Abstract
Obesity, insulin resistance, and metabolic syndrome continue to increase in prevalence. Hypertriglyceridemia is commonly associated and represents a valuable marker of metabolic syndrome. An increase in subcutaneous fat deposition places patients at risk for visceral adipose deposition in sites such as the liver, heart, and pancreas. Pancreatic steatosis in the setting of metabolic syndrome is a rapidly emerging entity whose clinical spectrum remains to be defined. Hypertriglyceridemia is an accepted cause of acute pancreatitis but its role in chronic pancreatic injury remains to be explored. We present 3 patients with chronic abdominal pain and pancreatic steatosis in the setting of underlying metabolic syndrome with hypertriglyceridemia. These cases were identified in one endoscopic ultrasonographer's practice over a 12-month period. Each patient had documented hypertriglyceridemia but no history of acute hypertriglyceride-induced pancreatitis. A history of significant alcohol exposure was carefully excluded. Each patient underwent endoscopic ultrasonography (EUS) which proved critical in delineating the spectrum of chronic pancreatic injury. Each of our patients had EUS documentation of pancreatic steatosis and sufficient criteria to establish a diagnosis of chronic pancreatitis. Intraductal pancreatic calculi were identified in all 3 patients. Our series suggests that in the setting of metabolic syndrome, chronic hypertriglyceridemia and pancreatic steatosis may be associated with chronic pancreatitis. We hypothesize that hypertriglyceridemia may provide a pathogenic role in the development of chronic pancreatic microinjury. In addition, each of our patients had EUS-documented pancreatic ductal lithiasis. To our review, these are novel findings which have yet to be reported. We believe that with an enhanced awareness, it is likely that the entity of metabolic syndrome with features of pancreatic steatosis and hypertriglyceridemia with their associated manifestations of chronic pancreatitis, including ductal lithiasis, will be widely appreciated.
Keywords: Metabolic pancreatitis, Pancreatic steatosis, Hypertriglyceridemia, Chronic pancreatitis, Metabolic syndrome
Background
Obesity, insulin resistance, and metabolic syndrome continue to increase in prevalence [1]. An increase in subcutaneous fat puts patients at risk for visceral adipose accumulation in sites such as the liver, pancreas, and heart leading to hepatic steatosis, insulin resistance, and coronary artery disease [2, 3]. Pancreatic steatosis is an increasingly appreciated finding, especially with endoscopic ultrasound (EUS) imaging. Pancreatic fat is closely related to metabolic syndrome and hepatic steatosis but its effect has been poorly understood, and its clinical implications remain vaguely defined [4, 5, 6, 7, 8]. Hypertriglyceridemia is clearly established as a cause of acute pancreatitis. However, hypertriglyceridemia has yet to be associated with pancreatic ductal lithiasis and chronic pancreatic injury.
Cases
Case 1
A 69-year-old Caucasian female was referred for chronic abdominal pain. She had been losing weight (11 lbs) unintentionally over a 2-month period. Abdominal CT scan revealed pancreatic duct (PD) dilation from neck to tail up to 1.2 cm with a 1-cm stone in the pancreatic neck. Minimal inflammation in the midportion of the body of the pancreas was noted along with a 2.8 × 2.9 cm cyst in the tail. Endoscopic ultrasound was pursued to further examine this PD stone and to rule out a malignant process.
Her past medical history includes obesity (221 lbs, BMI 41.75), type 2 diabetes mellitus, hypertriglyceridemia, hepatic steatosis, and hypertension. There is no family history of any gastrointestinal (GI) or autoimmune disorders. She has a 30-pack-year smoking history (none for 10 years) and rare social alcohol use. Labs were significant for a mild normocytic anemia and lipase which was within normal limits. Triglycerides were normal but had been documented to be > 1,000 mg/dL prior to the initiation of medical therapy.
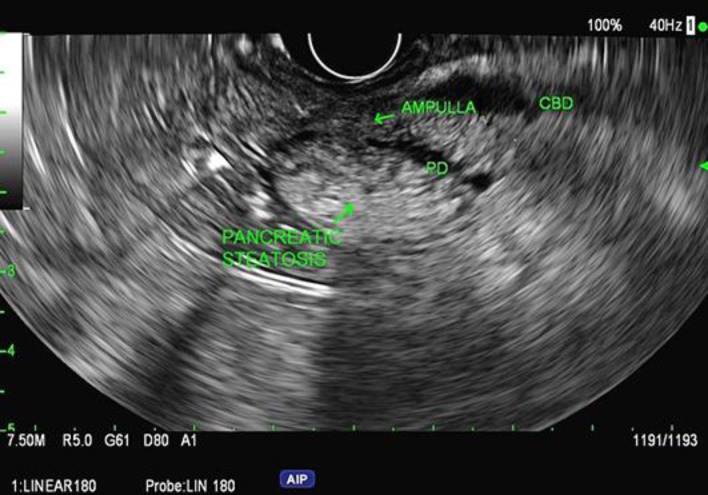
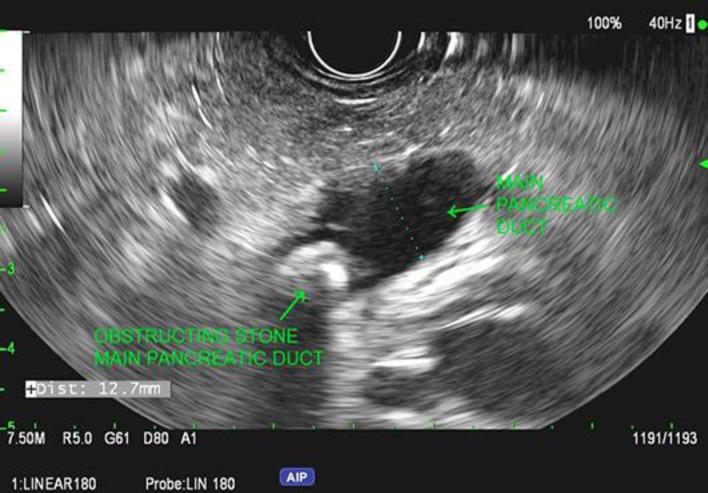
EUS demonstrated the pancreas to be hyperechoic consistent with steatosis (Fig. 1). The cyst which was noted on CT had resolved; however, there was significant PD dilation in the body and tail. The PD measured 12.7 mm, and the minor duct was dilated to 4.9 mm; both of them contained large calcified stones causing obstruction (Fig. 2). Hyperechoic foci with strands and focal hyperechoic duct walls were also present.
Fig. 1.
Example of pancreatic steatosis on endoscopic ultrasonography in patient 1. Patients 2 and 3 were found to have similar findings.
Fig. 2.
Pancreatic duct dilation with pancreatic duct lithiasis on endoscopic ultrasonography in patient 1.
Case 2
A 68-year-old Caucasian female was referred for evaluation of ongoing abdominal pain. Since her cholecystectomy, she developed vacillating epigastric pain associated with nausea, vomiting, and loose stools. CT scan revealed mild peripancreatic haziness suggestive of pancreatitis.
Her past medical history includes obesity (209 lbs, BMI 34.78), hypertension, hypertriglyceridemia, and hepatic steatosis. There is no family history of GI or autoimmune disorders and no tobacco or alcohol use. Fasting triglyceride level was 2,400 mg/dL. Labs were otherwise normal including IgG subclasses. EUS demonstrated hepatic and pancreatic steatosis with PD body dilation (to 4.5 mm) due to a calcified stone.
Case 3
A 75-year-old Caucasian female was referred for chronic epigastric abdominal pain, nausea, and vomiting with low-grade lipase elevations. EUS, at that time, revealed fatty pancreas and common bile duct dilation to 8 mm without calculi. Four months later, she again developed abdominal pain.
Her past medical history is significant for hypertension, hypertriglyceridemia, hypercholesterolemia, hepatic steatosis, and obesity (222 lbs, BMI 37.03). Surgical history is significant for bilateral hip arthroplasties and cholecystectomy. There is no family history of any GI or autoimmune disorders. She is a former smoker, but quit 40 years ago (1.25 pack-years), and uses alcohol on rare occasions. Laboratory studies were notable for high triglycerides (240 mg/dL).
Due to her recurrent abdominal pain, EUS was repeated. It revealed parenchyma which was hyperechoic, consistent with pancreatic steatosis. A PD stone and ductal dilation to 8 mm were identified. The common bile duct was again dilated to 9 mm but without choledocholithiasis or sludge. Several months later, the patient underwent removal of the PD stone due to persistent chronic abdominal pain.
Discussion
We believe pancreatic steatosis has pathogenic implications and may be related to hypertriglyceridemia as is true in other organs. Pancreatic steatosis has been found to promote metastasis and increase mortality in pancreatic adenocarcinoma as well as fistula formation after pancreatic mass resection [9, 10, 11]. One recent retrospective study identified fatty pancreas in 32 of 2,338 (1.4%) patients undergoing EUS over a 5-year period, with abdominal pain being the dominant indication for the procedure. Alcohol consumption and metabolic syndrome were significantly associated [12].
We present 3 patients with metabolic syndrome including variable elevations in triglycerides and chronic abdominal pain. EUS greatly expanded the extent of pancreatic injury by identifying PD stones, ductal dilation, hyperechoic strands and foci consistent with chronic pancreatitis by Rosemont criteria [13]. Despite these findings, none of our patients had a documented episode of acute pancreatitis. While we appreciate that acute episodes of pancreatitis may have occurred and eluded documentation, we suspect an element of chronic, subclinical, hypertriglyceride-induced, low-grade injury to be likely.
Hypertriglyceridemia leads to increased free fatty acids and inflammatory mediator release via pancreatic lipase release and enhanced lipolysis secondary to hyperchylomicronemia [14]. Pancreatic steatosis may provide enhanced substrate for pancreatic lipase and a potential for subclinical injury. Irrespective of mechanism, we believe the clinical presentation of our patients, along with EUS evidence of chronic calcific pancreatitis, suggests a possible role for chronic microinjury due to hypertriglyceridemia. A recent study by Pedersen et al. [15] demonstrated that pancreatitis can occur in the setting of lower than expected triglyceride levels. In this study of 116,550 individuals, it was found that pancreatitis can occur with triglyceride levels as low as 177 mg/dL [15]. Traditionally, the development of pancreatic insult has been associated with triglyceride levels > 1,000 mg/dL [14].
Our findings contrast those of Sepe et al. [4] who examined 250 patients with pancreatic steatosis on EUS and proposed no association between fatty pancreas and chronic pancreatitis but did distinguish metabolic syndrome as a strong risk factor for developing pancreatic steatosis. To our review, we provide the initial evidence that chronic calcific pancreatitis may be associated with metabolic syndrome and hypertriglyceridemia. While genetic testing was not performed in our patients, we believe hereditary pancreatitis to be unlikely given the lack of family history and advanced age of our patients. Nevertheless, we appreciate that a genetic predisposition may exist and may ultimately be defined. Our reported cases occurred over a 12-month period in one endoscopic ultrasonographer's practice which may imply that our findings may not be unique. We believe that such cases have been undiagnosed or misdiagnosed as either alcohol-induced, hereditary, or idiopathic.
Our series provides evidence for a potential pathogenic role of hypertriglyceridemia in the development of chronic pancreatic injury. As such injury may occur with modest elevations in triglycerides, treatment of the hypertriglyceridemia may result in clinically meaningful implications. We believe that with an enhanced awareness, it is likely that the entity of metabolic syndrome with features of pancreatic steatosis and hypertriglyceridemia leading to chronic pancreatic injury, with its associated complications, will be widely appreciated. Future studies are needed to validate our findings and to further explore the relationship of pancreatic steatosis and hypertriglyceridemia in the genesis of chronic pancreatic injury.
Statement of Ethics
The authors have no ethical conflicts to disclose.
Disclosure Statement
The authors have no conflicts of interest to declare.
References
- 1.Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ. Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Body Mass Index): National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9·1 million participants. Lancet. 2011 Feb;377((9765)):557–67. doi: 10.1016/S0140-6736(10)62037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goossens GH. The role of adipose tissue dysfunction in the pathogenesis of obesity-related insulin resistance. Physiol Behav. 2008 May;94((2)):206–18. doi: 10.1016/j.physbeh.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 3.Després JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006 Dec;444((7121)):881–7. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 4.Sepe PS, Ohri A, Sanaka S, Berzin TM, Sekhon S, Bennett G, et al. A prospective evaluation of fatty pancreas by using EUS. Gastrointest Endosc. 2011 May;73((5)):987–93. doi: 10.1016/j.gie.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 5.Lee JS, Kim SH, Jun DW, Han JH, Jang EC, Park JY, et al. Clinical implications of fatty pancreas: correlations between fatty pancreas and metabolic syndrome. World J Gastroenterol. 2009 Apr;15((15)):1869–75. doi: 10.3748/wjg.15.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hannukainen JC, Borra R, Linderborg K, Kallio H, Kiss J, Lepomäki V, et al. Liver and pancreatic fat content and metabolism in healthy monozygotic twins with discordant physical activity. J Hepatol. 2011 Mar;54((3)):545–52. doi: 10.1016/j.jhep.2010.07.029. [DOI] [PubMed] [Google Scholar]
- 7.van Geenen EJ, Smits MM, Schreuder TC, van der Peet DL, Bloemena E, Mulder CJ. Nonalcoholic fatty liver disease is related to nonalcoholic fatty pancreas disease. Pancreas. 2010 Nov;39((8)):1185–90. doi: 10.1097/MPA.0b013e3181f6fce2. [DOI] [PubMed] [Google Scholar]
- 8.Al-Haddad M, Khashab M, Zyromski N, Pungpapong S, Wallace MB, Scolapio J, et al. Risk factors for hyperechogenic pancreas on endoscopic ultrasound: a case-control study. Pancreas. 2009 Aug;38((6)):672–5. doi: 10.1097/MPA.0b013e3181a9d5af. [DOI] [PubMed] [Google Scholar]
- 9.Mathur A, Zyromski NJ, Pitt HA, Al-Azzawi H, Walker JJ, Saxena R, et al. Pancreatic steatosis promotes dissemination and lethality of pancreatic cancer. J Am Coll Surg. 2009 May;208((5)):989–94. doi: 10.1016/j.jamcollsurg.2008.12.026. [DOI] [PubMed] [Google Scholar]
- 10.de Castro SM, Busch OR, van Gulik TM, Obertop H, Gouma DJ. Incidence and management of pancreatic leakage after pancreatoduodenectomy. Br J Surg. 2005 Sep;92((9)):1117–23. doi: 10.1002/bjs.5047. [DOI] [PubMed] [Google Scholar]
- 11.Mathur A, Pitt HA, Marine M, Saxena R, Schmidt CM, Howard TJ, et al. Fatty pancreas: a factor in postoperative pancreatic fistula. Ann Surg. 2007 Dec;246((6)):1058–64. doi: 10.1097/SLA.0b013e31814a6906. [DOI] [PubMed] [Google Scholar]
- 12.Alkully T, Darr U, Renno A, Khan Z, Oraibi O, Ruzieh M, et al. Su1327 Endoscopic Ultrasound Findings of Fatty Pancreas; Incidence, Etiology, and Clinical Implication. Gastrointest Endosc. 2016 May;83((5)):AB353. [Google Scholar]
- 13.Catalano MF, Sahai A, Levy M, Romagnuolo J, Wiersema M, Brugge W, et al. EUS-based criteria for the diagnosis of chronic pancreatitis: the Rosemont classification. Gastrointest Endosc. 2009 Jun;69((7)):1251–61. doi: 10.1016/j.gie.2008.07.043. [DOI] [PubMed] [Google Scholar]
- 14.Scherer J, Singh VP, Pitchumoni CS, Yadav D. Issues in hypertriglyceridemic pancreatitis: an update. J Clin Gastroenterol. 2014 Mar;48((3)):195–203. doi: 10.1097/01.mcg.0000436438.60145.5a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pedersen SB, Langsted A, Nordestgaard BG. Nonfasting Mild-to-Moderate Hypertriglyceridemia and Risk of Acute Pancreatitis. JAMA Intern Med. 2016 Dec;176((12)):1834–42. doi: 10.1001/jamainternmed.2016.6875. [DOI] [PubMed] [Google Scholar]