Abstract
Immune system plays crucial role in body and lymph nodes are essential parts of this system for combating pathogens. However, no study has ever been conducted on morphometric development of sheep lymph nodes in fetal period. Thus, this study attempted to examine the morphometric characteristics of a number of important lymph nodes of some lymphocenters of sheep during fetal period. To this end, 60 pieces of sheep fetuses collected from Ahvaz slaughterhouse were fixated in 10% formalin and then divided into four categories based on crown-rump length (CRL) following gender and weight determinations. Mandibular, caudal superficial cervical (prescapular), caudal mediastinum, jejunal mesenteric and popliteal lymph nodes were evaluated in five lymphocenters of head, neck, thoracic cavity, abdominal viscera and pelvic limbs, respectively. In each sample, nodes formation was visually checked and in cases of nodes formation, they were measured in terms of weight, length, width and thickness and collected data were statistically analyzed. The longest and shortest fetal CRLs were found to be 48.50 cm and 3.50 cm, respectively. Gender had no effect on study parameters in 32 male and 28 female fetuses. Study of sheep fetuses’ lymph nodes revealed no macroscopic lymph node development by day 45, while all nodes were observable after the day 59. The shortest lymph node was mandibular node and the longest one was caudal mediastinum. Based on the results, it seemed that although the size of lymph nodes grows by age, this increase is not the same for all nodes and groups.
Key Words: Fetus, Lymph nodes, Morphometric development, Sheep
Introduction
Lymphatic vasculature plays key roles in both normal and pathological conditions. It is necessary for maintaining proper body fluid homeostasis and tissue fluid levels, provides a pathway for immune cells and antigen-presenting cells to lymphatic organs, transports fats and nutrients for the digestive tract and serves other functions too numerous to list here.1
Lymphatic channels arise by vasculogenesis and angiogenesis from venous precursor cells. In humans, lymphangiogenesis begins with the formation of bilateral sprouts from anterior cardinal veins at about day forty-two of development. Shortly after the establishment of the cardiovascular system, lymphatic vessels develop in a manner similar to that described for blood vessels. Initially, six primary lymph sacs develop in the late embryonic period. Paired jugular sacs develop lateral to the internal jugular veins, followed by a single retroperitoneal sac close to the root of the mesentery. An additional sac, the cisterna chyli, develops dorsal to the retroperitoneal sac. A pair of iliac sac also develops at the junction of the iliac vein. Drainage of lymph from the pelvic region and hind limbs occurs through the iliac sacs, while the retroperitoneal sac and cisterna chyli drain the viscera. At a late stage of lymphatic development, the lymphatic sacs become interconnected by a series of lymphatic vessels and a lymphatic drainage system becomes established.2
Apart from the cisterna chyli, the lymph sacs become converted into lymph nodes by aggregation of lymphoid tissue around the sacs. Mesenchymal cells surrounding the sacs infiltrate these structures and convert them into a network of lymph channels. Later in development, additional lymph nodes develop along the course of the lymphatic vessels throughout the body.2
Lymph nodes which are bean-shaped in ruminants may be compressed or even wide. They are grayish-yellow to brownish-red and are generally surrounded by low or high amounts of adipose tissue.3 These nodes vary in size in animals. Ruminants and carnivores have large lymph nodes; while horse and pig have relatively smaller ones.4 Mammals have a number of fixed lymph sites across their body receiving afferent lymph vessels from their respective region. Lymphocenters in mammals include cerebral nodes which are mandibular, parotid and retropharyngeal, cervical lymph nodes including superficial and deep cervical nodes, thoracic including axillary nodes and thoracic cavity covering dorsal and abdominal thoracic as well as mediastinal and bronchial nodes. Abdominal wall and pelvic limb include lumbar, sacroiliac, inguinal and ischiatic nodes and femoral and iliac nodes belong to pelvic limb lymph center; while visceral nodes consist of cranial and caudal celiac and mesenteric nodes.5
Lymph nodes are different and change in color and size during infection.6 Although a number of studies have been carried out on morphometric and histometrical characteristics of lymph nodes in mature and immature sheep,7-9 few studies have been conducted on the animal fetus lymph nodes.10-13 It has been reported that the means of morphometric development parameters of the lymph nodes in 4-month- and 5-month-old goat fetuses were slightly higher in males.10 The neutrality of gender on morphometric development and cranial tracheobronchial lymph nodes evolution in sheep fetuses has also been suggested previously.11 Further, it has been shown that ovine T lymphocyte precursors are detected for the first time on day 43 or 44 of life; while the first T cell concentrations are first seen on day 55.12
Considering the importance of immune system in body and the crucial roles played by lymph nodes in combating pathogens and the fact that no study has ever been undertaken on morphometric development of lymph nodes in sheep fetuses, this study attempted to look into morphometric development of the lymph nodes in the sheep (Ovis aries) during fetal period.
Materials and Methods
Sixty pieces of sheep fetuses were selected from all samples collected from Ahvaz slaughterhouse (capital of Khuzestan province, south western Iran). Based on the fetus size, 5% formalin was injected into the abdominal walls of selected samples to fix their abdomens. Then, all samples were inserted in 10% formalin containing dishes and then stored in the Anatomy and Embryology Hall of Ahvaz Faculty of Veterinary Medicine. The samples were removed and coded followed by weighing and gender and age determinations. To estimate the fetuses ages, crown-rump length (CRL) was measured using measurement tape and packing thread and then the age was computed via employing the age determination formula.14
The samples were divided into four categories based CRL:
1 st group: fetuses with CRL shorter than 15.00 cm (43 to 66 days of gestation).
2 nd group: fetuses with CRL between 15.00 to 25.00 cm (67 to 86 days of gestation).
3 rd group: fetuses with CRL between 25.00 to 35.00 cm (88 to 103 days of gestation).
4 th group: fetuses with CRL of 35.00 cm and longer (109 to 138 days of gestation).
After categorization, mandibular, caudal superficial cervical, caudal mediastinal, jejunal mesenteric and popliteal lymph nodes were assessed in five lympho-centers of head, neck, thoracic cavity, abdominal viscera and pelvic limb, respectively. In each sample, node formation was visually checked following target site dissection. Nodes which were observable and macroscopically separable were then measured in terms of weight, length, width and thickness using a pairs of scales (N90; Sartorius, Tokyo, Japan) and calipers (Guanglu Co., Guilin, China) if such formation was confirmed in the first place. Measurements were administered in three regions with the highest length, width and thickness and the mean of measurements was acquired. This was done at least twice by two individuals. The means of collected information were recorded in separate tables for each fetus.
The data were studied analytically and descriptively using SPSS (version 16.0; SPSS Inc., Chicago, USA). Data analysis was based on one-way, two-way, and repeated measurements ANOVA, correlation coefficient computation, LSD supplementary and Dunnette’s tests C. α=5% was the basis of statistical analysis.
Results
Sixty fetuses of sheep (Ovis aries), at different ages, including 28 (47.00%) females and 32 (53.00%) males were studied. The approximate age of the fetuses was estimated from the CRL (3.50 - 48.50 cm) and the minimum and maximum ages of the samples were 43 and 138 days, respectively. Regarding the age at which macroscopic observation of lymph nodes in sheep fetuses was took place, the studied nodes were observed in most samples, although there were some exceptions. For example, no lymph nodes were observed macroscopically in 43-day- and 44-day-old fetuses belonged to the first group and were the youngest of all samples (Fig. 1).
Fig. 1.
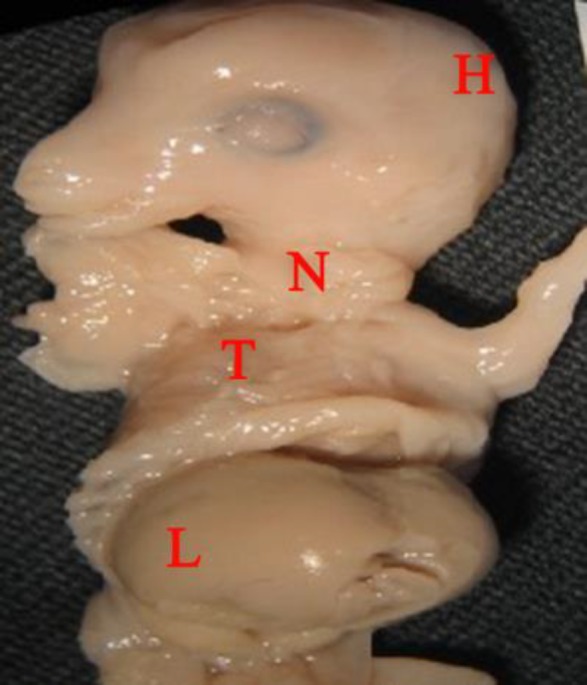
A male 43-day-old sheep fetus under the loupe, a ventrolateral view. Abdomen cut open, yet no observable lymph node. H: Head; N: Neck; T: Thorax; L: Liver
Moreover, jejunal mesenteric lymph nodes were macroscopically observed in 59-day-old fetuses. Macroscopically, eight fetuses (53.33%) belonging to the first group lacked jejunal mesenteric lymph nodes in which a 43-day-old fetus was the youngest; while a 57-day-old one was the oldest case in which no jejunal mesenteric lymph nodes were observed. Means standard deviations of the measured parameters for each studied lymph node have been presented in form of separate groups; while for pair nodes this has been done in separate groups as well as right and left sides (Tables 1 and 2).
Table 1.
Means standard deviations of the size of even lymph nodes (60 fetus).
| Lymph node | Groups | Position | Length (mm) | Width (mm) | Thickness (mm) | Weight (g) |
|---|---|---|---|---|---|---|
| Mandibular | 1 st Group | Right | 0.90 ± 0.63Ca | 0.67 ± 0.60Ca | 0.46 ± 0.33Da | 0.001 ± 0.00Ca |
| Left | 0.87 ± 0.86Ca | 0.65 ± 0.50Da | 0.37 ± 0.29Ca | 0.001 ± 0.00Ca | ||
| 2 nd Group | Right | 2.89 ± 1.07Ba | 2.10 ± 0.61Ba | 1.36 ± 0.35Ca | 0.001 ± 0.00BCa | |
| Left | 2.922 ± 0.90Ba | 1.90 ± 0.58Ca | 1.58 ± 0.43Ba | 0.001 ± 0.00BCa | ||
| 3 nd Group | Right | 3.76 ± 1.22Ba | 2.50 ± 0.75Ba | 1.68 ± 0.32Ba | 0.01 ± 0.00Ba | |
| Left | 2.50 ± 0.75Ba | 2.54 ± 0.80Ba | 1.76 ± 0.37Ba | 0.01 ± 0.00Ba | ||
| 4 th Group | Right | 5.90 ± 2.23Aa | 3.53 ± 0.83Aa | 2.06 ± 0.45Aa | 0.03 ± 0.02Aa | |
| Left | 5.33 ± 2.50Aa | 3.30 ± 1.06Aa | 2.16 ± 0.36Aa | 0.001 ± 0.00Ca | ||
| Prescapular | 1 st Group | Right | 2.79 ± 1.67Ca | 1.44 ± 1.24Da | 0.65 ± 0.60Da | 0.001 ± 0.00Ca |
| Left | 2.72 ± 1.46Da | 1.29 ± 1.07Da | 0.73 ± 0.64Da | 0.001 ± 0.00Ca | ||
| 2 nd Group | Right | 5.40 ± 1.80Ba | 3.63 ± 0.71Ca | 2.33 ± 064Ca | 0.03 ± 0.03Ca | |
| Left | 5.23 ± 1.76Ca | 3.83 ± 1.01Ca | 2.36 ± 0.51Ca | 0.03 ± 0.02Ca | ||
| 3 nd Group | Right | 9.96 ± 2.56Ba | 6.56 ± 1.60Ba | 3.40 ± 0.63Ba | 0.15 ± 0.09Ba | |
| Left | 8.88 ± 1.92Bb | 6.24 ± 1.79Ba | 3.27 ± 0.75Ba | 0.13 ± 0.08Ba | ||
| 4 th Group | Right | 14.86 ± 3.27Aa | 8.26 ± 2.01Aa | 4.13 ± 0.61Aa | 0.35 ± 0.14Aa | |
| Left | 13.26 ± 3.26Ab | 8.23 ± 1.80Aa | 4.30 ± 0.88Aa | 0.33 ± 0.14Aa | ||
| Popliteal | 1 st Group | Right | 1.20 ± 0.57Da | 0.85 ± 0.51Da | 0.46 ± 0.42Da | 0.001 ± 0.00Ca |
| Left | 1.12 ± 0.59Da | 0.64 ± 0.42Da | 0.36 ± 0.32Db | 0.001 ± 0.00Ca | ||
| 2 nd Group | Right | 2.16 ± 0.89Cc | 2.03 ± 0.69Ca | 1.73 ± 0.41Ca | 0.001 ± 0.00Ca | |
| Left | 2.23 ± 1.08Ca | 1.96 ± 0.61Ca | 1.70 ± 0.36Ca | 0.001 ± 0.00Ca | ||
| 3 nd Group | Right | 4.26 ± 1.27Ba | 3.40 ± 0.82Ba | 2.43 ± 0.49 Ba | 0.02 ± 0.01Ba | |
| Left | 4.06 ± 0.99Ba | 3.33 ± 0.97Ba | 2.43 ± 0.62Ba | 0.02 ± 0.01Ba | ||
| 4 th Group | Right | 6.50 ± 1.42Aa | 4.93 ± 0.99Aa | 3.13 ± 0.78Aa | 0.06 ± 0.03Aa | |
| Left | 6.60 ± 2.12Aa | 4.83 ± 0.81Aa | 3.26 ± 0.84Aa | 0.06 ± 0.03Aa |
Different lower case letters indicate significant differences between the sides (p < 0.05).
Different upper case letters indicate significant differences between the groups (p < 0.05).
Table 2.
Means standard deviations of the size of odd lymph nodes (60 fetuses).
| Lymph node | Groups | Position | Length (mm) | Width (mm) | Thickness (mm) | Weight (g) |
|---|---|---|---|---|---|---|
| Mediastinal | 1 st Group | Caudal | 6.12 ± 4.73D | 0.88 ± 0.69C | 0.61 ± 0.60C | 0.001 ± 0.00B |
| 2 nd Group | Caudal | 16.06 ± 5.27C | 2.30 ± 0.56B | 1.73 ± 0.41B | 0.04 ± 0.03B | |
| 3 nd Group | Caudal | 25.36 ± 3.01B | 3.26 ± 0.56A | 1.97 ± 0.34AB | 0.14 ± 0.14A | |
| 4 th Group | Caudal | 36.86 ± 12.60A | 3.70 ± 0.95A | 2.36 ± 0.58A | 0.22 ± 0.19Aa | |
| Jejunal | 1 st Group | Mesenteric1 | 7.78 ± 4.03C | 0.76 ± 0.60D | 0.42 ± 0.33D | 0.00 ± 0.00B |
| Mesenteric2 | 2.76 ± 1.58B | 1.16 ± 0.81C | 0.62 ± 0.43C | 0.001 ± 0.00B | ||
| Mesenteric3 | 6.75 ± 6.01AB | 1.65 ± 0.35C | 0.75 ± 0.35B | 0.001 ± 0.00B | ||
| 2 nd Group | Mesenteric1 | 9.71 ± 7.12C | 2.12 ± 0.40C | 1.44 ± 0.38C | 0.02 ± 0.02B | |
| Mesenteric2 | 7.45 ± 4.63B | 2.22 ± 0.46BC | 1.53 ± 0.55 B | 0.01 ± 0.00 B | ||
| Mesenteric3 | 3.43 ± 1.26B | 2.12 ± 0.79B | 1.37 ± 0.44BC | 0.01 ± 0.02B | ||
| 3 nd Group | Mesenteric1 | 24.16 ± 13.13B | 3.00 ± 0.75B | 1.80 ± 0.36B | 0.14 ± 0.20A | |
| Mesenteric2 | 7.04 ± 2.46B | 2.79 ± 0.94B | 1.76 ± 0.41B | 0.03 ± 0.02 B | ||
| Mesenteric3 | 5.70 ± 2.61AB | 2.95 ± 0.98B | 1.90 ± 0.61 AB | 0.02 ± 0.01 B | ||
| 4 th Group | Mesenteric1 | 30.93 ± 21.01A | 4.26 ± 1.57A | 2.10 ± 0.43 A | 0.21 ± 0.18A | |
| Mesenteric2 | 15.70 ± 10.96A | 4.29 ± 1.35A | 2.41 ± 0.55A | 0.09 ± 0.07A | ||
| Mesenteric3 | 7.90 ± 2.99A | 4.25 ± 1.08A | 2.20 ± 0.91A | 0.06 ± 0.05A |
Different letters indicate significant differences between the groups (p 0.05).
After checking the Tables 1 and 2 as well as comparing the means of the measured parameters for lymph nodes in different groups, it was revealed that nodes can be ranked in the following descending order (larger to smaller) in terms of each measurement parameter.
1 st Group:
Length: jejunal mesenteric, caudal mediastinal, prescapular, popliteal and mandibular lymph nodes.
Width: jejunal mesenteric, prescapular, caudal mediastinal, popliteal and mandibular lymph nodes.
Thickness: prescapular, jejunal mesenteric, caudal mediastinal, popliteal and mandibular lymph nodes.
Weight: since the precision of the pair of scales was 0.001 g, this figure was recorded.
2 nd Group:
Length: caudal mediastinal, jejunal mesenteric, prescapular, mandibular and popliteal lymph nodes.
Width: prescapular, caudal mediastinal, jejunal mesenteric, popliteal and mandibular lymph nodes.
Thickness: prescapular, caudal mediastinal, popliteal, jejunal mesenteric and mandibular lymph nodes.
Weight: caudal mediastinal and jejunal mesenteric lymph nodes. The remaining nodes were at the precision rate of the scales and a minimum of 0.001 g.
3 rd Group:
Length: caudal mediastinal, jejunal mesenteric, prescapular, popliteal and mandibular lymph nodes.
Width: prescapular, popliteal, caudal mediastinal, jejunal mesenteric and mandibular lymph nodes.
Thickness: prescapular, popliteal, caudal mediastinal, jejunal mesenteric and mandibular lymph nodes.
Weight: caudal mediastinal, jejunal mesenteric, prescapular, popliteal and mandibular lymph nodes.
4 th Group:
Length: caudal mediastinal, jejunal mesenteric, prescapular, popliteal and mandibular lymph nodes. Width: prescapular, popliteal, jejunal mesenteric, caudal mediastinal and mandibular lymph nodes.
Thickness: prescapular, popliteal, jejunal mesenteric, caudal mediastinal and mandibular lymph nodes.
Weight: prescapular, caudal mediastinal, jejunal mesenteric, popliteal and mandibular lymph nodes.
Based on the statistical tests and the content of Table 3, gender had no effect on the study parameters (p 0.05).
Table 3.
Means standard deviations of the sheep fetuses lymph node parameters based on gender. Female (F; 28 fetuses), male (M; 32 fetuses).
| Lymph node | Position |
Length (mm)
|
Width (mm)
|
Thickness (mm)
|
Weight (g)
|
||||
|---|---|---|---|---|---|---|---|---|---|
| F | M | F | M | F | M | F | M | ||
| Mandibular | Right | 3.42 ± 2.17 | 3.31 ± 2.39 | 2.21 ± 1.12 | 2.19 ± 1.35 | 1.41 ± 0.69 | 1.31 ± 0.82 | 0.01 ± 0.01 | 0.01 ± 0.01 |
| Left | 3.45 ± 1.97 | 3.03 ± 2.16 | 2.23 ± 1.23 | 1.90 ± 1.34 | 1.57 ± 0.73 | 1.34 ± 0.85 | 0.01 ± 0.01 | 0.01 ± 0.01 | |
| Prescapular | Right | 8.33 ± 4.77 | 8.18 ± 5.61 | 5.06 ± 2.51 | 4.90 ± 4.43 | 2.75 ± 1.25 | 2.52 ± 1.62 | 0.15 ± 0.13 | 0.17 ± 0.14 |
| Left | 7.75 ± 4.38 | 7.32 ± 4.74 | 5.22 ± 2.81 | 4.61 ± 3.15 | 2.70 ± 1.42 | 2.63 ± 1.57 | 0.15 ± 0.12 | 0.15 ± 0.12 | |
| Popliteal | Right | 3.48 ± 2.02 | 3.57 ± 2.57 | 2.91 ± 1.59 | 2.71 ± 1.82 | 1.90 ± 0.97 | 1.92 ± 1.28 | 0.03 ± 0.02 | 0.03 ± 0.02 |
| Left | 3.63 ± 2.61 | 3.39 ± 2.34 | 2.80 ± 1.60 | 2.59 ± 1.85 | 1.99 ± 1.06 | 1.87 ± 1.37 | 0.03 ± 0.02 | 0.03 ± 0.02 | |
| Mediastinal | Caudal | 21.69 ± 10.57 | 20.58 ± 15.82 | 2.50 ± 0.99 | 2.56 ± 1.51 | 1.7 ± 0.66 | 1.56 ± 0.92 | 0.16 ± 0.10 | 0.12 ± 0.10 |
| Jejunal | Mesenteric1 | 1.57 ± 18.89 | 15.49 ± 14.83 | 2.76 ± 1.61 | 2.27 ± 1.66 | 1.51 ± 0.63 | 1.33 ± 0.89 | 0.03 ± 0.01 | 0.08 ± 0.01 |
| Mesenteric2 | 7.91 ± 5.53 | 10.40 ± 9.49 | 2.74 ± 1.38 | 3.00 ± 1.46 | 1.73 ± 0.75 | 1.77 ± 0.74 | 0.04 ± 0.02 | 0.06 ± 0.05 | |
| Mesenteric3 | 5.20 ± 2.51 | 6.63 ± 3.43 | 2.75 ± 1.11 | 3.29 ± 1.39 | 1.75 ± 0.62 | 1.80 ± 0.89 | 0.04 ± 0.03 | 0.02 ± 0.01 | |
No statistically significant differences were observed in each row (p > 0.05).
Table 4 demonstrates the correlation between body weight and nodes weights in different groups (r in this Table represents correlation; while its magnitude is denoted by *). Significances or insignificances of differences between the weights of body and node have been specified via p 0.05 or p 0.05, respectively.
Table 4.
The correlation between the weights of body and node in different groups of studied sheep fetuses (60 pieces
| Node name | Position | 1 st Group | 2 nd Group | 3 rd Group | 4 th Group |
|---|---|---|---|---|---|
| Mandibular | Right | r= 0.88***** p < 0.001 |
r = 0.22** p > 0.05 |
r = 0.50*** p > 0.05 |
r = 0.45*** p > 0.05 |
| Left | r = 0.65***** p < 0.001 |
r = 0.41*** p > 0.05 |
r = 0.39** p > 0.05 |
r = 0.14* p > 0.05 |
|
| Prescapular | Right | r = 0.68**** p < 0.001 |
r = 0.52*** p < 0.05 |
r= 0.39** p > 0.05 |
r = 0.70**** p < 0.05 |
| Left | r = 0.78**** p < 0.001 |
r = 0.38** p > 0.05 |
r = 0.37** p > 0.05 |
r = 0.49*** p > 0.05 |
|
| Popliteal | Right | r = 0.69**** p < 0.001 |
r = 0.53*** p < 0.05 |
r = 0.39** p > 0.05 |
r = 0.51*** p > 0.05 |
| Left | r = 0.62**** p < 0.05 |
r = 0.44*** p > 0.05 |
r = 0.13* p > 0.05 |
r = 0.41*** p > 0.05 |
|
| Mediastinal | Caudal | r = 0.72**** p < 0.01 |
r = 0.30** p > 0.05 |
r = 0.13* p > 0.05 |
r = 0.41*** p > 0.05 |
| Jejunal | Mesenteric1 | r = 0.40*** p > 0.05 |
r = 0.41*** p > 0.05 |
r = 0.04* p > 0.05 |
r = 0.45*** p > 0.05 |
| Mesenteric2 | r = 0.86**** p > 0.05 |
r = 0.16* p > 0.05 |
r = 0.04* p > 0.05 |
r = 0.75**** p < 0.001 |
|
| Mesenteric3 | - - |
r = 0.51*** p > 0.05 |
r = 0.04*** p > 0.05 |
r = 0.12* p > 0.05 |
Very poor correlation;
poor correlation;
average correlation;
strong correlation;
very strong correlation.
Discussion
Forty-three- and 44-day-old fetuses in this research lacked any observable lymph node. Moreover, jejunal mesenteric lymph nodes were not macroscopically observable up to 57th day of life and were first detected on day 59. Parotid, mandibular, prescapular, prefemoral, mediastinal and mesenteric lymph nodes have been detected previously in goat fetuses aged 4 and 5 months which is consistent with the results of this study.10
In another study conducted in Poland on sheep fetuses, cranial tracheobronchial lymph node was studied in bronchial lymphocenters sites aged three to five months before the birth. It was concluded that it is not possible to observe all nodes macroscopically in fetuses younger than three months.11 In another study conducted on ovine lymphocyte antigens it was revealed that T lymphocyte precursors can be detected for the first time on day 43 or44 of life; while the first T cell concentrations are first seen on day 55; moreover, the first lymph follicles were detected in the sheep spleen on day 77 of life. Thus, it seems that the results of the present study are consistent with previous findings;12 the development of all lymph nodes in ovine fetuses is not complete by two months into the prenatal period which is consistent with embryological principles. This is because lymph sacs as precursors of lymph nodes are developed in the late embryonic stage and then are converted to lymph nodes through the concentrations of lymphoid tissues.15 Thus, it stands to reason that no lymph nodes were detected macro-scopically in sheep fetuses before 45th day of the gestation but all were observable from the day 59 onwards.
Considering the statistical comparisons drawn between the sheep fetuses’ groups, it was revealed that caudal mediastinal lymph node is the longest of all nodes, except in the first group where jejunal mesenteric is the longest. Regarding the width parameter, the prescapular node was the widest, except for the first group again where mesenteric came in the first rank. While in the 1st and the 2nd groups the weight had been recorded at 0.001 g given the precision of the pair of scales used, prescapular lymph node was the heaviest of all in 3rd and 4th groups. Therefore, it could be concluded that excluding length parameter, subscapular lymph node is the largest node in other respect, a fact which became more manifest as fetuses grew older. As for the length parameter, we could guess that mesenteric lymph will be replaced by mediastinal node once caudal mediastinal nodes have joined each other during advanced development of lymphoid system. As documented by veterinary anatomy references, the caudal mediastinum node is formed via connection of several consecutive mediastinal nodes and it is characteristically long.3,5 Moreover, the study of measured parameters in this research showed that mandibular lymph node is the smallest node in all cases and all study groups. Surprisingly, this did not change even as fetuses grew older. Although popliteal node was marginally smaller than mandibular one in the second group, this is not significant as mandibular node is the smallest in all other study groups. Accordingly, parotid, mandibular, pre-scapular, prefemoral, mediastinal and mesenteric lymph nodes have been measured in caprine fetuses aged 4 and 6 months and it has been stated that while caudal mediastinal is the longest, it is the prescapular node which is the largest in all other aspects which is completely consistent with the results of the current research. However, mesenteric node has been introduced as the smallest one in all parameters which is in contrast with the results of this research. Regardless of differences in species, it could be attributed to differences in this node choosing method. As already stated, mesenteric group has multiple nodes and a vast diversity. Furthermore, jejunal mesenteric was considered in this research; while researchers in the aforementioned study have just named the mesenteric nodes in their research and have not addressed the type and quantity of the nodes. In another research, the dimensions and weights of superficial lymph nodes in water buffalo (Bubalus bubalis) during adolescence and maturity have been evaluated. It was reported that mandibular node is the smallest among parotid, mandibular, prescapular, prefemoral, popliteal and superficial inguinal nodes in mature buffalos; while axillary node is the smallest in calves.7 Also, the prescapular node was the biggest in both age groups which is confirmed in the present study.7 The study in cattle aged 1 to 4 years old revealed that the largest and the smallest nodes were subscapular and mandibular nodes respectively among a pool of nodes which were mandibular, parotid, retropharyngeal, prescapular, sacral, prefemoral and popliteal.16 Also, prescapular, prefemoral and popliteal have been studied in Lori-Bakhtiari sheep and prescapular and popliteal nodes have been reported as the largest and smallest nodes, respectively.17 The findings confirm the results of this research as they did not include mandibular node in their research. It is interesting that popliteal node was the smallest node after mandibular one in a study performed on ovine fetuses.17 Moreover, prescapular, prefemoral and popliteal lymph nodes have been examined in mature dromedaries and prefemoral and popliteal lymph nodes have been reported as the largest and the smallest nodes, respectively. Since mediastinal and mandibular nodes were not studied in above mentioned research and considering the fact that the present research had not addressed prefemoral node, the results of research on dromedaries do not appear to contradict our findings. Species differences should be considered as well in this respect.18 In another research conducted on morphometric characteristics of lymph nodes in mature Wistar rats, the largest and the smallest nodes were accessory axillary and ischiatic nodes respectively among mandibular, deep and superficial cervical, true axillary, accessory axillary, femoral, inguinal, popliteal and ischiatic nodes.19 The results of latter study are different from the present research, mostly due to structural and performance differences between the lymph system of rats and sheep. Animals which were more similar to sheep achieved almost similar research findings. Moreover, this should also be considered that rats and sheep are not close to each other in animal categorization and thus limited similarities are expected between these two species.
Regarding the effects of the right side of studied pair nodes i.e. mandibular, prescapular and popliteal, this parameter was not found to exert significant effects, except for the length of prescapular node in 3rd and 4th groups and thickness in the 1st group where right nodes were significantly bigger. In a study conducted on five-month-old caprine fetuses, the means of morphometric parameters were marginally higher in the right node of pairs. Their findings are similar to the findings of this research in this respect. Similar results have been reported in Asami indigenous goats (Capra hircus).21
Morphometric development of right and left lymph nodes in sheep aged 4-6 months and 1 year has been studied previously and similar dimensions between these nodes have been reported. This is also consistent with the findings of the present research as no significant differences were detected between the parameters and most of the nodes in sheep fetuses.8
After studying the lymph nodes in Lori-Bakhtiari sheep, it has been reported that only the thickness of left side is higher than the right one; while other parameters are variable. In this research, the thickness of the right side of the popliteal groups in the first group was higher (different thicknesses between two sides) which is consistent with the previous findings,17 but it was in contrast with this statement that left-sided nodes were bigger. Previous reports on dromedary 18 and cattle 17 did not pay any particular attention to the effect of the side. In the former, the difference between the largest and the smallest node was associated with the type of the node and thus it could be concluded that the node growth is almost consistent and even for both sides, except for pathological reasons which may enlarge one side. However, individual, racial and species differences should not be ignored.
Regarding the age, this research showed positive effects of the age on most research parameters. In other words, morphometric dimensions of the lymph nodes would increase as the fetus would grow older. The only exception was the weight parameter, particularly for the small fetuses of the first and the second groups which could be attributed to the accuracy of the pair of scales used. This pair of scales had an accuracy of 0.001 g and thus could not accurately measure nodes lighter than 0.001 g. Previous studies conducted on Korean indigenous goats,9 caprine fetuses,10 cow,16 Lori-Bakhtiari sheep,17 and mixed-raced calves20 indicated enlarged dimensions of the lymph nodes as the animal aged. Thus, one can come to this conclusion that lymph nodes grow in size as the animal ages and as a result its lymph tissue develops better compared to younger ages. This has been demonstrated in histological studies where the thicknesses of lymph nodules and capsules were higher in 1-year-old sheep compared to their 4 to 6-month-old counterparts.8
Based on the administered statistical tests, gender had no effect on study parameters in 32 male and 28 female fetuses. It has been stated that the means of morphometric development parameters of the 4 and 5-month-old goat fetuses’ lymph nodes are slightly higher in males.10 The difference was reported between 2.00 to 5.00 mm. In contrast, the neutrality of gender on morphometric development and evolution of cranial tracheobronchial lymph nodes has also been suggested.11 However; larger lymph nodes have been reported in male cattle than female ones. Further, no significant variations between the sizes of lymph nodes in young buffaloes in both genders have been noted previously.7 Since the present research has not found any significant effect of gender on morphometric development of lymph nodes, the detected differences could be associated with the environment and the living conditions of the studied animals. However, more accurate interpretations require increased studies. Post-natal conditions in which there are higher chances of being exposed to different antigens, variations in the sizes of lymph nodes are likely in both genders.
Studying the correlation between the fetal weight parameter and the weight of lymph nodes showed direct correlation between these parameters, yet variable for different nodes and ranging from very poor to very strong. Interestingly, while a strong correlation was observed in the first group, it was average in the fourth group. In other words, there was a stronger relationship between the weights of the small fetuses and the nodes because as the fetus grows in age and accordingly gains more weight, in particular in the second half of gestation period, the weight increase of lymph nodes can’t match the speed at which the body gains weight. However, since lymph nodes have grown in proportion to the body’s growth, this could be a case of isometric growth. Of course, it demands for more studies. After making enquiries and searching the available literature and resources, there was not any evidence on the correlation between morphometric development of lymph nodes and age and thus it seems that this research is unique in terms of the vastness of analyses and the statistical comparisons made as well as the population of fetuses.
In conclusion, the study of sheep fetuses’ lymph nodes revealed not all lymphoid node developments are macroscopically detectable before the day 59 of birth.
Moreover, no macroscopic lymphoid node development was observed by day 45. Lymph nodes would grow in size as the fetus aged. This growth was the same for all nodes and groups, so that the smallest and the largest lymph nodes were prescapular and mandibular nodes, respectively; while the longest was the caudal mediastinal node. Based on the results, it seemed that although the size of lymph nodes grew by age, this increase was not the same for all nodes and all groups. In addition, gender had no effect on the research parameters. It seems that both sides grow evenly and at the same rate and the detected variations are limited and negligible.
Acknowledgment
Hereby, Research Deputy of Shahid Chamran University of Ahvaz will be highly appreciated for financial funding of this study.
Conflict of Interest
No conflict of interest is associated with this work.
References
- 1.Schoenwolf GC, Bleyl SB, Brauer PR, et al. Larson's human embryology. 5th ed. Philadelphia, USA: Churchill Livingstone ; 2015. p. 334. [Google Scholar]
- 2.McGeady TA, Quinn PJ, Fitzpatrick ES, et al. Veterinary embryology. UK: John Wiley & Sons ; 2017. pp. 341–342. [Google Scholar]
- 3.Schummer A, Wilkens H, Vollmerhus B, et al. The circulatory system, the skin and the cutaneous organs of the domestic mammals. Berlin: Germany: Verlag Paul Parey ; 1981. pp. 278–294. [Google Scholar]
- 4.Konig HE, Liebich HG. Veterinary anatomy of domestic mammals. 1st ed. Schattauer, Munich, Germany: Schattauer ; 2007. pp. 452–453. [Google Scholar]
- 5.Getty R. Sisson and Grossman's the anatomy of the domestic animals. 5th ed. New York, USA: WB Saunders ; pp. 1975–179. [Google Scholar]
- 6.Dyce KM, Sack WO, Wensing CJG. Textbook of veterinary anatomy. 3rd ed. Philadelphia, USA: WB Saunders; 2010. pp. 29–30. [Google Scholar]
- 7.Bagi AS, Vyas KN, Bhayani DM. Study on the various dimensions of superficial regional lymph nodes in young and adult Surti buffalo (Bubalus bubalis) Indian Vet J. 1992;69(2):172–175. [Google Scholar]
- 8.Panchal KM, Vyas KN, Vyas YL. Histomorphological study on secondary lymphoid organs (spleen, sub-lumbar lymph nodes and Peyer's patches) of Marwari sheep (Ovis aries) Indian Vet J. 1982;75(4):318–322. [Google Scholar]
- 9.Yoon YS, Shin JW, Lee JS. Age related morphological studies on hemal node and hemolymph node in Korean native goats. Korean J Vet Res. 1999;39(5):865–877. [Google Scholar]
- 10.Asha A, Maya S, Harshan KR, et al. Morphometric observation on lymph nodes in goat foetuses. J Vet Anim Sci. 2004;42:30–33. [Google Scholar]
- 11.Pospieszny N, Kleckowska J, Chroszcz A, et al. The morphology and development of the sheeps tracheobronchial cranial lymph nodes in prenatal period. Vet Med. 2002;5(2):#02. [Google Scholar]
- 12.Maddox JF, Makay CR, Brandon MR. Ontogeny of ovine lymphocytes an immunohistological study on the development of T lymphocytes in the sheep fetal spleen. Immunology. 1987;62(1):107–112. [PMC free article] [PubMed] [Google Scholar]
- 13.Zöltzer H. Initial lymphatics: Morphology and function of endothelial cells. Lymphology. 2003;36(1):7–25. [PubMed] [Google Scholar]
- 14.Noakes DE, Parkinson TJ, England GCW. Arthur’s veterinary reproduction and obstetrics. WB Saunders: London, UK; 2001. 68 pp. [Google Scholar]
- 15.Hyttel P, Sinowatz F, Vejlted M. Essentials of domestic animal embryology. Sanders : London, UK; 2010. p. 237. [Google Scholar]
- 16.Mohammadpour AA, Radmehr B, Al-e-Ahmad Dehkordi M. Morphological and morphometrical study of important lymph nodes of cow in meat inspection [Persian] Pajouhesh-va-Sazandegi. 2006;71:13–18. [Google Scholar]
- 17.Mohammadpour AA, Moshtaghi H, Naderi AR. Morphometry and histometrical study of prescapular, prefemoral and popliteal lymph nodes in Iranian Lori-Bakhtiari sheep [Persian] Pajouhesh-va-Sazandegi. 2010;86:47–52. [Google Scholar]
- 18.Raji R, Feizbakhsh H. Morphometrical study of prescapular, prefemoral and popliteal lymph node of one-humped camel (Camelus dromedaries) [Persian] Pajouhesh-va-Sazandegi. 2010;87:47–52. [Google Scholar]
- 19.Dabanoglu I, Baradakcioglu HE, Sevil F, et al. Morphometry of the lymph node excluding the thoracic and abdominal cavity in Wistar rats. Turk J Vet Anim Sci. 2004;28:775–778. [Google Scholar]
- 20.Gadre KM, Malik MR, Srivastava AM. Gross anatomical changes in abdominal lymph node cross bred calves with age and immune response. Indian J Anim Sci. 1986;56:623–633. [Google Scholar]
- 21.Sarma K, Kalita A, Suri S, et al. Gross anatomical observations on the superficial lymph nodes of Bakarwali goat (Capra hircus) Indian J Anim Sci. 2004;74:750–751. [Google Scholar]


