maffei L (2010). Reducing intracortical inhibition in the adult visual cortex promotes ocular dominance plasticity. Journal of Neuroscience 30, 361–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwerth RS, Smith EL, Duncan GC, Crawford ML & von Noorden GK (1986). Multiple sensitive periods in the development of the primate visual system. Science 232, 235–238. [DOI] [PubMed] [Google Scholar]
- He HY, Hodos W & Quinlan EM (2006). Visual deprivation reactivates rapid ocular dominance plasticity in adult visual cortex. Journal of Neuroscience 26, 2951–2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He HY, Ray B, Dennis K & Quinlan EM (2007). Experience-dependent recovery of vision following chronic deprivation amblyopia. Nature Neuroscience 10, 1134–1136. [DOI] [PubMed] [Google Scholar]
- Hensch TK, Fagiolini M, Mataga N, Stryker MP, Baekkeskov S & Kash SF (1998). Local GABA circuit control of experience-dependent plasticity in developing visual cortex. Science 282, 1504–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimel JA, Hermans JM, Sommeijer JP, Neuro-Bsik Mouse Phenomics Consortium & Levelt CN (2008). Genetic control of experience-dependent plasticity in the visual cortex. Genes, Brain and Behavior 7, 915–923. [DOI] [PubMed] [Google Scholar]
- Hess RF & Thompson B (2015). Amblyopia and the binocular approach to its therapy. Vision Research 114, 4–16. [DOI] [PubMed] [Google Scholar]
- Hofer SB, Mrsic-Flogel TD, Bonhoeffer T & Hübener M (2009). Experience leaves a lasting structural trace in cortical circuits. Nature 457, 3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Gu Y, Quinlan EM & Kirkwood A (2010). A refractory period for rejuvenating GABAergic synaptic transmission and ocular dominance plasticity with dark exposure. Journal of Neuroscience 30, 16636–16642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang ZJ, Kirkwood A, Pizzorusso T, Porcialtti V, Morales B, Bear MF & Maffei L (1999). BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell 98, 7965–7980. [DOI] [PubMed] [Google Scholar]
- Huang X, Stodieck SK, Goetze B, Cui L, Wong MH, Wenzel C, Hosand L, Dong Y, Löwel S & Schlüter OM (2015). Progressive maturation of silent synapses governs the duration of a critical period. Proceedings of the National Academy of Sciences of the United States of America 112, E3131–E3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN & LeVay S (1977). Plasticity of ocular dominance columns in monkey striate cortex. Philosophical Transactions of the Royal Society B: Biological Sciences 278, 377–409. [DOI] [PubMed] [Google Scholar]
- Iwai Y, Fagiolini Μ, Obata K & Hensch TK (2003). Rapid critical period induction by tonic inhibition in visual cortex. Journal of Neuroscience 23, 6695–6702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko M, Stellwagen D, Malenka RC & Stryker MP (2008). Tumor necrosis factor-alpha mediates one component of competitive, experience-dependent plasticity in developing visual cortex. Neuron 58, 673–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko M & Stryker M (2014). Sensory experience during locomotion promotes recovery of function in adult visual cortex. eLife 3, e02798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kameyama K, Sohya K, Ebina T, Fukuda A, Yanagawa Y & Tsumoto T (2010). Difference in binocularity and ocular dominance plasticity between GABAergic and excitatory cortical neurons. Journal of Neuroscience 30,1551–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan ES, Cooke SF, Komorowski RW, Chubykin AA, Thomazeau A, Khibnik LA, Gavornik JP & Bear MF (2016). Contrasting roles for parvalbumin expressing inhibitory neurons in two forms of adult visual cortical plasticity. Elife 5, e11450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katagiri H, Fagiolini M & Hensch TK (2007). Optimization of somatic inhibition at critical period onset in mouse visual cortex. Neuron 53, 805–812. [DOI] [PubMed] [Google Scholar]
- Kawato M, Lu ZL, Sagi D, Sasaki Y, Yu C & Watanabe T (2014). Perceptual learning–the past, present, and future. Vision Research 99, 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiorpes L (2015). Visual development in primates: Neural mechanisms and critical periods. Developmental Neurobiology 75, 1080–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiorpes L, Tang C & Movshon JA (2006). Sensitivity to visual motion in amblyopic macaque monkeys. Visual Neuroscience 23, 247–256. [DOI] [PubMed] [Google Scholar]
- Krishnan K, Wang BS, Lu J, Wang L, Maffei A, Cang J & Huang ZJ (2015). MeCP2 regulates the timing of critical period plasticity that shapes functional connectivity in primary visual cortex. Proceedings of the National Academy of Sciences of the United States of America 112, E4782–E4791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlman SJ, Olivas ND, Tring E, Ikrar T, Xu T & Trachtenberg JT (2013). A disinhibitory microcircuit initiates critical-period plasticity in the visual cortex. Nature 56, 908–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WC, Huang H, Feng G, Sanes JR, Brown EN, So PT & Nedivi E (2006). Dynamic remodeling of dendritic arbors in GABAergic interneurons of adult visual cortex. PLoS Biology 4, e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann K & Löwel S (2008). Age-dependent ocular dominance plasticity in adult mice. PLoS One 3, e3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennartsson A, Arner E, Fagiolini M, Saxena A, Andersson R, Takahashi H, Noro Y, Sng J, Sandelin A, Hensch TK & Carninci P (2015). Remodeling of retrotransposon elements during epigenetic induction of adult visual cortical plasticity by HDAC inhibitors. Epigenetics & Chromatin 8, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letzkus JJ, Wolff SB, Meyer EM, Tovote P, Courtin J, Herry C & Lüthi A (2011). A disinhibitory microcircuit for associative fear learning in the auditory cortex. Nature 480,331. [DOI] [PubMed] [Google Scholar]
- LeVay S, Stryker MP & Shatz CJ (1978). Ocular dominance columns and their development in layer IV of the cat’s visual cortex: A quantitative study. Journal of Comparative Neurology 179, 223–244. [DOI] [PubMed] [Google Scholar]
- Levi DM & Li RW (2009). Perceptual learning as a potential treatment for amblyopia: A mini-review. Vision Research 49, 2535–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi DM, Knill DC & Bavelier D (2015). Stereopsis and amblyopia: A mini-review. Vision Research 114, 17–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao DS, Krahe TE, Prusky GT, Medina AE & Ramoa AS (2004). Recovery of cortical binocularity and orientation selectivity after the critical period for ocular dominance plasticity. Journal of Neurophysiology 92, 2113–2121. [DOI] [PubMed] [Google Scholar]
- Masuda Y, Dumoulin SO, Nakadomari S & Wandell BA (2008). V1 projection zone signals in human macular degeneration depend on task, not stimulus. Cerebral Cortex 18, 2483–2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mataga N, Mizaguchi Y & Hensch TK (2004). Experience-dependent pruning of dendritic spines in visual cortex by tissue plasminogen activator. Neuron 44, 1031–1041. [DOI] [PubMed] [Google Scholar]
- Mataga N, Nagai N & Hensch TK (2002). Permissive proteolytic activity for visual cortical plasticity. Proceedings of the National Academy of Sciences of the United States of America 99, 7717–7721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maya Vetencourt JF, Sale A, Viegi A, Baroncelli L, De Pasquale R, O’Leary OF, Castrén E & Maffei L (2008). The antidepressant fluoxetine restores plasticity in the adult visual cortex. Science 18, 385–388. [DOI] [PubMed] [Google Scholar]
- McCurry CL, Shepherd JD, Tropea D, Wang KH, Bear MF & Sur M (2010). Loss of Arc renders the visual cortex impervious to the effects of sensory deprivation or experience. Nature Neuroscience 13, 450–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee AW, Yang Y, Fischer QS, Daw NW & Strittmatter SM (2005). Experience-driven plasticity of visual cortex limited by myelin and Nogo receptor. Science 309, 2222–2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell DE & Duffy KR (2014). The case from animal studies for balanced binocular treatment strategies for human amblyopia. Ophthalmic and Physiological Optics 34, 129–145. [DOI] [PubMed] [Google Scholar]
- Mitchell DE & MacKinnon S (2002). The present and potential impact of research on animal models for clinical treatment of stimulus deprivation amblyopia. Clinical and Experimental Optometry 85, 5–18. [DOI] [PubMed] [Google Scholar]
- Mitchell DE, MacNeill K, Crowder NA, Holman K & Duffy KR (2016). Recovery of visual functions in amblyopic animals following brief exposure to total darkness. Journal of Physiology 594, 149–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa JM, Ibanez-Tallon I, Crabtree GW, Sanchez R, Sali A, Role LW & Heintz N (1999). Lynx1, an endogenous toxin-like modulator of nicotinic acetylcholine receptors in the mammalian CNS. Neuron 23, 105–114. [DOI] [PubMed] [Google Scholar]
- Montey KL, Eaton NC & Quinlan EM (2013). Repetitive visual stimulation enhances recovery from severe amblyopia. Learning & Memory 20, 311–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montey KL & Quinlan EM (2011). Recovery from chronic monocular deprivation following reactivation of thalamocortical plasticity by dark exposure. Nature Communications 2, 317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishita H & Hensch TK (2008). Critical period revisited: Impact on vision. Current Opinion in Neurobiology 18, 101–107. [DOI] [PubMed] [Google Scholar]
- Morishita H, Miwa JM, Heintz N & Hensch TK (2010). Lynx1, a cholinergic brake, limits plasticity in adult visual cortex. Science 330, 1238–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy KM, Roumeliotis G, Williams K, Beston BR & Jones DG (2015). Binocular visual training to promote recovery from monocular deprivation. Vision Research 114, 68–78. [DOI] [PubMed] [Google Scholar]
- Movshon JA & Dürsteler MR (1977). Effects of brief periods of unilateral eye closure on the kitten’s visual system. Journal of Neurophysiology 40, 1255–1265. [DOI] [PubMed] [Google Scholar]
- Nagakura I, Van Wart A, Petravicz J, Tropea D & Sur M (2014). STAT1 regulates the homeostatic component of visual cortical plasticity via an AMPA receptor-mediated mechanism. Journal of Neuroscience 34, 10256–10263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niell CM & Stryker MP (2010). Modulation of visual responses by behavioral state in mouse visual cortex. Neuron 65, 472–A79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary TP, Kutcher MR, Mitchell DE & Duffy KR (2012). Recovery of neurofilament following early monocular deprivation. Frontiers in Systems Neuroscience 6, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson CR & Freeman RD (1975). Progressive changes in kitten striate cortex during monocular vision. Journal of Neurophysiology 38, 26–32. [DOI] [PubMed] [Google Scholar]
- Oray S, Majewska A & Sur M (2004). Dendritic spine dynamics are regulated by monocular deprivation and extracellular matrix degradation. Neuron 44, 1021–1030. [DOI] [PubMed] [Google Scholar]
- Pelkey KA, Barksdale E, Craig MT, Yuan X, Sukumaran M, Vargish GA, Mitchell RM, Wyeth MS, Petralia RS, Chittajallu R, Karlsson RM, Cameron HA, Murata Y, Colonnese MT, Worley PF & McBain CJ (2015). Pentraxins coordinate excitatory synapse maturation and circuit integration of parvalbumin interneurons. Neuron 85, 1257–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penzes P, Buonanno A, Passafaro M, Sala C & Sweet RA (2013). Developmental vulnerability of synapses and circuits associated with neuropsychiatric disorders. Journal of Neurochemistry 126, 165–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer C, Xue M, He M, Huang Z & Scanziani M (2013). Inhibition of inhibition in visual cortex: The logic of connections between molecularly distinct interneurons. Nature Neuroscience 16, 1068–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pi HJ, Hangya B, Kvitsiani D, Sanders JI, Huang ZJ & Kepecs A (2013). Cortical interneurons that specialize in disinhibitory control. Nature 503, 521–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzorusso T, Medini P, Berardi N, Chierzi S, Fawcett JW & Maffei L (2002). Reactivation of ocular dominance plasticity in the adult visual cortex. Science 298, 1248–1251. [DOI] [PubMed] [Google Scholar]
- Pizzorusso T, Medini P, Landi S, Baldini S, Berardi N & Maffei L (2006). Structural and functional recovery from early monocular deprivation in adult rats. Proceedings of the National Academy of Sciences of the United States of America 103, 8517–8522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusky GT & Douglas RM (2003). Developmental plasticity of mouse visual acuity. European Journal of Neuroscience 17, 167–173. [DOI] [PubMed] [Google Scholar]
- Putignano E, Lonetti G, Cancedda L, Ratto G, Costa M, Maffei L & Pizzorusso T (2007). Developmental downregulation of histone posttranslational modifications regulates visual cortical plasticity. Neuron 53, 747–759. [DOI] [PubMed] [Google Scholar]
- Quinlan EM, Philpot BD, Huganir RL & Bear MF (1999). Rapid, experience-dependent expression of synaptic NMDA receptors in visual cortex in vivo. Nature Neuroscience 2, 352–357. [DOI] [PubMed] [Google Scholar]
- Rico B & Marín O (2011). Neuregulin signaling, cortical circuitry development and schizophrenia. Current Opinion in Genetics & Development 21, 262–270. [DOI] [PubMed] [Google Scholar]
- Saiepour MH, Rajendran R, Omrani A, Ma WP, Tao HW, Heimel JA & Levelt CN (2015). Ocular dominance plasticity disrupts binocular inhibition-excitation matching in visual cortex. Current Biology 25, 713–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajo M, Ellis-Davies G & Morishita H (2016). Lynx1 limits dendritic spine turnover in the adult visual cortex. Journal of Neuroscience 36, 9472–9478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sale A, Maya Vetencourt JF, Medini P, Cenni MC, Baroncelli L, De Pasquale R & Maffei L (2007). Environmental enrichment in adulthood promotes amblyopia recovery through a reduction of intracortical inhibition. Nature Neuroscience 10, 679–681. [DOI] [PubMed] [Google Scholar]
- Sato M & Stryker MP (2008). Distinctive features of adult ocular dominance plasticity. Journal of Neuroscience 28, 10278–10286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawtell NB, Frenkel MY, Philpot BD, Nakazawa K, Tonegawa S & Bear MF (2003). NMDA receptor-dependent ocular dominance plasticity in adult visual cortex. Neuron 38, 977–985. [DOI] [PubMed] [Google Scholar]
- Scali M, Baroncelli L, Cenni MC, Sale A & Maffei L (2012). A rich environmental experience reactivates visual cortex plasticity in aged rats. Experimental Gerontology 47, 337–341. [DOI] [PubMed] [Google Scholar]
- Scholl B, Burge J & Priebe NL (2013). Binocular integration and disparity selectivity in mouse primary visual cortex. Journal of Neurophysiology 109, 3013–3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzkopf DS, Vorobyov V, Mitchell DE & Sengpiel F (2007). Brief daily binocular vision prevents monocular deprivation effects in visual cortex. European Journal of Neuroscience 25, 270–280. [DOI] [PubMed] [Google Scholar]
- Sengpiel F (2014). Plasticity of the visual cortex and treatment of amblyopia. Current Biology 24, R936–R940. [DOI] [PubMed] [Google Scholar]
- Shatz CJ & Stryker MP (1978). Ocular dominance in layer IV of the cat’s visual cortex and the effects of monocular deprivation. Journal of Physiology 281, 267–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shooner C, Hallum LE, Kumbhani RD, Ziemba CM, Garcia-Marin V, Kelly JG, Majaj NJ, Movshon JA & Kiorpes L (2015). Population representation of visual information in areas V1 and V2 of amblyopic macaques. Vision Research 114, 56–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silingardi D, Scali M, Belluomini G & Pizzorusso T (2010). Epigenetic treatments of adult rats promote recovery from visual acuity deficits induced by long-term monocular deprivation. European Journal of Neuroscience 31, 2185–2192. [DOI] [PubMed] [Google Scholar]
- Simons K (2005). Amblyopia characterization, treatment, and prophylaxis. Survey of Ophthalmology 50, 123–166. [DOI] [PubMed] [Google Scholar]
- Spatazza J, Lee HH, Di Nardo AA, Tibaldi L, Joliot A, Hensch TK & Prochiantz A (2013). Choroid-plexus-derived Otx2 homeoprotein constrains adult cortical plasticity. Cell Reports 3, 1815–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephany CÉ, Chan LL, Parivash SN, Dorton HM, Piechowicz M, Qiu S & McGee AW (2014). Plasticity of binocularity and visual acuity are differentially limited by nogo receptor. Journal of Neuroscience 34,11631–11640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stodieck SK, Greifzu F, Goetze B, Schmidt KF & Löwel S (2014). Brief dark exposure restored ocular dominance plasticity in aging mice and after a cortical stroke. Experimental Gerontology 60, 1–11. [DOI] [PubMed] [Google Scholar]
- Sugiyama S, Di Nardo AA, Aizawa S, Matsuo I, Volovitch M, Prochiantz A & Hensch TK (2008). Experience-dependent transfer of Otx2 homeoprotein into the visual cortex activates postnatal plasticity. Cell 134, 508–520. [DOI] [PubMed] [Google Scholar]
- Sun Y, Ikrar T, Davis MF, Gong N, Zheng Z, Luo ZD, Lai C, Mei L, Holmes TC, Gandhi SP &Xu X (2016). Neurogulin-1/ErbB4 signaling regulates visual cortical plasticity. Neuron 92, 160–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syken J, Grandpre T, Kanold PO & Shatz CJ (2006). PirB restricts ocular-dominance plasticity in visual cortex. Science 313, 1795–1800. [DOI] [PubMed] [Google Scholar]
- Taha S & Stryker MP (2002). Rapid ocular dominance plasticity requires cortical but not geniculate protein synthesis. Neuron 34, 425–436. [DOI] [PubMed] [Google Scholar]
- Takesian AE & Hensch TK (2013). Balancing plasticity/stability across brain development. Progress in Brain Research 207, 3–34. [DOI] [PubMed] [Google Scholar]
- Ting AK, Chen Y, Wen L, Yin DM, Shen C, Tao Y, Liu X, Xiong WC & Mei L (2011). Neuregulin 1 promotes excitatory synapse development and function in GABAergic interneurons. Journal of Neuroscience 31, 15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoizumi T, Miyamoto H, Yazaki-Sugiyama Y, Atapour N, Hensch TK & Miller KD (2013). A theory of the transition to critical period plasticity: Inhibition selectively suppresses spontaneous activity. Neuron 80, 51–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachtenberg JT & Stryker MP (2001). Rapid anatomical plasticity of horizontal connections in the developing visual cortex. Journal of Neuroscience 21, 3476–3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachtenberg JT, Trepel C & Stryker MP (2000). Rapid extragranular plasticity in the absence of thalamocortical plasticity in the developing primary visual cortex. Science 287, 2029–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tropea D, Majewska AK, Garcia R & Sur M (2010). Structural dynamics of synapses in vivo correlate with functional changes during experience-dependent plasticity in visual cortex. Journal of Neuroscience 30, 11086–11095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsirlin I, Colpa L, Goltz HC & Wong AM (2015). Behavioral training as new treatment for adult amblyopia: A meta-analysis and systemic review. Investigative Ophthalmology & Visual Science 56, 4061–A075. [DOI] [PubMed] [Google Scholar]
- van Versendaal D & Levelt CN (2016). Inhibitory interneurons in visual cortical plasticity. Cellular and Molecular Life Sciences 73, 3677–3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang BS, Sarnaik R & Cang J (2010). Critical period plasticity matches binocular orientation preference in the visual cortex. Neuron 65, 246–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesel TN & Hubel DH (1963). Single-cell responses in striate cortex of kittens deprived of vision in one eye. Journal of Neurophysiology 26, 1003–1017. [DOI] [PubMed] [Google Scholar]
- Wiesel TN & Hubel DH (1970). The period of susceptibility to the physiological effects of unilateral eye closure in kittens. Journal de Physiologie 206, 419–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang EJ, Lin EW & Hensch TK (2012). Critical period for acoustic preference in mice. Proceedings of the National Academy of Sciences of the United States of America 109, 17213–17220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yashiro K, Corlew R & Philpot BD (2005). Visual deprivation modifies both presynaptic glutamate release and the composition of perisynaptic/extrasynaptic NMDA receptors in adult visual cortex. Journal of Neuroscience 25, 11684–11692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazaki-Sugiyama Y, Kang S, Cåteau H, Fukai T & Hensch TK (2009). Bidirectional plasticity in fast-spiking GABA circuits by visual experience. Nature 462, 218–221. [DOI] [PubMed] [Google Scholar]
- Yu H, Majewska AK & Sur M (2011). Rapid experience-dependent plasticity of synapse function and structure in ferret visual cortex in vivo. Proceedings of the National Academy of Sciences of the United States of America 108, 21235–21240. [DOI] [PMC free article] [PubMed] [Google Scholar]