SUMMARY
Simon’s two-stage designs are very popular for phase II clinical trials. A literature review revealed that the inference procedures used with Simon’s designs almost always ignore the actual sampling plan used. Reported P values, point estimates and confidence intervals for the response rate are not usually adjusted for the design’s adaptiveness. In addition, we found that the actual sample size for the second stage is often different from that planned. We present here a method for inferences using both the planned and the actual sample sizes. The conventional and the preferred inference procedures usually yield similar P values and confidence intervals for the response rate. The conventional inference, however, may contradict the result of the corresponding hypothesis testing.
1. INTRODUCTION
Two-stage designs are commonly used in phase II clinical trials and especially in cancer clinical trials. Simon [1] has proposed two criteria, minimax and optimal, for selecting sample sizes and critical values for these two-stage designs. The maximum sample size and the expected sample size under H0 are minimized in the minimax and optimal designs, respectively. Simon’s two-stage designs have been gaining influence; his 1989 paper [1] has been cited more than 700 times. Since it was cited twice in 1991, the number of citations per year has increased steadily, to 50 times in 2001 and over 100 times in each of 2005 and 2006.
There have been a number of extensions proposed for Simon’s designs. These include consideration of toxicity [2, 3, 4, 5], inclusion of more than one treatment [6, 7, 8, 9], addition of the third stage [10], consideration of partial and complete responses [11, 12], and consideration of multiple strata [13]. Banerjee and Tsiatis [14] proposed to extend a Simon’s design by allowing different stage 2 sample sizes for different values of X1. Their more flexible designs have advantages over the original Simon’s designs with respect to the expected sample sizes under the null and alternative hypotheses. Others have considered optimality in 2 stage designs and proposed to improve on Simon’s designs [15, 16, 17].
Due to the adaptive nature of the design, the inference procedures for Simon’s designs are not straightforward. A maximum likelihood estimator of the response rate, number of positive responses / total number of patients, is biased [18, 19]. Confidence interval and P value should not be computed as if the data were obtained in a single stage. There are relatively few papers that discuss the inference procedures used with two-stage designs in phase II clinical trials. Whitehead [18] has studied the bias of the maximum likelihood estimator and has proposed a bias-reduced estimator that was further studied by Chang et al [19]. Jung and Kim [20] have provided a comprehensive study of estimators from a multi-stage design. For general group sequential designs, the inference procedures have been considered by Fairbanks and Madsen [21], Tsiatis, Rosner and Mehta [22], Lin, Wei and DeMets [23], Yi and Yu [24], Fan and DeMets [25], among others.
It could be argued that these two-stage designs are primarily for decision making and that estimation is a secondary objective of a phase II clinical trial. It is preferable, however, to compute a P value, a confidence interval and an estimate of the response rate at the termination of the trial. The latter two are especially useful when the design of a new phase III clinical trial is based on the findings of a phase II trial. As a matter of fact, our literature review revealed that the majority of studies using Simon’s two-stage designs report an estimate of the response rate with a confidence interval.
Our literature review also revealed that the actual sample size of stage 2 is often different from the planned stage 2 sample size. It is not straightforward to conduct a hypothesis testing when the stage 2 sample size is changed in a Simon’s design. We will further extend the inference procedures to handle the cases in which the actual sample size is different from the one planned.
In Section 2, a brief introduction of Simon’s designs will be given. We will apply the inference procedures developed for multi-stage group sequential designs to Simon’s designs in Section 3. Then in Section 4, we will present general inference procedures for Simon’s designs when stage 2 sample size is different from the one planned.
We have developed and made widely available a software program that makes inference from a Simon’s design based on the method discussed in this paper.
2. SIMON’S DESIGNS
In a study with a Simon’s design, the null hypothesis is concerned with a response rate, π. Without loss of generality we assume that a higher value of π is more favorable and write H0 : π ≤ π0. The power of the study is set at some π1 that is greater than π0.
A Simon’s design is usually indexed by four numbers that represent stage 1 sample size (n1), stage 1 critical value (r1), final sample size (nt), and final critical value (rt). In stage 1, a sample of size n1 is taken. Let X1 be the number of successes in stage 1. If X1 ≤ r1, the trial is stopped for futility; otherwise, an additional sample is taken until total of nt observations are obtained. Let X2 be the number of successes in stage 2, and let Xt = X1 + X2. If Xt ≤ rt, futility is concluded; otherwise efficacy is concluded by rejecting H0.
We depart from convention and use R1 ≡ r1 + 1 and Rt ≡ rt + 1 to denote the critical values of a Simon’s design. This new notation is a simpler one in certain extensions that we will consider in later sections. It will also be necessary to consider the stage 2 critical value, and we will use the notation, R2(x1), which is a function of X1 and can be written simply as R2(x1) ≡ Rt − x1 for R1 ≤ X1 < Rt, and 0 for Rt ≤ X1 in a usual Simon’s design.
To present the power function of a Simon’s design, we first introduce the conditional power of stage 2 given the stage 1 result, X1 = x1. It is Pπ[X2 ≥ R2(x1)] for X1 ≥ R1, where X2 ~ Binomial(n2, π). Throughout the paper, the notation Pπ[E] represents the probability of the event E at a specific π. We use the notation A(x1, π) to denote the conditional power, which can be written as:
| (1) |
If the trial is terminated in stage 1 because the results indicated futility (X1 < R1), we let A(x1, π) = 0. We note that the conditional power at π = π0 is the conditional type I error rate.
The power function of a Simon’s design is
| (2) |
A design, (n1, R1, nt, Rt), is usually chosen so that β(π0) ≤ α and β(π1) ≥ 1 − β.
3. INFERENCE PROCEDURES
Inference procedures for multi-stage designs have been discussed by many [21, 22, 23, 24, 25, 26], and we will review them in this section and extend them in the subsequent section.
3.1. P value
Suppose that the number of successes is X1 = x1 ≥ R1 and X2 = x2 for stage 1 and 2, respectively. It is a common practice to compute a P value at the end of the stage 2, incorrectly assuming that the data are collected in a single stage. Let us call this a conventional P value and denote it by pc. We can write
| (3) |
The summand of the right hand side of the above equation include impossible sample paths in which X1 < R1 and X2 = Xt − X1. A preferred P value may be
| (4) |
which does not include these impossible sample paths. Clearly, pc ≥ pp.
The P value is the probability of obtaining the result that is at least as extreme as the observed one under the null hypothesis. In (3) and (4), we are implicitly assuming that the larger value of Xt, regardless of X1 and X2, is more extreme. When X1 < R1 and futility is concluded in stage 1, the P value is Pπ0 [X1 ≥ x1]. Because we use the total number of successes to order the possible outcomes in (4), it is applicable only if n2 is a constant for all X1. In certain extensions of Simon’s design in which stage 2 sample size is not a constant in X1 (e.g., Banerjee and Tsiatis [14]), the P value in (4) is not applicable. We will also show in Section 4 that, when the realized stage 2 sample size is different from the one planned in a simple design with a constant n2, (4) may not be applicable.
3.2. Confidence Interval
A 95% two-sided confidence interval is reported in many of the papers that we have studied. It is not wrong to report a 95% two-sided confidence interval. If, however, it is desirable that a confidence interval and hypothesis testing be consistent, because Simon’s design is used in one-sided hypothesis testing, then a 95% one-sided confidence interval of the form, (πL, 1] for α = .05 is required. The method described below can be used to form a more common two-sided confidence interval of the form, (πL, πU). We will use a 90% two-sided confidence interval to allow consistency with the one-sided hypothesis testing at α = .05. In other words, we can interpret this confidence interval in a usual way: the two statements, “π0 is not contained in a confidence interval” and “H0 is rejected” are equivalent.
We can compute a P value for testing using (4) for any . A 90% two-sided confidence interval that we use is a collection of such that the corresponding P value is within [.05, .95]. This is a simple application of the method based on “stage-wise ordering” of group sequential methodology, and is a methodology that produces an interval [26, 27].
3.3. Point Estimate of the Response Rate
The maximum likelihood estimator of the response rate, π̂ = xt/nt if x1 ≥ R1 or π̂ = x1/n1 if x1 < R1, underestimates the true response rate in Simon’s designs that we are considering. When x1/n1 is larger than π, the trial tends to proceed to stage 2, and the upward bias tends to be corrected. On contrary, when x1/n1 is smaller than π, the trial tends to be terminated without a chance for the downward bias to be corrected.
A bias reduced estimator due to Whitehead [18], denoted here by π̂w, is the solution to Eπ̂w[π̂] = π̂ [19]. The characteristics of this and other estimators have been studied previously [20, 28]. Generally, it is more favorable than the maximum likelihood estimator in terms of the mean square errors.
3.4. Example
We will consider as an example a design (n1 = 10, R1 = 2, nt = 29, Rt = 6) that is the minimax design for π0 = .1, π1 = .3 with α = .05, β = .2. Suppose that we observe X1 = 2 and Xt = 6. This sample path leads to rejection of H0 in stage 2. We can compute for this example, pc = .064 and pp = .047 using (3) and (4). A 90% confidence interval is (.094, .368) with (3) and (.102, .401) with (4). The conventional P value (3) yields a P value that is greater than α and a confidence interval that contains π0 although H0 is rejected. And π̂ = .207, π̂w = .243.
4. EXTENDING OR SHORTENING THE STUDY
Our literature review reveals that the actual sample size is often different from the planned sample size in many studies that use a Simon’s design. Depending on the predicted and actual accrual rates and drop-out rates, the actual sample size may be larger or smaller.
Extending or shortening a study is simple in a single-stage design since it is easy to recalculate a new critical value or the correct P value and make the correct decision regarding H0. With a Simon’s two-stage design, however, extending or shortening a study is not as straightforward. A common practice to conduct a hypothesis testing is to compute the conventional P value that is based on incorrect distributional assumption, and use it to make a decision. Equivalently, the critical value is re-computed as if the new sample size were originally planned in a single stage design. Unlike the simple two-stage designs with no sample size change, where the preferred P value in (4) is always smaller than or equal to the conventional one in (3), it is now possible to make an incorrect decision and inflate the type I error rate. We will discuss in this section how to calculate the new critical value and how to make a new inference based on the actual sample size.
A subtle but critical issue is that the decision to use a different sample size must be made blinded to any part of the data including the stage 1 result. A legitimate situation in which a sample size is different from that planned can occur when the sample size is planned with anticipation of a certain number of uninformative drop-outs, but a different number of patients actually finish the study. On the other hand, it is not permissible to decide at the end of stage 1 (or during the stage 2) to extend the study because there are fewer positive responses than expected, or to shorten the study because there are more positive responses than expected. There may be designs that allow such adaptive changes and still protect type I error rates. In order to make a proper inference, an additional assumption is also required about the sample size that would have been used if a different number of successes were observed in stage 1 [29, 30]. We will only consider noninformative sample size change in this paper.
As a notational convention, we use a prime to indicate that the sample size has changed. For example, is the new sample size for stage 2 and is the number of successes among in stage 2.
4.1. Hypothesis Testing
We have introduced the term, conditional power, in Section 3.1 and have denoted it by A(x1, π), which is the probability of rejecting H0 in stage 2 given the result of stage 1. Rejecting H0 if X2 ≥ R2(x1) and rejecting H0 if cp(x1, x2, n2, π0) ≡ Pπ0 [X2 ≥ x2 | X1 = x1] ≤ A(x1, π0) are equivalent. We call cp(x1, x2, n2, π0) the conditional P value, which is the P value of stage 2 given the result of stage 1. The conditional P value is compared to the conditional type I error rate in decision making at termination of stage 2.
The conditional P value can be computed regardless of the sample size of the actual stage 2, and the decision to reject H0 can be made by comparing the conditional P value and conditional type I error rate evaluated at the observed x1. The same conclusion may be based on the new critical value, , which is defined as the largest R, such that
| (5) |
where . With the new critical value, , we define . Because we set the new critical value so that the new conditional type I error rate is always equal to or smaller than the original conditional type I error rate, the overall unconditional type I error rate is controlled.
An alternative method of hypothesis testing may be to re-compute the critical value as if the new sample size, , were planned originally. This involves a simple numerical search of the critical value that produces the type I error rate less than α given n1, R1 and . This method only attempts to control the unconditional type I error rate even though the stage 1 has already been terminated. If we apply the same reasoning and only attempt to control the unconditional type I error rate, we could have as much conditional type I error rate at the observed X1 = x1 as α/Pπ0 [X1 = x1], and 0 elsewhere. Then the result of computation of (2) at π = π0 with A(x1, π0) replaced by A′(x1, π0) would be at most α. The type I error rate, however, is not actually controlled because we would have used different critical values if X1 were different. In other words, this method fails to protect the conditional type I error rate, which is relevant and needs to be protected after stage 1. Thus we prefer the first method of re-computing the critical value (5) because it guarantees that the conditional type I error is protected given the stage 1 result.
When the new critical value, , is obtained from (5), the total number of positive responses, , necessary to reject H0 may be different for different values of X1 as the following example demonstrates.
We will consider a new design, (n1 = 19, R1 = 7, nt = 39, Rt = 17), as an example. This is the minimax design for π0 = .3, π1 = .5 with α = .05 and β = .2. Suppose that we observe X1 = 7 in stage 1. Then H0 would be rejected if the stage 2 P value is smaller than the conditional type I error rate, A(7, .3) = Pπ0 [X2 ≥ 10] = .0480. Further suppose that the stage 2 sample size is increased from the planned 20 to 23. If we observe 11 successes in these 23 stage 2 observations, the conditional P value is , and the null hypothesis should not be rejected. The critical value at X1 = 7 with n2 = 23 is from (5).
In a different scenario, suppose that we observe X1 = 10 in stage 1; the conditional type I error rate is A(10, .3) = .3920. With the new sample size, , the critical value is from (5). Thus in the first scenario, H0 is rejected with 19 or more positive responses, but in the second scenario, the number of necessary positive responses is 18.
In the second scenario above, the conditional type I error rate is .3920 at X1 = 10, and H0 would be rejected with a stage 2 P value that is less than or equal to .3920. When compared to the usual unconditional type I error rate, α, the conditional type I error rate sometimes seems very high. It may be unintuitive that H0 is rejected with a such a high (conditional) type I error rate. The unconditional type I error rate can be viewed in (2) as a weighted average of the conditional type I error rate. Thus, when Pπ0 [X1 = x1] is very small for a particular x1, the conditional type I error rate at this x1 may be very high, and the unconditional type I error rate is still protected.
4.2. Inference When Sample Size is Changed
As shown in 4.1, even when the stage 2 sample size is changed, hypothesis testing can be conducted with the conditional P value and conditional type I error rate. These conditional quantities may not be intuitive because they can not be compared directly to the usual unconditional type I error rate, α. Together with the original motivation for computing unconditional P value, confidence interval and an estimate of π, this compels us to make unconditional inference when the stage 2 sample size is changed.
The formula for P value in (4) needs to be extended because this formula is only valid when the stage 2 sample size is not changed. The unconditional P value may be written as follows:
| (6) |
where cp(x1, x2, n2, π0) is the conditional P value for testing H0 : π ≤ π0. It is only observed at one (x1, x2), but it needs to be extended to the entire range of X1 ∈ [R1, n1] to evaluate (6). When stage 2 sample size is constant and not changed (Section 3.1), this extension is based on Xt. When, however, stage 2 sample size is changed, we cannot extend the conditional P value based on Xt. It is demonstrated in Section 4.1 that the same value of Xt may or may not lead to rejection of H0 depending on the sample paths. Moreover, as per the work of Banerjee and Tsiatis [14], if the planned stage 2 sample size is not constant for different values of X1 extending the conditional P value based on Xt would not make sense.
We propose the following approach for extending cp(x1, x2, n2, π) to the potential values of X1. We find a conditional power function, A(x1, π*), for some π* that goes through the observed conditional P value. It requires solving numerically for π* such that A(x1, π*) = cp(x1, x2, n2, π0). This A(x1, π*) function can be extended to the potential values of x1 using (1) with the original n2 and the original R2. We propose that the sample paths that lead to the same π* have the same magnitude of evidence against H0. In other words, it is possible to order different sample paths with different x1 and the realized sample size for stage 2 by comparing π*. The smaller π*, the more extreme the evidence against H0. This ordering is coherent with the hypothesis testing procedure described in 4.1; the P value based on this ordering is smaller than the type I error rate if and only if the null hypothesis is rejected. To compute the P value, we replace cp(x1, x2, n2, π0) in (6) by A(x1, π*) so that
| (7) |
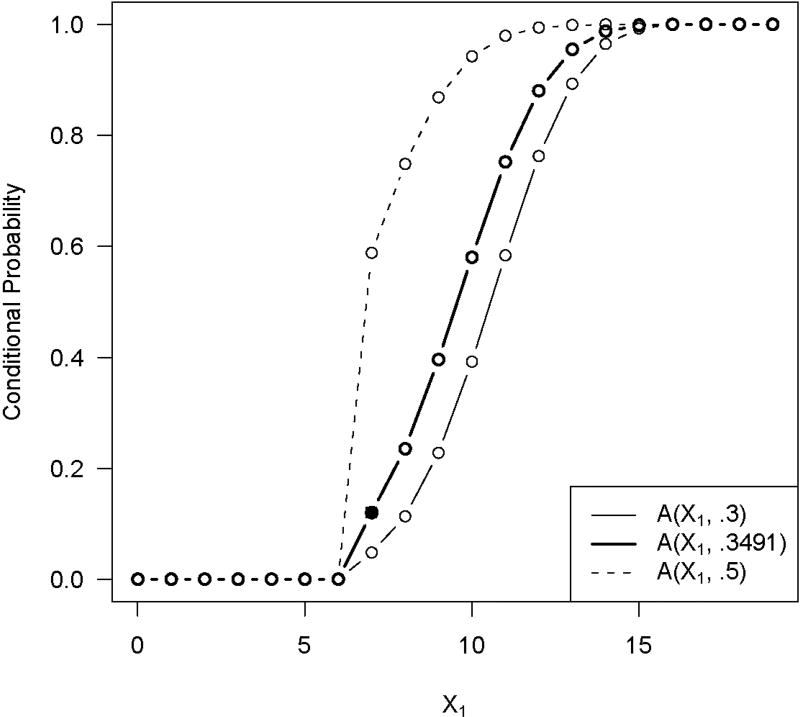
Suppose that we observe in the same example that X1 = 7 and . Then the conditional P value is .1201, and H0 is not rejected because the conditional type I error rate at X1 = 7 is .0480. We compute to find that π* = .3491. We can then extend the conditional power to the different potential values of X1. This is represented by the bold line, A(x1, .3491), in Figure 1 which also shows the conditional type I error rate, A(x1, .3), and the conditional power at the original alternative, A(x1, .5). Finally, we find the P value using (7) to be pp = .0828.
Figure 1.
Extension of the observed conditional P value to other x1 values based on π*. The solid line is the conditional type I error rate, and the dotted line is the conditional power under the alternative. The conditional P value is indicated by a solid circle. The bold line is the conditional power function that goes through this point.
A confidence interval and a reasonable point estimate of π can be obtained by “inverting the hypothesis testing.” A 90% confidence interval is a collection of such that would be rejected by the sample path. We can use (7) with to compute a P value for testing . We note that different π* are used for different values of . And the value of that makes the P value = .5 can be used as a heuristically reasonable estimate of π. The properties of this estimator in contrast with a simple estimator, , would need further exploration.
For the current example, X1 = 7 (n1 = 19) and , a 90% confidence interval for π is (.282, .546). And the value of which gives the P value = .5 in this example is .405.
5. DISCUSSION
The simplicity of Simon’s designs may account for their popularity. Our literature review revealed that, frequently, the inference procedures used with Simon’s designs are often not corrected for these designs’ adaptive nature.
Also, when the sample size is changed, the inference procedures become more complicated. In this paper, we have shown how to make a preferred inference taking into account the planned and actual sample sizes when a Simon’s design is used. When the actual sample size is the same as the planned sample size, the inference procedure is rather simple. When, however, the actual sample size is different from that planned, we need to take into consideration both the realized and the planned sample sizes when computing the P value and confidence interval of the response rate.
The concept of “conditional power” is well studied in the context of adaptive phase III clinical trials, and it is directly applicable in phase II methodologies that we have considered in this paper. When the sample size is changed, the critical value needs to be updated so that the conditional type I error rate is not inflated. As shown in this paper, more obvious methods of updating the design may not control type I error rate.
Finally, we have developed and made widely available a web-based program to compute a P value and a point estimate and a confidence interval for the response rate from data obtained from a Simon’s design.
Acknowledgments
The authors thank the anonymous reviewers for their thoughtful and through review and comments.
References
- 1.Simon R. Optimal two-stage designs for phase II clinical trials. Controlled Clinical Trials. 1989;10(1):1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 2.Bryant J, Day R. Incorporating toxicity considerations into the design of two-stage phase II clinical trials. Biometrics. 1995;51(4):1372–1383. [PubMed] [Google Scholar]
- 3.Conaway MR, Petroni GR. Design for phase II trials allowing for a trade-off between response and toxicity. Biometrics. 1996;52(4):1375–1386. [PubMed] [Google Scholar]
- 4.Thall PF, Cheng SC. Optimal two-stage designs for clinical trials based on safety and efficacy. Statistics in Medicine. 2001;20(7):1023–1032. doi: 10.1002/sim.717. [DOI] [PubMed] [Google Scholar]
- 5.Panageas KS, Smith A, Gönen M, Chapman PB. An optimal two-stage phase II design utilizing complete and partial response information separately. Controlled Clinical Trials. 2002;23(4):367–37. doi: 10.1016/s0197-2456(02)00217-9. [DOI] [PubMed] [Google Scholar]
- 6.Thall PF, Sung HG. Some extensions and applications of a Bayesian strategy for monitoring multiple outcomes in clinical trials. Statistics in Medicine. 1998;17(14):1563–1580. doi: 10.1002/(sici)1097-0258(19980730)17:14<1563::aid-sim873>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 7.Yao TJ, Venkatraman ES. Optimal two-stage design for a series of pilot trials of new agents. Biometrics. 1998;54(3):1183–1189. [PubMed] [Google Scholar]
- 8.Stallard N, Thall PF, Whitehead J. Decision theoretic designs for phase II clinical trials with multiple outcomes. Biometrics. 1999;55(3):971–977. doi: 10.1111/j.0006-341x.1999.00971.x. [DOI] [PubMed] [Google Scholar]
- 9.Steinberg SM, Venzon DJ. Early selection in a randomized phase II clinical trial. Statistics in Medicine. 2002;21(12):1711–1726. doi: 10.1002/sim.1150. [DOI] [PubMed] [Google Scholar]
- 10.Chen TT. Optimal three-stage designs for phase II cancer clinical trials. Statistics in Medicine. 1997;16(23):2701–2711. doi: 10.1002/(sici)1097-0258(19971215)16:23<2701::aid-sim704>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 11.Lin SP, Chen TT. Optimal two-stage designs for phase II clinical trials with differentiation of complete and partial responses. Communications in Statistics. 2000;29:923–940. [Google Scholar]
- 12.Lu Y, Jin H, Lamborn KR. A design of phase II cancer trials using total and complete response endpoints. Statistics in Medicine. 2005;24(20):3155–3170. doi: 10.1002/sim.2188. [DOI] [PubMed] [Google Scholar]
- 13.London WB, Chang MN. One- and two-stage designs for stratified phase II clinical trials. Statistics in Medicine. 2005;24(17):2597–2611. doi: 10.1002/sim.2139. [DOI] [PubMed] [Google Scholar]
- 14.Banerjee A, Tsiatis AA. Adaptive two-stage designs in phase II clinical trials. Statistics in Medicine. 25(19):3382–3395. doi: 10.1002/sim.2501. [DOI] [PubMed] [Google Scholar]
- 15.Chen TT, Ng TH. Optimal flexible designs in phase II clinical trials. Statistics in Medicine. 1998;17(20):2301–2312. doi: 10.1002/(sici)1097-0258(19981030)17:20<2301::aid-sim927>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 16.Shuster J. Optimal two-stage designs for single arm phase II cancer trials. Journal of Biopharmaceutical Statistics. 2002;12(1):39–51. doi: 10.1081/bip-120005739. [DOI] [PubMed] [Google Scholar]
- 17.Jung SH, Lee T, Kim KM, George SL. Admissible two-stage designs for phase II cancer clinical trials. Statistics in Medicine. 23(4):561–569. doi: 10.1002/sim.1600. [DOI] [PubMed] [Google Scholar]
- 18.Whitehead J. On the bias of maximum-likelihood-estimation following a sequential test. Biometrika. 1986;73(3):573–581. [Google Scholar]
- 19.Chang MN, Wieand HS, Chang VT. The bias of the sample proportion following a group sequential phase II clinical trial. Statistics in Medicine. 1989;8(5):563–570. doi: 10.1002/sim.4780080505. [DOI] [PubMed] [Google Scholar]
- 20.Jung SH, Kim KM. On the estimation of the binomial probability in multistage clinical trials. Statistics in Medicine. 2004;23(6):881–896. doi: 10.1002/sim.1653. [DOI] [PubMed] [Google Scholar]
- 21.Fairbanks K, Madsen R. P values for tests using a repeated significance test design. Biometrika. 1982;69(1):69–74. [Google Scholar]
- 22.Tsiatis AA, Rosner GL, Mehta CR. Exact confidence intervals following a group sequential test. Biometrics. 1984;40(3):797–803. [PubMed] [Google Scholar]
- 23.Lin DY, Wei LJ, DeMets DL. Exact statistical-inference for group sequential trials. Biometrics. 1991;47(4):1399–1408. [PubMed] [Google Scholar]
- 24.Yi C, Yu S. Estimation of a parameter and its exact confidence interval following sequential sample size reestimation trials. Biometrics. 2004;60(4):910–918. doi: 10.1111/j.0006-341X.2004.00246.x. [DOI] [PubMed] [Google Scholar]
- 25.Fan XY, DeMets DL. Conditional and unconditional confidence intervals following a group sequential test. Journal of Biopharmaceutical Statistics. 2006;16(1):107–122. doi: 10.1080/10543400500406595. [DOI] [PubMed] [Google Scholar]
- 26.Jennison C, Turnbull BW. Group Sequential Methods With Applications to Clinical Trials. London: Chapman & Hall; 1999. [Google Scholar]
- 27.Bather JA. Stopping rules and ordered families of distributions. Sequential Analysis. 1988;7:111–126. [Google Scholar]
- 28.Guo HY, Liu A. A simple and efficient bias-reduced estimator of response probability following a group sequential phase II trial. Journal of Biopharmaceutical Statistics. 2005;15(5):773–781. doi: 10.1081/BIP-200067771. [DOI] [PubMed] [Google Scholar]
- 29.Liu Q, Chi GY. On sample size and inference for two-stage adaptive designs. Biometrics. 2001;57(1):172–177. doi: 10.1111/j.0006-341x.2001.00172.x. [DOI] [PubMed] [Google Scholar]
- 30.Koyama T. Flexible designing of two-stage adaptive procedures in phase III clinical trials. to appear in. Contemporary Clinical Trials. doi: 10.1016/j.cct.2007.01.007. [DOI] [PubMed] [Google Scholar]