Abstract
Myocardial infarction is commonly considered as a leading cause of cardiovascular disease taking the lives of seven million people annually. Liver dysfunction is associated with cardiac diseases. The profile of abnormal liver functions in heart failure is not clearly defined. This study was designed to investigate the protective effects of betaine on liver injury after myocardial infarction induced by isoprenaline in rats. Forty-eight male rats were divided into four groups: the control group received normal diet and the experimental groups received 50, 150, and 250 mg kg-1 body weight of betaine daily through gastric gavages for 60 days. All of experimental and control groups experienced myocardial infarction, induced by subcutaneous injection of 100 mg kg-1 isoprenaline in two consecutive doses )8:00 AM to 8:00 PM). Liver enzymes including aspartate transaminase (AST) and alanine transaminase (ALT) were significantly reduced in the groups treated with betaine, compared with the control group. The total antioxidant capacity in the experimental groups, treated with betaine, showed a significant increase, compared with the control group. In the control group, severe lesions were created in the liver tissue, while degenerative changes of liver tissue significantly reduced in groups treated with different doses of betaine, showing the repair of liver tissue. Betaine decreased apoptosis in the experimental groups in comparison with the control group. Betaine showed a protective effect against biochemical and histological changes in liver tissue caused by the induction of myocardial infarction via isoprenaline injection.
Key Words: Apoptosis, Betaine, Isoprenaline, Liver, Myocardial infarction
Introduction
Myocardial infarction is one the most common cardiovascular disease affecting more than seven million people annually.1 It has been known for many years that the heart and the liver are intimately related. Thus, patients with acute and chronic heart failure develop manifestations of liver dysfunctions. A sudden and dramatic serum hepatic transaminase elevation in relation to cardiogenic shock indicates massive hepatocellular necrosis named ischemic hepatitis.2
Liver dysfunction is associated with cardiac diseases, such as myocardial infarction.3 Impaired blood flow and congestion play important roles in development of liver dysfunction.4 Since liver is a highly vascular organ, the damaging effects of heart failure on the liver are multifactorial including reduced blood flow to the liver, increased hepatic vein pressure, and reduced arterial saturation.5,6 Liver is a multifunctional organ that plays major roles in metabolism, biosynthesis, excretion, secretion, and detoxification. These processes require energy and turn the liver to an aerobic and oxygen-dependent organ.7 One-third of the blood that liver receives originates from the hepatic artery and two-third from the portal vein system.3 Liver cells contain enzymes that are released into the blood in various pathological conditions.8 Liver enzymes including gamma glutamyl transferase, alanine transaminase, aspartate transaminase and alkaline phosphatase, frequently used as markers of hepatic dysfunction and an enhanced indicator for cardiovascular risk.9
Oxidative stress balances the body in the production and elimination of reactive oxygen species (ROS) and reactive nitrogen species and decreases the production of antioxidants.10,11 Oxidative stress is usually associated with increased formation of ROS that plays a central role in cardiac physiology and pathophysiology. The ROS are molecules that have one or more unpaired electrons in their outer orbit, a state that greatly increases their reactivity.12High levels of ROS and oxidative stress, stimulates cell death through necrosis or apoptosis leading to tissue and cell damage.13 Oxidative stress is one of the pathological mechanisms leading to the initiation and progression of various liver diseases.14,15
Betaine (N,N,N-trimethyl glycine) is a methyl group donor that also affects lipid partitioning.16,17 Its metabolism links several metabolites that play an important role in the health of humans and other mammals, including choline (an important source of betaine), and homocysteine and methionine which are involved in its catabolism.18 Betaine is also known as an essential organic osmolyte that has protective effects on different types of liver cells.19
An important and common secondary effect of heart failure is liver dysfunction, but the importance and description of hepatic dysfunction in heart failures have rarely been studied. The histopathological assessments of liver in heart failure and the protective effects of betaine as an antioxidant on liver dysfunction caused by heart failure have not been investigated. Thus, the present study aimed to investigate the protective effects of betaine on liver injury caused by myocardial infarction via isoprenaline.
Materials and Methods
Animals. Forty-eight male Wistar rats with an average weight of 200 ± 10 g were divided into four groups of 12 rats. The rats were kept in standard cages with 12-hr light cycles, constant air temperature of 22-25 ˚C, and humidity of 55-60%. They were provided with free food and water. Before starting the test, the rats were kept in these conditions for one week to get used to the environment and the ethical considerations of working with animals were respected at all stages of the trial. The experiments were approved by the Institutional Ethics Committee of Urmia University of Medical Sciences, Urmia, Iran (ir.umsu.rec.1396.72; 22.04.2017). Oral betaine (Biochem, Lohne, Germany) was dissolved in distilled water. The doses of 50, 150, and 250 mg kg-1 given to the rats in the experimental groups daily through gastric gavages for 60 days. The control group received normal diet without betaine. Then all four groups received two doses of subcutaneous injection of 100 mg kg-1 isoprenaline (8:00AM and 8:00 PM) and developed infarction within 24 hr.20 A reduction in blood pressure considered as induction of myocardial infarction.21
Biochemical studies. Anesthesia was performed with intraperitoneal 50 mg kg-1 ketamine (Alfasan, Woerden, Netherlands) and 10 mg kg-1 xylazine (Alfasan).22 Cardiac blood was collected and kept for 15 min at room temperature. Serum was separated with a desktop centrifuge at 3000 rpm and kept at – 20 ˚C until biochemical tests were performed. The levels of alanine transaminase (ALT) and aspartate transaminase (AST) enzymes in the rats’ serum were measured according to the kit’s manufacturer instructions (Pars-Azmoon, Tehran, Iran) with auto-analyzer system (BT 3000; Biotecnica, Rome, Italy).
The total antioxidant capacity (TAC) was determined using TAC assay kit (Labor Diagnostika, Nordhorn, Germany). Serum samples from rats were used for TAC detection which the detection was based on peroxides reaction with peroxidase following color reaction of tetra methyl benzidine. After adding a stop solution, the blue color turned yellow and the optic density (OD) was measured over at wavelength of 450 nm by ELISA reader (Awareness Technology Inc., Palm City, USA).
Histological studies. Twelve hour after the last injection of isoprenaline, anesthesia was performed with the same dosage intraperitoneal ketamine and xylazine. The rat’s abdomen wall was incised and liver was removed and fixed in 10% formalin. After fixation, the tissues were passaged and embedded in paraffin. Then using the rotary microtome, slices were prepared with a thickness of 5 µm and stained by hematoxylin and eosin (H&E). The histological changes in the liver tissue were evaluated with an optical microscope (Carl Zeiss, Jena, Germany) in all treatment groups and compared with the control group.
Tunnel staining for apoptosis. To visualize the fragmented DNA after cell death (apoptosis), In Situ Cell Death Detection Kit, POD (Roche, Mannheim, Germany) was used. The tissue sections with a thickness of 5 µm were rinsed in xylene and descending grades of alcohol (absolute alcohol, 95%, 90%, 80% and 70%). The samples were then exposed to proteinase K for 10 min at 37 ˚C and then the tissue slides were incubated in TUNEL reaction solution at 37 ˚C for 60 min and then placed in anti-fluorescein POD antibody conjugated with horseradish peroxidase (Roche) solution for 30 min. Then, the samples were placed in 3'-Diaminobenzidine (DAB; Sigma-Aldrich, St. Louis, USA) for 5 to 10 min for color reactions and at the end stained with hematoxylin solution. After each step, the slides were rinsed in phosphate buffer pH=7.2 three times and the slides were then washed with ascending grades of alcohol (70%, 80%, 90%, 95%, and absolute alcohol). Finally, the number of apoptotic cells in liver tissue (nuclei in the microscopic field of view, observed in brown color) were counted using Image J software (version 1.47;NIH, Bethesda, USA).
Statistical analysis. In this study, data of liver enzymes and TAC were analyzed using GraphPad Prism (version 6.0; GraphPad software Inc., San Diego, USA). To compare different groups, one-way ANOVA was used. All the data were reported by mean ± SD and p < 0.05 was considered as significant.
Results
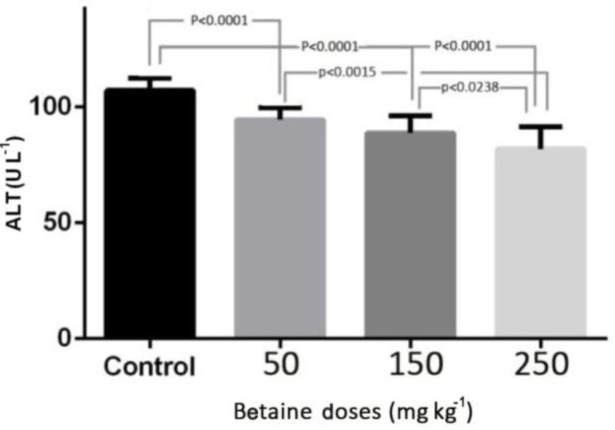
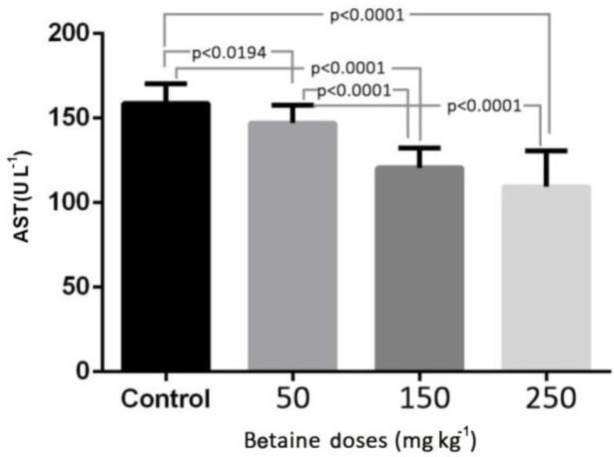
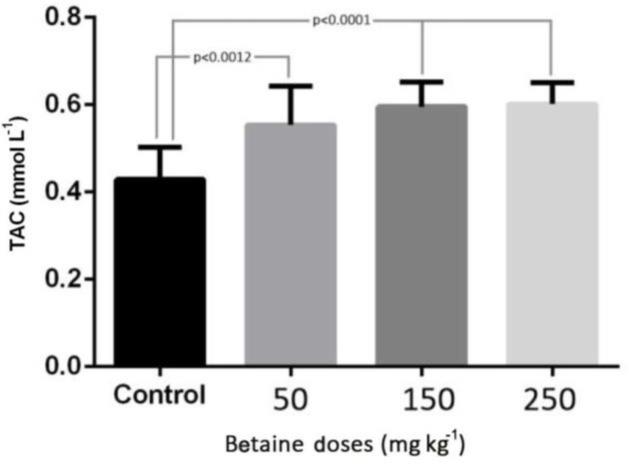
The levels of ALT, AST, and TAC in the experimental groups with betaine doses of 50, 150, and 250 mg kg-1 were compared with the control group. The results of this study revealed that induction of myocardial infarction significantly increased the levels of ALT, AST in the serum of control rats (p < 0.001). There was a significant reduction in the level of ALT in the experimental groups with different betaine doses, compared with the control group (p < 0.001). But there was no significant difference between the two groups treated with 50 and 150 mg kg-1 betaine (Fig. 1). The level of AST in the experimental groups treated with different doses of betaine showed a significant reduction compared with the control group (p < 0.001). However, there was no significant difference between the two groups treated with 150 and 250 mg kg-1 betaine (Fig. 2). The level of TAC in the experimental groups treated with different doses of betaine showed a significant increase compared with the control group (p < 0.001). However, there was no significant difference between the two groups treated with 50 and 150 mg kg-1 betaine, between the two groups treated with 150 and 250 mg kg-1 betaine, or between the two groups treated with 50 and 250 mg kg-1 betaine (Fig. 3).
Fig. 1.
The level of ALT enzyme in the experimental groups with betaine doses of 50, 150, 250 mg kg-1 compared with the control group after myocardial infarction induced by isoprenaline in rats. p < 0.05 represents significant differences among the groups
Fig. 2.
The level of AST enzyme in the experimental groups with betaine doses of 50, 150, 250 mg kg-1 compared with the control group after myocardial infarction induced by isoprenaline in rats. p < 0.05 represents significant differences among the groups
Fig.3.
The level of TAC in the experimental groups with betaine doses of 50, 150, 250 mg kg-1 compared with the control group after myocardial infarction induced by isoprenaline in rats
p < 0.05 represents significant differences among the groups.
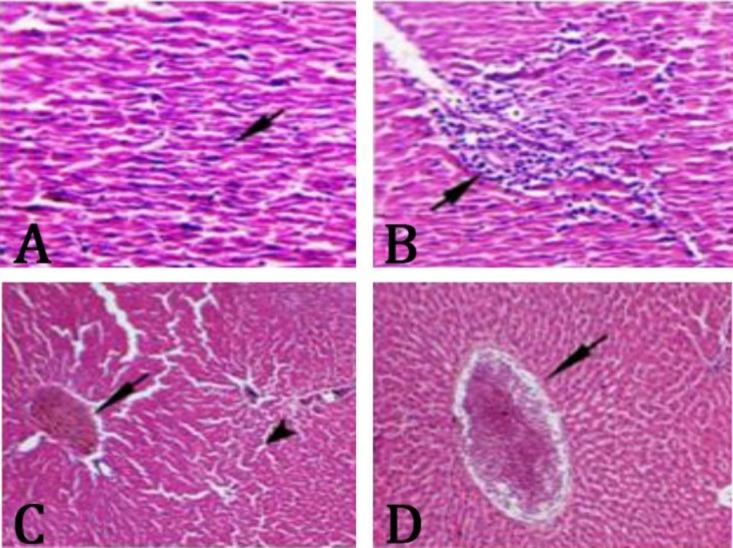
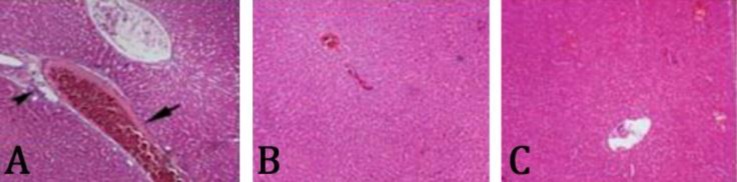
Histopathological findings. Results of histo-pathological examination of liver tissues stained with H&E showed that in the control group which had a myocardial infarction, necrotic cells with pyknotic nuclei, degenerated cytoplasm, mononuclear cells infiltration around the portal zone, congestion of central and portal veins, and sinusoid dilation were observed (Fig. 4). In the group treated with 50 mg kg-1 betaine, there were no cell necrosis and sinusoid dilation, but mild infiltration of mononuclear cells around the portal zone and portal vein congestion were observed (Fig. 5A). In groups treated with 150 and 250 mg kg-1 betaine, there were no necrotic cells, however, infiltration of mononuclear cells, portal and central vein congestion, or sinusoid dilation and tissue degenerative changes were substantially less than the control group and the liver tissue restored to normal and repair status (Figs. 5B and 5C).
Fig. 4.
A) Infiltration of mononuclear cells (arrow), B) cell necrosis (arrow), C) portal vein congestion (arrow), dilation of sinusoids (arrow tip), and D) central vein congestion (arrow) in the control group after myocardial infarction induced by isoprenaline in rats (H & E, 400×).
Fig. 5.
A) Portal vein congestion (arrow) and mild infiltration of inflammatory mononuclear cells (arrow tip) in experimental groups treated with 50 mg kg-1 betaine (H & E ×400). B) liver tissue of the groups receiving 150 mg kg-1 betaine, decreased portal congestion and tissue degenerative changes; and C) liver tissue of the group receiving 250 mg kg-1 betaine, striking decrease in tissue degenerative changes (H & E ,100×).
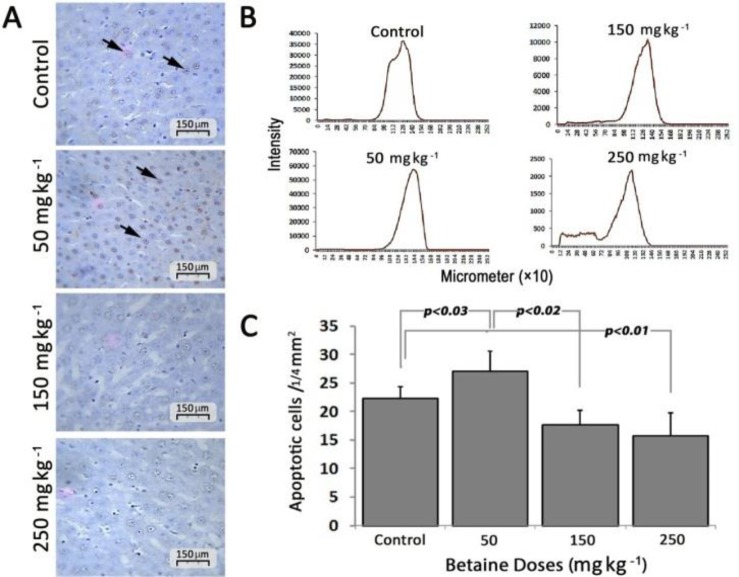
TUNEL staining results. Apoptotic cells with brown nuclei are shown by arrows (Fig.6A). The number of apoptotic cells in the group treated with 50 mg kg-1 betaine significantly increased compared with the control group (27.00 ± 3.60 vs 22.33 ± 2.08, respectively, p < 0.05). The number of apoptotic cells in the group treated with 50 mg kg-1 betaine was significantly higher than that of 150 and 250 mg kg-1 betaine group (17.66 ± 2.51 vs 15.66 ± 4.04, respectively; p < 0.05). The number of apoptotic cells in the groups treated with 150 and 250 mg kg-1 betaine was significantly less than the control group (p < 0.05). There was no significant difference in the number of apoptotic cells between the groups treated with 150 and 250 mg kg-1 betaine (Fig. 6C). The pixel-based intensity values for positive chromogenic reaction in each 10×253 µm of the liver in Figure 6B. Apoptotic cells in each 1.4 mm2 of tissue sections are presented as mean ± SD.
Fig. 6.
A) The histological sections from the liver, apoptotic cells are shown with arrows; B) Pixel based intensity values for positive reaction of chromogen (per 10 × 253 µm) of liver tissue; and C) The bar diagram of the number of apoptotic cells per 1/4 mm2. p <0.05 represents significant differences among the groups
Discussion
Cardiovascular diseases, including myocardial infarction, are the leading causes of death world-wide.23 According to the results of our research, administration of isoprenaline induce myocardial infarction in the rats. Isoprenaline, a synthetic catecholamine, and a beta-adrenergic agonist cause severe stress in myocardial cells and subsequently necrosis that resembles myocardial infarction in humans.24 Isoprenaline can cause a certain degree of damage to the heart clinical indicators, which are related to cardiac myocytes damages.25 Oxidative stress is the important etiopathological factor in isoprenaline induced myocardial necrosis.26
Acute cardiogenic liver injury is mostly associated with acute cardio circulatory failure resulting from acute myocardial infarction and replacement of hepatocytes with red blood cells extravasating from the sinusoids and necrosis/apoptosis.27 There is evaluation of cardiac and hepatic function with severe cardiac failure and liver injury are reported.28 Kubo and colleagues showed that liver dysfunction increased with higher severity of heart failure.29 Betaine is known as an important nutrient.30 Reportedly, betaine protects liver against alcohol damage.31-33 In a study by Junnila et al., betaine was found to be effective in preventing liver injury caused by tetrachloride carbon.34 Betaine, as a powerful antioxidant, has been shown to have positive effects on redox homeostasis during ischemia reperfusion of liver injury in rats.35 Dietary betaine supplementation could prove very effective in the treatment of liver diseases and other diseases associated with defective methylation.36
Liver dysfunction that causes liver tissue changes was reported as a complication of severe heart failure.8 The protective effects of betaine on liver tissue after myocardial infarction has not been studied properly. In a study it was reported that Tribulus terrestrisfruit aqueous extract protects heart and liver from beta adrenergic-stimulated cardio toxicity showed which can support isoproterenol-induced histological changes in liver and maintain the near normal architecture of liver tissues and they showed histological changes in catecholamine-induced cardiomyopathy are characterized by degeneration and necrosis of myocardial fiber, accumulation of inflammatory cells such as leukocytes, interstitial edema, lipid droplet and endocardial hemorrhage.8 In the present study it was shown that myocardial infarction induced changes in liver tissue and administration of betaine, as an antioxidant, had protective effects on liver by preventing the development of liver necrosis. Betaine reduced the formation of inflammatory mediators and improved tissue repair by reducing degenerative changes with the best protective effects in doses 150 and 250 mg kg-1. These findings were completely in agreement with the findings of Ganesan et al.37 They reported that the cardioprotective effects of betaine was related to its ability to maintain the myocardial energy status at high level by maintaining the activity of TCA cycle enzymes and the respiratory marker enzymes at near normalcy, and/or to its free radical scavenging ability against isoprenaline induced lipid peroxidation, which is primary responsible for the irreversible necrosis of the myocardial membrane. Hepatocellular damage is associated with increasing the levels of ALT and AST in serum and hepatocellular necrosis with marked elevation in serum AST/ALT increase in patients with acute heart dysfunction.38 Increased AST/ALT ratio due to apoptosis and necrosis in liver tissue was presented in this study. In the study by Kunutsor et al., liver enzymes were associated with the risk of cardiovascular diseases.39 Increased level of ALT and AST enzymes in heart failure due to liver injury is caused by reduced blood supply of the liver.40 It has been shown that the administration of isoprenaline to rats significantly increases the activity of ALT and AST enzymes.41 In the present study ALT and AST enzymes were increased in the control group which had infarction induced by isoprenaline. The plasma levels of these enzymes were reduced by administering different doses of betaine. The ability of betaine against oxidative stress depends on its highly lipotropic nature which readily pass across the lipid bilayer membrane and diffuse into intracellular compartment.42 Myocardial oxidative stress caused by isoprenaline has been suggested.8
The TAC of plasma is considered as a tool for medical diagnosis and treatment of different diseases.43 Yao et al. reported a decline in TAC following acute myocardial infarction that is consistent with the results of the present study.44 In a study by Kasap et al., it was showed that TAC level was lower in patients with acute myocardial infarction than the control group, but the difference was not significant.45 The results of this study showed that administration of betaine in different doses increased TAC level that could be due to the protective effect of betaine against oxidative stress related to the liver tissue. Cell death pattern of hepatocytes in liver injury caused by heart disease is in the form of apoptosis.46 Protective effects of betaine by preventing apoptosis of hepatocytes was approved.47 In a study by Graf et al., it was shown that betaine has strong protective effects against hepatocyte apoptosis induced by intra- and extra-cellular bile acids.19 In another study by Hagar et al., they showed that betaine had reno-protective effect against oxidative stress, inflammation and apoptosis associated with cisplatin therapy.48
TUNEL staining is detecting nuclear apoptosis in hepatocytes.49In the present study necrosis of hepatocytes was evident in the control group and the nuclei were pyknotic. In TUNEL test, the number of apoptotic cells in the control group were less than the group treated with 50 mg kg-1 betaine. In doses 150 and 250 mg kg-1, betaine prevented necrosis by protective effects on hepatocytes, indicating that betaine can also reduce the number of apoptotic cells in high doses. The results of this study revealed that betaine has protective effects on liver against apoptosis induced by myocardial infarction. For the first time Oberhammer et al. identified the association between apoptosis and elevation of serum transaminases by intravenous infusion of beta growth factor in rats and showed that apoptosis of hepatocytes and increased serum levels of ALT and AST happens together that are consistent with the findings of the present study.50
In conclusion, the results of the present study revealed that betaine has a protective effect on liver dysfunction and improves antioxidant activity and decreased apoptosis in hepatocytes after myocardial infarction.
Acknowledgments
This study was financially supported by a research grant from the Urmia University of Medical Science, Urmia, Iran.
Conflict of Interest
The authors have declared no conflict of interest.
References
- 1.Nichols M, Townsend N, Scarborough P, et al. Cardiovascular disease in Europe: Epidemiological update. Eur heart J. 2014;35(42):2950–2959. doi: 10.1093/eurheartj/ehu299. [DOI] [PubMed] [Google Scholar]
- 2.Møller S, Bernardi M. Interactions of the heart and the liver. Eur Heart J. 2013;34:2804–2811. doi: 10.1093/eurheartj/eht246. [DOI] [PubMed] [Google Scholar]
- 3.Soultati A, SP Dourakis A. Liver dysfunction in the intensive care unit. Ann Gastroenterol. 2007;18(1):35–45. [Google Scholar]
- 4.Biegus J, Zymlinski R, Sokolski M, et al. Liver function tests in patients with acute heart failure. Pol Arch Med Wewn. 2012;122(10):471–479. doi: 10.20452/pamw.1413. [DOI] [PubMed] [Google Scholar]
- 5.Ambrosy AP, Vaduganathan M, Huffman MD, et al. Clinical course and predictive value of liver function tests in patients hospitalized for worsening heart failure with reduced ejection fraction: an analysis of the EVEREST trial. Eur J Heart Fail. 2012;14(3):302–311. doi: 10.1093/eurjhf/hfs007. [DOI] [PubMed] [Google Scholar]
- 6.Giallourakis CC, Rosenberg PM, Friedman LS. The liver in heart failure. Clin Liver Dis. 2002;6(4):947–967. doi: 10.1016/s1089-3261(02)00056-9. [DOI] [PubMed] [Google Scholar]
- 7.Malhi H, Gores GJ, Lemasters JJ. Apoptosis and necrosis in the liver: Atale of two deaths? Hepatology. 2006;43(2 Suppl. 1):S31–44. doi: 10.1002/hep.21062. [DOI] [PubMed] [Google Scholar]
- 8.Rahmathulla SM, Sailaja K, Devi KL. Tribulus terrestris (I) protects heart and liver from beta adrenergic-stimulated cardiotoxicity: Biochemical and histological study in Wistar rats. Int J Drug Dev Res. 2013;5(1):264–270. [Google Scholar]
- 9.Pratt DS, Kaplan MM. Evaluation of abnormal liver-enzyme results in asymptomatic patients. N Engl J Med. 2000;342(17):1266–1271. doi: 10.1056/NEJM200004273421707. [DOI] [PubMed] [Google Scholar]
- 10.Apel K, Hirt H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol. 2004;55:373–399. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- 11.McCord JM. The evolution of free radicals and oxidative stress. Am J Med. 2000;108(8):652–659. doi: 10.1016/s0002-9343(00)00412-5. [DOI] [PubMed] [Google Scholar]
- 12.Di Filippo C, Cuzzocrea S, Rossi F, et al. Oxidative stress as the leading cause of acute myocardial infarction in diabetics. Cardiovasc Drug Rev. 2006;24(2):77–87. doi: 10.1111/j.1527-3466.2006.00077.x. [DOI] [PubMed] [Google Scholar]
- 13.Sanchez-Valle V, Chavez-Tapia NC, Uribe M, et al. Role of oxidative stress and molecular changes in liver fibrosis: A review. Curr Med Chem. 2012;19(28):4850–4860. doi: 10.2174/092986712803341520. [DOI] [PubMed] [Google Scholar]
- 14.Feng Y, Wang N, Ye X, et al. Hepatoprotective effect and its possible mechanism of Coptidis rhizoma aqueous extract on carbon tetrachloride-induced chronic liver hepatotoxicity in rats. J Ethnopharmacol. 2011;138(3):683–690. doi: 10.1016/j.jep.2011.09.032. [DOI] [PubMed] [Google Scholar]
- 15.Singal AK, Jampana SC, Weinman SA. Antioxidants as therapeutic agents for liver disease. Liver Int. 2011;31(10):1432–1448. doi: 10.1111/j.1478-3231.2011.02604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sakamoto A, Nishimura Y, Ono H, et al. Betaine and homocysteine concentrations in foods. Pediatr Int. 2002;44(4):409–413. doi: 10.1046/j.1442-200x.2002.01591.x. [DOI] [PubMed] [Google Scholar]
- 17.Barve A, Khan R, Marsano L, et al. Treatment of alcoholic liver disease. Ann Hepatol. 2008;7(1):5–15. [PubMed] [Google Scholar]
- 18.Lever M, George PM, Elmslie JL, et al. Betaine and secondary events in an acute coronary syndrome cohort. PLoS ONE. 2012;7(5):e37883. doi: 10.1371/journal.pone.0037883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Graf D, Kurz AK, Reinehr R, et al. Prevention of bile acid-induced apoptosis by betaine in rat liver. Hepatology. 2002;36(4 Pt 1):829–839. doi: 10.1053/jhep.2002.35536. [DOI] [PubMed] [Google Scholar]
- 20.Rahmathulla SM, Maruthi E, Bheemewsaraiah K, et al. Effect of Tribulus terrestris (L) on liver in isoproterenol-induced myocardial infarction. Int J Res Biochem Biophys. 2012;2(4):10–12. [Google Scholar]
- 21.Ghodratizadeh S, Rasmi Y, Khadem Ansari MH. Effect of betaine supplement on isoprenaline induced myocardial infarction and serum cathepsin G level in rat model. Urmia Med J. 2017;28(8):48–54. [Google Scholar]
- 22.Cuce G, Cetinkaya S, Koc T, et al. Chemoprotective effect of vitamin E in cyclophosphamide-induced hepato-toxicity in rats. Chem Biol Interact. 2015;232:7–11. doi: 10.1016/j.cbi.2015.02.016. [DOI] [PubMed] [Google Scholar]
- 23.Lee J, Jung Y, Park JY, et al. LC/MS-based polar metabolite profiling reveals gender differences in serum from patients with myocardial infarction. J Pharm Biomed Anal. 2015;115:475–486. doi: 10.1016/j.jpba.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 24.Mohanty I, Arya D, Gupta S. Dietary Curcuma longa protects myocardium against isoproterenol induced hemodynamic, biochemical and histopathological alternations in rats. Int J Appl. 2008;1(4):19–28. [Google Scholar]
- 25.Huang H, Geng Q, Yao H, et al. Protective effect of scutellarin on myocardial infarction induced by isoprenaline in rats. Iran J Basic Med Sci. 2018;21:267–276. doi: 10.22038/ijbms.2018.26110.6415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Noronha-Dutra AA, Steen EM, Woolf N. The early changes induced by isoproterenol in the endocardium and adjacent myocardium. Am J Pathol. 1984;114(2):231–239. [PMC free article] [PubMed] [Google Scholar]
- 27.Cagli K, Basar FN, Tok D, et al. How to interpret liver function tests in heart failure patients? Turk J Gastroenterol. 2015;26(3):197–203. doi: 10.5152/tjg.2015.0086. [DOI] [PubMed] [Google Scholar]
- 28.Fouad YM, Yehia R. Hepato-cardiac disorders. World J Hepatol. 2014;6(1):41–54. doi: 10.4254/wjh.v6.i1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kubo SH, Walter BA, John DH, et al. Liver function abnormalities in chronic heart failure Influence of systemic hemodynamics. Arch Intern Med. 1987;147(7):1227–1230. [PubMed] [Google Scholar]
- 30.Craig SA. Betaine in human nutrition. Am J Clin Nutr. 2004;80(3):539–549. doi: 10.1093/ajcn/80.3.539. [DOI] [PubMed] [Google Scholar]
- 31.Ji C, Kaplowitz N. Betaine decreases hyperhomo-cysteinemia, endoplasmic reticulum stress, and liver injury in alcohol-fed mice. Gastroenterology. 2003;124(5):1488–1499. doi: 10.1016/s0016-5085(03)00276-2. [DOI] [PubMed] [Google Scholar]
- 32.Ji C, Deng Q, Kaplowitz N. Role of TNF-alpha in ethanol-induced hyperhomocysteinemia and murine alcoholic liver injury. Hepatology. 2004;40(2):442–451. doi: 10.1002/hep.20309. [DOI] [PubMed] [Google Scholar]
- 33.Barak AJ, Beckenhauer HC, Mailliard ME, et al. Betaine lowers elevated s-adenosylhomocysteine levels in hepatocytes from ethanol-fed rats. J Nutr. 2003;133(9):2845–2848. doi: 10.1093/jn/133.9.2845. [DOI] [PubMed] [Google Scholar]
- 34.Junnila M, Barak AJ, Beckenhauer HC, et al. Betaine reduces hepatic lipidosis induced by carbon tetrachloride in Sprague-Dawley rats. Vet Hum Toxicol. 1998;40(5):263–266. [PubMed] [Google Scholar]
- 35.Hagar H, Al Malki W. Betaine supplementation protects against renal injury induced by cadmium intoxication in rats: role of oxidative stress and caspase-3. Environ Toxicol Pharmacol. 2014;37(2):803–811. doi: 10.1016/j.etap.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 36.Kharbanda KK, Mailliard ME, Baldwin CR, et al. Betaine attenuates alcoholic steatosis by restoring phosphatidylcholine generation via the phosphatidyl-ethanolamine methyltransferase pathway. J Hepatol. 2007;46(2):314–321. doi: 10.1016/j.jhep.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 37.Ganesan B, Rajesh R, Anandan R. Biochemical studies on the protective effect of betaine on mitochondrial function in experimentally induced myocardial infarction in rats. J Health Sci. 2007;53(6):671–681. [Google Scholar]
- 38.Alvarez AM, Mukherjee D. Liver abnormalities in cardiac diseases and heart failure. Int J Angiol. 2011;20(3):135–142. doi: 10.1055/s-0031-1284434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kunutsor SK, Apekey TA, Khan H. Liver enzymes and risk of cardiovascular disease in the general population: A meta-analysis of prospective cohort studies. Atherosclerosis. 2014;236(1):7–17. doi: 10.1016/j.atherosclerosis.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 40.van Deursen VM, Damman K, Hillege HL, et al. Abnormal liver function in relation to hemodynamic profile in heart failure patients. J Card Fail. 2010;16(1):84–90. doi: 10.1016/j.cardfail.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 41.Shinohara M, Ji C, Kaplowitz N. Differences in betaine-homocysteine methyltransferase expression, endo-plasmic reticulum stress response, and liver injury between alcohol-fed mice and rats. Hepatology. 2010;51(3):796–805. doi: 10.1002/hep.23391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kanbak G, Akyuz F, Inal M. Preventive effect of betaine on ethanol-induced membrane lipid composition and membrane ATPases. Arch Toxicol. 2001;75(1):59–61. doi: 10.1007/s002040000179. [DOI] [PubMed] [Google Scholar]
- 43.Bartosz G. Total antioxidant capacity. Adv Clin Chem. 2003;37:219–292. doi: 10.1016/s0065-2423(03)37010-6. [DOI] [PubMed] [Google Scholar]
- 44.Yao D, Vlessidis AG, Evmiridis NP, et al. Possible mechanism for nitric oxide and oxidative stress induced pathophysiological variance in acute myocardial infarction development: A study by a flow injection-chemiluminescence method. Anal Chim Acta. 2004;505(1):115–123. [Google Scholar]
- 45.Kasap S, Gonenc A, Sener DE, et al. Serum cardiac markers in patients with acute myocardial infarction: oxidative stress, C-reactive protein and N-terminal probrain natriuretic Peptide. J Clin Biochem Nutr. 2007;41(1):50–57. doi: 10.3164/jcbn.2007007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Herzer K, Kneiseler G, Bechmann LP, et al. Onset of heart failure determines the hepatic cell death pattern. Ann Hepatol. 2011;10(2):174–179. [PubMed] [Google Scholar]
- 47.Horio M, Ito A, Matsuoka Y, et al. Apoptosis induced by hypertonicity in Madin Darley canine kidney cells: Protective effect of betaine. Nephrol Dial Transplant. 2001;16(3):483–490. doi: 10.1093/ndt/16.3.483. [DOI] [PubMed] [Google Scholar]
- 48.Hagar H, Medany AE, Salam R, et al. Betaine supplementation mitigates cisplatin-induced nephrotoxicity by abrogation of oxidative/nitrosative stress and suppression of inflammation and apoptosis in rats. Exp Toxicol Pathol. 2015;67(2):133–141. doi: 10.1016/j.etp.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 49.Schoemaker MH, Gommans WM, Conde de la Rosa L, et al. Resistance of rat hepatocytes against bile acid-induced apoptosis in cholestatic liver injury is due to nuclear factor-kappa B activation. J Hepatol. 2003;39(2):153–161. doi: 10.1016/s0168-8278(03)00214-9. [DOI] [PubMed] [Google Scholar]
- 50.Oberhammer F, Bursch W, Tiefenbacher R, et al. Apoptosis is induced by transforming growth factor-beta 1 within 5 hours in regressing liver without significant fragmentation of the DNA. Hepatology. 1993;18(5):1238–1246. [PubMed] [Google Scholar]