Abstract
Background:
Cardiovascular magnetic resonance (CMR) imaging is an important modality that allows the assessment of regional myocardial function by measuring myocardial deformation parameters, such as strain and strain rate throughout the cardiac cycle. Feature tracking is a promising quantitative post-processing technique that is increasingly used. It is commonly applied to cine images, in particular steady-state free precession, acquired during routine CMR examinations.
Objective:
To review the studies that have used feature tracking techniques in healthy subjects or patients with cardiovascular diseases. The article emphasizes the advantages and limitations of feature tracking when applied to regional deformation parameters. The challenges of applying the techniques in clinics and potential solutions are also reviewed.
Results:
Research studies in healthy volunteers and/or patients either applied CMR-feature tracking alone to assess myocardial motion or compared it with either established CMR-tagging techniques or to speckle tracking echocardiography. These studies assessed the feasibility and reliability of calculating or determining global and regional myocardial deformation strain parameters. Regional deformation parameters are reviewed and compared. Better reproducibility for global deformation was observed compared with segmental parameters. Overall, studies demonstrated that circumferential was the most reproducible deformation parameter, usually followed by longitudinal strain; in contrast, radial strain showed high variability.
Conclusion:
Although feature tracking is a promising tool, there are still discrepancies in the results obtained using different software packages. This highlights a clear need for standardization of MRI acquisition parameters and feature tracking analysis methodologies. Validation, including physical and numerical phantoms, is still required to facilitate the use of feature tracking in routine clinical practice.
Background
There is a growing recognition that early detection of cardiac abnormalities could improve patient quality of life and reduce both morbidity and mortality. Extensive improvements and developments in cardiovascular magnetic resonance (CMR) sequences and post-processing techniques have been introduced to facilitate their use in clinical settings in order to improve the diagnostic accuracy of cardiovascular diseases (CVD) in its onset stage.
Recent extensive research has proven that global measures, such as ejection fraction, are only an indicator of global heart function and cannot be used to infer regional function, nor to detect any ventricle dysfunction at the very early stages of established diseases.1 Contrary to visual myocardial wall-deformation analysis, indices including strain, strain rate and torsion can be sensitive indicators of underlying myocardial contractile dysfunctions. Those indices can be derived from CMR-tagging images.2Figure 1 illustrates the different components of wall-deformation indices relative to cardiac anatomy. Tagging sequences use spatially selective saturation pulses to create dark lines on the myocardial tissue at the end diastole, with those lines persisting throughout part of, or all, the cardiac cycle.3 These techniques have since undergone extensive development and improvement for both imaging sequences4–6 and post-processing methods.7,8 CMR-tagging is now considered to be the gold standard for myocardial regional function assessment.9–11
Figure 1.
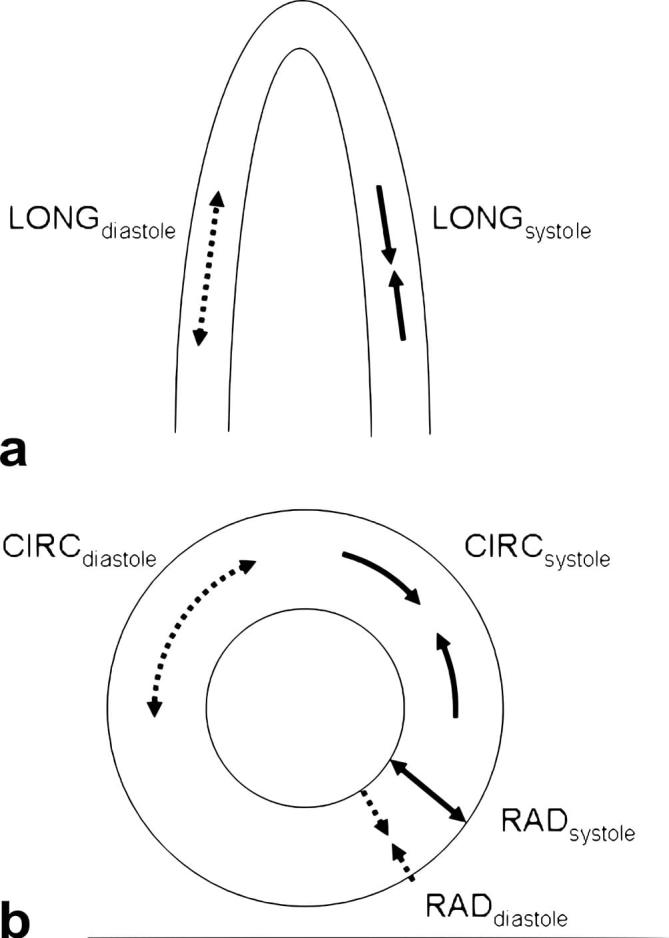
Myocardial deformation contains three strain components: circumferential, radial and longitudinal of the left ventricle; longitudinal (a), radial and circumferential (b). The direction of the deformation in diastole is shown as a dashed line and in systole shown as a solid line. The myocardial fibres shorten and lengthen in the three spatial directions: longitudinal, radial and circumferential. The strain can be calculated as the difference between myocardial fibre length (radial, circumferential and longitudinal) at end-diastole and at end-systole divided by the length at end-diastole, and expressed as a percentage (%).12
Feature tracking has been introduced to track myocardial motions, such as displacement and velocity, and derive cardiac deformation parameters, such as strain and strain rate in CMR. It tracks the tissue motion between the epicardial and endocardial borders throughout the cardiac cycle using optical flow methods, see the appendices for more information on feature tracking and tagging post-processing techniques.13–15 This article reviews the expanding field of feature tracking with a particular emphasis on clinical and multimodality comparative studies.
Results
Feature tracking (CMR-FT) studies
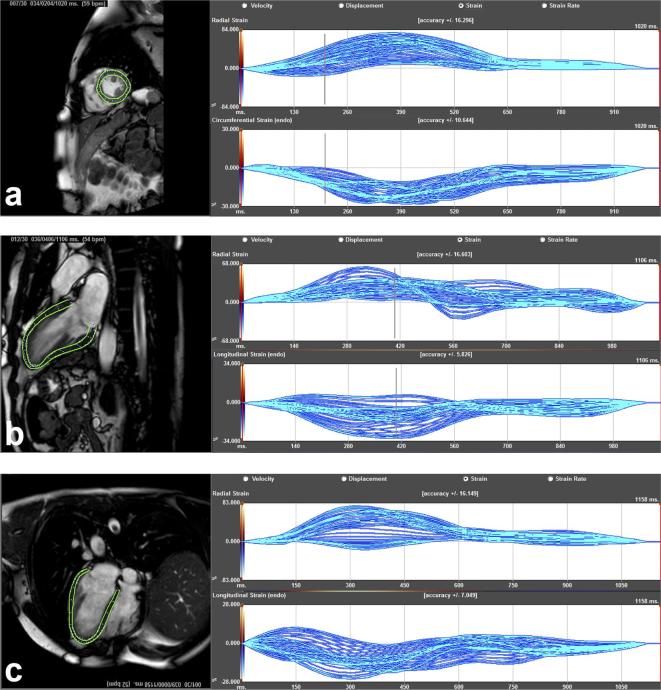
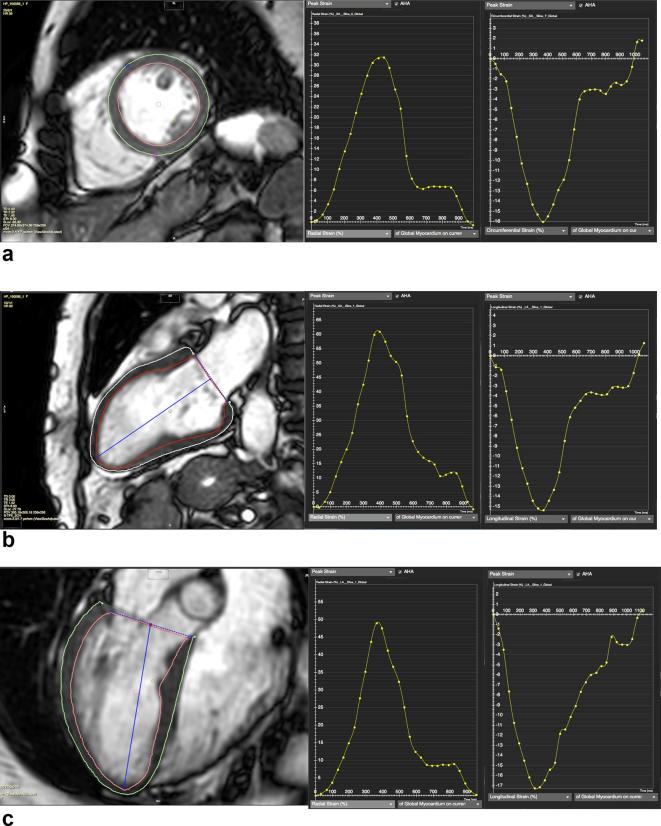
Cardiovascular magnetic resonance feature tracking (CMR-FT) is a quantitative post-processing technique that tracks myocardial tissue motion on steady-state free recession (SSFP) cine images, the most commonly used sequence in clinical cardiac function assessment. The first software package based on FT techniques was introduced by TomTec Imaging Systems GMbH (Munich, Germany) and has been used in most clinical studies published to date (Figure 2).14,16,17 More recent studies used a different FT software package: a tissue-tracking module within the CVI42 software (Circle Cardiovascular Imaging Inc. Calgary, Canada) (Figure 3). 18 A summary of studies using CMR-FT is given in Table 1.
Figure 2.
Example of FT analysis using Tomtec. Endocardial and epicardial contours of the LV are drawn on one frame and propagated throughout the cardiac cycle. (a) A short axis slice with endocardial and epicardial contours (left-hand side), and the corresponding radial (upper right-hand side) and circumferential strains (lower right-hand side). (b) A two-chamber view with endocardial and epicardial contours (left-hand side), with corresponding radial (upper right-hand side) and longitudinal strains (lower right-hand side). (c) A four-chamber view with endocardial and epicardial contours (left-hand side), and the corresponding radial (upper right-hand side) and longitudinal strains (lower right-hand side). Other deformation parameters such as velocity, displacement and strain rates can becalculated. FT, feature tracking; LV, left ventricle.
Figure 3.
Example of CVI42 FT analysis. The software semi-automatically defines the endocardial (red contour) and epicardial (green contour) LV contours throughout the cardiac cycle. (a) A short axis slice with delineated endocardial and epicardial contours (left-hand side) and the corresponding radial (middle) and circumferential strains (right-hand side). (b) A two-chamber long axis slice with delineated endocardial and epicardial contours (left-hand side) and the corresponding radial (middle) and longitudinal strains (right-hand side). (c) A four-chamber long axis slice with delineated endocardial and epicardial contours (left-hand side) and the corresponding radial (middle) and longitudinal strains (right hand side). Additional calculated parameters include velocity, displacement and strain rates. FT, feature tracking; LV, left ventricle.
Table 1.
Comparison between studies using CMR-FT technique
| Study | Strain parameters | Software | Healthy subjects | Subjects Disease studied | Main findings |
Limitations | |
|---|---|---|---|---|---|---|---|
| Positive | Negative | ||||||
| Schuster et al 201119 | RV & LV C, R, L Segmental, Global | Tomtec | 10 | - |
|
|
|
| Schuster et al 201314 | LV C, R Segmental | Tomtec CVI42 | - | 15 Ischaemic cardiomyopathy |
|
|
|
| Kowallick et al 201420 | LA L Global and segmental | Tomtec | 10 | 20 Hypertrophic cardiomyopathy (10) Heart failure (10) |
|
|
|
| Taylor et al 201421 | LV C, R Segmental | Tomtec | 55 | 108 Cardiomyopathy |
|
|
|
| Maret et al 200916 | LV R, L Global and segmental | Tomtec | – | 30 Presence of LV scar |
|
||
| Morton et al 201222 | LV R, L Global and segmental | Tomtec | 16 | - | CS most reproducible measure of LV |
|
|
C, circumferential; CS, circumferential strain; CVI42, Circle Cardiovascular Imaging Inc. Calgary, Canada; GCS, global circumferential strain; GLS, global longitudinal strain; GRS, global radial strain; L, longitudinal LA, left atrial; LS, longitudinal strain; LV, Left ventricle; R, radial; RS, radial strain; RV, right ventricle; Tomtec, TomTec Imaging Systems, Munich, Germany.
Some clinical studies were dedicated to assessing the reproducibility of FT by evaluating inter- and intraobserver reproducibility, whereas others applied FT to both healthy subjects and patients to quantify the difference in cardiac deformation parameters between those groups.17,20 Feature tracking can be applied to evaluate the function and the mechanics of all heart chambers: right ventricle (RV), left ventricle (LV) and atrial deformations.
CMR-FT was applied to detect quantitative motion changes at rest and stress of LV,14,19 as LV motion abnormalities detected by CMR post-processing techniques could be an early and sensitive tool for any contractile dysfunction. The quantitative wall parameters derived from cine images were assessed at rest and during dobutamine stress in healthy volunteers19 and in patients with ischaemic cardiomyopathy.14 CMR-FT demonstrated its ability to detect wall motion changes between rest and stress, where circumferential and radial strains increased significantly with dobutamine in both studies. However, there was no response to dobutamine in dysfunctional segments with scar in patients with ischaemic cardiomyopathy compared with non-dysfunctional segments. In stress studies, the more reproducible myocardial deformation parameter for inter- and intraobserver was circumferential strain.14,19 CMR-FT can then be used to assess strain measures at rest and stress and could provide a potential method for assessing wall contraction changes.
Heart failure and cardiomyopathies have also been evaluated using CMR-FT in particular hypertrophic cardiomyopathy.20 The ability of CMR-FT to differentiate between patients and healthy controls was evaluated in two studies.16,20 In hypertrophic cardiomyopathy and heart-failure patients, both left atrium longitudinal strain (22.1 and 16.3%) and strain rate (0.9 and 0.8 s−1) were lower than in healthy subjects (strain 29.1% and strain rate 1.1 s−1).20 Scarred segments showed lower contractile function, radial displacement, radial velocity, radial strain and longitudinal strain values compared with non-scar segments. Radial strain was shown to be the best parameter to discriminate between scarred segments and non-scarred ones.16
Diseases of the aorta have also been given a great deal of attention in clinical research, in particular coarctation of the aorta.17,23 Repaired COA patients were assessed using CMR-FT compared with normal subjects.17 Global radial strain and global longitudinal strain were decreased in patients, while global circumferential strain was preserved compared with normal subjects. In the presence of hypertrophy, global longitudinal strain was significantly reduced; this could be used as an indicator of early LV dysfunction.
A study carried out by Maret et al assessed the ability of the CMR-FT technique to detect scar defined with gadolinium-enhanced CMR of LV.16 Scarred segments showed lower functional measurements than distant segments. Myocardial function can also be measured by FT-motion parameters, such as velocity and displacement of a specific myocardial point or segment. Myocardial wall contractility will be reduced in the presence of scar and as a consequence of reduced myocardial blood flow.
CMR-FT applications were not limited to CVD patients but also included healthy subjects to assess interstudy reproducibility at global and segmental levels. Circumferential strain was found to be the most reproducible component (coefficient of variation (CV) = 20.3%), whereas reproducibility for radial strain was poor (CV = 27.2%).22 In another study, both inter- and intra-observer variability was best for circumferential strain at rest while observer-variability did not significantly increase with stress.19
To evaluate whether inter-study reproducibility is affected by physiological variations, 16 healthy volunteers underwent CMR examinations three times on the same day: the first scan was conducted after fasting, the second scan immediately after the first scan, and the last examination was a non-fasting scan in the afternoon. No diurnal variation was observed.22 Global measures showed no significant difference among the three repeated scans, as opposed to segmental measures, which were significant for radial strain.
Comparison between CMR-FT and CMR-tagging
There are currently two main CMR post-processing techniques that have been applied in order to quantify regional myocardial function: analysis of CMR tagging and CMR-FT using functional cine images.20,21,24 Regional myocardial deformation strain is a sensitive measure for detecting onset stages of myocardial dysfunctions and can be derived from CMR-FT and CMR-tagging techniques. CMR-FT and CMR-tagging techniques can help in early identification of myocardial dysfunctions. These techniques could prove important for clinical risk management, starting treatment and helping in therapy decision-making.2,25 CMR-FT is increasingly being used in studies to assess its potential in routine clinical evaluation, as CMR-FT analysis computes strain from routinely performed SSFP cine images without the need to acquire any additional CMR sequences. However, CMR-FT requires standardization of MRI acquisition and post-processing protocols to reduce any possible discrepancies between studies beside inherent natural physiological variability between healthy subjects.26 As for CMR-tagging, tagged lines fade out towards the end of the cardiac cycle making them difficult to track using post-processing techniques.27 Few studies have compared CMR-FT to CMR-tagging in healthy subjects or patients to diagnose subtle myocardial motion abnormalities. The number of subjects in each study needs to be taken into account when comparisons are being made with other studies. A summary of the studies is given in Table 2.
Table 2.
Comparison between studies using CMR-FT and tagging techniques
| Study | Strain parameters | Software | Healthy subjects | Subjects disease studied | Main findings |
Limitations | |
|---|---|---|---|---|---|---|---|
| Positive | Negative | ||||||
| Hor et al 201026 | LV C Global and segmental | TomTec HARP | 42 | 19 Duchenne muscular dystrophy (DMD) |
|
|
|
| Harrild DM et al 200911 | LV C | 13 | 11 Hypertrophic cardiomyopathy |
|
|
||
| Augustine et al 201328 | C, R, L Global and segmental | TomTec CIMTag2D | 145 | - | Some variation in strain with gender: longitudinal strain higher and radial lower in females |
|
|
| Singh et al 201429 | C, L Global and segmental | TomTec InTag | - | 18 aortic stenosis (AS) |
|
|
|
| Wu et al 201430 | LV C Segmental | TomTec MASS | 10 | 20 left bundle branch block (10) hypertrophic cardiomyopathy (10) |
|
|
|
| Moody et al 20142 | LV C, L Global | TomTec CIMTag2D | 35 | 10 dilated cardiomyopathy |
|
|
|
C, circumferential; CS, circumferential strain; L, longitudinal; LS, longitudinal strain; LV, left ventricle; R, radial; SCS, systolic circumferential strain; T2P-SCS, time to-peak-systolic circumferentialstrain.
Tomtec, MR FT analysis (TomTec Imaging Systems, Munich, Germany). Tagging analysis: HARP = (Diagnosoft, Palo Alto, California). CIMTag2D = (CIMTag2D v.7, Auckland MRI Research Group, New Zealand). InTag = (Creatis, Lyon, France) and MASS= (Medis, Leiden, Netherlands).
Muscular dystrophies such as Duchenne Muscular Dystrophy were the subject of regional myocardial function assessment using both FT and tagging techniques.26 The study included healthy volunteers and a large population of Duchenne Muscular Dystrophy patients of different age groups and severity; when strain values from the mid-left ventricular short-axis slice were compared between the two techniques, the mean circumferential strain was highly correlated. This study showed that the two techniques were comparable.
Comparison between the two techniques was also carried out in cardiomyopathies.2,11,30 One study compared the techniques in both healthy subjects and hypertrophic cardiomyopathy patients.11 The results showed a closer agreement in time-to-peak circumferential strain than in the magnitude of strain peak between both techniques. A second study compared the techniques in healthy volunteers, patients with left bundle branch block and hypertrophic cardiomyopathy.30 The segmental peak and time-to-peak for systolic circumferential strains were assessed, and both the intra- and inter-observer reproducibility were evaluated. This study demonstrated that absolute values of peak systolic circumferential strain are higher with CMR-FT than with tissue tagging. There was also a significant difference in mean peak systolic circumferential strain values between the populations studied. The inter- and intra-observer agreements were both lower with CMR-FT than with tagging.
While most studies11,26 focused solely on systolic deformation parameters, a study by Moody et al2 compared both techniques in short and long axis views, both in systole and diastole, in healthy subjects and patients with dilated cardiomyopathy. The study showed a good agreement between CMR-FT and CMR-tagging techniques for systolic global circumferential strain (−22.7 ± 6.2% vs −22.5 ± 6.9%, bias = 0.2 ± 4%, p = 0.8) respectively and early diastolic global circumferential strain rate (1.21 ± 0.44 s−1 vs 1.07 ± 0.3 s−1, bias = −0.14 ± 0.34 s−1). There was an acceptable agreement for systolic global longitudinal strain (−18.1 ± 5% vs −16.7 ± 4.8%, bias = 1.3 ± 3.8%, p = 0.03) in healthy subjects. In dilated cardiomyopathy patients, the difference between both techniques was not significant (−9.7 ± 4.5% vs −8.8 ± 3.9%, p = 0.44), whereas the agreement for early diastolic global longitudinal strain rate was poor and the difference between both techniques was significant (p < 0.001) in healthy subjects. Overall, there was an acceptable agreement between systolic and diastolic strains for some parameters measured by both techniques in both groups. However, the study only included 35 healthy subjects and 10 dilated cardiomyopathy patients; this could have had an impact on the statistical results, and should be considered when comparing this study to other studies with larger population sizes.
A different study was carried out to compare the two techniques for diastolic and systolic strain measurements in patients with aortic stenosis.29 In this study, the strain parameters were consistently higher with FT than with tagging. Furthermore, the interstudy reproducibility for circumferential peak systolic strain was excellent with FT and good with tagging, whereas the reproducibility for circumferential peak end diastolic strain rate was good only with basal and mid-slices.
Finally, FT and tagging were compared in healthy adults.28 For global measurement of strain, there was a good agreement between both techniques with circumferential strain, but this was not the case with radial and longitudinal strains. Reproducibility showed the same trends with reasonable inter-observer variability for circumferential measures. The study showed some variation in strain with gender: longitudinal strain values were higher in females, whereas radial values were higher in males.
There are obvious limitations in comparison studies that could explain the published disparities and disagreements in results. CMR-FT studies have been published by numerous centres using heterogeneous equipment (including field strength) and sequence acquisition parameters (temporal resolution, spatial resolution, slice orientation etc.). All these differences can affect the reported results and unfortunately, few studies include detailed limitations and reproducibility data. Although MRI acquisition parameters (temporal resolution, spatial resolution, slice orientation etc.) could be made as close as possible for both tagging and SSFP sequences, they are not identical.30,31 There were also differences in external parameters such as population demographics (population size, age, gender, heart rate, race, etc...).32
Comparison between CMR-FT and echocardiography
The calculation of strain and strain rate always depends on image quality; this can have an effect on the reliability and reproducibility of deformation parameters derived from echocardiographic images. Echocardiography is limited by acquisition angle and operator dependence.27,33 CMR is increasingly the method of choice because of its wide field-of-view, better image quality and reproducibility.34 A few clinical studies have compared echocardiography and CMR-FT in patients and healthy subjects to evaluate the clinical usefulness of the latter in assessing myocardial deformation parameters.35,36 A summary of studies comparing CMR-FT to echocardiography is given in Table 3.
Table 3.
Comparison between studies using CMR-FT and echocardiography
| Study | Strain parameters | Software | Healthy subjects | Subjects disease studied | Main findings |
Limitations | |
|---|---|---|---|---|---|---|---|
| Positive | Negative | ||||||
| Kempny et al 201237 | RV & LV C, R, L Global and segmental | TomTec Tomtec (STE) | 25 | 28 Tetralogy of Fallot |
|
|
|
| Padiyath et al 201335 | RV & LV C, R, L Global and segmental | TomTec Tomtec (2DE) | 20 | 20 Tetralogy of Fallot |
|
|
|
| Onishi et al 201336 | R Segmental | TomTec Tomtec | 72 Dyssynchrony |
|
|
||
| Orwat et al 201434 | L, C Global | TomTec Tomtec | 20 | 20 patients with left ventricular hypertrophy cardiomyopathy (HCM) |
|
|
|
C, circumferential; CS, circumferential strain; CSR, circumferential strain rate; GCS, global circumferential strain; GRS, global radialstrain; GLS, global longitudinal strain; L, longitudinal; LS, longitudinalstrain; LV, left ventricle; R, radial; RS, radialstrain; RV = right ventricle; TOF, tetralogy of fallot.
Tomtec = MR feature tracking analysis. Echocardiography FT: Tomtec(2DE)=2D Echocardiography analysis. Tomtec (STE) = Speckle Trackinganalysis. (TomTec Imaging Systems, Munich, Germany).
Most comparative studies have focussed on adult congenital heart disease, in particular Tetralogy of Fallot (TOF).35,37 A study was carried out in adult TOF patients and healthy subjects comparing CMR-FT to speckle tracking echocardiography (STE).37 There was a close agreement between global longitudinal and circumferential LV strains measured by CMR-FT and STE techniques, but the agreement was poor for global radial LV strain. There was also a good agreement between both techniques for global longitudinal RV strain. Inter-observer agreement for both techniques was similar for LV global longitudinal strain; however, CMR-FT showed better inter-observer reproducibility for LV circumferential and radial strains and RV global longitudinal strain. There was no significant difference between TOF patients and healthy subjects in LV circumferential strain (−23.5 ± 6vs−22±3.9%, p = 0.28) with CMR-FT, while LV longitudinal strain (−19.2 ± 4vs−21.3±3.3%, p = 0.048) and LV radial strain (22 ± 8.9 vs 28 ± 11.3%, p = 0.2) were found to be lower in patients. Furthermore, RV longitudinal strain was lower in patients compared to healthy subjects (18.3 ± 4.3 vs 24.1 ± 4%, p = 0.0001).37
The agreement between CMR-FT and STE techniques were also assessed for LV and RV global longitudinal, radial and circumferential strains in TOF patients.35 LV global circumferential and longitudinal strains had the best inter-modality agreement, whereas poorer intermodalities and interobserver variability were found for global radial strain, contrary to what was observed for radial strain in a previous study.37 When comparing TOF patients to healthy subjects, LV global circumferential, radial and longitudinal strains and RV global longitudinal strain were lower in patients compared to healthy subjects; this is in line with previously reported data.37
The feasibility of CMR-FT technique was assessed in patients with dyssynchrony.36 There was a reasonable agreement in radial dyssynchrony in patients with more marked dyssynchrony between CMR-FT and STE. The results showed a significant increase in radial myocardial contraction and circumferential strain after stent implantation. The feasibility of CMR-FT technique compared to echocardiography was also assessed in healthy subjects and patients with left ventricle hypertrophy cardiomyopathy.34 CMR-FT-derived strain and strain rate correlated well with echocardiography, and consequently could become an alternative to echocardiography for assessing myocardial deformation parameters in clinical settings in the future.
Discussion
An increasing number of research studies are using feature tracking and comparing it to tagging techniques or echocardiography in both patients and healthy subjects. Some studies have proved the usefulness of feature tracking for evaluating myocardial deformation indices and differentiating between healthy and disease states. As summarized in Tables 1–3, the number of subjects vary between studies, so that comparison between those studies is affected by the number of subjects, with a subsequent impact on statistical results.37 The feature tracking technique was used to assess regional cardiac function by calculating myocardial deformation parameters and their variation with age, gender and different cardiac dysfunction conditions.
The detection of motion abnormalities in the early stage of CVD is of great importance for an accurate diagnosis. Feature tracking provides a quantitative assessment of left ventricular motion,14,19 and can therefore be a sensitive tool to detect contractile dysfunction. Significant changes between rest and dobutamine stress were detected by FT technique in ischaemic cardiomyopathy, with no response to dobutamine in dysfunctional parts with scar.14 FT can distinguish scarred segments from distant ones as scarred segments showed lower functional measures.16
Global strain measures proved to be more reproducible than regional results.20,22,35 The potential benefit of global myocardial strain assessment has been shown to be a sensitive indicator of RV function in TOF patients.35 In another study that assessed inter-reproducibility in TOF patients, a close agreement was found between global LV and RV global strain measures.37The most consistently reproducible strain components were global longitudinal and global circumferential strains, whereas large variations were observed in global radial strain.14,19
Despite the increasing number of published studies in feature tracking, there is still an obvious lack of comparison, standardization and validation studies. Therefore, results of these studies have highlighted discrepancies between the different FT software packages available. Unlike speckle tracking echocardiography,38,39 CMR-FT has not gone through standardization and validation in physical or numerical phantom and/or animal models in order to validate it as a routine clinical tool. It is of paramount importance to understand the origin of these discrepancies in CMR-FT results. Consequently, in order to validate and compare the different FT software, it would be ideal to develop a “ground truth” numerical phantom. Such a phantom would also allow for the optimization of clinical applications. Feature tracking software providers should aim to reach a consensus for the validation and standardization of reliable deformation parameters and MRI acquisitions and analysis of post-processing methods.
Conclusion
The current review summarized the main results, reproducibility, and clinical applications of feature tracking studies, as well as their limitations, while also suggesting important possible avenues for future work.
Although comparative studies with tagging and echocardiography are a necessary step in validating CMR-FT, only numerical phantoms could give an absolute answer when evaluating different algorithms. Ideally, synthetic images mimicking known LV motions should be used to validate and compare the different FT software packages. This approach has already yielded significant results in validating speckle tracking in echocardiography.40 Additionally, companies offering feature tracking software should be encouraged to release their algorithms to help with the understanding of differences between vendors and to assist in reaching a consensus on the best method of analysis.39 Standardizing MRI acquisition parameters for FT analysis will also be crucial to its wider acceptance in routine clinical practice.
Appendix A1
A1: CMR Tagging
The first CMR-tagging sequence was introduced in the late eighties by Zerhouni.A1 CMR-tagging is based on the application of spatially selective saturation pulses perpendicular to the imaging plane, which cause a saturation of the magnetization along one (line tagging) or two (grid tagging) spatial directions. The intersection of the selected slice and imaging plane create visible dark lines (low signal intensity) on the myocardial tissue before image acquisition. CMR-tagging acquisition sequences have since undergone extensive development and improvements.A1,A2 Different post-processing techniques exist to extract and track myocardial tagging lines’ deformation from consecutive frames and calculate local and global parameters such as displacement or velocity throughout the entire cardiac cycle.A3 The most common CMR-tagging post-processing approaches are listed below.
-
(1)
Active contour: This semi-automated method, introduced in 1994,A4 uses an active shape model that delineates the image contours in a region of interest in the LV. A deformable spline is constrained by image forces which pulls it iteratively towards the LV and tagged lines’ contours until the delineating contour matches the LV boundaries or tagged lines.A5
-
(2)
Optical flow: This method determines motion by tracking and detecting the displacement vector (image velocity) of the different image signal intensities and image features (tagged and non-tagged tissues) as they move throughout the cardiac cycle.A6 Myocardial deformation is calculated from the corresponding 2D motion field.
-
(3)
Sinusoidal analysis: This method extracts motion from CMR-tagging images based on a sinusoidal approach. Image intensity distribution of each pixel in the tagging image is modelled as a moving sine wave with local frequency and amplitude. The displacement is assessed at subpixel accuracy, making it highly accurate.A7
-
(4)
Volumetric modelling: To allow three-dimensional detection of the tagged lines, a set of tagged short-axis and long-axis slices are used to compute 3D myocardial deformation and rotation parameters.A8
-
(5)
Finite element modelling: This method reconstructs 3D myocardial motion from CMR-tagging images without prior detection of the boundaries and tagging lines locations. A model is used to define the heart shape and motion. Model tagging points are generated as a material surface, which defines the location of the tagged lines. The difference between the model tagging points and images’ tagging lines is extracted and minimized to allow the model to deform the tagging lines.A9
Appendix A2
A2: Feature tracking
In 2011, the CMR-FT technique was introduced as a quantitative post-processing technique for cine SSFP sequences that are acquired as part of routine clinical cardiac examinations.A10 The fundamental principle of the feature tracking method is based on optical flow to extract spatiotemporal image features, such as varying image signal intensities, local textures and patterns from the cine images. The technique can then track anatomical features, such as epicardial and endocardial borders and myocardial tissue, in consecutive cine image frames by searching for the most comparable features in a local neighbourhood (defining a local voxel search window).
Current FT software packages are semi-automated and rely on an operator to manually delineate the initial endocardial and epicardial contours, usually on the end-diastolic cardiac phase. This frame then serves as the initial time point from which all motion parameters are calculated. Myocardial deformation parameters such as displacement, velocity, strain and strain rates can be computed at local and global levels.A11
FT was initially developed for 2D cine images but can easily be extended to 3D cine images based on the same principles. The details of how tracking is implemented in different FT-software packages are not always known and this might affect the quality and accuracy of the tracking and of the derived strain measurements. Furthermore, results are also affected by CMR imaging sequence parameters, such as temporal and spatial resolutions, and image quality, in particular signal-to-noise ratio.
Contributor Information
Haifa M Almutairi, Email: haifa.1401@hotmail.com; h.m.h.almutairi@qmul.ac.uk.
Redha Boubertakh, Email: r.boubertakh@qmul.ac.uk.
Marc E Miquel, Email: m.e.miquel@qmul.ac.uk.
Steffen E Petersen, Email: s.e.petersen@qmul.ac.uk.
References
- 1.Lorca MCN, Haraldsson H, Ordovas KG, . Ventricular Mechanics. Magn Reson Imaging Clin N Am 2015; 23: 7–13. doi: 10.1016/j.mric.2014.08.005 [DOI] [PubMed] [Google Scholar]
- 2.Moody WE, Taylor RJ, Edwards NC, Chue CD, Umar F, Taylor TJ, et al. Comparison of magnetic resonance feature tracking for systolic and diastolic strain and strain rate calculation with spatial modulation of magnetization imaging analysis. J Magn Reson Imaging 2015; 41: 1000–12. doi: 10.1002/jmri.24623 [DOI] [PubMed] [Google Scholar]
- 3.Zerhouni EA, Parish DM, Rogers WJ, Yang A, Shapiro EP. Human heart: tagging with MR imaging--a method for noninvasive assessment of myocardial motion. Radiology 1988; 169: 59–63. doi: 10.1148/radiology.169.1.3420283 [DOI] [PubMed] [Google Scholar]
- 4.Axel L, Dougherty L. MR imaging of motion with spatial modulation of magnetization. Radiology 1989; 171: 841–5. doi: 10.1148/radiology.171.3.2717762 [DOI] [PubMed] [Google Scholar]
- 5.Mosher TJ, Smith MB. A DANTE tagging sequence for the evaluation of translational sample motion. Magn Reson Med 1990; 15: 334–9. doi: 10.1002/mrm.1910150215 [DOI] [PubMed] [Google Scholar]
- 6.Fischer SE, McKinnon GC, Maier SE, Boesiger P. Improved myocardial tagging contrast. Magn Reson Med 1993; 30: 191–200. doi: 10.1002/mrm.1910300207 [DOI] [PubMed] [Google Scholar]
- 7.Kraitchman DL, Young AA, Chang CN, Axel L. Semi-automatic tracking of myocardial motion in MR tagged images. IEEE Trans Med Imaging 1995; 14: 422–33. doi: 10.1109/42.414606 [DOI] [PubMed] [Google Scholar]
- 8.Osman NF, Kerwin WS, McVeigh ER, Prince JL. Cardiac motion tracking using CINE harmonic phase (HARP) magnetic resonance imaging. Magn Reson Med 1999; 42: 1048–60. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Young AA, Axel L, Dougherty L, Bogen DK, Parenteau CS. Validation of tagging with MR imaging to estimate material deformation. Radiology 1993; 188: 101–8. doi: 10.1148/radiology.188.1.8511281 [DOI] [PubMed] [Google Scholar]
- 10.Edvardsen T, Gerber BL, Garot J, Bluemke DA, Lima JA, Smiseth OA. Quantitative assessment of intrinsic regional myocardial deformation by Doppler strain rate echocardiography in humans: validation against three-dimensional tagged magnetic resonance imaging. Circulation 2002; 106: 50–6. doi: 10.1161/01.CIR.0000019907.77526.75 [DOI] [PubMed] [Google Scholar]
- 11.Harrild DM, Han Y, Geva T, Zhou J, Marcus E, Powell AJ. Comparison of cardiac MRI tissue tracking and myocardial tagging for assessment of regionalventricular strain. Int J Cardiovasc Imaging 2012; 28: 2009–18. doi: 10.1007/s10554-012-0035-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abraham TP, Dimaano VL, Liang HY. Role of tissue Doppler and strain echocardiography in current clinical practice. Circulation 2007; 116: 2597–609. doi: 10.1161/CIRCULATIONAHA.106.647172 [DOI] [PubMed] [Google Scholar]
- 13.Hor KN, Baumann R, Pedrizzetti G, Tonti G, Gottliebson WM, Taylor M. Magnetic resonance derived Myocardial strain assessment using feature tracking. J Vis Exp 2011; 48: 2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schuster A, Paul M, Bettencourt N, Morton G, Chiribiri A, Ishida M, et al. Cardiovascular magnetic resonance myocardial feature tracking for quantitative viability assessment in ischemic cardiomyopathy. Int J Cardiol 2013; 166: 413–20. doi: 10.1016/j.ijcard.2011.10.137 [DOI] [PubMed] [Google Scholar]
- 15.Pedrizzetti G, Claus P, Kilner PJ, Nagel E. Principles of cardiovascular magnetic resonance feature tracking and echocardiographic speckle tracking for informed clinical use. J Cardiovasc Magn Reson 2016; 18: 51. doi: 10.1186/s12968-016-0269-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maret E, Todt T, Brudin L, Nylander E, Swahn E, Ohlsson JL, et al. Functional measurements based on feature tracking of cine magnetic resonance images identify left ventricular segments with myocardial scar. Cardiovasc Ultrasound 2009; 7: 53. doi: 10.1186/1476-7120-7-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kutty S, Rangamani S, Venkataraman J, Li L, Schuster A, Fletcher SE, et al. Reduced global longitudinal and radial strain with normal left ventricular ejection fraction late after effective repair of aortic coarctation: a CMR feature tracking study. Int J Cardiovasc Imaging 2013; 29: 141–50. doi: 10.1007/s10554-012-0061-1 [DOI] [PubMed] [Google Scholar]
- 18.Almutairi HM, Zemrak F, Treibel TA, Sado D, Boubertakh R, Miquel ME, et al. A comparison of cardiac motion analysis software packages: application to left ventricular deformation analysis in hypertensive patients. J Cardiovasc Magn Reson 2015; 17(Suppl 1): P57: P57. doi: 10.1186/1532-429X-17-S1-P57 [DOI] [Google Scholar]
- 19.Schuster A, Kutty S, Padiyath A, Parish V, Gribben P, Danford DA, et al. Cardiovascular magnetic resonance myocardial feature tracking detects quantitative wall motion during dobutamine stress. J Cardiovasc Magn Reson 2011; 13: 58. doi: 10.1186/1532-429X-13-58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kowallick JT, Kutty S, Edelmann F, Chiribiri A, Villa A, Steinmetz M, et al. Quantification of left atrial strain and strain rate using Cardiovascular Magnetic Resonance myocardial feature tracking: a feasibility study. J Cardiovasc Magn Reson 2014; 16: 60. doi: 10.1186/s12968-014-0060-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taylor RJ, Umar F, Moody WE, Meyyappan C, Stegemann B, Townend JN, et al. Feature-tracking cardiovascular magnetic resonance as a novel technique for the assessment of mechanical dyssynchrony. Int J Cardiol 2014; 175: 120–5. doi: 10.1016/j.ijcard.2014.04.268 [DOI] [PubMed] [Google Scholar]
- 22.Morton G, Schuster A, Jogiya R, Kutty S, Beerbaum P, Nagel E. Inter-study reproducibility of cardiovascular magnetic resonance myocardial feature tracking. J Cardiovasc Magn Reson 2012; 14: 43. doi: 10.1186/1532-429X-14-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lossnitzer D, Bellsham-Revell H, Bell A, Schuster A, Hussain T, Botnar RM, et al. Speckle tracking for Cardiac MRI in patients Pre and Post dilation and stent implantation of Aortic coarctation. J Cardiovasc Magn Reson 2012; 14(Suppl 1): P125. doi: 10.1186/1532-429X-14-S1-P125 [DOI] [Google Scholar]
- 24.Swoboda PP, Larghat A, Zaman A, Fairbairn TA, Motwani M, Greenwood JP, et al. Reproducibility of myocardial strain and left ventricular twist measured using complementary spatial modulation of magnetization. J Magn Reson Imaging 2014; 39: 887–94. doi: 10.1002/jmri.24223 [DOI] [PubMed] [Google Scholar]
- 25.Donal E, Mascle S, Brunet A, Thebault C, Corbineau H, Laurent M, et al. Prediction of left ventricular ejection fraction 6 months after surgical correction of organic mitral regurgitation: the value of exercise echocardiography and deformation imaging. Eur Heart J Cardiovasc Imaging 2012; 13: 922–30. doi: 10.1093/ehjci/jes068 [DOI] [PubMed] [Google Scholar]
- 26.Hor KN, Gottliebson WM, Carson C, Wash E, Cnota J, Fleck R, et al. Comparison of magnetic resonance feature tracking for strain calculation with harmonic phase imaging analysis. JACC Cardiovasc Imaging 2010; 3: 144–51. doi: 10.1016/j.jcmg.2009.11.006 [DOI] [PubMed] [Google Scholar]
- 27.Shehata ML, Cheng S, Osman NF, Bluemke DA, Lima JA. Myocardial tissue tagging with cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2009; 11: 55. doi: 10.1186/1532-429X-11-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Augustine D, Lewandowski AJ, Lazdam M, Rai A, Francis J, Myerson S, et al. Global and regional left ventricular myocardial deformation measures by magnetic resonance feature tracking in healthy volunteers: comparison with tagging and relevance of gender. J Cardiovasc Magn Reson 2013; 15: 8. doi: 10.1186/1532-429X-15-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh A, Steadman CD, Khan JN, Horsfield MA, Bekele S, Nazir SA, et al. Intertechnique agreement and interstudy reproducibility of strain and diastolic strain rate at 1.5 and 3 Tesla: a comparison of feature-tracking and tagging in patients with aortic Stenosis. J Magn Reson Imaging 2015; 41: 1129–37. doi: 10.1002/jmri.24625 [DOI] [PubMed] [Google Scholar]
- 30.Wu L, Germans T, Güçlü A, Heymans MW, Allaart CP, van Rossum AC. Feature tracking compared with tissue tagging measurements of segmental strain by cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2014; 16: 10. doi: 10.1186/1532-429X-16-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petitjean C, Rougon N, Cluzel P. Assessment of myocardial function: a review of quantification methods and results using tagged MRI. J Cardiovasc Magn Reson 2005; 7: 501–16. doi: 10.1081/JCMR-200053610 [DOI] [PubMed] [Google Scholar]
- 32.Moore CC, Lugo-Olivieri CH, McVeigh ER, Zerhouni EA. Three-dimensional systolic strain patterns in the normal human left ventricle: characterization with tagged MR imaging. Radiology 2000; 214: 453–66. doi: 10.1148/radiology.214.2.r00fe17453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amundsen BH, Helle-Valle T, Edvardsen T, Torp H, Crosby J, Lyseggen E, et al. Noninvasive myocardial strain measurement by speckle tracking echocardiography: validation against sonomicrometry and tagged magnetic resonance imaging. J Am Coll Cardiol 2006; 47. doi: 10.1016/j.jacc.2005.10.040 [DOI] [PubMed] [Google Scholar]
- 34.Orwat S, Kempny A, Diller GP, Bauerschmitz P, Bunck ACh, Maintz D, et al. Cardiac magnetic resonance feature tracking: a novel method to assess myocardial strain. Comparison with echocardiographic speckle tracking in healthy volunteers and in patients with left ventricular hypertrophy. Kardiol Pol 2014; 72: 363–71. doi: 10.5603/KP.a2013.0319 [DOI] [PubMed] [Google Scholar]
- 35.Padiyath A, Gribben P, Abraham JR, Li L, Rangamani S, Schuster A, et al. Echocardiography and cardiac magnetic resonance-based feature tracking in the assessment of myocardial mechanics in tetralogy of Fallot: an intermodality comparison. Echocardiography 2013; 30: 203–10. doi: 10.1111/echo.12016 [DOI] [PubMed] [Google Scholar]
- 36.Onishi T, Saha SK, Ludwig DR, Onishi T, Marek JJ, Cavalcante JL, et al. Feature tracking measurement of dyssynchrony from cardiovascular magnetic resonance cine acquisitions: comparison with echocardiographic speckle tracking. J Cardiovasc Magn Reson 2013; 15: 95. doi: 10.1186/1532-429X-15-95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kempny A, Fernández-Jiménez R, Orwat S, Schuler P, Bunck AC, Maintz D, et al. Quantification of biventricular myocardial function using cardiac magnetic resonance feature tracking, endocardial border delineation and echocardiographic speckle tracking in patients with repaired tetralogy of Fallot and healthy controls. J Cardiovasc Magn Reson 2012; 14: 32. doi: 10.1186/1532-429X-14-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Farsalinos KE, Daraban AM, Ünlü S, Thomas JD, Badano LP, Voigt JU. Head-to-Head Comparison of Global Longitudinal Strain Measurements among Nine Different Vendors: The EACVI/ASE Inter-Vendor Comparison Study. J Am Soc Echocardiogr 2015; 28: 1171–81. doi: 10.1016/j.echo.2015.06.011 [DOI] [PubMed] [Google Scholar]
- 39.Voigt JU, Pedrizzetti G, Lysyansky P, Marwick TH, Houle H, Baumann R, et al. Definitions for a common standard for 2D speckle tracking echocardiography: consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur Heart J Cardiovasc Imaging 2015; 16: 1–11. doi: 10.1093/ehjci/jeu184 [DOI] [PubMed] [Google Scholar]
- 40.D'hooge J, Barbosa D, Gao H, Claus P, Prater D, Hamilton J, et al. Two-dimensional speckle tracking echocardiography: standardization efforts based on synthetic ultrasound data. Eur Heart J Cardiovasc Imaging 2016; 17: 693–701. doi: 10.1093/ehjci/jev197 [DOI] [PubMed] [Google Scholar]
References
- A1.Zerhouni EA, Parish DM, Rogers WJ, Yang A, Shapiro EP. Human heart: tagging with MR imaging--a method for noninvasive assessment of myocardial motion. Radiology 1988; 169: 59–63. doi: 10.1148/radiology.169.1.3420283 [DOI] [PubMed] [Google Scholar]
- A2.Moody WE, Taylor RJ, Edwards NC, Chue CD, Umar F, Taylor TJ, et al. Comparison of magnetic resonance feature tracking for systolic and diastolic strain and strain rate calculation with spatial modulation of magnetization imaging analysis. J Magn Reson Imaging 2015; 41: 1000–12. doi: 10.1002/jmri.24623 [DOI] [PubMed] [Google Scholar]
- A3.Mosher TJ, Smith MB. A DANTE tagging sequence for the evaluation of translational sample motion. Magn Reson Med 1990; 15: 334–9. doi: 10.1002/mrm.1910150215 [DOI] [PubMed] [Google Scholar]
- A4.Guttman MA, Prince JL, McVeigh ER. Tag and contour detection in tagged MR images of the left ventricle. IEEE Trans Med Imaging 1994; 13: 74–88. doi: 10.1109/42.276146 [DOI] [PubMed] [Google Scholar]
- A5.Guttman MA, Zerhouni EA, McVeigh ER. Analysis of cardiac function from MR images. IEEE Comput Graph Appl 1997; 17: 30–8. doi: 10.1109/38.576854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- A6.Barron JL, Fleet DJ, Beauchemin SS. Performance of optical flow techniques. Int J Comput Vis 1994; 12: 43–77. doi: 10.1007/BF01420984 [DOI] [Google Scholar]
- A7.Arts T, Prinzen FW, Delhaas T, Milles JR, Rossi AC, Clarysse P. Mapping displacement and deformation of the heart with local sine-wave modeling. IEEE Trans Med Imaging 2010; 29: 1114–23. doi: 10.1109/TMI.2009.2037955 [DOI] [PubMed] [Google Scholar]
- A8.O'Dell WG, Moore CC, Hunter WC, Zerhouni EA, McVeigh ER. Three-dimensional myocardial deformations: calculation with displacement field fitting to tagged MR images. Radiology 1995; 195: 829–35. doi: 10.1148/radiology.195.3.7754016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- A9.Young AA. Model tags: direct three-dimensional tracking of heart wall motion from tagged magnetic resonance images. Med Image Anal 1999; 3: 361–72. doi: 10.1016/S1361-8415(99)80029-2 [DOI] [PubMed] [Google Scholar]
- A10.Hor KN, Baumann R, Pedrizzetti G, Tonti G, Gottliebson WM, Taylor M. Magnetic resonance derived Myocardial strain assessment using feature tracking. J Vis Exp 2011; 48: 2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- A11.Pedrizzetti G, Claus P, Kilner PJ, Nagel E. Principles of cardiovascular magnetic resonance feature tracking and echocardiographic speckle tracking for informed clinical use. J Cardiovasc Magn Reson 2016; 18: 51. doi: 10.1186/s12968-016-0269-7 [DOI] [PMC free article] [PubMed] [Google Scholar]