Abstract
Introduction
Parasitic diseases that pose a threat to human life include leishmaniasis – caused by protozoan parasite Leishmania species. Existing drugs have limitations due to deleterious side effects like teratogenicity, high cost and drug resistance. These call for the need to have an insight into the therapeutic aspects of disease.
Areas covered
We have identified different drug targets, via. molecular, imuunological, metabolic as well as by system biology approaches. We bring these promising drug targets into light so that they can be explored to their maximum. In an effort to bridge the gaps between existing knowledge and future prospects of drug discovery, we have compiled interesting studies on drug targets thereby paving the way for establishment of better therapeutic aspects.
Expert opinion
Advancements in technology sheds light on many unexplored pathways. Further probing of well established pathways, led to the discovery of new drug targets.This review is a comprehensive report on current and emerging drug targets, with emphasis on several metabolic targets, organellar biochemistry, salvage pathways, epigenetics, kinome, etc. Identification of new targets can contribute significantly towards strengthening the pipeline for disease elimination.
Keywords: leishmaniasis, drug discovery, drug targets, mitochondria, metabolic pathways
Introduction
Leishmaniasis is a group of manifestations ranging from cutaneous to most severe visceral forms, caused by protozoan parasite Leishmania species. Visceral form of disease, caused by Leishmania donovani, characterised by prolonged fever (>2 weeks), weight loss, anemia, and splenomegaly. India, Bangladesh, Sudan, South Sudan, Brazil and Ethiopia together contribute for more than 90% of the global disease burden with 0.3 million new VL cases reported each year. Leishmania parasites cycle between two hosts- human and sandfly, in two distinct life stages, as flagellar promastigote or amastigote form. The promastigotes from sandfly are injected into the human upon blood feeding where they are phagocytosed by circulating monocytes, dendritic cells and/or neutrophils. Once inside the phagosome these parasites undergo differentiation to non-flagellar amastigote. These amastigotes divide several times until bursting of host cells to infect another cell.
There was a collaborative association of the governments of India, Bangladesh and Nepal for elimination of this disease in 2005[1, 2]. This memorandum was later renewed in 2014, upon consideration of several underestimated parameters while reporting new cases. With the main focus on early case detection, diagnosis, treatment, as well as vector management. VL elimination program has been extended to 2020 with the establishment of new taskforce to facilitate attainment of the set goal [2]. There are several challenges to make the target a realistic goal including the drug resistance, toxicity issues related with current therapeutic options, asymptomatic carriers, inadequate knowledge on vector biome and non-availability of vaccine. Therefore, coordinated monitoring at different levels of implementation, proper case management, social awareness, active case detection and strong partnership among stakeholders could serve to make the way easy.
Recent years have experienced changes in terms of flow of funds to support drug discovery. The complex life cycle of the parasite also includes several checkpoints that might be exploited towards drug development. Additionally, technological advances in the field of clinical research and the availability of complete genome sequence of Leishmania [4], have further strengthened the way towards the drug discovery. This review is an effort towards comprehensive understanding of current chemotherapeutic options, its limitations and recent developments in drug designing and discovery. An insight into the proteins from different biological pathways attribute towards the identification of potential drug targets.
Current therapeutic option
The current treatment option for leishmaniasis relies solely on chemotherapy, indeed, the lack of effective and affordable drugs has led the attention of the scientific community towards drug research and development of therapeutic options. The WHO approved treatment regimen for antimonial was a 30-day treatment that costed between 120USD to 150USD with cost effective disease intervention strategy [5-7]. However, later the long hospitalization period, cardiotoxicity [8], cirrhosis, pancreatic toxicity [9] and emergence of high proportions of drug resistant cases [10-12] led to the use of pentamidine in early 1980s as a second line therapy for refractory cases but it remained unaffordable for most patients. The high costs and toxicity issues raised public health concerns for safe and cost effective option [13], leading towards the emergence of amphotericin B and its lipid formulation as the second line therapy. However, the failure of antimonials and pentamidine along with the emergence of drug resistancen made amphotericin B as the first line drug in Bihar in 1990s.
Indeed, limited number of registered drugs for leishmaniasis with the high costs of treatment, toxicity and drug resistance remain significant challenges for health authorities. This led to the concept of drug repurposing, where the clinically approved drugs used for treatment of other diseases can be used for leishmaniasis. Repurposed drugs included the conventional drugs- amphotericin B, paromomycin, and miltefosine. Amphotericin B deoxycholate (AmBD) has been used as antifungal agent later used for treatment of visceral leishmaniasis (VL) in India [14, 15]. The liposomal formulation (AmBisome; Gilead Sciences) of the drug has been efficacious for several fungal infections and beneficial in patients with renal impairment as well as neutropenia [16], later in 1997 it was approved for the treatment of leishmaniasis [17].
Miltefosine, only approved oral drug for leishmaniasis [18] was originally discovered for its anti-cancer properties [19, 20]. However, its use was limited due to the high cost, teratogenic potential and differential drug susceptibility in different clinical isolates [21]. Paromomycin, a broad spectrum aminoglycoside antibiotic, has been used for treatment of bacterial infections, also found effective against protozoal infections as giardiasis, amoebiasis [22] and later against leishmaniasis in 1960s [23-25] It is approved in India for the treatment of VL. However, being aminoglycoside, it poses the risk of development of drug resistance, if used as monotherapy. Drug repurposing has also provided us with delamanid, approved anti-tubercular drug, with good efficacy in experimental leishmaniasis [26-28]. Multi-centre phase III trial suggested for use of paromomycin as registered drug for VL in India [29], but its efficacy was poor in Sudanese population where parasite clearance was below 50% [29]. Sitamaquine was another orally administrable drug with good efficacy in Indian[30] and Kenyan[31] population but significant nephrotoxicity, associated with the drug, abandoned its use. The growing issue of drug resistance called upon the use of combination therapy (miltefosine, antimonials, AmB and parmomycin in different combinations) which has been known to act synergistically and efficacious at lower doses.
Inspite of the large population suffering from disease, the funds for the research and development of treatment options has been limited. One or the other limitations associated with current chemotherapeutic options calls for an urgent need for development of newer therapeutic options. Therefore, significant research in the field of therapeutic development has led towards the study of several target molecules for disease intervention.
Novel therapeutic options and drug discovery
Despite the remarkable researches for development of drug targets, chemotherapy remains to be the mainstay of successful treatment. As leishmaniasis affects poorest of poor with meagre returns on investments, pharmaceutical industry has low interest in development of new antilieshmanial drugs. In 2000, only 0.1% contribution in health research was reported for malaria, leishmaniasis, trypanosomiasis and tuberculosis, while the estimated global burden for these diseases was 5% [32]. A sense of urgency for disease management led the policymakers to frame objective for assessment of new options. The drug discovery process from identification of active compound to its establishment as clinical candidate has been an arduous task; however, it remains to be the main goal of VL elimination programme. This section of the review aims to look into the researches in determining the novel candidates as drug targets.
Ongoing developments:Phytoproducts/Compounds as therapeutic agents
Phytotherapy is the study of the use of extracts from natural origin as medicines or health promoting agents. There has been growing interest in utilization of medicinal plants for treatment of different diseases [33]. Medicinal plants have further been a choice amongst general masses as a safe and inexpensive option for different ailments.
Historically, use of medicines for treating different diseases began with the use of herbs. During ancient times, in Mediterranean civilization, healing was associated with the use of herbs. Later advances in chemical sciences provided a platform for isolation and extraction of components of plant origin. Phytomedicines are the medicinal products derived from the plant extracts. These phytomedicines usually have complex chemical composition attributed to the presence of chemical groups including-alkaloids, phenylpropanoid, flavonoids, terepenoids and sterols [34-36]. Phytotherapy, these days is flourishing in search for low cost anti-leishmanial agents with fewer side effects and chemical diversity of plant extracts make them pharmacologically relevant for use as drug. Many research communities have discovered anti-parasitic drugs, however, leishmaniasis has received less attention. Therefore, search of novel therapeutic options remains to be of high priority for treating this neglected tropical disease. In last few decades, many new evidences for the use of medicinal plants for treatment of leishmaniasis have been reported. The noteworthy researches in this field remain to be the use of Kalanchoe pinnata, with triterpenes, sterols and flavonoids as the major constituents [37]; table 1 summarizes the major secondary metabolites/compounds with potent antileishmanial activity. The leishmanicidal activity was reported [38] to be similar to the meglumine antimoniate upon oral administration without any toxic side effects. The anti-parasitic activity has been attributed to macrophage mediated elevated levels of nitric oxide [39]. Studies from human CL model have reported significant reduction in lesion size without any alteration in serum transaminases, urea, alkaline phosphatase[40].
Table 1.
Anti-leishmanial activities of some plant products/compounds.
| Plant products/ compounds |
Standard Drug | Plant | Species | References |
|---|---|---|---|---|
| Oxylipin (Polyacetylene) Flavonoids | Oxylipin (3S)-16, 17-didehydrofalcarinol | Tridax procumbens | L.mexicana | [304] |
| Flavonoids | Luteolin | Vitex negundo | L.donovani | [305] |
| Quercetin | Fagopyrum esculentum | L.donovani | [306] | |
| Quercetin and its derivatives | Kalanchoe pinnata | L.amazonensis | [307] | |
| Quercetin | Kalanchoe pinnata | L. chagasi | [308] | |
| Ethanolic extract | Piper betle L. | L.donovani | [309] | |
| Methanolic extract | Piper betle L. | L.donovani | [310] | |
| 2′′-acetylpetiolaroside | Delphinium staphisagria | L. infantum and L.braziliensis | [311] | |
| Guaianolide | Tanacetum parthenium (L.) Schultz Bip |
L. amazonensis | [312] | |
| Quercetin3-O-methyl ether | Strychnos pseudoquina | L. amazonensis | [313] | |
| Plant extract | Bidens pilosa L. and Punica granatum L | L. amazonensis | [314] | |
| Alkaloids | N-Methyltetrahydroberberinium iodide | Enantia chlorantha | L.donovani | [315] |
| Ancistroealaine A | Ancistrocladus ealaensis | L.donovani | [316] | |
| Quinoline alkaloid | Galipea longiflora | L.amazonensis | [317] | |
| 2-n-Propylquinoline | L.amazonensis | [318] | ||
| Julocrotine, a glutarimide alkaloid | Croton pullei var. Glabrio | L. amazonensis | [319] | |
| Methanol extract | Nuphar lutea | L.major | [320] | |
| Quinolinic alkaloid | Galipea longiflora | L.amazonensis | [321] | |
| Ethanolic extract | Senna Spectabilis | L.major | [322] | |
| Bisbenzylisoquinoline | Cissampelos sympodialis Eichl. | L. chagasi | [323] | |
| Furoquinoline alkaloids | Helietta apiculata | L.amazonensis | [324] | |
| Securinega alkaloids | Margaritaria nobilis | L.amazonensis | [325] | |
| Dicentrinone | Duguetia furfuracea | [326] | ||
| Terepenoids | Triterepenoid extract | Trichosanthes dioica | L.donovani | [327] |
| Sesquiterepene lactone-rich dicholomethane fraction | Tanacetum parthenium | L.amazonensis | [328] | |
| Oleanolic- and ursolic-containing titerpenic fraction | Baccharis uncinella | L.amazonensis and L.braziliensis | [329] | |
| Taxodione | Salvia deserta | L.donovani | [330] | |
| Artemisinin | Artemisia annua | L.major | [331] | |
| Abietane diterpenoids | Taxodium distichum | L.donovani and L.amazonensis | [332] | |
| trans-dehydrocrotonin | Croton cajucara Benth. | L.amazonensis | [333] | |
| Chalcones | Licochalcone A | Chinese liquorice | L.major | [334] |
| L. donovani | [335] | |||
| 20 naturally occurring chalcones | Piper aduncum | L. donovani | [336] | |
| Adunchalcone | Piper aduncum L. | L.amazonensis, L.braziliensis and L.chagasi | [337] | |
| Dihydrochalcones | Piper elongatum | L.braziliensis | [338] | |
| Quinones | Plumbagin | Pera benensis | L.amazonensis | [339, 340] |
| 3,3_-Biplumbagin | ||||
| 1-Acetylbenzoisochromanquinone | Cephaelis camponutans | L.donovani | [341] | |
| N-Methylliriodendronine | Stephania dinklagei | L.donovani | [342] | |
| 2-methyl-5 -(3′-methyl-but-2′-enyloxy)-[1,4]naphthoquinone (1) | Plumbago zeylanica | L.donovani | [343] | |
| Methanolic extract | Nigella sativa L | L.tropica and L.infantum | [344] | |
| Saponin | Hederagenin | Ivy Hedera helix | L.tropica and L.infantum | [345] |
| α-Hederin | ||||
| β-Hederin Hederacolchiside A1 |
Hedera colchica | L.mexicana | [346] | |
| Maesasaponin V.3 and VI.2 |
Maesa balansae Maesa lanceolata |
L.infantum | [347] | |
| Maesabalides III Maesabalides IV |
Maesa balansae | L.infantum | [348] | |
| Lignan | Diphyllin | Haplophyllum bucharicum | L.infantum | [349] |
| Neolignan 2,3-dihydrobenzofuran | Propolis | L.amazonensis | [350] | |
| 4-Aminoquinoline | AMQ-j | L.amazonensis | [351] | |
| [352] | ||||
| N-cinnamoylated amioquinoline | L.infantum | |||
| [353] | ||||
| 7-cholro 4- quinolinylhydraz one | L.amazonensis | |||
| 8-hydroxyquinoline | [354] | |||
| 1,2,3-triazole salts (Bioisosteres) |
L.amazonensi s,L.braziliensi s,L.infantum |
[355] | ||
| L.amazonensis | ||||
| N-benzyl- 1-Hbenzimidazole | L.mexicana, L.donovani, L.braziliensis | [356] |
Naphthoquinone are another class of secondary metabolite from plant with potential anti-leishmanial activity. Plumbagin, naphthoquinone has been reported to inhibit trypanothione reductase from L.donovani and induces mitochondria mediated cell death [41]. Aloe vera leaf exudate has also been established to have potential antileishmanial activity against different Leishmania spp. by triggering programmed cell death [42]. The list of antileishmanial compounds does not end here; there are plethoras of plant derived products/chemical compounds which have shown potential antileishmanial activity that remains beyond the scope of current article.
Nanotechnology based therapeutic options
In recent years nanotechnology has revolutionized the field of drug designing and development. It has been a promising tool in parasitic diseases where the parasite recrudescence against conventional chemotherapeutic options. Nanotechnology has provided a platform for drug development in two ways: design of drug delivery systems and nano-formulation of drugs which are easy targets for phagocytosis by macrophages therefore, causing targeted delivery of drug. Nanotechnology has managed to invent nanoparticles for drug delivery as carriers, liposomal formulation of amphotericin B reduces its toxicity profile [43] Their biodegradability and non-immunogenic properties make them suitable candidate for research and therapeutic applications. Initially, nanotechnology approach for drug delivery used nanodisks impregnated with amphotericin B, polymeric nanoparticles loaded with pentamidine etc. Gold nanoparticles conjugated with quercetin was found to effective in sodium stibogluconate and paramomycin resistant parasites [44]. Nanotechnology has further improved the conventional leishmanization approach utilizing the liposome-protamine-DNA nanoparticle with immunostimulatory CpG for inducing TH1 type immune response in BALB/c during L.major infection [45]. On the other hand, the conventional autoclaved parasite formulation of vaccine has been modified with the nano-formulation, where autoclaved L.major with PLGA and CpG showed highest IgG2a/IgG1 ratio and IFN-γ production induced strong immune responses against parasite in BALB/c mice [46]. Also, there are several micro- and nano-immunostimulatory adjuvants which have been used to enhance vaccine delivery and served as candidate leishmaniasis vaccine [47]. In this context, plasmid DNA encoding KMP-11 with poly(lactic-co-glycolic acid)(PLGA) nanoparticle elicited strong cellular and innate immune responses in the form of high levels of IFN- γ and TNF-α and significantly decreased parasite loads at infection site therefore, encouraging nano-based systems for vaccine development in mice model enhancing innate immune responses [48, 49]. Additionally, chitosan nanoparticles in combination with superoxide dismutase increased TH1 immune response therefore, providing a great option for development of single dose nanovaccine against leishmania infection [50]. One of the remarkable breakthroughs has been the high efficacy of nanoformulation of amphotericin-B with good safety profile and low production cost establishing it as a better alternative to conventional amphotericin B [51]. Recently, amphotericin B formulation with engineered poly (propylene-imine) dendrimeric nanoconjugate has been reported to have higher parasiticidal activity with reduced toxicity for macrophages as reported from in vitro and in vivo assays [52]. Nanoliposomal formulation of 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP) with soluble leishmania antigen (SLA) has been documented to enhance TH1 type of immune response and induces protection against L.major infection. Similarly, engineered DOTAP has been reported as conjugated nanoparticle with amastigote class I nuclease, second generation vaccine candidate improved the TH1 response and therefore, serving as promising candidate for vaccine development against CL [53]. Thus, nanotechnology has illuminated the way towards vaccine and drug development by improving its therapeutic proficiency.
Ongoing developments and emerging targets
Metabolic pathways as therapeutic target
Current chemotherapeutic drugs are known to target various metabolic pathways. Pentavalent antimonials interfere with DNA replication, fatty acid oxidation, ADP phosphorylation and glycolysis. Amphotericin B acts to target ergosterol in parasite cell membrane. Miltefosine induces apoptosis, parmomycin inhibits cytochrome C in mitochondria and pentamidine reduces membrane potential and inhibits topoisomerase in mitochondria. However, currently, available options suffer from limitations of high cost, side effects and drug resistance and calls for alternative options. The conventional drugs target different biomolecules, indeed, drugs with greatest efficacy are directed against protein targets [54]. Indeed, the major concern for therapeutic target remains the identification of degree of homology between the host and parasite proteins and therefore, selecting inhibitors which reacts with parasite protein without damaging the host system [55]. The drugs directed against the energy metabolism remained to be the choice that included many protein targets for therapeutics. Figure 1 provides an overview of five decades of studies targeting different metabolic pathways in Leishmania. Glycosome and mitochondria are main energy production houses being the sites for glycolysis and Kreb’s cycle as well as oxidative phosphorylation, respectively.
Figure 1.
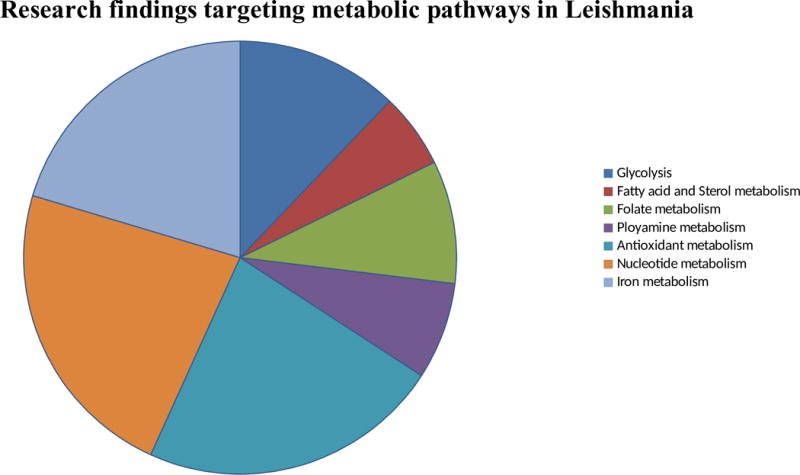
Overview of different metabolic pathways studied in last five decades in Leishmania. The pie chart represents different researches in the field of Leishmania throughout the world targeting different metabolic pathways.
Indeed, blocking of any step of glycolysis causes an arrest of the glycolytic influx and thus, parasite killing. This was also demonstrated in T.brucei where glucose starvation or incubation with glucose transporter inhibitor led to parasite death within minutes. Similarly, pentalactone or bromopyruvate, inhibitors of glyceraldehyde-3-phosphate dehydrognase and inhibitors of pyruvate killed the parasite but these studies suffered limitation since, these inhibitors blocked the human counterpart. Despite the major contribution of mitochondria-mediated breakdown of fatty acids in Leishmania life cycle, glycolysis remains an important process. Structure of enolase has not been deciphered while few atypical residues have reported which could serve as drug target. Pyruvate kinase structure has been determined in L.mexicana with unambiguous selectivity feature which makes it a candidate for drug targeting. Similarly, glyceraldehyde-3-phosphate dehydrogenase has been depicted with 30% homology with human counterpart making it an important candidate for drug designing. Most of the enzymes of glycolytic pathways are localised inside glycosomes and this compartmentalisation promotes parasite survival, and thus, inhibitors designed for preventing their transport by blocking membrane transporters responsible for glucose flux through the glycosome can serve as a drug target.
Mitochondria, vital organ for parasite survival, serves as target for several drugs. The insight into the mechanism of action of drugs emphasises the role of this organelle as a key determinant for disease pathogenesis. The proteins in mitochondria have been derived from two sources- nucleus and small part encoded by mitochondria itself. Currently available studies on trypanosomatid mitochondria are scarce, however, the available set of findings established the essential role for this organelle and its peculiarities as compared to human counterpart making it an attractive candidate for drug development. Experimental evidences suggest mitochondria targeting by conventional chemotherapeutic approaches. Amphotericin B results in membrane permeability and rapid decline in mitochondrial membrane potential, likewise, pentamidine also destroys membrane potential [56], miltefosine inhibits cytochrome C oxidase [57]. On the other hand, Leishmania transmembrane redox system differs from mammalian cells in being less sensitive to cholroquine and more sensitive towards niclosamide [58] therefore, suggesting the possibility of membrane electron transport and proton pumping to act as an attractive therapeutic target.
A number of researches for the antileishmanial agents led to establishment of chalcones as potential drug which targets ultrastructure and functions of mitochondria [59, 60], later its ability to inhibit fumarate reductase[61] has established it as potential drug target. Endochin-like quinolones (ELQs) has been another potent inhibitor of cytochrome bc 1 in Plasmodium [62]. It has been shown to have toxic effects on amastigotes of L.donovani and L.mexicana, however, hydroxynaphthoquinone buparvaquone acts as more potent inhibitor of electron transport, ATP production and parasite growth [63] raising concern for targeting cytochrome bc1 as potential therapeutic option.
Other inhibitors of mitochondria included benzophenone-derived bisphosphonium salt which targeted complex II [64]; and anti-malarial compound artemisinin, have shown anti-leishmanial activity by inducing apoptosis [65] Hydroxynapththoquinone atovaquone inhibits L.infantum [66]; tafenoquine, analogue of primaquine, causes mitochondrial dysfunction by inhibiting cytochrome c reductase (complex III) decreasing oxygen consumption and mitochondrial membrane potential and ultimately apoptosis [67].
Mitochondria remains to be the principal site for fatty acid metabolism where 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) reductase from L.donvani [68] has been established as potential drug target. Similarly another drug target, fatty acyl-CoA ligase regulates cellular homeostasis of lipid, differentially regulated in antimony resistant L.donovani [69] affirming its potential as a drug target. Sterol biosynthesis has been important constituent for cellular functions and maintenance of cell structure. The major sterols in trypanosomatids are ergosterol and 24-methyl sterol, essential for growth and viability. This makes, sterol and fatty acid metabolic pathway, attractive drug targets. Several other enzymes involved in sterol biosynthesis has been reported for their potential as drug target including- squalene synthase [70], squalene epoxide [71], farnesyl diphosphate synthase [72, 73], sterol methyl transferase[74, 75], sterol 14 alpha demethylase[76, 77] and many more as demonstrated in different chemical inhibition studies. Recent reports have suggested combination targeting where two steps in sterol biosynthesis are targeted at once using imipramine and miconazole[78].
Edelfosine has recently been demonstrated as target drug for disrupting the mitochondrial membrane potential by recruiting F0-F1 ATPase in the lipid raft, driving DNA disruption and thus serving as novel drug target for the treatment of leishmaniasis[79].
Polyamine metabolism remains another important candidate pathway for the drug designing. Polyamines are strongly associated with cell survival, growth and proliferation. Arginase is the first enzyme in polyamine biosynthetic pathway [80]. Other enzyme as potent drug targets include ornithine decarboylase[81, 82], S-adenosylmethionine decarboxylase(S-AdoMet inhibitor CGP40215A, 1996) [83], spermidine synthase [84], trypanothione synthetase [85, 86], trypanothione reductase [87], tryparedoxin peroxidase [88], deoxyhypusine synthase [89], and deoxyhypusine hydroxylase[90].Polyamine transporters-putrescine transporters, DAB, LmPOT1 etc. [91-93].
Folate metabolism constitutes another crucial biochemical pathway essential for parasite survival [94]. The potential drug targets from folate pathway include pteridine reductase, dihydrofolate reductase-thymidylate synthase [95, 96], folylpolyglutamate synthetase [97] and serine hydoxymethyl transferase [98].
Antimicrobial peptides as therapeutic target
Antimicrobial peptides (AMPs) are multifunctional, cationic protein weapons of innate immune system in wide range of species. These are usually expressed at low levels but upregulated upon infection or inflammation [99]. It constitutes the most primitive but important immune defense armory because of its rapid action, broad target range, amphipathicity and flexibility of utilization in conjunction with existing regimens [100]. The AMPs serves many functions as chemotactic agents for leukocytes, interact with the microbial membrane inducing autophagic [101], necrotic or apoptotic cell death [102]. These are structurally diverse peptides, with high content of basic amino acid residues while certain subsets are also rich in cysteine allowing for their functionality[103]. In spite of its wellknown role to destabilize the membrane, it also penetrates intracellular organelles and has pleiotropic effect on bioenergetic function of cells [104, 105]. Several AMPs have antileishmanial activity including dermaseptin[106], phylloseptin [107], bombinins[108], temporins [109], spinigerin [110] and magainins[111]. Other class of AMPs expressed in mammals include cathelicidin (Protegrin-1,SMAP-18,-27) [101] and defensin [112] which influence host inflammatory responses by serving as chemokines or inducing chemokine secretion by other cells leading to migration of neutrophils, monocytes and macrophages. Chemokines have also been known for their antimicrobial peptide activity on pathogens termed as kinocidins [113-115]. Further, several other AMPs from different species have been known to effect Leishmania [116]. However, the clever parasite overcomes this innate barrier of AMPs by employing leishmanolysin (gp63) as documented from knock-out studies in mice [117]. It is unlikely that leishmanolysin has significant effect on Leishmania resistance against AMPs as the parasite resides in phagolysosome. These AMPs have been known for inducing cell death in the parasite using different mechanisms. These AMPs break the membrane potential, equilibrate the internal as well as the external pH without perturbing the cell membrane and alters ATP content as documented from in vitro as well as in vivo studies [105, 111, 118]. The AMPs have less activity in LPG-deficient as well as late stationary phase parasites (LPG enriched) suggestive of the LPG mediated alteration in the membrane as regulator of AMP activity. They induce formation of vacuoles without any alteration in their activities upon caspase-blocking indicating for their ability to cause autophagy mediated cell death in parasites [101]. While other class of AMPs from human salivary gland causes disruption of mitochondrial membrane potential, decreased oxygen consumption and ATP depletion[119]. Therefore, it is well understood that different classes of AMPs disrupts parasite membrane, target mitochondria [119] and delocalize intracellular calcium crucial for inducing cell death by affecting mitochondrial activity. Intracellular AMPs affect calcium reserves in parasites (acidocalcisomes, glycosomes and/or endoplasmic reticulum) [120]. These researches have provided reasonable basis for the development of AMP based therapeutic targets as suggested from the preliminary evidences from animal studies [112, 121].
However, there are a number of AMPs which have been screened against Leishmania, the picture of development of AMPs based anti-leishmanial agent still remains elusive because of limited studies on amastigote form of parasite and intracellular nature of the parasite making it difficult for access by AMPs. Additionally, given the differences in surface architecture of two forms of parasite, there always remains a need for developing/screening AMPs against the amastigotes. However, studies from mice model reported the absence of cathelicidin-AMP associated with exacerbated infection [122], therefore, augumentation of intracellular AMP expression may serve as novel therapeutic approach. AMPs can also be targeted in the vector where parasite grows and differentiates [123] so, understanding of role of vector AMPs in parasite dissemination remains a crucial checkpoint for intervention of parasite transmission from insect to humans and therefore, this could serve as a major breakthrough in controlling the number of new cases and asymptomatic carriers of disease.
Proteasome and cell cycle as therapeutic target
Proteasome is multi-subunit protein complex in the cytoplasm and nucleolus, which regulates cellular protein turnover and degradation of misfolded proteins. It has two main components- the core particle and the regulatory subunit. Proteins destined for degradation are conjugated with ubiquitin (in eukaryoes) or ubiquitin-like protein (pup) (in prokaryotes) [124]. These proteins are then recognised by regulatory proteins flanking the proteolytic core of proteasome. These proteins then unfold polyubiquitinated proteins, threading it through the narrow opening of the core subunit upon the removal of ubiquitin and pup chains [125] [126]. The ubiquitin-proteasome system from protozoans has been widely studied [127]. The study of proteasome as drug target dates back to the 90s when use of proteasome inhibitors altered the cell growth, development and differentiation of the parasite in concentration-dependent manner [128]. In vitro assays in L.donovani with 2 1M MG-132 or 5 1M suggested for induction of programmed cell death in parasite while the surviving parasites were short with rounded apical ends and damaged mitochondria [129]. Treatment with conventional proteasome inhibitor, lactacystin, caused sharp decline in the parasite viability in the macrophages [130]. Phenotypic screening of three million compounds and hit-to-lead optimization against Leishmania donovani, Trypanosoma cruzi and Trypanosoma brucei identified efficacious compound for VL, CL, Chagas disease and trypanosomiasis targeting parasite proteasome [131]. A high-throughput proteomic study at Genomics Institute of the Novartis Research Foundation (GNF), led to the identification of a compound termed GNF5343, GNF6702 with good efficacy for L.donovani, T.brucei and T.cruzi cultures [131].
There are reports from mitochondrial protein, Ufm1 from Leishmania, has been suggested as a protein drug target [132] which has shown remarkable similarities between mammalian and Leishmania conjugation system. This has fueled the idea for use of anticancer drugs that target ubiquitin pathway as a key for development of the antiparasitic drug which has been suggested as protein drug target. The anti-parasitic effect of disruption of this pathway leads to decreased parasite survival therefore, increasing its recognition as a potential therapeutic tool. Deubiquitinases are another key regulator of ubiquitin-proteasome pathway, exploited as anti-cancer weapon has further been of interest for development of antileishmanial drug because of the ability of the parasite to obstruct host protein degradation system for its survival [133] provides impetus for utilizing this pathway as therapeutic option.
There are varieties of diseases with defective protein kinase signaling, which is usually required for progression of cell cycle therefore, paving the way for use of specific inhibitors to target these molecules for drug development. Leishmania has tightly controlled cell cycle program regulated by cyclin-dependent kinases (CDKs). Several small chemicals targeting CDKs are currently under clinical trial including alvocidib[134], seliciclib [135] etc. Due to their pivotal role in cell cycle these proteins serves as attractive target for drug discovery. The studies from mammalian and parasite system have revealed for many homologues in parasites e.g. CRK3 [136]. The protein kinases from cyclin-dependent kinase, cAMP-dependent kinase and mitogen activated protein kinase families are major target molecules from the parasite.
CRK3 in Leishmania is essential for proliferation and has been targeted for drug development with the use of chemical inhibitors [137]. The report from other cell cycle target from Leishmania is aurora kinases, essential for cell division has been established by bioinformatic as well as immunoproteomic approaches as drug target whose inhibition led to aberrant changes in cell cycle progression and parasite viability [138].
Histone acetyl transferase and histone deacetylases are another group of important molecules required for regulating gene transcription, cell cycle progression and essential for parasite survival. It has been reported to have therapeutic potential by pharmacological studies [139]. SIR2 family proteins are another interesting candidate for parasite survival and stage specific as documented in genetic deletion studies [140]. Recent findings on Leishmania glycogen synthase have revealed for its role in cell cycle progression therefore, drugs targeting this enzyme has therapeutic potential [141].
Metacaspases, known for their apoptotic function remains one the key components of the actively replicating amastigote and promastigote. However, its overexpression has been associated with growth retardation, changes in ploidy and impaired cytokinesis. Indeed, essentiality of metacaspases for segregation of the nucleus and kinetoplast apart from its role in programmed cell death, and absence of mammalian counterpart [142] makes this enzyme potential candidate with therapeutic application.
Secretory proteins/secretion pathway based therapeutic targets
Leishmania secretes a wide array of proteins in the extracellular milieu to cope with the hostile macrophage environment [143-146]. These secretory proteins are trafficked through eukaryotic secretion pathways where proteins are folded in endoplasmic reticulum and transported through the golgi for secretion outside the cell. So, processing and secretion of virulence factors remains crucial for parasite survival in the host [147].
Endoplasmic reticulum (ER) remains the key organelle for the secretory pathway, where the folding and packaging of the proteins is done for its delivery to respective sites. It also serves as the check point for the misfolded proteins which then is destined for the ubiquitin-proteasome system for their degradation.
Any kind of insult to ER activates unfolded protein response (UPR), which leads to activation of transmembrane proteins-Atf6, Ire1 and PERK. Computational modeling analysis revealed the involvement of PERK in L.donovani infection without measureable changes in the UPR-specific expression of BiP (ER chaperone) and increased sensitivity towards ER stress-inducing drugs as compared to host macrophage, and these findings indicate ER stress as a pathway of therapeutic interest [148]. In addition to the UPR, there is extremely well coordinated quality control machinery of ubiquitin-proteasome system in cytosol termed as the ER associated degradation (ERAD), which degrades misfolded peptides upon extraction from ER [149]. However, the protozoan parasites contain minimal ERAD network therefore, these parasites are highly sensitive to inhibition of protein quality control system components. Recently, ER stress on L.amazonensis infection was associated with TLR2 dependent XBP1 formation that ultimately induced IFN-β expression and altered the oxidative response of infected macrophages causing parasite proliferation [150]. Thus, targeting this transcription factor can have potential therapeutic implications.
The PERK/ATF4 pathway plays an important role in protein homeostasis and is exploited by the parasite to establish infection. PERK signalling also diminishes translation and induces ATF4 expression which drives cell survival or induces cell death. PERK/eIF2α/ATF4 signaling is known to induce upregulation of cytoprotective autophagy genes, such as ATG5 and ATG7, which promotes cellular survival [151] and parasite infection [152]. Chemical inhibition studies for screening of potential drugs identified GSK2606414 targeting the kinase domain of PERK. It has already been used as an anti-cancer agent [153], and targeting these pathways can have therapeutic implications.
There are significant numbers of secretory proteins produced by the parasite during host invasion. Some of these secretory products are important virulence factors, and processing and transport of these proteins outside the cell [147] comprises a crucial component of parasite life cycle in the host. A number of proteins involved in protein folding and quality control of ER-mediated chaperoning activity involves-calreticulin [154], BiP [155] and protein disulfide isomerase (PDI) [156]. Calreticulin constitutes critical players of ER quality control of secretory proteins. However, its overexpression is associated with decreased survival inside macrophage, and altering the function of ER chaperone [157] affects protein secretion and thus a potential candidate for drug discovery.
ER also remains an important constituent of parasitophorous vacuole (site of parasite residence) as documented by blocking the N-ethylmaleimide-sensitive factor attachment protein receptors (SNAREs) which causes fusion of early secretory vesicles. Blocking SNARE does not affect ER morphology but significantly reduced parasite replication [158] therefore, providing clues for utilizing SNARE complex as therapeutic option for targeting the disease.
On the other hand involvement of protein disulfide isomerase (PDI), a key molecule in regulating ER quality control process of protein processing [159] has also been suggested as one of the demanding drug targets whose depletion is associated with cytotoxic effects and ultimately apoptosis [160, 161]. Chemical inhibition studies has further shed light on the essential role of these chaperones as regulator of parasite survival [160]. Signal peptide peptidase (SPP) is amongst other important candidates, due to its high selectivity for misfolded/unstable proteins for degradation [162], and makes them a choice for drug target. As a pathway for identification of drug targets, the ER mediated pathway of protein processing has not been much explored therefore, there remains a need to study the role of ER proteins which serves as the key regulators of parasite life cycle. This would be of great help in laying the base for the drug designing and therapeutic intervention.
Epigenetic manipulations as therapeutic option
Parasitic organisms have evolved the strategy to promote their survival in the host by playing with the host transcriptional machinery. Alteration in the host transcriptome and proteome profiles using different weapons of parasite origin leads to disease pathology.
In recent times, epigenetics has gained attention of scientists, where heritable changes in the gene expression does not involve modification of core DNA sequence but depends on alteration of either DNA/histones. This alteration of DNA/histone affects the chromatin structure and gene expression. These modifications in addition to the changes in DNA methylation subverts the host cellular responses and drives the expression of genes favoring the growth and survival of the pathogen. These heritable long term changes promote disease pathogenesis and persistence of the pathogen within the host. In this section we discuss how epigenetic changes are brought about by the parasite, its implications in immune regulation and as a therapeutic target. The changes in DNA methylation attenuated NFкB1-mediated pro-inflammatory signaling [163], inflammatory reposnses [164], apoptosis, pathogen induced signalling and changes in host behavior. However, limited evidences are available for epigenetic effects of Leishmania infection on host cell, with recent research suggesting for epigenetic reprogramming of macrophage during L.donovani infection [165].
Recent years have experienced a plethora of researches focusing on the epigenetic phenomenon in understanding disease biology. Epigenetic analysis provides fast, reversible and ready access to phenotypic changes which shapes the host-parasite interactions. These changes are brought about by the histone modifications, transcriptional and post-translational regulatory circuits by altering the histone-DNA interactions. The remodelling of chromatin by T.gondii downregulated TNF-α [166], altered STAT1 mediated IFN-γ responses [167]. Moreover, the host epigenetics has also been known to be altered by microbial secretory products as reported from Listeria [168], influenza virus [169] and Legionella [170] studies. Diverse effectors from microbial agents have been known to inhibit cellular machinery including MAPK, IFN and transcription factor NF-кB signaling [171] as reported from Mycobacterium [172], influenza virus [169]. Therefore, providing compelling evidences for the pathogen mediated induction of epigenetic modification in the host.
Recently, non-coding RNA (nc RNAs) and miRNAs have also been reported as the regulators of epigenetic processes by DNA silencing by post-transcriptional regulation [173]. Epigenetic control of pathogen virulence has been well known from studies including Salmonella, where lack of DNA adenine methyltransferase led to impaired invasion capacities, envelope instability, reduced motility in the pathogen [174]; Plasmodium switches its protein expression to overcome host immune responses [175].
In the light of limited data available for the effect of epigenetic changes in the host macrophages and most of the evidences, available till date, are correlational, and pathogenic proteins mediated changes in the host epigenetics are still to be described. The scope for targetting the parasite-mediated changes in the host epigenome, and directly modulating the host epigenome, arresting the pathogen developmental switches inside host or limiting their virulency with the use of chemical inhibitors, RNAi (RNA intrerference), gene knockout, etc. could opens up new avenues for targeting histone modifying enzymes, DNA methyltransferases and chromatin therefore, providing the science with the new concept of “epigenetic therapy”, which may serve to deal with the issues related with drug resistance.
Iron homeostasis as therapeutic target
Iron is one of the crucial components of the cell which cycles between ferrous and ferric forms enabling the organisms for performing several life sustaining biological processes. Iron forms active part of diverse proteins such as aconitase, ribonucleotide reductase, cytochromes and Fe-S proteins of electron transport chain [176]. Additionally, it forms an active ingredient of collagen, tyrosine catecholamines [177] as well as immune responses in mammals. Iron homeostasis is stringently regulated in prokaryotes as well as eukaryotes. Leishmania has LIT1 (Leishmania iron transporter) as one of the crucial iron transporters [178], expressed in iron deficient environment and serves to increase iron flux. This serves as a trigger for parasite differentiation. Genetic deletion studies revealed the replication defect inside macrophages and avirulent state of the parasite[179]. Recently, LIT1 up-regulation has been associated with increased super oxide dismutase activity and reactive oxygen species therefore, regulating parasite differentiation[180]. Genome researches have led to the identification of LFR1, membrane protein with heme, FAD and NADPH-binding sites in transmembrane regions. Together with LIT1 it equips Leishmania with inorganic iron acquisition pathway. LFR1 accounts for ferric reductase activity and deletion studies have reported its role in parasite differentiation [181]. These findings suggested for therapeutically targeting these transporters for disease intervention.
Iron is also a constituent of mitochondrial superoxide dismutase which mediates parasite protection and redox signalling as demonstrated by RNAi-studies. Genetic deletion studies further validated their role in driving susceptibility of parasite to ROS-induced stress and differentiation[182]. Thus, this molecule should be considered as drug target. Bioinformatics and florescence microscopy based study revealed LmABCB3 as important mitochondrial target molecule. It is ATP-binding cassette (ABC) half-transporter with metal binding domain responsible for mitochondria dependent heme and iron-sulfur cluster synthesis. Thus, LmABCB3 transporter is essential for parasite survival, and represents novel target for combating leishmaniasis [183].
LABCG2, another ABC subfamily transporter, plays vital role in parasite virulence. It is required for externalization of phosphatidylserine, as an adaptive strategy for macrophage invasion. Mutation studies from this transporter indicated for decreased pathogenesis as well as virulence of the parasite [184]. Moreover, evidences from susceptibility and biotinylation assays reported the overexpression of LABCG2 in antimony resistant isolates as documented by sequestering metal-thiol conjugates [185]. Similarly, LABCG1 was also found as the key player working hand in hand with LABCG2 in regulating metacyclogenesis, infectivity, oxidative stress and autophagy [186]. Thus, these transporters could be used as therapeutic targets, and with these, cure of drug resistance is also a possibility. LHR1 remains another important target molecule of iron homeostatic pathway of parasite. Fluorescence tagging and genetic deletion studies has unveiled the role of this transporter in parasite survival [187, 188] and virulency [189], targeting this transporter for therapeutic purpose can be effective in these intervention of trypanosomatid diseases.
LMIT1, mitochondrial iron transporter identified in Leishmania has been found to be crucial for parasite viability and differentiation. The parasites with reduced LMIT1 expression showed growth defects, severe defects in iron content and susceptibility for ROS. The involvement of this iron-dependent transporter in mitochondrial redox balance and parasite virulence opening horizon for targeting iron metabolism for therapeutic benefit [190].
Other host based iron homeostatic regulators include-DMT-1(Divalent metal transporter) [191], Tf/TfR (transferrin/transferring receptor)[192], hepcidin[193], ferroportin [194] and IL-6 receptor [195] which can be selectively targeted for therapeutic benefits.
Kinome based therapeutic targets
Kinome represents the complete set of protein kinases encoded by the genome. Protein kinases serve as an important arm of cellular programming. Post-genomic era has experienced technological advancements which has served as the landmark in understanding the disease biology and unraveled the relative contribution of parasite genes in disease progression [196, 197]. In-depth genomic and functional analyses have suggested phosphoinositol kinase (PIK) pathway as popular targets for development of drugs in the treatment of several diseases. Recent years have experienced a plethora of chemical and natural products targeting specific protein kinases, several are under clinical trials.
Protein kinases have a crucial role to play, in parasite life cycle as discussed in recent articles including mitogen-activated protein (MAP) kinases, PI3 kinases, NF-кB signalling [198-201]. On the other hand, complement receptors also play important role in activation of protein kinases in turn regulating host immune responses.
Cyclin-dependent kinases (CDK) are most studied amongst kinases as a target for leishmaniasis. As already discussed above, CDKs play important regulator of cell cycle, therefore, inhibitors targeting the ATP binding to the catalytic site of CDKs [202], could serve as potent drug targets. In this context, flavopiridol is worth mentioning as it is the first CDK inhibitor that reached clinical trial. Recently, Abl-family of kinases (discovered as oncogene in Abelson leukemia virus) have been reported by Wetzel et al. as non-receptor kinases[203]. These kinases have been known to play important role in cell-cell contact, cytoskeletal rearrangement during phagocytosis and cell motility [204]. The chemical inhibition studies and knock down studies has further elaborated the role of these kinases during infection. The combination of Imatinib (Abl-family kinase inhibitor) with conventional chemotherapeutic choices could have a potential role in controlling parasite. Abl-family kinases during Shigella [205], Chlamydia [206] and Mycobacterium infection [207] have already been established. MAPKs are signal transducers that regulate cytoskeletal rearrangements, proliferation, differentiation, immune responses and diversity of other cellular responses.The MAPKs displays conserved residues for their regulatory roles. The cascade of events initiates with MAPK activation, followed by its phosphorylation and involvement of different transcription factors that alters gene expression [208] and epigenetic modifications by affecting histone remodeling [209], JNK(c-Jun activated kinases) [210] and p38 stress-response MAPKs [211]. Although modest body of work has been reported, however, utilizing the phylogenetic differences between MAPKs of parasites and host as well as the upstream MAPK regulators can be of great relevance in developing new therapeutic options. LmaMPK7 is the only MAPK known for controlling the parasite virulence, in its active form [212] while decreased LdMAPK1 has been associated with antimony resistance [213]. Therefore, these studies lay the foundation stone for exploiting this MAPK for development of the noninfective parasite which can be of relevance as an immune boosting strategy for controlling parasite. Protein kinase regulated by RNA constitutes another class of kinases involved in promoting parasite burden during L.amazonensis infection [214] Similar studies from Mycobacterium infection [215] suggest designing of inhibitor based approaches for targeting this protein kinase towards therapeutic advantage. However, reports from L.major infection were contrasting with the L.amazonensis dataset [216], and this could be attributed to different host-parasite interaction and downstream signaling. Other kinases include casein kinase which remains important drug target due to its essence for parasite survival and virulency [217, 218]. NEK kinases [219, 220], CLK and DYRK are other protein kinases [221] are potential candidates as drug targets. Several CDK family members including cdc2-related kinases(CRKs) [222], tyrosine kinase[223], glycogen synthase kinase [224], PI3 kinase, Src kinases [225], JNK [226] are other potential drug target.
Calcium homeostasis as therapeutic target
Calcium remains an important component of cellular homeostatic machinery essential for cell viability for all organisms ranging from mammals to parasites and non-mammals [227, 228]. Any kind of perturbation in the calcium homeostasis severely affects the cell viability, leading to cell death [120, 229]. Calcium also plays crucial role in protozoan parasites by regulating flagellar, ciliary movements [230], exocytosis and regulator of several enzymatic activities including adenylate cyclase [231], cAMP phosphodiestrase [232], protein kinases[233] and guanylate cyclase [234].
In trpanosomatids, the levels of calcium regulated at cytoplasmic level where the regulatory control is under: ER, mitochondria and acidocalcisome[235]. At the plasma membrane level, Ca+2-ATPase, membrane channel is regulated by calmodulin in trypanosomatids [236, 237], another transporter in internal membrane of mitochondria drives calcium-ion accumulation [236, 238]. All these system of homeostatic machinery work in coordination for maintaining the intracellular calcium equilibrium to serve as signalling messenger[227]. Calcium has also been known to play crucial role in differentiation of the parasite and its thermotolerance [239-241].
The alteration in the calcium levels are sensed by calcineurin which serves to activate the signaling cascade required for parasite differentiation and adaptation to cellular stress. However, genetic deletion of calcineurin led to devastating effects including changes in membrane fluidity, unfolded protein response along with the loss of virulence [242].
ER remains the largest storage reservoir of calcium with different calcium influx and efflux pathways. Calreticulin is calcium storage protein in ER [232], SERCA (Sarcoplasmic reticulum Ca +2-ATPase) has been demonstrated in different trypanosomatids which serves as a virulence factor in Leishmania [243] could act as a potential therapeutic target. Calcium sensitive protein aequorin in the nuclear membrane has been known to mediate the flux of calcium in the nucleus of trypanosomatids[244]. Also, the nuclear membrane shows the presence of proteins that stain for BiP, calreticulin and TcSCA [245]. Mitochondria possesses calcium uniporter as already mentioned above, also, literature has evidences for calcium-uptake and release channels in trypanosomatids [238, 246, 247].
Acidocalcisome constitutes another important target site for the storage of calcium, pyrophosphates, polyphosphates and other elements [235, 248]. It has PMCA-type Ca +2-ATPase responsible for calcium influx [235], while Ca +2/H+ exchanger has been demonstrated to be responsible for calcium efflux [249]. Calcium ionophores play important role in host-parasite interaction as demonstrated in T.cruzi infection, regulation of several protein kinases[221, 250] and parasite differentiation [251].
Noteworthy role of calcium in regulating parasite life cycle emphasizes for targeting calcium transporters could have therapeutic implications. In this regard, calcium channel blockers- fendiline, mibefradil and lidoflazine, etc. were found to have potent antileishmanial activities. However, the combination of these calcium blockers with the conventional chemotherapeutic options further elicited additive effect for clearing off the parasite by inducing mitochondrial depolarization, increased reactive oxygen species generation without plasma membrane disruption [252]. Therefore, use of calcium channel blockers has suggested for their utility as therapeutic candidates alone or in combination with chemotherapy for treating leishmaniasis. Likewise, bepridil has shown good efficacy[253],verapamil in combination with meglumine antimoniate has synergistic effect on parasite clearance[254].
Mitochondria remain the site for electron transport chain where four complexes coordinating the entire process. Rotenone-insensitive, NADH: quinone oxidoreductase constitutes the complex I in Leishmania and Trypanosoma without any human counterpart[255, 256]. The reports from various researches have suggested for the presence of complex II, and limited involvement of electron transport between complex I-III, makes succinate, primary electron donor for energy production. It has been characterised in Leishmania [257] and Trypanosoma [258], its absence in mammalian mitochondria makes it potential candidate for the drug designing.
Early efforts to target ETC dated back to the 90s when complex III (cytochrome bc 1 complex) was analysed and amino acid differences in ubiquinone binding with the human counterpart made it an interesting target for drug development [259]. Complex IV has subunits encoded by nuclear and mitochondrial DNA [260], present in both Leishmania (Ldp27) [261] and Trypanosoma (Tb11.0400) [262], correlated with infectiousness of the parasite. Trypanosomatid ETC involves alternative oxidase as terminal electron acceptor in ETC, lost during evolution in mammals[263], does not participate in generating proton motive force across mitochondrial membrane [264].
Other therapeutic targets
Leishmania undergoes preadaptation to the environmental conditions of the host macrophage. Autophagy constitutes an important component of parasite life cycle which involves cathepsins and cysteine peptidases for controlling protein turnover crucial step in parasite development and differentiation [265]. This established these molecules as therapeutic target for drug development.
Immunotherapy is the hottest area of research for disease intervention these days. It involves the host-directed immunotherapies, that targets the immune and inflammatory pathways for alleviating disease pathology [266]; cytokine based immunoregulatory therapy[267]; cell based therapy using dendritic cells, mesenchymal cell, etc. [268-271] and immune checkpoint blockade as well as monoclonal antibody based immunotherapy [272-274]. Combination therapy with monoclonal antibody has shown promising results in clearing off parasite burden even at a suboptimal dose of conventional chemotherapeutic approach [272]. Indeed, immunotherapy is in itself a broad research area which is beyond the scope of this article.
Recently, nicotinamidase, enzyme involved in assimilation of nicotinamide, nicotinic acid, nicotinamide riboside to synthesize NAD+ by salvage pathway. It constitutes a key component of parasite development and virulence, add-back and mutational studies has attracted the scientific community for designing novel inhibitors and utilize this molecule as a therapeutic target [275]. Leishmania utilizes purine pathway from mammalian system using different nucleoside transporters. Phosphoribosyltransferases constitutes a key component of purine salvage pathway including three main enzymes: hypoxanthine-guanine phosphoribosyltransferase, xanthine phosphoribosyltransferase and adenine phosphoribosyltransferase [276, 277]. Leishmania has several enzymes for breakdown of host nucleosides, nucleotide and nucleic acid before incorporation into its purine pools. Cloning, expression and immunolocalization has led to characterization of several nucleoside hydrolases [278-280], several nucleases/nucleotidases that generates free nucleosides [281] thereby making the purine accessible for translocation through transporters into the parasite.
Several transporters designated for purine acquisition include: LdNT1 [285], LdNT2 [283, 284], LdNT3 and LdNT4 [285]. Nucleobase/proton symporters transporters from L.major –LmaNT3 and LmaNT4 [286], demonstrated for their important role in parasite viability inside macrophage therefore, providing clues for the necessity of targeting the salvage pathway and acquisition systems for therapeutic purpose.
Glycoconjugates, present another interesting target molecule for therapeutic intervention as they are the key player in parasite survival and infectivity. Lipophosphoglycan (LPG) and proteophosphoglycan (PPG) are phosphoglycans with host immune subversion properties [287, 288], genetic deletion and mutation studies [289] established their importance as therapeutic target. Therefore, the functional role of these glycosylphosphatidylinositol (GPI) molecules and their biosynthetic pathways served as an essential factor for parasite virulence [290] opening avenues for their exploitation for drug designing and discovery. Tubulins are another target molecule of interest for therapeutic intervention [291].
DNA topoisomerases plays vital role in DNA replication, repair, transcription and recombination forms another candidate for drug discovery. It has gained attention in recent years after success of camptothecin for cancer treatment when camptothecin analogs –topotecan, gimatecan and irinotecan were shown to exhibit anti-leishmanial activity [292].
Recent study by Abidin et al. reports the activation of hematopoietic stem cells by the parasite, which in turn promotes infection. These finding suggest hematopoesis as a potential therapeutic target in leishmaniasis [293].
Apoptotic pathway serves as regulator of multitude of physiological processes ranging from cell division to cell death. Several proteases, metacaspases and chemical inhibitors have been proven to possess anti-leishmanial activity [294-296], also they play roles in determinant of host-parasite interaction as well as disease pathogenesis[297]. Therefore, the apoptotic pathway can serve as interesting therapeutic target for drug development.
Multiscale mathematical modeling and simulation have also led to the discovery of IPC synthase, a sphingolipid synthase present in acidic macrophage phagolysosome. It forms an essential component of disease pathogenesis and serves as another vital candidate for drug discovery therefore, interactome analysis can serve for the drug designing [298].
Cholesterol forms another important therapeutic target for leishmaniasis as documented by cyclodextrin-based alterations in membrane cholesterol levels [299]. Sterol 14α-demethylase also forms an important therapeutic target for antiparasitic chemotherapy [300].
Autophagy associated peptidases also constitutes the class of therapeutic targets involved in protein turnover and remodelling, therefore constituting an important component of development, differentiation and virulence of parasites[301].
The parasite derived toll-like receptors (TLRs) ligands also forms target for therapeutics and vaccine development. Several membrane protein transporters, glucose transporters, sugar-nucleotide and purine transporters [302] are also potential targets for drug development. The list of candidate molecules does not end here there are many more under evaluation and others with unknown functions have yet to be explored.
Expert opinion and Conclusion
In this review we have analyzed the recent developments in search for the candidate molecules for their potential use as therapeutic targets. The drawback associated with current chemotherapeutic approach led to the introduction of the single dose liposomal amphotericin B which showed satisfying treatment outcomes. This was the major breakthrough in drug development as to overcome the issues related to the development of drug resistance because of suboptimal drug concentrations and patient non-compliance. Additionally, combination therapies [303] have been expected to be efficacious in preventing disease recrudescence and provide better patient compliance. It has paved the way for targetting multiple pathways in the parasite by inducing synergistic effect for clearing off the infection. However, the identification of combination regimen with characteristic features of good tolerance, minimal need of clinical observation and ambulatory care will be the priority for drug development. Therefore, discovery and development of new therapeutic tools remain highest priority.
The technical advances in nanotechnology and system biology have contributed to development of the drug designing and delivery systems with fairly good treatment outcomes in different models. Plant products and chemical compounds have been raising flags as potential and inexpensive alternatives for different forms of leishmaniasis which has been expected to consolidate the needs of low-income patients. Similarly, immunomodulatory and host-directed immunotherapies have further expanded the horizon for the multifaceted approach for disease cure. It appears that systematic molecular and cell biology approaches, drug repurposing, screening of off-the-shelf drugs have served for identification of novel candidates for drug discovery. Last decade has experienced boom in the immunotherapy and immunochemotherapy defines the remarkable progress in the research for therapeutics. However, variability in the potency of host responses to different pathogens needs to be standardized which calls for heavy investments from government and industries. The search for new therapeutic targets continued and led to the study of metabolic pathway as drug targets for therapeutic benefit. There are several pathways as the targets for the development of therapeutic tool several of which have being documented for their ability to improve clinical symptoms and decrease parasite load. Mitochondrial targeted approaches have significantly gained the interest of the scientific communities however, there are many other potential pathways that have emerged in recent years. Therefore, there always remains an urgent need for the development of therapeutic option with potential of cure or reverse severity of clinical manifestation. Leishmaniasis elimination program in developing nations has got support from international community for ensured treatment of disease has been the helping hands for the local authorities for drafting the roadmaps for combating the disease. This has led to steep decline in the number of cases reported per year, however, the achievement of elimination goal still requires efforts at ground level for following up the past cases to monitor the proper clinical cure as well as prevention of disease transmission. In order to bolster the drug development, identification of new targets can contribute significantly towards strengthening the pipeline for disease elimination. This is expected to reap rewards in the form of leishmaniasis free nations in coming years.
Highlights.
The conventional chemotherapy approaches are associated with toxic side effects which called for the development of alternative approaches.
The technical advancement in the post-genomic era has explored many new drug targets.
The advances in metabolomics, system biology and nanotechnology approaches has led to better understanding of parasite biology therefore, better chances for identification of drug targets and their therapeutic potential.
Footnotes
Declaration of Interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict.
References
**Considerable interest
* Special interest
- 1.Bhattacharya SK, et al. Elimination of leishmaniasis (kala-azar) from the Indian subcontinent is technically feasible & operationally achievable. Indian J Med Res. 2006;123(3):195–6. [PubMed] [Google Scholar]
- 2.Organization WH. Kala-Azar elimination programme: report of a WHO consultation of partners, Geneva, Switzerland, 10-11 February 2015. World Health Organization; 2015. [Google Scholar]
- 3.Aad G, et al. Combined Measurement of the Higgs Boson Mass in pp Collisions at sqrt[s]=7 and 8 TeV with the ATLAS and CMS Experiments. Phys Rev Lett. 2015;114(19):191803. doi: 10.1103/PhysRevLett.114.191803. [DOI] [PubMed] [Google Scholar]
- 4.Ivens AC, et al. The genome of the kinetoplastid parasite, Leishmania major. Science. 2005;309(5733):436–442. doi: 10.1126/science.1112680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sundar S, et al. Short-course, cost-effective treatment with amphotericin B-fat emulsion cures visceral leishmaniasis. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2000;94(2):200–204.ris. doi: 10.1016/s0035-9203(00)90277-3. [DOI] [PubMed] [Google Scholar]
- 6.Murray HW. Clinical and experimental advances in treatment of visceral leishmaniasis. Antimicrobial Agents and Chemotherapy. 2001;45(8):2185–2197. doi: 10.1128/AAC.45.8.2185-2197.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Griekspoor A, Sondorp E, Vos T. Cost-effectiveness analysis of humanitarian relief interventions: visceral leishmaniasis treatment in the Sudan. Health policy and planning. 1999;14(1):70–76. doi: 10.1093/heapol/14.1.70. [DOI] [PubMed] [Google Scholar]
- 8.Sundar S, et al. A cluster of cases of severe cardiotoxicity among kala-azar patients treated with a high-osmolarity lot of sodium antimony gluconate. The American journal of tropical medicine and hygiene. 1998;59(1):139–143. doi: 10.4269/ajtmh.1998.59.139. [DOI] [PubMed] [Google Scholar]
- 9.Gasser RA, Jr, et al. Pancreatitis induced by pentavalent antimonial agents during treatment of leishmaniasis. Clinical infectious diseases. 1994;18(1):83–90. doi: 10.1093/clinids/18.1.83. [DOI] [PubMed] [Google Scholar]
- 10*.Thakur C, et al. Do the diminishing efficacy and increasing toxicity of sodium stibogluconate in the treatment of visceral leishmaniasis in Bihar, India, justify its continued use as a first-line drug? An observational study of 80 cases. Annals of Tropical Medicine & Parasitology. 1998;92(5):561–569. doi: 10.1080/00034989859258. First reporting on the issues related with the toxicity of sodium stibogluconate for treatment of VL. [DOI] [PubMed] [Google Scholar]
- 11.Rijal S, et al. Sodium stibogluconate cardiotoxicity and safety of generics. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2003;97(5):597–598. doi: 10.1016/s0035-9203(03)80043-3. [DOI] [PubMed] [Google Scholar]
- 12.Sundar S. Drug resistance in Indian visceral leishmaniasis. Tropical Medicine & International Health. 2001;6(11):849–854. doi: 10.1046/j.1365-3156.2001.00778.x. [DOI] [PubMed] [Google Scholar]
- 13.Sundar S. Treatment of visceral leishmaniasis. Medical microbiology and immunology. 2001;190(1):89–92. doi: 10.1007/s004300100088. [DOI] [PubMed] [Google Scholar]
- 14.Hu E, et al. Identification of novel isoform-selective inhibitors within class I histone deacetylases. Journal of Pharmacology and Experimental Therapeutics. 2003;307(2):720–728. doi: 10.1124/jpet.103.055541. [DOI] [PubMed] [Google Scholar]
- 15.Saravolatz LD, et al. Amphotericin B: time for a new “gold standard”. Clinical Infectious Diseases. 2003;37(3):415–425. doi: 10.1086/376634. [DOI] [PubMed] [Google Scholar]
- 16.Sundar S, Chakravarty J. Liposomal amphotericin B and leishmaniasis: dose and response. Journal of global infectious diseases. 2010;2(2):159. doi: 10.4103/0974-777X.62886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17*.Meyerhoff A. US Food and Drug Administration approval of AmBisome (liposomal amphotericin B) for treatment of visceral leishmaniasis. Clinical Infectious Diseases. 1999;28(1):42–48. doi: 10.1086/515085. First report on the use of AmBisome for treatment of VL. [DOI] [PubMed] [Google Scholar]
- 18.Berman J. Treatment of leishmaniasis with miltefosine: 2008 status. Expert opinion on drug metabolism & toxicology. 2008;4(9):1209–1216. doi: 10.1517/17425255.4.9.1209. [DOI] [PubMed] [Google Scholar]
- 19.Croft SL, Coombs GH. Leishmaniasis–current chemotherapy and recent advances in the search for novel drugs. Trends in parasitology. 2003;19(11):502–508. doi: 10.1016/j.pt.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 20**.Dorlo TP, et al. Miltefosine: a review of its pharmacology and therapeutic efficacy in the treatment of leishmaniasis. Journal of Antimicrobial Chemotherapy. 2012;67(11):2576–2597. doi: 10.1093/jac/dks275. Established the experimental proof of the pharmacology and therapeutic efficacy of miltefosine for the treatment of leishmaniasis. [DOI] [PubMed] [Google Scholar]
- 21.Prajapati VK, et al. In vitro antileishmanial drug susceptibility of clinical isolates from patients with Indian visceral leishmaniasis—status of newly introduced drugs. The American journal of tropical medicine and hygiene. 2012;87(4):655–657. doi: 10.4269/ajtmh.2012.12-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davidson RN, den Boer M, Ritmeijer K. Paromomycin. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2009;103(7):653–660. doi: 10.1016/j.trstmh.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 23.Kellina O. A study of experimental cutaneous leishmaniasis in white mice. Meditsinskaia parazitologiia i parazitarnye bolezni. 1960;30:684–691. [PubMed] [Google Scholar]
- 24.Neal R. The effect of antibiotics of the neomycin group on experimental cutaneous leishmaniasis. Annals of Tropical Medicine & Parasitology. 1968;62(1):54–62. doi: 10.1080/00034983.1968.11686529. [DOI] [PubMed] [Google Scholar]
- 25.Neal R, et al. The sensitivity of Leishmania species to aminosidine. Journal of Antimicrobial Chemotherapy. 1995;35(5):577–584. doi: 10.1093/jac/35.5.577. [DOI] [PubMed] [Google Scholar]
- 26*.Patterson S, et al. The anti-tubercular drug delamanid as a potential oral treatment for visceral leishmaniasis. eLife. 2016;5:e09744. doi: 10.7554/eLife.09744. Research reported for the use of anti-tubercular drug in the treatment of leishmaniasis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sundar S, et al. Trial of oral miltefosine for visceral leishmaniasis. The Lancet. 1998;352(9143):1821–1823. doi: 10.1016/S0140-6736(98)04367-0. [DOI] [PubMed] [Google Scholar]
- 28**.Sundar S, et al. Oral miltefosine for Indian visceral leishmaniasis. N Engl J Med. 2002;2002(347):1739–1746. doi: 10.1056/NEJMoa021556. Study by Sundar et al.for establishing the ground for the use of oral miltefosine in treatment of visceral leishmaniasis in India. [DOI] [PubMed] [Google Scholar]
- 29.Sundar S, et al. Injectable paromomycin for visceral leishmaniasis in India. New England Journal of Medicine. 2007;356(25):2571–2581. doi: 10.1056/NEJMoa066536. [DOI] [PubMed] [Google Scholar]
- 30.Jha TK, et al. A phase II dose-ranging study of sitamaquine for the treatment of visceral leishmaniasis in India. The American journal of tropical medicine and hygiene. 2005;73(6):1005–1011. [PubMed] [Google Scholar]
- 31.Wasunna M, et al. AMERICAN JOURNAL OF TROPICAL MEDICINE AND HYGIENE. AMER SOC TROP MED & HYGIENE 8000 WESTPARK DR; STE 130, MCLEAN, VA 22101 USA: 2005. A Phase II dose-rising study of sitamaquine for the treatment of visceral leishmaniasis in Kenya. [PubMed] [Google Scholar]
- 32.Pink R, et al. Opportunities and challenges in antiparasitic drug discovery. Nature reviews Drug discovery. 2005;4(9):727. doi: 10.1038/nrd1824. [DOI] [PubMed] [Google Scholar]
- 33.Basso LA, et al. The use of biodiversity as source of new chemical entities against defined molecular targets for treatment of malaria, tuberculosis, and T-cell mediated diseases: a review. Memórias do Instituto Oswaldo Cruz. 2005;100(6):475–506. doi: 10.1590/s0074-02762005000600001. [DOI] [PubMed] [Google Scholar]
- 34.Iwu M, Jackson J, Schuster B. Medicinal plants in the fight against leishmaniasis. Parasitology today. 1994;10(2):65–68. doi: 10.1016/0169-4758(94)90398-0. [DOI] [PubMed] [Google Scholar]
- 35.Rocha L, et al. A review of natural products with antileishmanial activity. Phytomedicine. 2005;12(6):514–535. doi: 10.1016/j.phymed.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 36.Wang J, Peng Q, Guoyu G. New compounds of natural resources in 2008. African Journal of Biotechnology. 2009;8(18) [Google Scholar]
- 37.Lorenzi H, Matos FJ, Francisco JM. Plantas medicinais no Brasil: nativas e exóticas. 2002 [Google Scholar]
- 38.Da Silva S, et al. Therapeutic effect of oral Kalanchoe pinnata leaf extract in murine leishmaniasis. Acta Tropica. 1995;60(3):201–210. doi: 10.1016/0001-706x(95)00128-2. [DOI] [PubMed] [Google Scholar]
- 39.Da-Silva S, Costa S, Rossi-Bergmann B. The anti-leishmanial effect of Kalanchoe is mediated by nitric oxide intermediates. Parasitology. 1999;118(6):575–582. doi: 10.1017/s0031182099004357. [DOI] [PubMed] [Google Scholar]
- 40.Torres-Santos E, et al. Toxicological analysis and effectiveness of oral Kalanchoe pinnata on a human case of cutaneous leishmaniasis. Phytotherapy research. 2003;17(7):801–803. doi: 10.1002/ptr.1242. [DOI] [PubMed] [Google Scholar]
- 41.Awasthi BP, et al. Plumbagin, a plant-derived naphthoquinone metabolite induces mitochondria mediated apoptosis-like cell death in Leishmania donovani: an ultrastructural and physiological study. Apoptosis. 2016;21(8):941–953. doi: 10.1007/s10495-016-1259-9. [DOI] [PubMed] [Google Scholar]
- 42.Dutta A, et al. Aloe vera leaf exudate induces a caspase-independent cell death in Leishmania donovani promastigotes. Journal of medical microbiology. 2007;56(5):629–636. doi: 10.1099/jmm.0.47039-0. [DOI] [PubMed] [Google Scholar]
- 43.Date AA, Joshi MD, Patravale VB. Parasitic diseases: liposomes and polymeric nanoparticles versus lipid nanoparticles. Advanced drug delivery reviews. 2007;59(6):505–521. doi: 10.1016/j.addr.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 44.Das S, et al. One pot synthesis of gold nanoparticles and application in chemotherapy of wild and resistant type visceral leishmaniasis. Colloids and Surfaces B: Biointerfaces. 2013;107:27–34. doi: 10.1016/j.colsurfb.2013.01.061. [DOI] [PubMed] [Google Scholar]
- 45.Alavizadeh SH, et al. The role of liposome–protamine–DNA nanoparticles containing CpG oligodeoxynucleotides in the course of infection induced by Leishmania major in BALB/c mice. Experimental parasitology. 2012;132(3):313–319. doi: 10.1016/j.exppara.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 46*.Tafaghodi M, Khamesipour A, Jaafari MR. Immunization against leishmaniasis by PLGA nanospheres encapsulated with autoclaved Leishmania major (ALM) and CpG-ODN. Parasitology research. 2011;108(5):1265–1273. doi: 10.1007/s00436-010-2176-4. Intresting report of the combination of nanoparticles with autoclaved parasites for vaccine development. [DOI] [PubMed] [Google Scholar]
- 47.Badiee A, et al. Micro/nanoparticle adjuvants for antileishmanial vaccines: present and future trends. Vaccine. 2013;31(5):735–749. doi: 10.1016/j.vaccine.2012.11.068. [DOI] [PubMed] [Google Scholar]
- 48.Santos DM, et al. Towards development of novel immunization strategies against leishmaniasis using PLGA nanoparticles loaded with kinetoplastid membrane protein-11. International journal of nanomedicine. 2012;7:2115. doi: 10.2147/IJN.S30093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49*.Santos DM, et al. PLGA nanoparticles loaded with KMP-11 stimulate innate immunity and induce the killing of Leishmania. Nanomedicine: Nanotechnology, Biology and Medicine. 2013;9(7):985–995. doi: 10.1016/j.nano.2013.04.003. Reported for use of nanoparticle based vaccine for stimulating the innate immune in addtion to the parasite killing. [DOI] [PubMed] [Google Scholar]
- 50.Danesh-Bahreini MA, et al. Nanovaccine for leishmaniasis: preparation of chitosan nanoparticles containing Leishmania superoxide dismutase and evaluation of its immunogenicity in BALB/c mice. International journal of nanomedicine. 2011;6:835. doi: 10.2147/IJN.S16805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Manandhar KD, et al. Infectious Diseases and Nanomedicine II. Springer; 2014. Nanonization increases the antileishmanial efficacy of amphotericin B: an ex vivo approach; pp. 77–91. [DOI] [PubMed] [Google Scholar]
- 52.Jain K, et al. Surface-engineered dendrimeric nanoconjugates for macrophage-targeted delivery of amphotericin B: formulation development and in vitro and in vivo evaluation. Antimicrobial agents and chemotherapy. 2015;59(5):2479–2487. doi: 10.1128/AAC.04213-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fakhraee F, et al. Coadminstration of L. major amastigote class I nuclease (rLmaCIN) with LPD nanoparticles delays the progression of skin lesion and the L. major dissemination to the spleen in BALB/c mice-based experimental setting. Acta tropica. 2016;159:211–218. doi: 10.1016/j.actatropica.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 54.Hopkins AL, Groom CR. Opinion: The druggable genome. Nature reviews Drug discovery. 2002;1(9):727. doi: 10.1038/nrd892. [DOI] [PubMed] [Google Scholar]
- 55.Verlinde CL, et al. Glycolysis as a target for the design of new anti-trypanosome drugs. Drug Resistance Updates. 2001;4(1):50–65. doi: 10.1054/drup.2000.0177. [DOI] [PubMed] [Google Scholar]
- 56.Lee N, et al. Programmed cell death in the unicellular protozoan parasite Leishmania. Cell death and differentiation. 2002;9(1):53. doi: 10.1038/sj.cdd.4400952. [DOI] [PubMed] [Google Scholar]
- 57.Luque-Ortega JR, Rivas L. Miltefosine (hexadecylphosphocholine) inhibits cytochrome c oxidase in Leishmania donovani promastigotes. Antimicrobial agents and chemotherapy. 2007;51(4):1327–1332. doi: 10.1128/AAC.01415-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.DATTA G, BERA T. Transplasma membrane electron transport in Leishmania donovani promastigotes. Journal of Eukaryotic Microbiology. 2002;49(1):24–29. doi: 10.1111/j.1550-7408.2002.tb00335.x. [DOI] [PubMed] [Google Scholar]
- 59.Zhai L, et al. The antileishmanial activity of novel oxygenated chalcones and their mechanism of action. Journal of antimicrobial chemotherapy. 1999;43(6):793–803. doi: 10.1093/jac/43.6.793. [DOI] [PubMed] [Google Scholar]
- 60.Zhai L, et al. The antileishmanial agent licochalcone A interferes with the function of parasite mitochondria. Antimicrobial agents and chemotherapy. 1995;39(12):2742–2748. doi: 10.1128/aac.39.12.2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen M, et al. Inhibition of Fumarate Reductase inLeishmania major and L. donovani by Chalcones. Antimicrobial agents and chemotherapy. 2001;45(7):2023–2029. doi: 10.1128/AAC.45.7.2023-2029.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stickles AM, et al. Subtle changes in endochin-like quinolone structure alter the site of inhibition within the cytochrome bc1 complex of Plasmodium falciparum. Antimicrobial agents and chemotherapy. 2015;59(4):1977–1982. doi: 10.1128/AAC.04149-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ortiz D, et al. Targeting the cytochrome bc1 complex of Leishmania parasites for discovery of novel drugs. Antimicrobial agents and chemotherapy. 2016;60(8):4972–4982. doi: 10.1128/AAC.00850-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Luque-Ortega JR, et al. New benzophenone-derived bisphosphonium salts as leishmanicidal leads targeting mitochondria through inhibition of respiratory complex II. Journal of medicinal chemistry. 2010;53(4):1788–1798. doi: 10.1021/jm901677h. [DOI] [PubMed] [Google Scholar]
- 65.Sen R, et al. Artemisinin triggers induction of cell-cycle arrest and apoptosis in Leishmania donovani promastigotes. Journal of medical microbiology. 2007;56(9):1213–1218. doi: 10.1099/jmm.0.47364-0. [DOI] [PubMed] [Google Scholar]
- 66.Cauchetier E, et al. Characterisation of atovaquone resistance in Leishmania infantum promastigotes. International journal for parasitology. 2002;32(8):1043–1051. doi: 10.1016/s0020-7519(02)00065-6. [DOI] [PubMed] [Google Scholar]
- 67.Carvalho L, et al. Tafenoquine, an antiplasmodial 8-aminoquinoline, targets Leishmania respiratory complex III and induces apoptosis. Antimicrobial agents and chemotherapy. 2010;54(12):5344–5351. doi: 10.1128/AAC.00790-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dinesh N, et al. Exploring Leishmania donovani 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGR) as a potential drug target by biochemical, biophysical and inhibition studies. Microbial pathogenesis. 2014;66:14–23. doi: 10.1016/j.micpath.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 69.Kaur J, et al. Bioinformatic analysis of Leishmania donovani long-chain fatty acid-CoA ligase as a novel drug target. Molecular biology international. 2011;2011 doi: 10.4061/2011/278051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rodrigues JCF, et al. In vitro activities of ER-119884 and E5700, two potent squalene synthase inhibitors, against Leishmania amazonensis: antiproliferative, biochemical, and ultrastructural effects. Antimicrobial agents and chemotherapy. 2008;52(11):4098–4114. doi: 10.1128/AAC.01616-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.VANNIER-SANTOS MA, et al. Alterations induced by the antifungal compounds ketoconazole and terbinafine in Leishmania. Journal of eukaryotic microbiology. 1995;42(4):337–346. doi: 10.1111/j.1550-7408.1995.tb01591.x. [DOI] [PubMed] [Google Scholar]
- 72.Martin MB, et al. Bisphosphonates Inhibit the Growth of Trypanosoma b rucei, Trypanosoma c ruzi, Leishmania d onovani, Toxoplasma g ondii, and Plasmodium f alciparum: A Potential Route to Chemotherapy. Journal of medicinal chemistry. 2001;44(6):909–916. doi: 10.1021/jm0002578. [DOI] [PubMed] [Google Scholar]
- 73.Docampo R, Moreno SN. The acidocalcisome as a target for chemotherapeutic agents in protozoan parasites. Current pharmaceutical design. 2008;14(9):882–888. doi: 10.2174/138161208784041079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Magaraci F, et al. Azasterols as Inhibitors of Sterol 24-Methyltransferase in Leishmania Species and Trypanosoma c ruzi. Journal of medicinal chemistry. 2003;46(22):4714–4727. doi: 10.1021/jm021114j. [DOI] [PubMed] [Google Scholar]
- 75.Lorente SO, et al. Novel azasterols as potential agents for treatment of leishmaniasis and trypanosomiasis. Antimicrobial agents and chemotherapy. 2004;48(8):2937–2950. doi: 10.1128/AAC.48.8.2937-2950.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McCall LI, et al. Targeting ergosterol biosynthesis in Leishmania donovani: essentiality of sterol 14alpha-demethylase. PLoS neglected tropical diseases. 2015;9(3):e0003588. doi: 10.1371/journal.pntd.0003588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lepesheva GI, et al. VFV as a new effective CYP51 structure-derived drug candidate for Chagas disease and visceral leishmaniasis. The Journal of infectious diseases. 2015;212(9):1439–1448. doi: 10.1093/infdis/jiv228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Andrade-Neto VV, et al. Imipramine alters the sterol profile in Leishmania amazonensis and increases its sensitivity to miconazole. Parasites & vectors. 2016;9(1):183. doi: 10.1186/s13071-016-1467-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Villa-Pulgarín JA, et al. Mitochondria and lipid raft-located F O F 1-ATP synthase as major therapeutic targets in the antileishmanial and anticancer activities of ether lipid edelfosine. PLoS Neglected Tropical Diseases. 2017;11(8) doi: 10.1371/journal.pntd.0005805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Silva ER, et al. Novel selective inhibitor of Leishmania (Leishmania) amazonensis arginase. Chemical biology & drug design. 2015;86(5):969–978. doi: 10.1111/cbdd.12566. [DOI] [PubMed] [Google Scholar]
- 81.Boitz JM, et al. Leishmania donovani ornithine decarboxylase is indispensable for parasite survival in the mammalian host. Infection and immunity. 2009;77(2):756–763. doi: 10.1128/IAI.01236-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Haimeur A, et al. Elevated levels of polyamines and trypanothione resulting from overexpression of the ornithine decarboxylase gene in arsenite-resistant Leishmania. Molecular microbiology. 1999;34(4):726–735. doi: 10.1046/j.1365-2958.1999.01634.x. [DOI] [PubMed] [Google Scholar]
- 83.Brun R, et al. In vitro trypanocidal activities of new S-adenosylmethionine decarboxylase inhibitors. Antimicrobial agents and chemotherapy. 1996;40(6):1442–1447. doi: 10.1128/aac.40.6.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Baumann R, et al. Suppression of both antimony-susceptible and antimony-resistant Leishmania donovani by a bis (benzyl) polyamine analog. Antimicrobial agents and chemotherapy. 1990;34(5):722–727. doi: 10.1128/aac.34.5.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wyllie S, Cunningham ML, Fairlamb AH. Dual action of antimonial drugs on thiol redox metabolism in the human pathogen Leishmania donovani. Journal of Biological Chemistry. 2004;279(38):39925–39932. doi: 10.1074/jbc.M405635200. [DOI] [PubMed] [Google Scholar]
- 86.Roberts SC, et al. Leishmania donovani polyamine biosynthetic enzyme overproducers as tools to investigate the mode of action of cytotoxic polyamine analogs. Antimicrobial agents and chemotherapy. 2007;51(2):438–445. doi: 10.1128/AAC.01193-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Khan MOF. Trypanothione reductase: a viable chemotherapeutic target for antitrypanosomal and antileishmanial drug design. Drug target insights. 2007;2:129. [PMC free article] [PubMed] [Google Scholar]
- 88.Wilkinson SR, et al. RNA interference identifies two hydroperoxide metabolizing enzymes that are essential to the bloodstream form of the African trypanosome. Journal of Biological Chemistry. 2003;278(34):31640–31646. doi: 10.1074/jbc.M303035200. [DOI] [PubMed] [Google Scholar]
- 89.Chawla B, et al. Identification and characterization of a novel deoxyhypusine synthase in Leishmania donovani. Journal of biological chemistry. 2010;285(1):453–463. doi: 10.1074/jbc.M109.048850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chawla B, et al. A unique modification of the eukaryotic initiation factor 5A shows the presence of the complete hypusine pathway in Leishmania donovani. PLoS One. 2012;7(3):e33138. doi: 10.1371/journal.pone.0033138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Balaña-Fouce R, Ordóñez D, Alunda J. Putrescine transport system in Leishmania infantum promastigotes. Molecular and biochemical parasitology. 1989;35(1):43–50. doi: 10.1016/0166-6851(89)90140-0. [DOI] [PubMed] [Google Scholar]
- 92.Basselin M, Coombs GH, Barrett MP. Putrescine and spermidine transport in Leishmania. Molecular and biochemical parasitology. 2000;109(1):37–46. doi: 10.1016/s0166-6851(00)00234-6. [DOI] [PubMed] [Google Scholar]
- 93.Vannier-Santos MA, et al. The putrescine analogue 1, 4-diamino-2-butanone affects polyamine synthesis, transport, ultrastructure and intracellular survival in Leishmania amazonensis. Microbiology. 2008;154(10):3104–3111. doi: 10.1099/mic.0.2007/013896-0. [DOI] [PubMed] [Google Scholar]
- 94.Fox JT, Stover PJ. Folate-mediated one-carbon metabolism. Vitamins & hormones. 2008;79:1–44. doi: 10.1016/S0083-6729(08)00401-9. [DOI] [PubMed] [Google Scholar]
- 95.Ouellette M, et al. Pterin transport and metabolism in Leishmania and related trypanosomatid parasites. International journal for parasitology. 2002;32(4):385–398. doi: 10.1016/s0020-7519(01)00346-0. [DOI] [PubMed] [Google Scholar]
- 96.Nare B, et al. New approaches to Leishmania chemotherapy: pteridine reductase 1 (PTR1) as a target and modulator of antifolate sensitivity. Parasitology. 1997;114(7):101–110. [PubMed] [Google Scholar]
- 97.El Fadili A, Kündig C, Ouellette M. Characterization of the folylpolyglutamate synthetase gene and polyglutamylation of folates in the protozoan parasite Leishmania. Molecular and biochemical parasitology. 2002;124(1):63–71. doi: 10.1016/s0166-6851(02)00163-9. [DOI] [PubMed] [Google Scholar]
- 98.Stover P, Schirch V. The metabolic role of leucovorin. Trends in biochemical sciences. 1993;18(3):102–106. doi: 10.1016/0968-0004(93)90162-g. [DOI] [PubMed] [Google Scholar]
- 99.Yount NY, Yeaman MR. Multidimensional signatures in antimicrobial peptides. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(19):7363–7368. doi: 10.1073/pnas.0401567101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hancock RE. Cationic peptides: effectors in innate immunity and novel antimicrobials. The Lancet infectious diseases. 2001;1(3):156–164. doi: 10.1016/S1473-3099(01)00092-5. [DOI] [PubMed] [Google Scholar]
- 101.Bera A, et al. Induction of autophagic cell death in Leishmania donovani by antimicrobial peptides. Molecular and biochemical parasitology. 2003;127(1):23–35. doi: 10.1016/s0166-6851(02)00300-6. [DOI] [PubMed] [Google Scholar]
- 102.Brogden KA. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nature reviews. Microbiology. 2005;3(3):238. doi: 10.1038/nrmicro1098. [DOI] [PubMed] [Google Scholar]
- 103.Ganz T, Lehrer RI. Defensins. Current opinion in immunology. 1994;6(4):584–589. doi: 10.1016/0952-7915(94)90145-7. [DOI] [PubMed] [Google Scholar]
- 104.Luque-Ortega JR, et al. Human antimicrobial peptide histatin 5 is a cell-penetrating peptide targeting mitochondrial ATP synthesis in Leishmania. The FASEB Journal. 2008;22(6):1817–1828. doi: 10.1096/fj.07-096081. [DOI] [PubMed] [Google Scholar]
- 105.Luque-Ortega JR, et al. In Vivo Monitoring of Intracellular ATP Levels inLeishmania donovani Promastigotes as a Rapid Method To Screen Drugs Targeting Bioenergetic Metabolism. Antimicrobial agents and chemotherapy. 2001;45(4):1121–1125. doi: 10.1128/AAC.45.4.1121-1125.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hernandez C, et al. Functional and structural damage in Leishmania mexicana exposed to the cationic peptide dermaseptin. European journal of cell biology. 1992;59:414–414. [PubMed] [Google Scholar]
- 107.Kückelhaus SA, et al. Antiplasmodial and antileishmanial activities of phylloseptin-1, an antimicrobial peptide from the skin secretion of Phyllomedusa azurea (Amphibia) Experimental parasitology. 2009;123(1):11–16. doi: 10.1016/j.exppara.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 108.Mangoni ML, et al. Effect of natural L-to D-amino acid conversion on the organization, membrane binding, and biological function of the antimicrobial peptides bombinins H. Biochemistry. 2006;45(13):4266–4276. doi: 10.1021/bi052150y. [DOI] [PubMed] [Google Scholar]
- 109.Abbassi F, et al. Antibacterial and leishmanicidal activities of temporin-SHd, a 17-residue long membrane-damaging peptide. Biochimie. 2013;95(2):388–399. doi: 10.1016/j.biochi.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 110.Sardar A, et al. Spinigerin induces apoptotic like cell death in a caspase independent manner in Leishmania donovani. Experimental parasitology. 2013;135(4):715–725. doi: 10.1016/j.exppara.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 111.Guerrero E, et al. Role of positional hydrophobicity in the leishmanicidal activity of magainin 2. Antimicrobial agents and chemotherapy. 2004;48(8):2980–2986. doi: 10.1128/AAC.48.8.2980-2986.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.McGwire BS, et al. Killing of African trypanosomes by antimicrobial peptides. The Journal of infectious diseases. 2003;188(1):146–152. doi: 10.1086/375747. [DOI] [PubMed] [Google Scholar]
- 113.Yount NY, et al. Structural correlates of antimicrobial efficacy in IL-8 and related human kinocidins. Biochimica et Biophysica Acta (BBA)-Biomembranes. 2007;1768(3):598–608. doi: 10.1016/j.bbamem.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 114.Wolf M, Moser B. Antimicrobial activities of chemokines: not just a side-effect? Frontiers in immunology. 2012;3 doi: 10.3389/fimmu.2012.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yung SC, Murphy PM. Antimicrobial chemokines. Frontiers in immunology. 2012;3 doi: 10.3389/fimmu.2012.00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.McGwire BS, Kulkarni MM. Interactions of antimicrobial peptides with Leishmania and trypanosomes and their functional role in host parasitism. Experimental parasitology. 2010;126(3):397–405. doi: 10.1016/j.exppara.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 117.Yao C, Donelson JE, Wilson ME. The major surface protease (MSP or GP63) of Leishmania sp. Biosynthesis, regulation of expression, and function. Molecular and biochemical parasitology. 2003;132(1):1–16. doi: 10.1016/s0166-6851(03)00211-1. [DOI] [PubMed] [Google Scholar]
- 118.Mangoni ML, et al. Temporins, small antimicrobial peptides with leishmanicidal activity. Journal of Biological Chemistry. 2005;280(2):984–990. doi: 10.1074/jbc.M410795200. [DOI] [PubMed] [Google Scholar]
- 119.Luque-Ortega JR, et al. Human antimicrobial peptide histatin 5 is a cell-penetrating peptide targeting mitochondrial ATP synthesis in Leishmania. FASEB J. 2008;22(6):1817–28. doi: 10.1096/fj.07-096081. [DOI] [PubMed] [Google Scholar]
- 120.Moreno SN, Docampo R. Calcium regulation in protozoan parasites. Current opinion in microbiology. 2003;6(4):359–364. doi: 10.1016/s1369-5274(03)00091-2. [DOI] [PubMed] [Google Scholar]
- 121.Alberola J, et al. Safety and efficacy of antimicrobial peptides against naturally acquired leishmaniasis. Antimicrobial agents and chemotherapy. 2004;48(2):641–643. doi: 10.1128/AAC.48.2.641-643.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kulkarni MM, et al. Mammalian antimicrobial peptide influences control of cutaneous Leishmania infection. Cellular microbiology. 2011;13(6):913–923. doi: 10.1111/j.1462-5822.2011.01589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Katz O, et al. Adenosine, AMP, and protein phosphatase activity in sandfly saliva. The American journal of tropical medicine and hygiene. 2000;62(1):145–150. doi: 10.4269/ajtmh.2000.62.145. [DOI] [PubMed] [Google Scholar]
- 124.Jastrab JB, Darwin KH. Bacterial proteasomes. Annual review of microbiology. 2015;69:109–127. doi: 10.1146/annurev-micro-091014-104201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Burns KE, et al. “Depupylation” of prokaryotic ubiquitin-like protein from mycobacterial proteasome substrates. Molecular cell. 2010;39(5):821–827. doi: 10.1016/j.molcel.2010.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Komander D, Clague MJ, Urbé S. Breaking the chains: structure and function of the deubiquitinases. Nature reviews. Molecular cell biology. 2009;10(8):550. doi: 10.1038/nrm2731. [DOI] [PubMed] [Google Scholar]
- 127.Sinha A, Sarkar S. Ubiquitin-Proteasome System-a target to control pathogenic protozoa. Formatex 2013. 2013:764–773. [Google Scholar]
- 128.Gonzalez J, et al. Proteasome activity is required for the stage-specific transformation of a protozoan parasite. Journal of Experimental Medicine. 1996;184(5):1909–1918. doi: 10.1084/jem.184.5.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Raina P, Kaur S. Knockdown of LdMC1 and Hsp70 by antisense oligonucleotides causes cell-cycle defects and programmed cell death in Leishmania donovani. Molecular and cellular biochemistry. 2012;359(1–2):135–149. doi: 10.1007/s11010-011-1007-y. [DOI] [PubMed] [Google Scholar]
- 130.Silva-Jardim I, Horta MF, Ramalho-Pinto FJ. The Leishmania chagasi proteasome: role in promastigotes growth and amastigotes survival within murine macrophages. Acta tropica. 2004;91(2):121–130. doi: 10.1016/j.actatropica.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 131.Khare S, et al. Proteasome inhibition for treatment of leishmaniasis, Chagas disease and sleeping sickness. Nature. 2016;537(7619):229–233. doi: 10.1038/nature19339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gannavaram S, et al. Mitochondrial associated ubiquitin fold modifier-1 mediated protein conjugation in Leishmania donovani. PLoS One. 2011;6(1):e16156. doi: 10.1371/journal.pone.0016156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Azevedo CS, et al. Revealing a Novel Otubain-Like Enzyme from Leishmania infantum with Deubiquitinating Activity toward K48-Linked Substrate. Front Chem. 2017;5:13. doi: 10.3389/fchem.2017.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zeidner JF, et al. Randomized multicenter phase II study of flavopiridol (alvocidib), cytarabine, and mitoxantrone (FLAM) versus cytarabine/daunorubicin (7+3) in newly diagnosed acute myeloid leukemia. Haematologica. 2015;100(9):1172–9. doi: 10.3324/haematol.2015.125849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zeidner JF, Karp JE. Clinical activity of alvocidib (flavopiridol) in acute myeloid leukemia. Leuk Res. 2015;39(12):1312–8. doi: 10.1016/j.leukres.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 136.Grant KM, et al. The crk3 Gene of Leishmania mexicanaEncodes a Stage-regulated cdc2-related Histone H1 Kinase That Associates with p12 cks1. Journal of Biological Chemistry. 1998;273(17):10153–10159. doi: 10.1074/jbc.273.17.10153. [DOI] [PubMed] [Google Scholar]
- 137.Grant KM, et al. Inhibitors of Leishmania mexicana CRK3 cyclin-dependent kinase: chemical library screen and antileishmanial activity. Antimicrobial agents and chemotherapy. 2004;48(8):3033–3042. doi: 10.1128/AAC.48.8.3033-3042.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chhajer R, et al. Leishmania donovani Aurora kinase: A promising therapeutic target against visceral leishmaniasis. Biochimica et Biophysica Acta (BBA)-General Subjects. 2016;1860(9):1973–1988. doi: 10.1016/j.bbagen.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 139.Darkin-Rattray SJ, et al. Apicidin: a novel antiprotozoal agent that inhibits parasite histone deacetylase. Proceedings of the National Academy of Sciences. 1996;93(23):13143–13147. doi: 10.1073/pnas.93.23.13143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Vergnes B, et al. Targeted disruption of cytosolic SIR2 deacetylase discloses its essential role in Leishmania survival and proliferation. Gene. 2005;363:85–96. doi: 10.1016/j.gene.2005.06.047. [DOI] [PubMed] [Google Scholar]
- 141.Xingi E, et al. 6-Br-5methylindirubin-3′ oxime (5-Me-6-BIO) targeting the leishmanial glycogen synthase kinase-3 (GSK-3) short form affects cell-cycle progression and induces apoptosis-like death: exploitation of GSK-3 for treating leishmaniasis. International journal for parasitology. 2009;39(12):1289–1303. doi: 10.1016/j.ijpara.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 142.Ambit A, et al. An essential role for the Leishmania major metacaspase in cell cycle progression. Cell death and differentiation. 2008;15(1):113. doi: 10.1038/sj.cdd.4402232. [DOI] [PubMed] [Google Scholar]
- 143.Bates PA, Dwyer DM. Biosynthesis and secretion of acid phosphatase by Leishmania donovani promastigotes. Molecular and biochemical parasitology. 1987;26(3):289–296. doi: 10.1016/0166-6851(87)90081-8. [DOI] [PubMed] [Google Scholar]
- 144.Shakarian AM, Dwyer DM. The Ld Cht1 gene encodes the secretory chitinase of the human pathogen Leishmania donovani. Gene. 1998;208(2):315–22. doi: 10.1016/s0378-1119(98)00011-0. [DOI] [PubMed] [Google Scholar]
- 145.Webb JR, et al. Human and murine immune responses to a novel Leishmania major recombinant protein encoded by members of a multicopy gene family. Infect Immun. 1998;66(7):3279–89. doi: 10.1128/iai.66.7.3279-3289.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Silverman JM, et al. Proteomic analysis of the secretome of Leishmania donovani. Genome Biol. 2008;9(2):R35. doi: 10.1186/gb-2008-9-2-r35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.McConville MJ, et al. Secretory pathway of trypanosomatid parasites. Microbiology and Molecular Biology Reviews. 2002;66(1):122–154. doi: 10.1128/MMBR.66.1.122-154.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Gosline SJ, et al. Intracellular eukaryotic parasites have a distinct unfolded protein response. PLoS One. 2011;6(4):e19118. doi: 10.1371/journal.pone.0019118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Smith MH, Ploegh HL, Weissman JS. Road to ruin: targeting proteins for degradation in the endoplasmic reticulum. Science. 2011;334(6059):1086–1090. doi: 10.1126/science.1209235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Dias-Teixeira KL, et al. The integrated endoplasmic reticulum stress response in Leishmania amazonensis macrophage infection: the role of X-box binding protein 1 transcription factor. The FASEB Journal. 2016;30(4):1557–1565. doi: 10.1096/fj.15-281550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Luo J-Q, Chen D-W, Yu B. Upregulation of amino acid transporter expression induced by L-leucine availability in L6 myotubes is associated with ATF4 signaling through mTORC1-dependent mechanism. Nutrition. 2013;29(1):284–290. doi: 10.1016/j.nut.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 152.Cyrino LT, et al. In vivo and in vitro Leishmania amazonensis infection induces autophagy in macrophages. Tissue and Cell. 2012;44(6):401–408. doi: 10.1016/j.tice.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 153.Axten JM, et al. Discovery of 7-methyl-5-(1-{[3-(trifluoromethyl) phenyl] acetyl}-2, 3-dihydro-1 H-indol-5-yl)-7 H-pyrrolo [2, 3-d] pyrimidin-4-amine (GSK2606414), a potent and selective first-in-class Inhibitor of protein kinase R (PKR)-like endoplasmic reticulum kinase (PERK) Journal of medicinal chemistry. 2012;55(16):7193–7207. doi: 10.1021/jm300713s. [DOI] [PubMed] [Google Scholar]
- 154.Joshi M, et al. Isolation and characterization of Leishmania donovani calreticulin gene and its conservation of the RNA binding activity. Molecular and biochemical parasitology. 1996;81(1):53–64. doi: 10.1016/0166-6851(96)02676-x. [DOI] [PubMed] [Google Scholar]
- 155.Bangs JD, et al. A Soluble Secretory Reporter System in Trypanosoma brucei STUDIES ON ENDOPLASMIC RETICULUM TARGETING. Journal of Biological Chemistry. 1996;271(31):18387–18393. doi: 10.1074/jbc.271.31.18387. [DOI] [PubMed] [Google Scholar]
- 156.Hong B, Soong L. Identification and enzymatic activities of four protein disulfide isomerase (PDI) isoforms of Leishmania amazonensis. Parasitology research. 2008;102(3):437–446. doi: 10.1007/s00436-007-0784-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Debrabant A, et al. Expression of calreticulin P-domain results in impairment of secretory pathway in Leishmania donovani and reduced parasite survival in macrophages. International journal for parasitology. 2002;32(11):1423–1434. doi: 10.1016/s0020-7519(02)00134-0. [DOI] [PubMed] [Google Scholar]
- 158.Canton J, et al. Disruption of the fusion of Leishmania parasitophorous vacuoles with ER vesicles results in the control of the infection. Cellular microbiology. 2012;14(6):937–948. doi: 10.1111/j.1462-5822.2012.01767.x. [DOI] [PubMed] [Google Scholar]
- 159.Padilla A, et al. An atypical protein disulfide isomerase from the protozoan parasite Leishmania containing a single thioredoxin-like domain. Journal of Biological Chemistry. 2003;278(3):1872–1878. doi: 10.1074/jbc.M210322200. [DOI] [PubMed] [Google Scholar]
- 160.Achour YB, et al. Identification of a disulfide isomerase protein of Leishmania major as a putative virulence factor. Infection and Immunity. 2002;70(7):3576–3585. doi: 10.1128/IAI.70.7.3576-3585.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ben Khalaf N, et al. Leishmania major protein disulfide isomerase as a drug target. Parasitology Research. 2012;110(5):1911–1917. doi: 10.1007/s00436-011-2717-5. [DOI] [PubMed] [Google Scholar]
- 162.Harbut MB, et al. Targeting the ERAD pathway via inhibition of signal peptide peptidase for antiparasitic therapeutic design. Proceedings of the National Academy of Sciences. 2012;109(52):21486–21491. doi: 10.1073/pnas.1216016110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Pacis A, et al. Bacterial infection remodels the DNA methylation landscape of human dendritic cells. Genome research. 2015;25(12):1801–1811. doi: 10.1101/gr.192005.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Cizmeci D, et al. Mapping epigenetic changes to the host cell genome induced by Burkholderia pseudomallei reveals pathogen-specific and pathogen-generic signatures of infection. Scientific reports. 2016;6 doi: 10.1038/srep30861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165**.Marr AK, et al. Leishmania donovani infection causes distinct epigenetic DNA methylation changes in host macrophages. PLoS pathogens. 2014;10(10):e1004419. doi: 10.1371/journal.ppat.1004419. First report establishing the role of host epigenetics in Leishmania infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Leng J, et al. Toxoplasma gondii prevents chromatin remodeling initiated by TLR-triggered macrophage activation. The Journal of Immunology. 2009;182(1):489–497. doi: 10.4049/jimmunol.182.1.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lang C, et al. Impaired chromatin remodelling at STAT1-regulated promoters leads to global unresponsiveness of Toxoplasma gondii-infected macrophages to IFN-γ. PLoS pathogens. 2012;8(1):e1002483. doi: 10.1371/journal.ppat.1002483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Eskandarian HA, et al. A role for SIRT2-dependent histone H3K18 deacetylation in bacterial infection. Science. 2013;341(6145):1238858. doi: 10.1126/science.1238858. [DOI] [PubMed] [Google Scholar]
- 169.Marazzi I, et al. Suppression of the antiviral response by an influenza histone mimic. Nature. 2012;483(7390):428. doi: 10.1038/nature10892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Rolando M, et al. Legionella pneumophila effector RomA uniquely modifies host chromatin to repress gene expression and promote intracellular bacterial replication. Cell host & microbe. 2013;13(4):395–405. doi: 10.1016/j.chom.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 171.Ribet D, Cossart P. Post-translational modifications in host cells during bacterial infection. FEBS letters. 2010;584(13):2748–2758. doi: 10.1016/j.febslet.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 172.Pennini ME, et al. Mycobacterium tuberculosis 19-kDa lipoprotein inhibits IFN-γ-induced chromatin remodeling of MHC2TA by TLR2 and MAPK signaling. The Journal of Immunology. 2006;176(7):4323–4330. doi: 10.4049/jimmunol.176.7.4323. [DOI] [PubMed] [Google Scholar]
- 173.Sabin LR, Delás MJ, Hannon GJ. Dogma derailed: the many influences of RNA on the genome. Molecular cell. 2013;49(5):783–794. doi: 10.1016/j.molcel.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Marinus MG, Casadesus J. Roles of DNA adenine methylation in host–pathogen interactions: mismatch repair, transcriptional regulation, and more. FEMS microbiology reviews. 2009;33(3):488–503. doi: 10.1111/j.1574-6976.2008.00159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Volz JC, et al. PfSET10, a Plasmodium falciparum methyltransferase, maintains the active var gene in a poised state during parasite division. Cell host & microbe. 2012;11(1):7–18. doi: 10.1016/j.chom.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 176.Aisen P, Enns C, Wessling-Resnick M. Chemistry and biology of eukaryotic iron metabolism. The international journal of biochemistry & cell biology. 2001;33(10):940–959. doi: 10.1016/s1357-2725(01)00063-2. [DOI] [PubMed] [Google Scholar]
- 177.Chung J, Wessling-Resnick M. Molecular mechanisms and regulation of iron transport. Critical reviews in clinical laboratory sciences. 2003;40(2):151–182. doi: 10.1080/713609332. [DOI] [PubMed] [Google Scholar]
- 178.Jacques I, Andrews NW, Huynh C. Functional characterization of LIT1, the Leishmania amazonensis ferrous iron transporter. Molecular and biochemical parasitology. 2010;170(1):28–36. doi: 10.1016/j.molbiopara.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Huynh C, Sacks DL, Andrews NW. A Leishmania amazonensis ZIP family iron transporter is essential for parasite replication within macrophage phagolysosomes. Journal of Experimental Medicine. 2006;203(10):2363–2375. doi: 10.1084/jem.20060559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Mittra B, et al. Iron uptake controls the generation of Leishmania infective forms through regulation of ROS levels. Journal of Experimental Medicine. 2013;210(2):401–416. doi: 10.1084/jem.20121368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Flannery AR, et al. LFR1 ferric iron reductase of Leishmania amazonensis is essential for the generation of infective parasite forms. Journal of Biological Chemistry. 2011;286(26):23266–23279. doi: 10.1074/jbc.M111.229674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Mittra B, et al. The iron-dependent mitochondrial superoxide dismutase SODA promotes Leishmania virulence. Journal of Biological Chemistry. 2017 doi: 10.1074/jbc.M116.772624. p. jbc. M116. 772624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Martínez-García M, et al. LmABCB3, an atypical mitochondrial ABC transporter essential for Leishmania major virulence, acts in heme and cytosolic iron/sulfur clusters biogenesis. Parasites & vectors. 2016;9(1):7. doi: 10.1186/s13071-015-1284-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Campos-Salinas J, et al. LABCG2, a new ABC transporter implicated in phosphatidylserine exposure, is involved in the infectivity and pathogenicity of Leishmania. PLoS neglected tropical diseases. 2013;7(4):e2179. doi: 10.1371/journal.pntd.0002179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Perea A, et al. The LABCG2 transporter from the protozoan parasite Leishmania is involved in antimony resistance. Antimicrobial agents and chemotherapy. 2016;60(6):3489–3496. doi: 10.1128/AAC.02813-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Manzano JI, et al. Leishmania LABCG1 and LABCG2 transporters are involved in virulence and oxidative stress: functional linkage with autophagy. Parasites & vectors. 2017;10(1):267. doi: 10.1186/s13071-017-2198-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Huynh C, et al. Heme uptake by Leishmania amazonensis is mediated by the transmembrane protein LHR1. PLoS pathogens. 2012;8(7):e1002795. doi: 10.1371/journal.ppat.1002795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Renberg RL, et al. The heme transport capacity of LHR1 determines the extent of virulence in Leishmania amazonensis. PLoS neglected tropical diseases. 2015;9(5):e0003804. doi: 10.1371/journal.pntd.0003804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Miguel DC, et al. Heme uptake mediated by LHR1 is essential for Leishmania amazonensis virulence. Infection and immunity. 2013;81(10):3620–3626. doi: 10.1128/IAI.00687-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190**.Mittra B, et al. A trypanosomatid iron transporter that regulates mitochondrial function is required for Leishmania amazonensis virulence. PLoS pathogens. 2016;12(1):e1005340. doi: 10.1371/journal.ppat.1005340. Interesting report on the crucial role of iron transporter mediated parasite virulence. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Urrutia P, et al. Inflammation alters the expression of DMT1, FPN1 and hepcidin, and it causes iron accumulation in central nervous system cells. Journal of neurochemistry. 2013;126(4):541–549. doi: 10.1111/jnc.12244. [DOI] [PubMed] [Google Scholar]
- 192.D’Alessio F, Hentze MW, Muckenthaler MU. The hemochromatosis proteins HFE, TfR2, and HJV form a membrane-associated protein complex for hepcidin regulation. Journal of hepatology. 2012;57(5):1052–1060. doi: 10.1016/j.jhep.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 193.Nemeth E, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. science. 2004;306(5704):2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 194.Abboud S, Haile DJ. A novel mammalian iron-regulated protein involved in intracellular iron metabolism. Journal of Biological Chemistry. 2000;275(26):19906–19912. doi: 10.1074/jbc.M000713200. [DOI] [PubMed] [Google Scholar]
- 195.Nemeth E, et al. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. Journal of Clinical Investigation. 2004;113(9):1271. doi: 10.1172/JCI20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Parsons M, et al. Comparative analysis of the kinomes of three pathogenic trypanosomatids: Leishmania major, Trypanosoma brucei and Trypanosoma cruzi. BMC Genomics. 2005;6:127. doi: 10.1186/1471-2164-6-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.El-Sayed NM, et al. Comparative genomics of trypanosomatid parasitic protozoa. Science. 2005;309(5733):404–409. doi: 10.1126/science.1112181. [DOI] [PubMed] [Google Scholar]
- 198.Martinez PA, Petersen CA. Chronic infection by Leishmania amazonensis mediated through MAPK ERK mechanisms. Immunologic research. 2014;59(1–3):153–165. doi: 10.1007/s12026-014-8535-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Rotella DP. Recent results in protein kinase inhibition for tropical diseases. Bioorganic & medicinal chemistry letters. 2012;22(22):6788–6793. doi: 10.1016/j.bmcl.2012.09.044. [DOI] [PubMed] [Google Scholar]
- 200.Mol M, Patole MS, Singh S. Immune signal transduction in leishmaniasis from natural to artificial systems: role of feedback loop insertion. Biochimica et Biophysica Acta (BBA)-General Subjects. 2014;1840(1):71–79. doi: 10.1016/j.bbagen.2013.08.018. [DOI] [PubMed] [Google Scholar]
- 201.Reinhard K, et al. The role of NF-κB activation during protection against Leishmania infection. International Journal of Medical Microbiology. 2012;302(4):230–235. doi: 10.1016/j.ijmm.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 202.Engh RA, Bossemeyer D. Structural aspects of protein kinase control—role of conformational flexibility. Pharmacology & therapeutics. 2002;93(2):99–111. doi: 10.1016/s0163-7258(02)00180-8. [DOI] [PubMed] [Google Scholar]
- 203.Wetzel DM, McMahon-Pratt D, Koleske AJ. The Abl and Arg kinases mediate distinct modes of phagocytosis and are required for maximal Leishmania infection. Molecular and cellular biology. 2012;32(15):3176–3186. doi: 10.1128/MCB.00086-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Bradley WD, Koleske AJ. Regulation of cell migration and morphogenesis by Abl-family kinases: emerging mechanisms and physiological contexts. Journal of cell science. 2009;122(19):3441–3454. doi: 10.1242/jcs.039859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Burton EA, Plattner R, Pendergast AM. Abl tyrosine kinases are required for infection by Shigella flexneri. The EMBO journal. 2003;22(20):5471–5479. doi: 10.1093/emboj/cdg512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Elwell CA, et al. RNA interference screen identifies Abl kinase and PDGFR signaling in Chlamydia trachomatis entry. PLoS pathogens. 2008;4(3):e1000021. doi: 10.1371/journal.ppat.1000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Napier RJ, et al. Imatinib-sensitive tyrosine kinases regulate mycobacterial pathogenesis and represent therapeutic targets against tuberculosis. Cell host & microbe. 2011;10(5):475–485. doi: 10.1016/j.chom.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Seger R, Krebs EG. The MAPK signaling cascade. The FASEB journal. 1995;9(9):726–735. [PubMed] [Google Scholar]
- 209.Yang SH, Sharrocks AD, Whitmarsh AJ. Transcriptional regulation by the MAP kinase signaling cascades. Gene. 2003;320:3–21. doi: 10.1016/s0378-1119(03)00816-3. [DOI] [PubMed] [Google Scholar]
- 210.Kyriakis JM, et al. The stress-activated protein kinase subfamily of c-Jun kinases. Nature. 1994;369(6476):156–160. doi: 10.1038/369156a0. [DOI] [PubMed] [Google Scholar]
- 211.Whitmarsh AJ. A central role for p38 MAPK in the early transcriptional response to stress. BMC biology. 2010;8(1):47. doi: 10.1186/1741-7007-8-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Morales MA, Pescher P, Späth GF. Leishmania major MPK7 protein kinase activity inhibits intracellular growth of the pathogenic amastigote stage. Eukaryotic cell. 2010;9(1):22–30. doi: 10.1128/EC.00196-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Garg M, et al. Downregulation of mitogen-activated protein kinase 1 of Leishmania donovani field isolates is associated with antimony resistance. Antimicrobial agents and chemotherapy. 2012;56(1):518–525. doi: 10.1128/AAC.00736-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Pereira RM, et al. Novel role for the double-stranded RNA-activated protein kinase PKR: modulation of macrophage infection by the protozoan parasite Leishmania. The FASEB Journal. 2010;24(2):617–626. doi: 10.1096/fj.09-140053. [DOI] [PubMed] [Google Scholar]
- 215.Wu K, et al. Improved control of tuberculosis and activation of macrophages in mice lacking protein kinase R. PloS one. 2012;7(2):e30512. doi: 10.1371/journal.pone.0030512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Faria MS, et al. Role of protein kinase R in the killing of Leishmania major by macrophages in response to neutrophil elastase and TLR4 via TNFα and IFNβ. The FASEB Journal. 2014;28(7):3050–3063. doi: 10.1096/fj.13-245126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Durieu E, et al. From drug screening to target deconvolution: a target-based drug discovery pipeline using Leishmania casein kinase 1 isoform 2 to identify compounds with antileishmanial activity. Antimicrobial agents and chemotherapy. 2016;60(5):2822–2833. doi: 10.1128/AAC.00021-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Dan-Goor M, et al. Identification of a secreted casein kinase 1 in Leishmania donovani: effect of protein over expression on parasite growth and virulence. PloS one. 2013;8(11):e79287. doi: 10.1371/journal.pone.0079287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Gale M, Parsons M. A Trypanosoma brucei gene family encoding protein kinases with catalytic domains structurally related to Nek1 and NIMA. Molecular and biochemical parasitology. 1993;59(1):111–121. doi: 10.1016/0166-6851(93)90012-m. [DOI] [PubMed] [Google Scholar]
- 220.Gale M, Carter V, Parsons M. Translational control mediates the developmental regulation of the Trypanosoma brucei Nrk protein kinase. Journal of Biological Chemistry. 1994;269(50):31659–31665. [PubMed] [Google Scholar]
- 221.Parsons M, et al. Comparative analysis of the kinomes of three pathogenic trypanosomatids: Leishmania major, Trypanosoma brucei and Trypanosoma cruzi. BMC genomics. 2005;6(1):127. doi: 10.1186/1471-2164-6-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Tu X, Wang CC. The involvement of two cdc2-related kinases (CRKs) in Trypanosoma brucei cell cycle regulation and the distinctive stage-specific phenotypes caused by CRK3 depletion. Journal of Biological Chemistry. 2004;279(19):20519–20528. doi: 10.1074/jbc.M312862200. [DOI] [PubMed] [Google Scholar]
- 223.Sanderson L, Yardley V, Croft SL. Activity of anti-cancer protein kinase inhibitors against Leishmania spp. Journal of Antimicrobial Chemotherapy. 2014;69(7):1888–1891. doi: 10.1093/jac/dku069. [DOI] [PubMed] [Google Scholar]
- 224.Amata E, et al. Identification of “Preferred” Human Kinase Inhibitors for Sleeping Sickness Lead Discovery. Are Some Kinases Better than Others for Inhibitor Repurposing? ACS infectious diseases. 2016;2(3):180–186. doi: 10.1021/acsinfecdis.5b00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Wetzel DM, et al. The Src kinases Hck, Fgr and Lyn activate Arg to facilitate IgG-mediated phagocytosis and Leishmania infection. J Cell Sci. 2016;129(16):3130–3143. doi: 10.1242/jcs.185595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Valenciano AL, et al. Discovery and antiparasitic activity of AZ960 as a Trypanosoma brucei ERK8 inhibitor. Bioorganic & medicinal chemistry. 2016;24(19):4647–4651. doi: 10.1016/j.bmc.2016.07.069. [DOI] [PubMed] [Google Scholar]
- 227.Benaim G. Intracellular calcium signaling and regulation in Leishmania. Molecular and Immune Mechanism in the Pathogenesis of Cutaneous Leishmaniasis. 1996;5:89–106. [Google Scholar]
- 228.Garcia CR, et al. Plasmodium in the postgenomic era: new insights into the molecular cell biology of malaria parasites. International review of cell and molecular biology. 2008;266:85–156. doi: 10.1016/S1937-6448(07)66003-1. [DOI] [PubMed] [Google Scholar]
- 229.Zhivotovsky B, Orrenius S. Calcium and cell death mechanisms: a perspective from the cell death community. Cell calcium. 2011;50(3):211–221. doi: 10.1016/j.ceca.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 230.Machemer H, de Peyer JE. Analysis of ciliary beating frequency under voltage clamp control of the membrane. Cytoskeleton. 1982;2(S1):205–210. doi: 10.1002/cm.970020739. [DOI] [PubMed] [Google Scholar]
- 231.VOORHEIS HP, MARTIN BR. Characteristics of the Calcium-Mediated Mechanism Activating Adenylate Cyclase in Trypanosoma brucei. The FEBS Journal. 1981;116(3):471–477. doi: 10.1111/j.1432-1033.1981.tb05360.x. [DOI] [PubMed] [Google Scholar]
- 232.Téllez-iñón MT, et al. Calmodulin and Ca2+-dependent cyclic AMP phosphodiesterase activity in Trypanosoma cruzi. Molecular and biochemical parasitology. 1985;17(2):143–153. doi: 10.1016/0166-6851(85)90013-1. [DOI] [PubMed] [Google Scholar]
- 233.Gundersen RE, Nelson D. A novel Ca2+-dependent protein kinase from Paramecium tetraurelia. Journal of Biological Chemistry. 1987;262(10):4602–4609. [PubMed] [Google Scholar]
- 234.Kakiuchi S, et al. Ca2+-dependent modulator proteins from Tetrahymena pyriformis, sea anemone, and scallop and guanylate cyclase activation. Journal of Biological Chemistry. 1981;256(1):19–22. [PubMed] [Google Scholar]
- 235.Docampo R, Moreno SN. The acidocalcisome. Molecular and biochemical parasitology. 2001;114(2):151–159. doi: 10.1016/s0166-6851(01)00246-8. [DOI] [PubMed] [Google Scholar]
- 236.Benaim G, Bermudez R, Urbina JA. Ca2+ transport in isolated mitochondrial vesicles from Leishmania braziliensis promastigotes. Molecular and biochemical parasitology. 1990;39(1):61–68. doi: 10.1016/0166-6851(90)90008-a. [DOI] [PubMed] [Google Scholar]
- 237.Benaim G, et al. A calmodulin-stimulated Ca2+ pump in plasma-membrane vesicles from Trypanosoma brucei; selective inhibition by pentamidine. Biochemical Journal. 1993;296(3):759–763. doi: 10.1042/bj2960759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Docampo R, Vercesi A. Ca2+ transport by coupled Trypanosoma cruzi mitochondria in situ. Journal of Biological Chemistry. 1989;264(1):108–111. [PubMed] [Google Scholar]
- 239.Sarkar D, Bhaduri A. Temperature-induced rapid increase in cytoplasmic free Ca2+ in pathogenic Leishmania donovani promastigotes. FEBS letters. 1995;375(1–l2):83–86. doi: 10.1016/0014-5793(95)01161-7. [DOI] [PubMed] [Google Scholar]
- 240.Lu HG, et al. Intracellular Ca2+ pool content and signaling and expression of a calcium pump are linked to virulence in Leishmania mexicana amazonesis amastigotes. Journal of Biological Chemistry. 1997;272(14):9464–9473. doi: 10.1074/jbc.272.14.9464. [DOI] [PubMed] [Google Scholar]
- 241.Prasad A, et al. Ca2+ signaling in the transformation of promastigotes to axenic amastigotes of Leishmania donovani. Molecular and cellular biochemistry. 2001;224(1):39–44. doi: 10.1023/a:1011965109446. [DOI] [PubMed] [Google Scholar]
- 242.Naderer T, Dandash O, McConville MJ. Calcineurin is required for Leishmania major stress response pathways and for virulence in the mammalian host. Molecular microbiology. 2011;80(2):471–480. doi: 10.1111/j.1365-2958.2011.07584.x. [DOI] [PubMed] [Google Scholar]
- 243.Rodriguez NM, et al. Overexpression of the Leishmania amazonensis Ca2+-ATPase gene lmaa1 enhances virulence. Cellular microbiology. 2002;4(2):117–126. doi: 10.1046/j.1462-5822.2002.00175.x. [DOI] [PubMed] [Google Scholar]
- 244.Xiong ZH, Ruben L. Trypanosoma brucei: the dynamics of calcium movement between the cytosol, nucleus, and mitochondrion of intact cells. Experimental parasitology. 1998;88(3):231–239. doi: 10.1006/expr.1998.4249. [DOI] [PubMed] [Google Scholar]
- 245.Furuya T, et al. TcSCA complements yeast mutants defective in Ca2+ pumps and encodes a Ca2+-ATPase that localizes to the endoplasmic reticulum of Trypanosoma cruzi. Journal of Biological Chemistry. 2001;276(35):32437–32445. doi: 10.1074/jbc.M104000200. [DOI] [PubMed] [Google Scholar]
- 246.Vercesi AE, Docampo R, Moreno SN. Energization-dependent Ca2+ accumulation in Trypanosoma brucei bloodstream and procyclic trypomastigotes mitochondria. Molecular and biochemical parasitology. 1992;56(2):251–257. doi: 10.1016/0166-6851(92)90174-i. [DOI] [PubMed] [Google Scholar]
- 247.Vercesi A, Docampo R. Ca2+ transport by digitonin-permeabilized Leishmania donovani. Effects of Ca2+, pentamidine and WR-6026 on mitochondrial membrane potential in situ. Biochemical Journal. 1992;284(2):463–467. doi: 10.1042/bj2840463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Ruiz FA, Rodrigues CO, Docampo R. Rapid Changes in Polyphosphate Content within Acidocalcisomes in Response to Cell Growth, Differentiation, and Environmental Stress inTrypanosoma cruzi. Journal of Biological Chemistry. 2001;276(28):26114–26121. doi: 10.1074/jbc.M102402200. [DOI] [PubMed] [Google Scholar]
- 249.VERCESI AE, DOCAMPO R. Sodium-proton exchange stimulates Ca2+ release from acidocalcisomes of Trypanosoma brucei. Biochemical Journal. 1996;315(1):265–270. doi: 10.1042/bj3150265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Gómez ML, et al. Protein kinase C in Trypanosoma cruzi epimastigote forms: partial purification and characterization. Molecular and biochemical parasitology. 1989;36(2):101–108. doi: 10.1016/0166-6851(89)90182-5. [DOI] [PubMed] [Google Scholar]
- 251.Lammel EM, et al. Trypanosoma cruzi: involvement of intracellular calcium in multiplication and differentiation. Experimental parasitology. 1996;83(2):240–249. doi: 10.1006/expr.1996.0070. [DOI] [PubMed] [Google Scholar]
- 252.Reimão JQ, et al. Investigation of Calcium Channel Blockers as Antiprotozoal Agents and Their Interference in the Metabolism of Leishmania (L.) infantum. Evidence-Based Complementary and Alternative Medicine. 2016;2016 doi: 10.1155/2016/1523691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Reimão JQ, et al. In vitro and experimental therapeutic studies of the calcium channel blocker bepridil: detection of viable Leishmania (L.) chagasi by real-time PCR. Experimental parasitology. 2011;128(2):111–115. doi: 10.1016/j.exppara.2011.02.021. [DOI] [PubMed] [Google Scholar]
- 254.Shokri A, et al. The effect of verapamil on in vitro susceptibility of promastigote and amastigote stages of Leishmania tropica to meglumine antimoniate. Parasitology research. 2012;110(3):1113–1117. doi: 10.1007/s00436-011-2599-6. [DOI] [PubMed] [Google Scholar]
- 255.Chen M, et al. Purification and enzymatic activity of an NADH-fumarate reductase and other mitochondrial activities of Leishmania parasites. Apmis. 2001;109(12):801–808. doi: 10.1034/j.1600-0463.2001.091201.x. [DOI] [PubMed] [Google Scholar]
- 256.Abrahamsen MS, et al. Complete genome sequence of the apicomplexan, Cryptosporidium parvum. Science. 2004;304(5669):441–445. doi: 10.1126/science.1094786. [DOI] [PubMed] [Google Scholar]
- 257.Santhamma KR, Bhaduri A. Characterization of the respiratory chain of Leishmania donovani promastigotes. Molecular and biochemical parasitology. 1995;75(1):43–53. doi: 10.1016/0166-6851(95)02510-3. [DOI] [PubMed] [Google Scholar]
- 258.Turrens JF. Possible role of the NADH-fumarate reductase in superoxide anion and hydrogen peroxide production in Trypanosoma brucei. Molecular and biochemical parasitology. 1987;25(1):55–60. doi: 10.1016/0166-6851(87)90018-1. [DOI] [PubMed] [Google Scholar]
- 259.Srivastava IK, Rottenberg H, Vaidya AB. Atovaquone, a broad spectrum antiparasitic drug, collapses mitochondrial membrane potential in a malarial parasite. Journal of Biological Chemistry. 1997;272(7):3961–3966. doi: 10.1074/jbc.272.7.3961. [DOI] [PubMed] [Google Scholar]
- 260.Horváth A, et al. Leishmania tarentolae: a parallel isolation of cytochrome bc1 and cytochrome c oxidase. Experimental parasitology. 2000;96(3):160–167. doi: 10.1006/expr.2000.4564. [DOI] [PubMed] [Google Scholar]
- 261.Dey R, et al. Characterization of a Leishmania stage-specific mitochondrial membrane protein that enhances the activity of cytochrome c oxidase and its role in virulence. Molecular microbiology. 2010;77(2):399–414. doi: 10.1111/j.1365-2958.2010.07214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Zíková A, et al. Structural and functional association of Trypanosoma brucei MIX protein with cytochrome c oxidase complex. Eukaryotic cell. 2008;7(11):1994–2003. doi: 10.1128/EC.00204-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Chaudhuri M, Ott RD, Hill GC. Trypanosome alternative oxidase: from molecule to function. Trends in parasitology. 2006;22(10):484–491. doi: 10.1016/j.pt.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 264.Mather MW, Henry KW, Vaidya AB. Mitochondrial drug targets in apicomplexan parasites. Current drug targets. 2007;8(1):49–60. doi: 10.2174/138945007779315632. [DOI] [PubMed] [Google Scholar]
- 265.Singh PP, Chakraborty P. Malaria: autophagy as a potential therapeutic target. Journal of Pharmacy and Pharmacology. 2016;4:298–306. [Google Scholar]
- 266.Hancock RE, Nijnik A, Philpott DJ. Modulating immunity as a therapy for bacterial infections. Nature reviews Microbiology. 2012;10(4):243. doi: 10.1038/nrmicro2745. [DOI] [PubMed] [Google Scholar]
- 267.Murray HW, et al. Determinants of response to interleukin-10 receptor blockade immunotherapy in experimental visceral leishmaniasis. The Journal of infectious diseases. 2003;188(3):458–464. doi: 10.1086/376510. [DOI] [PubMed] [Google Scholar]
- 268.Manna PP, et al. Cellular therapy by allogeneic macrophages against visceral leishmaniasis: role of TNF-alpha. Cell Immunol. 2014;290(1):152–63. doi: 10.1016/j.cellimm.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 269.Ghosh M, et al. Dendritic cell-based immunotherapy combined with antimony-based chemotherapy cures established murine visceral leishmaniasis. J Immunol. 2003;170(11):5625–9. doi: 10.4049/jimmunol.170.11.5625. [DOI] [PubMed] [Google Scholar]
- 270.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105(4):1815–22. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 271.Kuci S, et al. CD271 antigen defines a subset of multipotent stromal cells with immunosuppressive and lymphohematopoietic engraftment-promoting properties. Haematologica. 2010;95(4):651–9. doi: 10.3324/haematol.2009.015065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Faleiro RJ, et al. Combined Immune Therapy for the Treatment of Visceral Leishmaniasis. PLoS Negl Trop Dis. 2016;10(2):e0004415. doi: 10.1371/journal.pntd.0004415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Novais FO, et al. CD8+ T cell cytotoxicity mediates pathology in the skin by inflammasome activation and IL-1beta production. PLoS Pathog. 2017;13(2):e1006196. doi: 10.1371/journal.ppat.1006196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Anjili C, et al. Effects of anti-Leishmania monoclonal antibodies on the development of Leishmania major in Phlebotomus duboscqi (Diptera: Psychodidae) East Afr Med J. 2006;83(2):72–8. [PubMed] [Google Scholar]
- 275.Gazanion E, et al. The Leishmania nicotinamidase is essential for NAD+ production and parasite proliferation. Mol Microbiol. 2011;82(1):21–38. doi: 10.1111/j.1365-2958.2011.07799.x. [DOI] [PubMed] [Google Scholar]
- 276.Marr JJ, Berens RL, Nelson DJ. Purine metabolism in Leishmania donovani and Leishmania braziliensis. Biochim Biophys Acta. 1978;544(2):360–71. doi: 10.1016/0304-4165(78)90104-6. [DOI] [PubMed] [Google Scholar]
- 277.Tuttle JV, Krenitsky TA. Purine phosphoribosyltransferases from Leishmania donovani. J Biol Chem. 1980;255(3):909–16. [PubMed] [Google Scholar]
- 278.Koszalka GW, Krenitsky TA. Nucleosidases from Leishmania donovani. Pyrimidine ribonucleosidase, purine ribonucleosidase, and a novel purine 2′-deoxyribonucleosidase. J Biol Chem. 1979;254(17):8185–93. [PubMed] [Google Scholar]
- 279.Shi W, Schramm VL, Almo SC. Nucleoside hydrolase from Leishmania major. Cloning, expression, catalytic properties, transition state inhibitors, and the 2.5-a crystal structure. J Biol Chem. 1999;274(30):21114–20. doi: 10.1074/jbc.274.30.21114. [DOI] [PubMed] [Google Scholar]
- 280.Cui L, Rajasekariah GR, Martin SK. A nonspecific nucleoside hydrolase from Leishmania donovani: implications for purine salvage by the parasite. Gene. 2001;280(1–2):153–62. doi: 10.1016/s0378-1119(01)00768-5. [DOI] [PubMed] [Google Scholar]
- 281.Gottlieb M, Dwyer DM. Protozoan parasite of humans: surface membrane with externally disposed acid phosphatase. Science. 1981;212(4497):939–41. doi: 10.1126/science.7233189. [DOI] [PubMed] [Google Scholar]
- 282.Carter NS, Landfear SM, Ullman B. Nucleoside transporters of parasitic protozoa. Trends Parasitol. 2001;17(3):142–5. doi: 10.1016/s1471-4922(00)01806-7. [DOI] [PubMed] [Google Scholar]
- 283.Liu W, et al. Functional characterization of nucleoside transporter gene replacements in Leishmania donovani. Mol Biochem Parasitol. 2006;150(2):300–7. doi: 10.1016/j.molbiopara.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Carter NS, et al. Cloning of a novel inosine-guanosine transporter gene from Leishmania donovani by functional rescue of a transport-deficient mutant. J Biol Chem. 2000;275(27):20935–41. doi: 10.1074/jbc.M002418200. [DOI] [PubMed] [Google Scholar]
- 285.Carter NS, et al. Adaptive responses to purine starvation in Leishmania donovani. Mol Microbiol. 2010;78(1):92–107. doi: 10.1111/j.1365-2958.2010.07327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Ortiz D, et al. An acid-activated nucleobase transporter from Leishmania major. J Biol Chem. 2009;284(24):16164–9. doi: 10.1074/jbc.M109.006718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Sacks DL, et al. The role of phosphoglycans in Leishmania-sand fly interactions. Proc Natl Acad Sci U S A. 2000;97(1):406–11. doi: 10.1073/pnas.97.1.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Turco SJ, Descoteaux A. The lipophosphoglycan of Leishmania parasites. Annu Rev Microbiol. 1992;46:65–94. doi: 10.1146/annurev.mi.46.100192.000433. [DOI] [PubMed] [Google Scholar]
- 289.Spath GF, et al. Lipophosphoglycan is a virulence factor distinct from related glycoconjugates in the protozoan parasite Leishmania major. Proc Natl Acad Sci U S A. 2000;97(16):9258–63. doi: 10.1073/pnas.160257897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Ferguson MA. Glycosylphosphatidylinositol biosynthesis validated as a drug target for African sleeping sickness. Proceedings of the National Academy of Sciences. 2000;97(20):10673–10675. doi: 10.1073/pnas.97.20.10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Bhargava SR, Chatterji BP. Emergence of Tubulin as a Vaccine against Parasitic Infections. Intellectual Property Rights: Open Access. 2014 [Google Scholar]
- 292.Carballeira NM, et al. Synthesis of marine α-methoxylated fatty acid analogs that effectively inhibit the topoisomerase IB from Leishmania donovani with a mechanism different from that of camptothecin. Marine drugs. 2013;11(10):3661–3675. doi: 10.3390/md11103661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Abidin BM, et al. Infection-adapted emergency hematopoiesis promotes visceral leishmaniasis. PLoS Pathog. 2017;13(8):e1006422. doi: 10.1371/journal.ppat.1006422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Carvalho L, et al. Tafenoquine, an antiplasmodial 8-aminoquinoline, targets leishmania respiratory complex III and induces apoptosis. Antimicrob Agents Chemother. 2010;54(12):5344–51. doi: 10.1128/AAC.00790-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Monte Neto RL, et al. Morphological and physiological changes in Leishmania promastigotes induced by yangambin, a lignan obtained from Ocotea duckei. Exp Parasitol. 2011;127(1):215–21. doi: 10.1016/j.exppara.2010.07.020. [DOI] [PubMed] [Google Scholar]
- 296.Mukherjee P, et al. Apoptosis-like death in Leishmania donovani promastigotes induced by diospyrin and its ethanolamine derivative. Int J Antimicrob Agents. 2009;34(6):596–601. doi: 10.1016/j.ijantimicag.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 297.Lüder CG, et al. Impact of protozoan cell death on parasite-host interactions and pathogenesis. Parasites & vectors. 2010;3(1):116. doi: 10.1186/1756-3305-3-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Mandlik V, et al. Biological network modeling identifies IPCS in Leishmania as a therapeutic target. Integr Biol (Camb) 2012;4(9):1130–42. doi: 10.1039/c2ib20037f. [DOI] [PubMed] [Google Scholar]
- 299.Pucadyil TJ, Chattopadhyay A. Cholesterol: a potential therapeutic target in Leishmania infection? Trends Parasitol. 2007;23(2):49–53. doi: 10.1016/j.pt.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 300.Lepesheva GI, Waterman MR. Sterol 14alpha-demethylase (CYP51) as a therapeutic target for human trypanosomiasis and leishmaniasis. Curr Top Med Chem. 2011;11(16):2060–71. doi: 10.2174/156802611796575902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Crauwels P, et al. Apoptotic-like Leishmania exploit the host’s autophagy machinery to reduce T-cell-mediated parasite elimination. Autophagy. 2015;11(2):285–97. doi: 10.1080/15548627.2014.998904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Carrey EA. Key enzymes in the biosynthesis of purines and pyrimidines: their regulation by allosteric effectors and by phosphorylation. Biochem Soc Trans. 1995;23(4):899–902. doi: 10.1042/bst0230899. [DOI] [PubMed] [Google Scholar]
- 303.Sundar S, et al. Comparison of short-course multidrug treatment with standard therapy for visceral leishmaniasis in India: an open-label, non-inferiority, randomised controlled trial. The Lancet. 2011;377(9764):477–486. doi: 10.1016/S0140-6736(10)62050-8. [DOI] [PubMed] [Google Scholar]
- 304.Martín-Quintal Z, et al. The leishmanicidal effect of (3S)-16, 17-didehydrofalcarinol, an oxylipin isolated from Tridax procumbens, is independent of NO production. Phytotherapy research. 2010;24(7):1004–1008. doi: 10.1002/ptr.3052. [DOI] [PubMed] [Google Scholar]
- 305.Kedzierski L, Zhu Y, Handman E. Leishmania vaccines: progress and problems. Parasitology. 2006;133(S2):S87–S112. doi: 10.1017/S0031182006001831. [DOI] [PubMed] [Google Scholar]
- 306.Handman E. Leishmaniasis: current status of vaccine development. Clinical microbiology reviews. 2001;14(2):229–243. doi: 10.1128/CMR.14.2.229-243.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Gomes DC, et al. Effectiveness of the immunomodulatory extract of Kalanchoe pinnata against murine visceral leishmaniasis. Parasitology. 2010;137(4):613–8. doi: 10.1017/S0031182009991405. [DOI] [PubMed] [Google Scholar]
- 308.Muzitano MF, et al. Quercitrin: an antileishmanial flavonoid glycoside from Kalanchoe pinnata. Planta Med. 2006;72(1):81–3. doi: 10.1055/s-2005-873183. [DOI] [PubMed] [Google Scholar]
- 309.Sarkar A, et al. An ethanolic extract of leaves of Piper betle (Paan) Linn mediates its antileishmanial activity via apoptosis. Parasitol Res. 2008;102(6):1249–55. doi: 10.1007/s00436-008-0902-y. [DOI] [PubMed] [Google Scholar]
- 310.Misra P, et al. Pro-apoptotic effect of the landrace Bangla Mahoba of Piper betle on Leishmania donovani may be due to the high content of eugenol. J Med Microbiol. 2009;58(Pt 8):1058–66. doi: 10.1099/jmm.0.009290-0. [DOI] [PubMed] [Google Scholar]
- 311.Ramirez-Macias I, et al. Leishmanicidal activity of nine novel flavonoids from Delphinium staphisagria. ScientificWorldJournal. 2012;2012:203646. doi: 10.1100/2012/203646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.da Silva BP, et al. Antileishmanial activity of a guaianolide from Tanacetum parthenium (L.) Schultz Bip. Parasitol Int. 2010;59(4):643–6. doi: 10.1016/j.parint.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 313.Lage PS, et al. Strychnos pseudoquina and Its Purified Compounds Present an Effective In Vitro Antileishmanial Activity. Evid Based Complement Alternat Med. 2013;2013:304354. doi: 10.1155/2013/304354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Garcia M, et al. Screening of medicinal plants against Leishmania amazonensis. Pharm Biol. 2010;48(9):1053–8. doi: 10.3109/13880200903485729. [DOI] [PubMed] [Google Scholar]
- 315.Vennerstrom JL, et al. Berberine derivatives as antileishmanial drugs. Antimicrob Agents Chemother. 1990;34(5):918–21. doi: 10.1128/aac.34.5.918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Bringmann G, et al. Ancistroealaines A and B, two new bioactive naphthylisoquinolines, and related naphthoic acids from Ancistrocladus ealaensis. J Nat Prod. 2000;63(11):1465–70. doi: 10.1021/np000247+. [DOI] [PubMed] [Google Scholar]
- 317.Fournet A, et al. In vivo efficacy of oral and intralesional administration of 2-substituted quinolines in experimental treatment of new world cutaneous leishmaniasis caused by Leishmania amazonensis. Antimicrob Agents Chemother. 1996;40(11):2447–51. doi: 10.1128/aac.40.11.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Fournet A, et al. 2-substituted quinoline alkaloids as potential antileishmanial drugs. Antimicrob Agents Chemother. 1993;37(4):859–63. doi: 10.1128/aac.37.4.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Guimaraes LR, et al. Activity of the julocrotine, a glutarimide alkaloid from Croton pullei var. glabrior, on Leishmania (L.) amazonensis. Parasitol Res. 2010;107(5):1075–81. doi: 10.1007/s00436-010-1973-0. [DOI] [PubMed] [Google Scholar]
- 320.El-On J, et al. Antileishmanial activity in Israeli plants. Ann Trop Med Parasitol. 2009;103(4):297–306. doi: 10.1179/136485909X440827. [DOI] [PubMed] [Google Scholar]
- 321.Calla-Magarinos J, et al. Quinolinic alkaloids from Galipea longiflora Krause suppress production of proinflammatory cytokines in vitro and control inflammation in vivo upon Leishmania infection in mice. Scand J Immunol. 2013;77(1):30–8. doi: 10.1111/sji.12010. [DOI] [PubMed] [Google Scholar]
- 322.de Albuquerque Melo GM, et al. Leishmanicidal activity of the crude extract, fractions and major piperidine alkaloids from the flowers of Senna spectabilis. Phytomedicine. 2014;21(3):277–81. doi: 10.1016/j.phymed.2013.09.024. [DOI] [PubMed] [Google Scholar]
- 323.Cavalcanti da Silva E, et al. Antileishmanial activity of warifteine: a bisbenzylisoquinoline alkaloid isolated from Cissampelos sympodialis Eichl. (Menispermaceae) Scientific World Journal. 2012;2012:516408. doi: 10.1100/2012/516408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Ferreira ME, et al. Antileishmanial activity of furoquinolines and coumarins from Helietta apiculata. Phytomedicine. 2010;17(5):375–8. doi: 10.1016/j.phymed.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 325.Moraes LS, et al. Leishmanicidal Activity of (+)-Phyllanthidine and the Phytochemical Profile of Margaritaria nobilis (Phyllanthaceae) Molecules. 2015;20(12):22157–69. doi: 10.3390/molecules201219829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.da Silva DB, et al. The antitumoral, trypanocidal and antileishmanial activities of extract and alkaloids isolated from Duguetia furfuracea. Phytomedicine. 2009;16(11):1059–63. doi: 10.1016/j.phymed.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 327.Bhattacharya S, Biswas M, Haldar PK. The triterpenoid fraction from Trichosanthes dioica root exhibits in vitro antileishmanial effect against Leishmania donovani promastigotes. Pharmacognosy Res. 2013;5(2):109–12. doi: 10.4103/0974-8490.110540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Rabito MF, et al. In vitro and in vivo antileishmania activity of sesquiterpene lactone-rich dichloromethane fraction obtained from Tanacetum parthenium (L.) Schultz-Bip. Exp Parasitol. 2014;143:18–23. doi: 10.1016/j.exppara.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 329.Yamamoto ES, et al. Treatment with triterpenic fraction purified from Baccharis uncinella leaves inhibits Leishmania (Leishmania) amazonensis spreading and improves Th1 immune response in infected mice. Parasitol Res. 2014;113(1):333–9. doi: 10.1007/s00436-013-3659-x. [DOI] [PubMed] [Google Scholar]
- 330.Bufalo J, et al. Antimicrobial and Antileishmanial Activities of Diterpenoids Isolated from the Roots of Salvia deserta. Planta Med. 2016;82(1–2):131–7. doi: 10.1055/s-0035-1557875. [DOI] [PubMed] [Google Scholar]
- 331.Yang DM, Liew FY. Effects of qinghaosu (artemisinin) and its derivatives on experimental cutaneous leishmaniasis. Parasitology. 1993;106(Pt 1):7–11. doi: 10.1017/s0031182000074758. [DOI] [PubMed] [Google Scholar]
- 332.Naman CB, et al. Antileishmanial and Cytotoxic Activity of Some Highly Oxidized Abietane Diterpenoids from the Bald Cypress, Taxodium distichum. J Nat Prod. 2016;79(3):598–606. doi: 10.1021/acs.jnatprod.5b01131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Lima GS, et al. Antileishmanial activity and trypanothione reductase effects of terpenes from the Amazonian species Croton cajucara Benth (Euphorbiaceae) Phytomedicine. 2015;22(12):1133–7. doi: 10.1016/j.phymed.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 334.Chen M, et al. Licochalcone A, a novel antiparasitic agent with potent activity against human pathogenic protozoan species of Leishmania. Antimicrob Agents Chemother. 1993;37(12):2550–6. doi: 10.1128/aac.37.12.2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Zhai L, et al. The antileishmanial activity of novel oxygenated chalcones and their mechanism of action. J Antimicrob Chemother. 1999;43(6):793–803. doi: 10.1093/jac/43.6.793. [DOI] [PubMed] [Google Scholar]
- 336.Kayser O, Kiderlen AF. In vitro leishmanicidal activity of naturally occurring chalcones. Phytother Res. 2001;15(2):148–52. doi: 10.1002/ptr.701. [DOI] [PubMed] [Google Scholar]
- 337.Dal Picolo CR, et al. Antileishmanial activity evaluation of adunchalcone, a new prenylated dihydrochalcone from Piper aduncum L. Fitoterapia. 2014;97:28–33. doi: 10.1016/j.fitote.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 338.Hermoso A, et al. Antileishmanial activities of dihydrochalcones from piper elongatum and synthetic related compounds. Structural requirements for activity. Bioorg Med Chem. 2003;11(18):3975–80. doi: 10.1016/s0968-0896(03)00406-1. [DOI] [PubMed] [Google Scholar]
- 339.Fournet A, et al. Biological and chemical studies of Pera benensis, a Bolivian plant used in folk medicine as a treatment of cutaneous leishmaniasis. J Ethnopharmacol. 1992;37(2):159–64. doi: 10.1016/0378-8741(92)90074-2. [DOI] [PubMed] [Google Scholar]
- 340.Fournet A, et al. Effect of natural naphthoquinones in BALB/c mice infected with Leishmania amazonensis and L. venezuelensis. Trop Med Parasitol. 1992;43(4):219–22. [PubMed] [Google Scholar]
- 341.del Rayo Camacho M, et al. In vitro antiprotozoal and cytotoxic activities of some alkaloids, quinones, flavonoids, and coumarins. Planta Med. 2004;70(1):70–2. doi: 10.1055/s-2004-815460. [DOI] [PubMed] [Google Scholar]
- 342.Camacho MR, et al. Oxoaporphine alkaloids and quinones from Stephania dinklagei and evaluation of their antiprotozoal activities. Planta Med. 2000;66(5):478–80. doi: 10.1055/s-2000-8597. [DOI] [PubMed] [Google Scholar]
- 343.Mishra BB, et al. An antileishmanial prenyloxy-naphthoquinone from roots of Plumbago zeylanica. Nat Prod Res. 2013;27(4–5):480–5. doi: 10.1080/14786419.2012.696254. [DOI] [PubMed] [Google Scholar]
- 344.Mahmoudvand H, et al. Leishmanicidal and cytotoxic activities of Nigella sativa and its active principle, thymoquinone. Pharm Biol. 2015;53(7):1052–7. doi: 10.3109/13880209.2014.957784. [DOI] [PubMed] [Google Scholar]
- 345.Majester-Savornin B, et al. Saponins of the ivy plant, Hedera helix, and their leishmanicidic activity. Planta Med. 1991;57(3):260–2. doi: 10.1055/s-2006-960086. [DOI] [PubMed] [Google Scholar]
- 346.Ridoux O, et al. In vitro antileishmanial activity of three saponins isolated from ivy, alpha-hederin, beta-hederin and hederacolchiside A(1), in association with pentamidine and amphotericin B. Phytother Res. 2001;15(4):298–301. doi: 10.1002/ptr.723. [DOI] [PubMed] [Google Scholar]
- 347.Foubert K, et al. LC-MS analysis of 13,28-epoxy-oleanane saponins in Maesa spp. extracts with antileishmanial activity. Phytochem Anal. 2009;20(2):159–67. doi: 10.1002/pca.1112. [DOI] [PubMed] [Google Scholar]
- 348.Germonprez N, et al. In vitro and in vivo anti-leishmanial activity of triterpenoid saponins isolated from Maesa balansae and some chemical derivatives. J Med Chem. 2005;48(1):32–7. doi: 10.1021/jm031150y. [DOI] [PubMed] [Google Scholar]
- 349.Di Giorgio C, et al. In vitro antileishmanial activity of diphyllin isolated from Haplophyllum bucharicum. Planta Med. 2005;71(4):366–9. doi: 10.1055/s-2005-864106. [DOI] [PubMed] [Google Scholar]
- 350.de Castro Oliveira LG, et al. In Vitro Effects of the Neolignan 2,3-Dihydrobenzofuran Against Leishmania Amazonensis. Basic Clin Pharmacol Toxicol. 2017;120(1):52–58. doi: 10.1111/bcpt.12639. [DOI] [PubMed] [Google Scholar]
- 351.Antinarelli LM, et al. 4-Aminoquinoline Derivatives as Potential Antileishmanial Agents. Chem Biol Drug Des. 2015;86(4):704–14. doi: 10.1111/cbdd.12540. [DOI] [PubMed] [Google Scholar]
- 352.Vale-Costa S, et al. N-cinnamoylated aminoquinolines as promising antileishmanial agents. Antimicrob Agents Chemother. 2013;57(10):5112–5. doi: 10.1128/AAC.00557-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Antinarelli LM, et al. Aminoquinoline compounds: Effect of 7-chloro-4-quinolinylhydrazone derivatives against Leishmania amazonensis. Exp Parasitol. 2016;171:10–16. doi: 10.1016/j.exppara.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 354.Costa Duarte M, et al. An effective in vitro and in vivo antileishmanial activity and mechanism of action of 8-hydroxyquinoline against Leishmania species causing visceral and tegumentary leishmaniasis. Vet Parasitol. 2016;217:81–8. doi: 10.1016/j.vetpar.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 355.Stroppa PHF, et al. Effect of 1,2,3-triazole salts, non-classical bioisosteres of miltefosine, on Leishmania amazonensis. Bioorg Med Chem. 2017;25(12):3034–3045. doi: 10.1016/j.bmc.2017.03.051. [DOI] [PubMed] [Google Scholar]
- 356.Nieto-Meneses R, et al. In vitro activity of new N-benzyl-1H-benzimidazol-2-amine derivatives against cutaneous, mucocutaneous and visceral Leishmania species. Exp Parasitol. 2018;184:82–89. doi: 10.1016/j.exppara.2017.11.009. [DOI] [PubMed] [Google Scholar]


