Abstract
A patient who has achieved resolution of acute hepatitis B and acquired anti-HBs would get protective immunity against hepatitis B virus (HBV). However, reactivation of HBV could happen if the patient was exposed to an immunocompromised state by using immunosuppressive drugs or chemotherapeutic agents. That is because cccDNA could reside within hepatocytes after recovery of acute hepatitis B. Therefore, guidelines for hepatitis B recommend the use of prophylactic antiviral agents such as entecavir or tenofovir in patients with anti-HBc IgG. The reactivation of hepatitis B without exposure to an immunocompromised state is very rare and only 1 case has been reported in the world to date. An 82-year-old male patient visited Dankook University Hospital because of high aspartate transaminase, alanine aminotransferase, and total bilirubin. He had shown HBsAg negative/anti-HBs positive when he had blood test examinations 1 year previously. However, the present blood test revealed HBsAg positive/anti-HBs negative and a high titer of HBV DNA (814,815 copies/mL). He had undergone vertebroplasty 5 years previously and had no other medical history. Other blood and radiological examinations failed to show other diseases that could affect host immunity. He started antiviral treatment with entecavir. However, he passed away because of deteriorated hepatic function and hepatorenal syndrome 20 days after admission. It is very rare that a patient with anti-HBs would develop hepatic failure and pass away without trigger factors. Here, we report the case with a literature review.
Keywords: Hepatitis B virus, Reactivation, Hepatic failure, Resolution
Introduction
There are two methods by which a subject can acquire anti-HBs. One is resolution of acute hepatitis B, and the second is vaccination for hepatitis B. Hepatitis B virus (HBV) reactivation cannot be developed in patients who acquired anti-HBs after vaccination although they received immunosuppressive treatment. However, a patient who has achieved resolution of acute hepatitis B can suffer HBV reactivation if the patient is in a condition of immunocompromised state. So, prophylactic antiviral treatment is recommended in patients with HBsAg negative/anti-HBc IgG positive/anti-HBs positive if they are scheduled to use high-risk immunosuppressive drugs such as rituximab [1]. HBV reactivation does not occur without immunosuppressive therapy or immunocompromised diseases. Only one case has been reported where HBV was reactivated in a patient with HBsAg negative/anti-HBs positive although there was no immunosuppressive treatment. The authors suggested that the case might be associated with a coronary artery bypass graft performed 9 months previously [2]. We experienced a rare case of HBV reactivation in a patient with HBsAg negative/anti-HBs positive without immunosuppressive treatment or immunocompromised state. The patient developed hepatic failure and expired 20 days after admission. Here, we report this rare case with a literature review.
Case Report
An 82-year-old male was admitted because of high aspartate transaminase (AST), alanine aminotransferase (ALT), and total bilirubin on blood tests. He had taken ferrous sulfate for 5 months prescribed at a family medicine clinic because of iron deficiency anemia, and a liver function test was normal at that time. He had complained of nausea and anorexia 1 month previously. Blood test showed that AST and ALT were 348 and 334 IU/L, respectively. Abdominal computed tomography showed normal liver shape without ascites (Fig. 1a). Although hospital admission was recommended by a family medicine doctor, the patient refused and insisted on a repeated blood test after a while. The blood test after 1 month showed that AST was 786 IU/L, ALT 964 IU/L, and total bilirubin 26 mg/dL. The patient was admitted to the gastroenterology department of Dankook University Hospital.
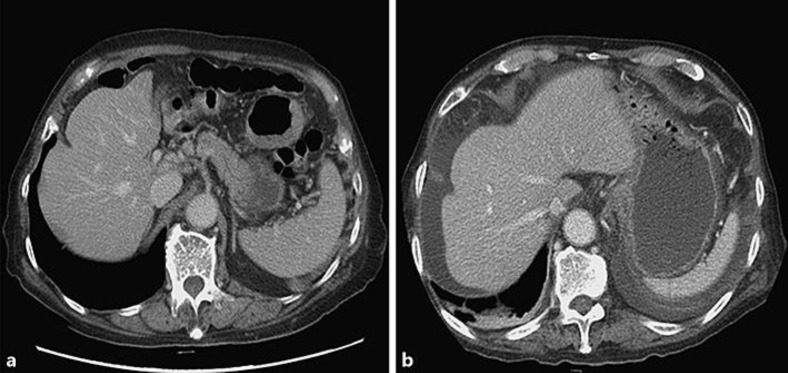
Fig. 1.
Computed tomography imaging of the liver. a The liver showed normal shape without ascites 1 month previously. b A moderate amount of ascites was found as well as newly developed pleural effusion compared to the previous computed tomography. However, the liver still showed normal shape without definite surface irregularity.
He had undergone vertebroplasty 5 years previously. The blood test performed before the operation had shown normal liver function, with HBsAg negative/anti-HBs positive. He also had blood tests during routine examinations 1 year previously and the results were AST 20 IU/L, ALT 13 IU/L, and HBsAg negative/anti-HBs positive. He had not been diagnosed with diabetes mellitus, hypertension, or tuberculosis. He refused to take any other medications such as immunomodulative or immunosuppressive drugs.
The blood tests performed after admission showed slightly improved liver function test with AST 430 IU/L, ALT 667 IU/L, total bilirubin 24.5 mg/dL, and direct bilirubin 20.8 mg/dL. However, severely impaired liver function was suspected with the result of INR 2.49 and albumin 2.3 g/dL. Hemoglobin and platelets were decreased at 10.9 g/dL and 67,000/mm3, respectively, compared to the result 1 month previously (13.3 g/dL, 187,000/mm3). BUN and creatinine were slightly elevated to 22.9 mg/dL and 1.25 mg/dL. Anti-HIV and anti-HCV showed negative results, but viral marker for HBV showed the opposite result compared to the past result. It showed HBsAg positive/anti-HBs negative. Other viral markers for hepatitis B were anti-HBc IgM, anti-HBc IgG, and anti-HBe positive/HBeAg negative. We first started entecavir 0.5 mg once daily while awaiting the HBV PCR result. After 5 days, HBV PCR was measured to 814,815 copies/mL and we continued with entecavir 0.5 mg. We did other examinations to exclude other causes of acute hepatitis, including anti-HAV IgM, herpes simplex virus PCR, Epstein-Barr virus PCR, cytomegalovirus PCR, and hepatitis E virus PCR; however, they were all negative. The blood tests for autoimmune hepatitis were also negative. α-Fetoprotein was elevated to 97 ng/mL but there was no hepatic mass on 3D dynamic liver computed tomography. A moderate amount of ascites was found as well as pleural effusion without definite surface irregularity (Fig. 1b). A liver biopsy was performed 5 days after admission because liver function was not improved with AST 261 IU/L, ALT 314 IU/L, total bilirubin 36.53 mg/dL, and INR 2.44, although he had taken entecavir. Liver biopsy showed chronic hepatitis with severe lobular activity, severe periportal activity, bridging necrosis, and some fibrosis (Fig. 2). We could not find other causes of acute hepatitis on laboratory and histological study, so he continued to take entecavir. As time went by, AST and ALT decreased to 89 IU/L and 90 IU/L, respectively, but other liver function results were not improved with total bilirubin 32.7 mg/dL, albumin 2.8 g/dL, and INR 3.28. At 19 days after admission, BUN and creatinine increased to 76.9 mg/dL and 2.0 mg/dL, respectively. Urine output was decreased and blood pressure decreased. Although we applied norepinephrine and Glypressin, albumin and oliguria were aggravated and the consciousness of the patient became comatose. We recommended continuous renal replacement therapy but the family of the patient refused it. The patient expired 20 days after admission.
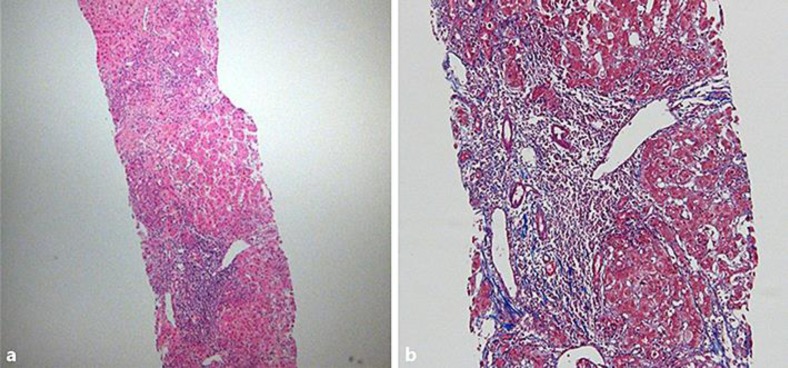
Fig. 2.
Microscopic findings of needle liver biopsy. Chronic hepatitis with severe lobular activity, severe periportal activity, bridging necrosis, and some fibrosis was found. Masson's trichrome stain. Original magnification, ×40 (a), ×100 (b).
Discussion
HBV exists within the nucleus as cccDNA after acute hepatitis B had been resolved and anti-HBs has been acquired. Then, it can cause HBV replication acting as a template for gene transcription [3, 4]. This replication usually happens in conditions such as immunosuppressive therapy, diseases with immunocompromised state, and organ transplantation that could decrease host immunity. Therefore, the latest HBV guideline of the EASL recommend pre-emptive or prophylactic antiviral therapy in patients with negative HBsAg and positive anti-HBc IgG if they are scheduled to receive immunosuppressive therapy [1]. Pre-emptive therapy refers to starting entecavir or tenofovir treatment in the case of HBsAg seroreversion or detectable HBV DNA for monitoring HBsAg and/or HBV DNA every 1–3 months during and after immunosuppression. Antiviral prophylaxis means that antiviral treatment should be started in HBsAg negative/anti-HBc positive patients who are scheduled to receive rituximab-containing regimens, regardless of their anti-HBs and serum HBV DNA status [5].
If the patient is HBsAg negative/anti-HBs positive, the possibility of HBV reactivation without trigger factors such as immunosuppressive therapy, disease with immunocompromised state, or organ transplantation is very rare. Only 1 case has been reported in the world to date [6]. He was a 73-year-old patient who had coronary artery bypass grafting 9 months previously. He was HBsAg negative/anti-HBs positive in the past, which changed to HBsAg positive/anti-HBs positive/HBeAg positive. HBV DNA was detected as 6.4 log10 copies/mL. HBsAg disappeared and HBV DNA decreased to an undetectable level after taking entecavir.
It is difficult to understand the mechanism whereby HBV reactivation was developed without trigger factors because of its rarity. The previously reported patient underwent coronary artery bypass graft 9 months previously and the major operation was known as a factor causing various immunological disturbances [2]. Circulating numbers of all lymphocytes were significantly decreased after surgery. The severity and duration of the reduction in cell numbers were strongly related to the degree of surgery. They usually returned to the preoperative state by postoperative day 7.
Other factors including aging, cancer (hematological malignancy and solid tumor), arteriosclerosis, diabetes mellitus, and malnutrition have been suggested as trigger factors causing decreased immunity [6]. The present patient had undergone vertebroplasty 5 years previously. However, his immunity was less likely to be affected by the operation because it was not a major operation and was performed a long time ago. There was also no evidence of diabetes mellitus and malignancy. The only thing suspected as a trigger factor was old age (82 years of age).
The Taormina expert meeting in 2008 defined occult hepatitis B as a case where HBV DNA is detected in liver biopsy regardless of the presence of HBV DNA in serum in patients with HBsAg negative [7]. Now, however, occult hepatitis B is generally defined as the presence of serum HBV DNA with HBsAg negative because a liver biopsy is not so easy to perform. The titer of HBV DNA is usually low [8, 9]. In patients with chronic hepatitis B, HBsAg is converted about 0.5–0.8% per year, but most of them have HBV DNA within the liver [10, 11].
The prevalence of occult hepatitis B is different according to the prevalence of chronic hepatitis B. The prevalence is as high as 41–90% in high-prevalence areas and 5–20% in low-prevalence areas [12, 13]. Anti-HBc IgG could predict the presence of occult hepatitis B. The prevalence of occult hepatitis B in a study using liver biopsy was 56% in anti-HBc positive, 31% in anti-HBc IgG positive/anti-HBs positive, and 14% in anti-HBc negative patients. The present patient showed HBsAg negative/anti-HBs positive, so we did not test for HBV DNA. Therefore, occult hepatitis B could not be completely excluded because of anti-HBc IgG positivity. However, it is also very rare that HBV reactivation would occur in a patient with occult hepatitis B without trigger factors.
The presence of anti-HBc IgM is useful to distinguish between acute and chronic hepatitis B infection [14]. The present patient could be suspected to have a new hepatitis B viral infection because he revealed anti-HBc IgM. But some chronic hepatitis B patients can show anti-HBc IgM after reactivation. It was not sure that the present case was infected with another new HBV. However, it could be suggested that HBV reactivation of cccDNA occurred instead of a new hepatitis B viral infection because anti-HBs changed to negativity after reactivation
In conclusion, the present case showed that HBV reactivation in a patient with anti-HBs can develop without trigger factors. This kind of reactivation is very rare and only one case has been reported to date. We hope that the present case may be helpful in understanding HBV reactivation without trigger factors.
Conclusion
If patients acquire anti-HBs after acute hepatitis B, they have immunity against HBV. However, immunosuppressive agents such as rituximab can cause reactivation of chronic hepatitis B. Reactivation of HBV without trigger factors and the development of hepatic failure in a patient with anti-HBs is very rare. The present patient did not have immunosuppressive treatment or immunocompromised status, except that he was 82 years of age. He developed hepatic failure and passed away. This is the first case that a patient passed away due to HBV reactivation without trigger factors.
Statement of Ethics
Patient consent was obtained.
Disclosure Statement
The authors declare no conflict of interest.
References
- 1.Lampertico P, Agarwal K, Berg T, Buti M, Janssen HL, Papatheodoridis G, et al. European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu; European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017 Aug;67((2)):370–98. doi: 10.1016/j.jhep.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 2.Lennard TW, Shenton BK, Borzotta A, Donnelly PK, White M, Gerrie LM, et al. The influence of surgical operations on components of the human immune system. Br J Surg. 1985 Oct;72((10)):771–6. doi: 10.1002/bjs.1800721002. [DOI] [PubMed] [Google Scholar]
- 3.Raimondo G, Caccamo G, Filomia R, Pollicino T. Occult HBV infection. Semin Immunopathol. 2013 Jan;35((1)):39–52. doi: 10.1007/s00281-012-0327-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levrero M, Pollicino T, Petersen J, Belloni L, Raimondo G, Dandri M. Control of cccDNA function in hepatitis B virus infection. J Hepatol. 2009 Sep;51((3)):581–92. doi: 10.1016/j.jhep.2009.05.022. [DOI] [PubMed] [Google Scholar]
- 5.Cholongitas E, Tziomalos K, Pipili C. Management of patients with hepatitis B in special populations. World J Gastroenterol. 2015 Feb;21((6)):1738–48. doi: 10.3748/wjg.v21.i6.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kamitsukasa H, Iri M, Tanaka A, Nagashima S, Takahashi M, Nishizawa T, et al. Spontaneous reactivation of hepatitis B virus (HBV) infection in patients with resolved or occult HBV infection. J Med Virol. 2015 Apr;87((4)):589–600. doi: 10.1002/jmv.24115. [DOI] [PubMed] [Google Scholar]
- 7.Raimondo G, Allain JP, Brunetto MR, Buendia MA, Chen DS, Colombo M, et al. Statements from the Taormina expert meeting on occult hepatitis B virus infection. J Hepatol. 2008 Oct;49((4)):652–7. doi: 10.1016/j.jhep.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 8.Allain JP. Occult hepatitis B virus infection. Transfus Clin Biol. 2004 Feb;11((1)):18–25. doi: 10.1016/j.tracli.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 9.De Mitri MS, Cassini R, Bernardi M. Hepatitis B virus-related hepatocarcinogenesis: molecular oncogenic potential of clear or occult infections. Eur J Cancer. 2010 Aug;46((12)):2178–86. doi: 10.1016/j.ejca.2010.03.034. [DOI] [PubMed] [Google Scholar]
- 10.Liaw YF, Sheen IS, Chen TJ, Chu CM, Pao CC. Incidence determinants and significance of delayed clearance of serum HBsAg in chronic hepatitis B virus infection: a prospective study. Hepatology. 1991 Apr;13((4)):627–31. [PubMed] [Google Scholar]
- 11.Loriot MA, Marcellin P, Walker F, Boyer N, Degott C, Randrianatoavina I, et al. Persistence of hepatitis B virus DNA in serum and liver from patients with chronic hepatitis B after loss of HBsAg. J Hepatol. 1997 Aug;27((2)):251–8. doi: 10.1016/s0168-8278(97)80168-7. [DOI] [PubMed] [Google Scholar]
- 12.Conjeevaram HS, Lok AS. Occult hepatitis B virus infection: a hidden menace? Hepatology. 2001 Jul;34((1)):204–6. doi: 10.1053/jhep.2001.25225. [DOI] [PubMed] [Google Scholar]
- 13.Kim YS, Jang JY, Eun SH, Cheon YK, Kim YS, Moon JH, et al. Detection of Intrahepatic HBV DNA in HBsAg-negative liver diseases (in Korean) Korean J Hepatol. 2006 Jun;12((2)):201–8. [PubMed] [Google Scholar]
- 14.Rodella A, Galli C, Terlenghi L, Perandin F, Bonfanti C, Manca N. Quantitative analysis of HBsAg, IgM anti-HBc and anti-HBc avidity in acute and chronic hepatitis B. J Clin Virol. 2006 Nov;37((3)):206–12. doi: 10.1016/j.jcv.2006.06.011. [DOI] [PubMed] [Google Scholar]