Abstract
Objectives
Focusing on the advanced non-small cell lung cancer (NSCLC) patients without driver mutations can elucidate the clinical impact of COPD on treatment outcomes. The present study evaluated the effects of COPD on the overall survival of driver mutation-negative NSCLC patients undergoing conventional chemotherapy as the first-line treatment.
Patients and methods
Medical records of stage IIIB and IV NSCLC patients from January 2008 to December 2015 from six university hospitals were reviewed.
Results
The total study population consisted of 197 patients; 92 (46.7%) were COPD patients and 105 (53.3%) were non-COPD patients. The median survival in the non-COPD group was 11.5 months, compared to 9.2 months in the COPD group. Univariate analysis showed that old age (>70 years), high Eastern Cooperative Oncology Group status score (2–3), squamous cell histology, and COPD were risk factors for mortality. The presence of COPD was a significant prognostic factor in univariate analysis (hazard ratio [HR], 1.402; p=0.037), but not in multivariate analysis (HR, 1.275; p=0.144). Subgroup analysis of 143 smokers showed that COPD was a significant prognostic factor on multivariate analysis (HR, 1.726; p=0.006). In 154 stage IV patients, COPD was also a prognostic factor in multivariate analysis (HR, 1.479; p=0.039).
Conclusion
COPD had a negative impact on overall survival in the stage IV NSCLC and smoker NSCLC patients who underwent conventional chemotherapy.
Keywords: non-small cell lung cancer, chronic obstructive pulmonary disease, smoker, overall survival
Introduction
Lung cancer is a leading cause of death worldwide.1,2 Lung cancer and chronic obstructive pulmonary disease (COPD) are interrelated diseases, and several epidemiological studies have shown that patients with COPD have an increased risk of developing lung cancer.3–6 The prevalence of COPD was reported to be 40%–70% in lung cancer patients, and COPD is a significant risk factor for the development of lung cancer.7,8 Furthermore, COPD and emphysema were reported to be independent risk factors for the development of lung cancer.6,9
The results of previous studies regarding the impact of COPD on the prognosis of lung cancer were inconclusive. Gao et al’s recent meta-analysis of 26 papers showed that COPD and emphysema were predictors of poor survival in patients with lung cancer, with poorer overall survival in lung cancer patients with COPD.10 A study by Izquierdo et al showed that COPD did not influence overall survival in cases of advanced lung cancer.11 Negative effects of COPD on the postoperative outcomes of early lung cancer patients undergoing lobectomy have been reported.12,13 Several previous studies showed that COPD was not a prognostic factor for overall survival in non-small cell lung cancer (NSCLC) patients.14–17
Most previous studies evaluating the correlation between COPD and lung cancer were performed in populations who were heterogeneous in terms of lung cancer stage and treatment modalities.16,18 Technical developments over the past decade have afforded options for targeted therapy of NSCLC patients, and median survival is longer in NSCLC patients with driver mutations compared to those without such mutations.19 However, the proportion of patients positive for driver mutations is relatively small, and conventional chemotherapy, mostly platinum-based regimens, is still the mainstay first-line treatment for advanced NSCLC.20,21
The impact of COPD on the outcomes of patients with advanced lung cancer could vary by the proportion of patients with driver mutations, the proportion receiving targeted therapy, and the proportion with advanced lung cancer. Furthermore, few studies have focused on advanced NSCLC patients undergoing conventional chemotherapy. In this regard, evaluating the influence of COPD on the outcome of NSCLC patients undergoing conventional chemotherapy, after excluding the patients positive for driver mutations, could elucidate the clinical impact of COPD on the treatment of advanced NSCLC. This study was performed to evaluate the effects of COPD on the overall survival of NSCLC patients undergoing conventional chemotherapy as the first-line treatment.
Patients and methods
Patient selection
Advanced lung cancer patients (stage IIIB and IV), diagnosed with NSCLC between January 2008 and December 2015, were selected among the records of six university hospitals: Seoul St Mary’s Hospital, Yeouido St Mary’s Hospital, Incheon St Mary’s Hospital, Bucheon St Mary’s Hospital, St Paul’s Hospital, and Uijeongbu St Mary’s Hospital. Patients who underwent a baseline pulmonary function test were included in this study.
This evaluation focused on patients with advanced NSCLC at diagnosis who did not undergo surgery. Patients with stage I, II, and IIIA NSCLC were excluded. Furthermore, patients who underwent stereotactic body radiation therapy or targeted therapy, such as erlotinib, gefitinib, or crizotinib, as the first-line treatment were also excluded. Patients with epidermal growth factor receptor (EGFR) and anaplastic lymphoma kinase (ALK) mutations were also excluded. Patients who underwent conventional chemotherapy as the first-line treatment for lung cancer, and who did not meet the above exclusion criteria, were included in the study. The 7th American Joint Committee on Cancer tumor, node, metastasis classification was used to stage the lung cancer.
The median follow-up duration for surviving patients was 23.9 months (range: 6.1–68.0 months), and the median survival time of censored patients was 7.8 months (range: 1.1–87.9 months).
Chemotherapy
First-line chemotherapy included cisplatin or carboplatin in combination with one of the following agents: pemetrexed (nonsquamous histology), vinorelbine, gemcitabine, or pacli-taxel. Patients who received pemetrexed maintenance therapy after induction by the same agent were included in the study. Patients’ age, performance status, and underlying disease were also taken into consideration with respect to selection of the chemotherapy regimen. Physicians carefully observed the toxicity of chemotherapy, and the regimens were changed or stopped if toxicity occurred. Adverse events (AEs) were evaluated according to the National Cancer Institute Common Terminology Criteria for Adverse Events, NCICTC 3.0.22 In the event of a grade 3 AE, the dose was reduced until it had dropped to grade 0 or 1, and the treatment was restarted at one dose level lower. The dose was adjusted if any of the following AEs occurred: 1) absolute neutrophil count nadir <500/mm3 for 4 days or more; 2) neutropenia with fever; 3) thrombocytopenia with a bleeding event or requirement for platelet transfusion; 4) platelet nadir <50,000/mm3 for 4 days or more; or 5) grade 3 or higher non-hematological toxicity, except nausea, vomiting, or alopecia.
Definition of COPD and spirometric values as prognostic factors
COPD was defined according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines: forced expiratory volume in 1 s (FEV1)/forced vital capacity (FVC) <0.7. Morris reference equation, which is widely used in Asian population, was used to calculate the normal predictive values for spirometric data.23
Statistical analysis
Comparison of continuous variables between the COPD and non-COPD groups was performed by the unpaired t-test. Pearson’s χ2 test was used to compare categorical variables between the two groups.
Kaplan–Meier survival curves were used to evaluate the overall survival of patients with lung cancer, and differences were assessed by the log-rank test. The follow-up time for survival analysis was defined as the time (in months) from diagnosis of lung cancer to the date of death or the date of the last outpatient visit. Independent risk factors for mortality were analyzed using a Cox proportional hazards regression model after adjustment for confounding variables. All variables analyzed using a Cox regression model were independent variables.
Only variables that were statistically significant in univariate analysis were entered into the multivariate analysis. Both univariate and multivariate analyses of overall survival are performed using Cox regression model. Hazard ratios (HRs) and 95% confidence intervals (95% CIs) were estimated. Subgroup analyses of never-smokers and patients with stage IIIB cancer were not performed due to the small numbers of patients. For all tests, p<0.05 was considered to indicate statistical significance. Statistical analyses were performed using SPSS for Windows software (ver. 18.0; SPSS Inc., Chicago, IL, USA).
Ethics statement
The study was approved by the ethics committee at each center (XC16RIMI0084P). The requirement for informed consent was waived due to the retrospective nature of the data. All the data accessed was de-identified.
Results
Demographic and clinical characteristics
The total study population consisted of 197 patients; 92 (46.7%) were in the COPD group and 105 (53.3%) were in the non-COPD group. Table 1 shows the comparison of clinicopathological parameters between the two groups. The mean age of the COPD group patients was 69.5±9.4 years and that of the non-COPD group was 64.7±10.4 years (p=0.001). The proportion of male patients was significantly higher in the COPD group (85.9%) than in the non-COPD group (p=0.002). The percentage of ever-smokers was significantly higher in the COPD group than in the non-COPD group (81.5% vs 64.8%, respectively; p=0.009).
Table 1.
Baseline clinical characteristics of the study patients
| Non-COPD (n=105) (n, %) | COPD (n=92) (n, %) | p-value | |
|---|---|---|---|
| Sex | |||
| Male | 70 (66.7) | 79 (85.9) | 0.002 |
| Age (years) | 64.7±10.4 | 69.5±9.4 | 0.001 |
| Stage | 0.235 | ||
| IIIB | 21 (20.0) | 25 (27.2) | |
| IV | 84 (80.0) | 67 (72.8) | |
| BMI (kg/m2) | 22.9±3.6 | 23.1±3.2 | 0.747 |
| Smoking | 0.009 | ||
| Never-smoker | 37 (35.2) | 17 (18.5) | |
| Ever-smoker | 68 (64.8) | 75 (81.5) | |
| ECOG | 0.515 | ||
| 0–1 | 85 (81.0) | 71 (77.2) | |
| 2–3 | 20 (19.0) | 21 (22.8) | |
| Pathology | 0.001 | ||
| Squamous | 24 (22.9) | 46 (50.0) | |
| Non-squamous | 81 (77.1) | 46 (50.0) | |
| FEV1 (L) | 2.18±0.91 | 1.76±0.61 | <0.001 |
| FEV1 (% predicted) | 87.93±21.70 | 71.55±21.97 | <0.001 |
| FVC (L) | 2.78±0.91 | 3.08±1.04 | 0.118 |
| FVC (% predicted) | 82.10±20.54 | 86.84±21.82 | 0.029 |
| FEV1/FVC (% predicted) | 77.39±6.11 | 57.65±9.19 | <0.001 |
| DLCO (abs) | 16.43±15.87 | 16.65±16.22 | 0.926 |
| DLCO (%) | 76.19±20.99 | 73.38±20.89 | 0.400 |
Abbreviations: Abs, absolute; BMI, body mass index; COPD, chronic obstructive pulmonary disease; DLCO, diffusing capacity of the lung for carbon monoxide; ECOG, Eastern Cooperative Oncology Group; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity.
The proportion of patients with stage IV cancer was higher than stage IIIB in both the groups; however, there were no significant differences in disease stage between the two groups. More patients had Eastern Cooperative Oncology Group (ECOG) performance status scores 0 or 1 in the non-COPD group compared to the COPD group, but the difference was not significant (p=0.515). The proportion of squamous carcinoma was significantly higher in the COPD group than in the non-COPD group (50% vs 22.9%, respectively; p=0.001).
The mean absolute FEV1 and predicted percentage of FEV1 were 1.76±0.61 L and 71.55%±21.97% in the COPD group, respectively; the respective values in the non-COPD group were 2.18±0.91 L and 87.93%±21.70% (p=0.001).
Prognostic factors for survival in the study population
Univariate analysis showed that older age (>70 years), high ECOG score (2–3), squamous cell histology, and COPD (Figure 1) were significant. In multivariate analysis, poor ECOG performance status score and histology were significant prognostic factors; however, the presence of COPD was not (HR, 1.275, 95% CI, 0.884–1.719; p=0.144). The median survival period of non-COPD patients was 11.5 months, compared to 9.2 months in the COPD group (Table 2).
Figure 1.
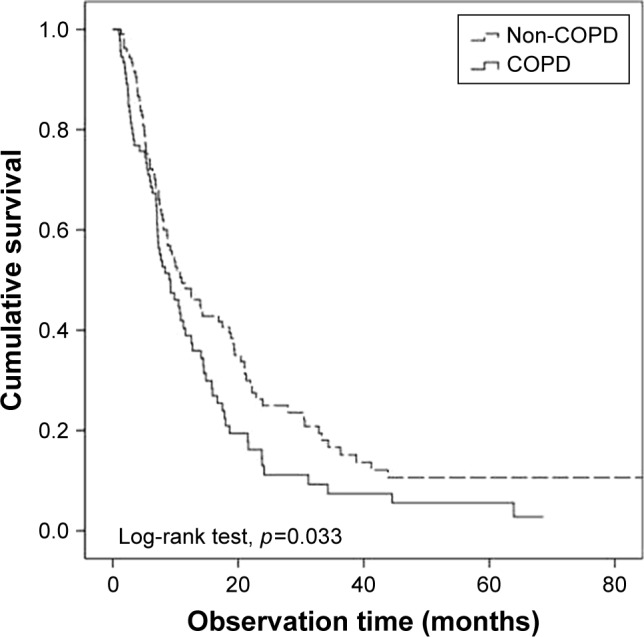
Overall survival of non-small cell lung cancer patients: COPD group versus non-COPD group.
Abbreviation: COPD, chronic obstructive pulmonary disease.
Table 2.
Univariate analysis of clinical variables affecting overall survival of the patients
| Parameter | Hazard ratio | 95% confidence interval | Median survival (months) | p-value |
|---|---|---|---|---|
| Sex | 0.371 | |||
| Male | 1.188 | 0.819–1.710 | 8.7 | |
| Female | 1 | 14.3 | ||
| ECOG | <0.001 | |||
| 0–1 | 1 | 11.5 | ||
| 2–3 | 2.337 | 1.587–3.441 | 5.4 | |
| Age (years) | 0.023 | |||
| ≤70 | 1 | 11.6 | ||
| >70 | 1.461 | 1.058–2.018 | 7.3 | |
| Stage | 0.757 | |||
| IIIB | 1.060 | 0.732–1.535 | 8.4 | |
| IV | 1 | 1 | 10.7 | |
| BMI (kg/m2) | 0.623 | |||
| <23 | 1.083 | 0.787–1.492 | 9.2 | |
| ≥23 | 1 | 10.8 | ||
| COPD | 0.037 | |||
| None | 1 | 11.5 | ||
| Yes | 1.402 | 1.020–1.927 | 9.2 | |
| Smoking | 0.398 | |||
| None | 1 | 13.9 | ||
| Yes | 1.161 | 0.821–1.640 | 8.7 | |
| Histology | 0.004 | |||
| Squamous | 1.641 | 1.173–2.295 | 7.8 | |
| Non-squamous | 1 | 12.5 |
Abbreviations: BMI, body mass index; ECOG, Eastern Cooperative Oncology Group; COPD, chronic obstructive pulmonary disease.
Subgroup analysis
Ever-smoker
Subgroup analysis of 143 smokers showed that COPD, poor ECOG, and old age were significant in univariate analysis (Figure 2). In multivariate analysis, COPD (HR, 1.726, 95% CI, 1.170–2.540; p=0.006) and poor ECOG (HR, 2.271; p<0.001) were significant prognostic factors (Table 3).
Figure 2.

Overall survival of non-small cell lung cancer patients according to the presence of COPD in smoker patients.
Abbreviation: COPD, chronic obstructive pulmonary disease.
Table 3.
Univariate and multivariate analyses of prognostic factors for overall survival in smokers (n=154)
| Parameter | Univariate analysis
|
Multivariate analysis
|
||||
|---|---|---|---|---|---|---|
| Hazard ratio | 95% confidence interval | p-value | Hazard ratio | 95% confidence interval | p-value | |
| Presence of COPD | 0.019 | 0.006 | ||||
| COPD | 1.585 | 1.080–2.327 | 1.726 | 1.170–2.54 | ||
| Non-COPD | 1 | 1 | ||||
| Male | 1.443 | 0.876–2.379 | 0.150 | |||
| ECOG | 0.001 | <0.001 | ||||
| 0–1 | 1 | 1 | ||||
| 2–3 | 2.303 | 1.433–3.702 | 2.602 | 1.603–4.221 | ||
| Squamous | 1.567 | 1.067–2.302 | 0.022 | 1.316 | 0.881–1.964 | 0.180 |
| Age (years) | 0.009 | 0.144 | ||||
| ≤70 | 1 | 1 | ||||
| >70 | 1.667 | 1.134–2.448 | 1.355 | 0.902–2.036 | ||
| Stage | 0.543 | |||||
| IIIB | 1 | |||||
| IV | 0.877 | 0.576–1.337 | ||||
| BMI (kg/m2) | 0.929 | |||||
| <23 | 1.017 | 0.696–1.488 | ||||
| ≥23 | 1 | |||||
Abbreviations: COPD, chronic obstructive pulmonary disease; ECOG, Eastern Cooperative Oncology Group; BMI, body mass index; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity.
Stage IV cancer
Among the 197 patients included in this study, 154 were diagnosed with stage IV cancer. In univariate analysis, COPD was significant (HR, 1.530, 95% CI, 1.054–2.222; p=0.025) (Figure 3) along with ECOG and old age. COPD was a prognostic factor (HR, 1.479, 95% CI, 1.020–2.145; p=0.039), along with ECOG, in multivariate analysis (Table 4).
Figure 3.
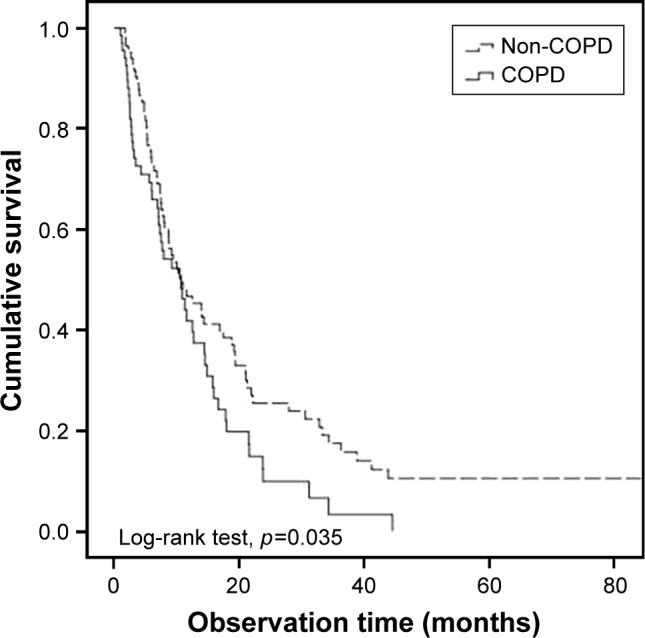
Overall survival of non-small cell lung cancer patients according to the presence of COPD in stage IV non-small cell lung cancer patients.
Abbreviation: COPD, chronic obstructive pulmonary disease.
Table 4.
Univariate and multivariate analyses of prognostic factors for overall survival in stage IV NSCLC patients (n=151)
| Parameter | Univariate analysis
|
Multivariate analysis
|
||||
|---|---|---|---|---|---|---|
| Hazard ratio | 95% confidence interval | p-value | Hazard ratio | 95% confidence interval | p-value | |
| Presence of COPD | 0.025 | 0.039 | ||||
| COPD | 1.530 | 1.054–2.222 | 1.479 | 1.020–2.145 | ||
| Non-COPD | 1 | 1 | ||||
| Male | 1.103 | 0.741–1.641 | 0.630 | |||
| ECOG | ,0.001 | <0.001 | ||||
| 0–1 | 1 | 1 | ||||
| 2–3 | 2.335 | 1.507–3.617 | 2.271 | 1.466–3.519 | ||
| Squamous | 1.365 | 0.916–2.035 | 0.126 | |||
| Age (years) | 0.012 | 0.189 | ||||
| ≤70 | 1 | 1 | ||||
| >70 | 1.616 | 1.113–2.348 | 1.306 | 0.877–1.944 | ||
| Smoker | 1.080 | 0.735–1.588 | 0.694 | |||
| BMI (kg/m2) | 0.737 | |||||
| <23 | 0.939 | 0.649–1.358 | ||||
| ≥23 | 1 | |||||
Abbreviations: COPD, chronic obstructive pulmonary disease; ECOG, Eastern Cooperative Oncology Group; BMI, body mass index; NSCLC, non-small cell lung cancer.
Discussion
The results of the present study indicated that COPD does not have a clinical impact on overall survival in driver mutation-negative NSCLC patients undergoing conventional chemotherapy. However, COPD was a significant prognostic factor in the smoker and stage IV cancer subgroups. From the previous studies, few reports have suggested that the mortality rate of lung cancer is higher among patients with prior COPD compared to non-COPD lung cancer patients.10,24 However, specifically for NSCLC subtype, studies on the impact of COPD on the survival of cancer patients presented inconclusive results. While the presence of emphysema affects the overall survival of patients,14 COPD was not a prognostic factor in advanced NSCLC patients with significant smoking experiences.16
In this study, we focused on advanced NSCLC patients who underwent conventional chemotherapy as first-line treatment, as most patient populations in previous studies were heterogeneous in terms of cancer stage or treatment modality,16,25 or focused on early lung cancer requiring surgery.13,26,27 Furthermore, NSCLC patients with driver mutations were also excluded as prognosis can differ between patients undergoing targeted therapy versus those who did not.19 COPD-related NSCLC cases show a low prevalence of EGFR and ALK mutations compared to non-COPD patients,28 and targeted therapy can influence patient outcomes.
COPD was a significant prognostic factor in the smoker subgroup, but not in the overall patient population (which included 54 never-smokers). COPD is an obstructive lung disease that is closely associated with tobacco smoking;29 we believe that the impact of COPD on overall survival would be much more clear after excluding never-smokers from the analysis. A previous study on the impact of COPD in NSCLC patients with a smoking history showed shorter overall survival of COPD patients compared to their non-COPD counterparts, although the difference was not statistically significant.16 COPD patients with more than 10 years of tobacco smoking history experience more rapid decline of lung function, compared to never-smokers.30,31 We assume that the decline of lung function and associated frequent exacerbations of COPD would make patients less tolerable to active anticancer treatment.32,33
Furthermore, COPD significantly influenced overall survival in the stage IV cancer patients. Although not shown in the results, the proportion of squamous cell carcinoma was higher in the stage IIIB group (51.4%) than in the stage IV group (31.1%) with statistical significance (p=0.005). Compared to adenocarcinoma type, squamous cell lung cancer is more centrally located.34 Central airway invasion can lead to localized airway obstruction, which can in turn be diagnosed as COPD from initial spirometry tests. However, airway obstruction is one among the many characteristic features of COPD. The proinflammatory microenvironment and progressive decline of lung function, which are other characteristic features of COPD,35 would render lung cancer treatment more difficult.36 After excluding the stage IIIB cancer patients, a separate analysis of the stage IV cancer patients would have better reflected the impact of COPD on the overall survival of NSCLC patients.
Although not a significant prognostic factor for overall patient population in multivariate analysis, COPD was a statistically significant prognostic factor in the smoker and stage IV subgroups. It may not be a powerful prognostic factor as ECOG performance status, histology, or cancer stage, but our study results suggested that it has an indirect negative impact on the overall survival of advanced NSCLC patients. First, we assume that lung cancer with COPD is a different phenotype, which shows a more aggressive course compared to non-COPD lung cancer. In addition to the effect of smoking, COPD may have other characteristics associated with the mortality of lung cancer. A prospective study on never-smokers showed that prior physician-diagnosed emphysema was significantly associated with lung cancer mortality.37 Chronic inflammation in COPD results in increased DNA damage, which leads to a greater probability of lung carcinogenesis,38,39 and the proinflammatory microenvironment of COPD makes treatment of lung cancer more difficult.36 Second, the presence of COPD is associated with other comorbidities, such as cardiovascular diseases, which can affect patient survival.40 A recent study in Denmark indicated that cardiovascular comorbidity was a significant comorbidity in NSCLC patients, along with diabetes and COPD.25 Finally, AEs associated with chemotherapy are more likely to develop in NSCLC patients with emphyse-matous lungs. Tamiya et al reported that NSCLC patients with emphysematous lungs are 4.95 times more likely to develop interstitial lung disease associated with docetaxel when compared to patients without this change.41
The study had some limitations. First, only a small number of patients with severe COPD were included in the analysis. More than 70% of study patients had an ECOG performance status of 0–1, and the patients with severe airway outflow limitations tended to receive supportive care. Less than 10% of patients were of GOLD grade 3–4 (results not shown). However, the majority of lung cancer patients with COPD have mild to moderate airway obstruction,42 so the evaluation of the clinical impact of COPD would not be significantly influenced by the paucity of cases of severe COPD. Second, we did not identify other comorbidities. Cardiovascular disease, which frequently accompanies COPD, may have contributed to decreased overall survival among the patients with COPD in our study population. Third, natural aging could have contributed to diagnosis of COPD among elderly patients. Using a fixed ratio for the definition of airflow limitation is presumed to be inducing overdiagnosis among older individuals.43 Finally, the location and size of the lung mass were not described. COPD defined as FEV1/FVC <70% may reflect lung cancer burden and the involvement of the central airway can be reflected in a decrease in FEV1.
Although the direct impact of COPD could not be determined, the results presented here suggest that the impact of COPD cannot be ignored in advanced NSCLC patients undergoing conventional chemotherapy especially in smoker and stage IV patients.
Conclusion
COPD was not a significant prognostic factor for advanced NSCLC patients. However, COPD had a negative impact on the overall survival of the NSCLC patients in the smoker and stage IV subgroups. Further studies with larger study population are required to clarify the impact of COPD in both stage IV and smoker NSCLC patients under active lung cancer treatment.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.McErlean A, Ginsberg MS. Epidemiology of lung cancer. Semin Roentgenol. 2011;46(3):173–177. doi: 10.1053/j.ro.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Wasswa-Kintu S, Gan WQ, Man SF, Pare PD, Sin DD. Relationship between reduced forced expiratory volume in one second and the risk of lung cancer: a systematic review and meta-analysis. Thorax. 2005;60(7):570–575. doi: 10.1136/thx.2004.037135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilson DO, Weissfeld JL, Balkan A, et al. Association of radiographic emphysema and airflow obstruction with lung cancer. Am J Respir Crit Care Med. 2008;178(7):738–744. doi: 10.1164/rccm.200803-435OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Powell HA, Iyen-Omofoman B, Baldwin DR, Hubbard RB, Tata LJ. Chronic obstructive pulmonary disease and risk of lung cancer: the importance of smoking and timing of diagnosis. J Thorac Oncol. 2013;8(4):e34–e35. doi: 10.1097/JTO.0b013e31828950e3. [DOI] [PubMed] [Google Scholar]
- 6.Smith BM, Pinto L, Ezer N, Sverzellati N, Muro S, Schwartzman K. Emphysema detected on computed tomography and risk of lung cancer: a systematic review and meta-analysis. Lung Cancer. 2012;77(1):58–63. doi: 10.1016/j.lungcan.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 7.Loganathan RS, Stover DE, Shi W, Venkatraman E. Prevalence of COPD in women compared to men around the time of diagnosis of primary lung cancer. Chest. 2006;129(5):1305–1312. doi: 10.1378/chest.129.5.1305. [DOI] [PubMed] [Google Scholar]
- 8.Buist AS, McBurnie MA, Vollmer WM, et al. International variation in the prevalence of COPD (the BOLD Study): a population-based prevalence study. Lancet. 2007;370(9589):741–750. doi: 10.1016/S0140-6736(07)61377-4. [DOI] [PubMed] [Google Scholar]
- 9.Wang H, Yang L, Zou L, et al. Association between chronic obstructive pulmonary disease and lung cancer: a case-control study in Southern Chinese and a meta-analysis. PLoS One. 2012;7(9):e46144. doi: 10.1371/journal.pone.0046144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao YH, Guan WJ, Liu Q, et al. Impact of COPD and emphysema on survival of patients with lung cancer: a meta-analysis of observational studies. Respirology. 2016;21(2):269–279. doi: 10.1111/resp.12661. [DOI] [PubMed] [Google Scholar]
- 11.Izquierdo JL, Resano P, El Hachem A, Graziani D, Almonacid C, Sanchez IM. Impact of COPD in patients with lung cancer and advanced disease treated with chemotherapy and/or tyrosine kinase inhibitors. Int J Chron Obstruct Pulmon Dis. 2014;9:1053–1058. doi: 10.2147/COPD.S68766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bugge A, Lund MB, Brunborg C, Solberg S, Kongerud J. Survival after surgical resection for lung cancer in patients with chronic obstructive pulmonary disease. Ann Thorac Surg. 2016;101(6):2125–2131. doi: 10.1016/j.athoracsur.2015.12.057. [DOI] [PubMed] [Google Scholar]
- 13.Yoshida Y, Kage H, Murakawa T, et al. Worse prognosis for stage IA lung cancer patients with smoking history and more severe chronic obstructive pulmonary disease. Ann Thorac Cardiovasc Surg. 2015;21(3):194–200. doi: 10.5761/atcs.oa.14-00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gullon JA, Suarez I, Medina A, Rubinos G, Fernandez R, Gonzalez I. Role of emphysema and airway obstruction in prognosis of lung cancer. Lung Cancer. 2011;71(2):182–185. doi: 10.1016/j.lungcan.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 15.Ueda K, Jinbo M, Li TS, Yagi T, Suga K, Hamano K. Computed tomography-diagnosed emphysema, not airway obstruction, is associated with the prognostic outcome of early-stage lung cancer. Clin Cancer Res. 2006;12(22):6730–6736. doi: 10.1158/1078-0432.CCR-06-1196. [DOI] [PubMed] [Google Scholar]
- 16.Lee SJ, Lee J, Park YS, et al. Impact of chronic obstructive pulmonary disease on the mortality of patients with non-small-cell lung cancer. J Thorac Oncol. 2014;9(6):812–817. doi: 10.1097/JTO.0000000000000158. [DOI] [PubMed] [Google Scholar]
- 17.Omote N, Hashimoto N, Morise M, et al. Impact of mild to moderate COPD on feasibility and prognosis in non-small cell lung cancer patients who received chemotherapy. Int J Chron Obstruct Pulmon Dis. 2017;12:3541–3547. doi: 10.2147/COPD.S149456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang JY, Jian ZH, Ndi Nfor O, et al. The impact of coexisting asthma, chronic obstructive pulmonary disease and tuberculosis on survival in patients with lung squamous cell carcinoma. PLoS One. 2015;10(7):e0133367. doi: 10.1371/journal.pone.0133367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kris MG, Johnson BE, Berry LD, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA. 2014;311(19):1998–2006. doi: 10.1001/jama.2014.3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Souquet PJ, Chauvin F, Boissel JP, et al. Polychemotherapy in advanced non small cell lung cancer: a meta-analysis. Lancet. 1993;342(8862):19–21. doi: 10.1016/0140-6736(93)91882-m. [DOI] [PubMed] [Google Scholar]
- 21.Non-small Cell Lung Cancer Collaborative Group Chemotherapy in non-small cell lung cancer: a meta-analysis using updated data on individual patients from 52 randomised clinical trials. Non-small Cell Lung Cancer Collaborative Group. BMJ. 1995;311(7010):899–909. [PMC free article] [PubMed] [Google Scholar]
- 22.Division of Cancer Treatment and Diagnosis, National Cancer Institute, National Institutes of Health and Department of Health and Human Services Cancer Therapy Evaluation Program, Common Terminology Criteria for Adverse Events, version 3.0. 2006 [Google Scholar]
- 23.Morris JF, Koski A, Johnson LC. Spirometric standards for healthy nonsmoking adults. Am Rev Respir Dis. 1971;103(1):57–67. doi: 10.1164/arrd.1971.103.1.57. [DOI] [PubMed] [Google Scholar]
- 24.Kiri VA, Soriano J, Visick G, Fabbri L. Recent trends in lung cancer and its association with COPD: an analysis using the UK GP Research Database. Prim Care Respir J. 2010;19(1):57–61. doi: 10.4104/pcrj.2009.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iachina M, Jakobsen E, Moller H, et al. The effect of different comorbidities on survival of non-small cells lung cancer patients. Lung. 2015;193(2):291–297. doi: 10.1007/s00408-014-9675-5. [DOI] [PubMed] [Google Scholar]
- 26.Sekine Y, Suzuki H, Yamada Y, Koh E, Yoshino I. Severity of chronic obstructive pulmonary disease and its relationship to lung cancer prognosis after surgical resection. Thorac Cardiovasc Surg. 2013;61(2):124–130. doi: 10.1055/s-0032-1304543. [DOI] [PubMed] [Google Scholar]
- 27.Kuo CH, Wu CY, Lee KY, et al. Chronic obstructive pulmonary disease in stage I non-small cell lung cancer that underwent anatomic resection: the role of a recurrence promoter. COPD. 2014;11(4):407–413. doi: 10.3109/15412555.2013.838946. [DOI] [PubMed] [Google Scholar]
- 28.Lim JU, Yeo CD, Rhee CK, et al. Chronic obstructive pulmonary disease-related non-small-cell lung cancer exhibits a low prevalence of EGFR and ALK driver mutations. PLoS One. 2015;10(11):e0142306. doi: 10.1371/journal.pone.0142306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Forey BA, Thornton AJ, Lee PN. Systematic review with meta-analysis of the epidemiological evidence relating smoking to COPD, chronic bronchitis and emphysema. BMC Pulm Med. 2011;11:36. doi: 10.1186/1471-2466-11-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lange P, Colak Y, Ingebrigtsen TS, Vestbo J, Marott JL. Long-term prognosis of asthma, chronic obstructive pulmonary disease, and asthma-chronic obstructive pulmonary disease overlap in the Copenhagen City Heart study: a prospective population-based analysis. Lancet Respir Med. 2016;4(6):454–462. doi: 10.1016/S2213-2600(16)00098-9. [DOI] [PubMed] [Google Scholar]
- 31.Ramirez-Venegas A, Sansores RH, Quintana-Carrillo RH, et al. FEV1 decline in patients with chronic obstructive pulmonary disease associated with biomass exposure. Am J Respir Crit Care Med. 2014;190(9):996–1002. doi: 10.1164/rccm.201404-0720OC. [DOI] [PubMed] [Google Scholar]
- 32.Omori H, Nonami Y, Morimoto Y. Effect of smoking on FEV decline in a cross-sectional and longitudinal study of a large cohort of Japanese males. Respirology. 2005;10(4):464–469. doi: 10.1111/j.1440-1843.2005.00727.x. [DOI] [PubMed] [Google Scholar]
- 33.Donaldson GC, Seemungal TA, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002;57(10):847–852. doi: 10.1136/thorax.57.10.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paris C, Benichou J, Saunier F, et al. Smoking status, occupational asbestos exposure and bronchial location of lung cancer. Lung Cancer. 2003;40(1):17–24. doi: 10.1016/s0169-5002(02)00538-x. [DOI] [PubMed] [Google Scholar]
- 35.Byun MK, Cho EN, Chang J, Ahn CM, Kim HJ. Sarcopenia correlates with systemic inflammation in COPD. Int J Chron Obstruct Pulmon Dis. 2017;12:669–675. doi: 10.2147/COPD.S130790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sekine Y, Hata A, Koh E, Hiroshima K. Lung carcinogenesis from chronic obstructive pulmonary disease: characteristics of lung cancer from COPD and contribution of signal transducers and lung stem cells in the inflammatory microenvironment. Gen Thorac Cardiovasc Surg. 2014;62(7):415–421. doi: 10.1007/s11748-014-0386-x. [DOI] [PubMed] [Google Scholar]
- 37.Turner MC, Chen Y, Krewski D, Calle EE, Thun MJ. Chronic obstructive pulmonary disease is associated with lung cancer mortality in a prospective study of never smokers. Am J Respir Crit Care Med. 2007;176(3):285–290. doi: 10.1164/rccm.200612-1792OC. [DOI] [PubMed] [Google Scholar]
- 38.Caramori G, Adcock IM, Casolari P, et al. Unbalanced oxidant-induced DNA damage and repair in COPD: a link towards lung cancer. Thorax. 2011;66(6):521–527. doi: 10.1136/thx.2010.156448. [DOI] [PubMed] [Google Scholar]
- 39.Sohal SS, Reid D, Soltani A, et al. Evaluation of epithelial mesenchymal transition in patients with chronic obstructive pulmonary disease. Respir Res. 2011;12:130. doi: 10.1186/1465-9921-12-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feary JR, Rodrigues LC, Smith CJ, Hubbard RB, Gibson JE. Prevalence of major comorbidities in subjects with COPD and incidence of myocardial infarction and stroke: a comprehensive analysis using data from primary care. Thorax. 2010;65(11):956–962. doi: 10.1136/thx.2009.128082. [DOI] [PubMed] [Google Scholar]
- 41.Tamiya A, Naito T, Miura S, et al. Interstitial lung disease associated with docetaxel in patients with advanced non-small cell lung cancer. Anticancer Res. 2012;32(3):1103–1106. [PubMed] [Google Scholar]
- 42.Hashimoto N, Matsuzaki A, Okada Y, et al. Clinical impact of prevalence and severity of COPD on the decision-making process for therapeutic management of lung cancer patients. BMC Pulm Med. 2014;14:14. doi: 10.1186/1471-2466-14-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Colak Y, Afzal S, Nordestgaard BG, Vestbo J, Lange P. Young and middle-aged adults with airflow limitation according to lower limit of normal but not fixed ratio have high morbidity and poor survival: a population-based prospective cohort study. Eur Respir J. 2018;51(3):1702681. doi: 10.1183/13993003.02681-2017. [DOI] [PubMed] [Google Scholar]


