Abstract
The differential diagnosis of a girl presenting with primary amenorrhoea includes numerous conditions. Often, patients of 46XY disorder of sex development (DSD) are reared as girl and present with primary amenorrhoea. Their further evaluation to reach the final diagnosis is often a great challenge. In this article, we report a challenging case of 46XY DSD presented with primary amenorrhoea. Patient had spontaneous breast development which initially confused the diagnosis to complete androgen insensitivity syndrome. However, low testosterone suggested against this possibility and further evaluation revealed hormonal findings consistent with 17α hydroxylase/17,20 lyase (CYP17A1) deficiency. Patient had 46XY karyotype and in consistence with hormonal findings patient was found to have a likely pathogenic homozygous c.1345C>T (p.Arg449Cys) variation in exon 8 of CYP17A1.
Keywords: adrenal disorders, endocrinology
Background
The differential diagnosis of a girl presenting with primary amenorrhoea includes numerous conditions.1 Often, patients with 46XY disorder of sex development (DSD) are reared as girl and present with primary amenorrhoea.1 Their diagnosis may go unnoticed unless karyotype testing is obtained. Further evaluation of a patient with 46XY DSD presenting with primary amenorrhoea to reach the final diagnosis is often a great challenge.2 In this article, we discuss the challenges encountered in the evaluation of a patient with 46XY DSD presented with primary amenorrhoea.
Case presentation
A 19-year-old reared as girl, presented with primary amenorrhoea. She had spontaneous breast development and except for the use of some unknown ayurvedic medication for 1 month for delayed menarche, no other hormonal supplements were used. She was born of a third-degree consanguineous marriage. She is the third in birth order and has two elder brothers and a younger sister (18 years); all of whom had normal pubertal development. There was no family history of ambiguous genitalia or hypertension. There was no history of chemotherapy or radiotherapy.
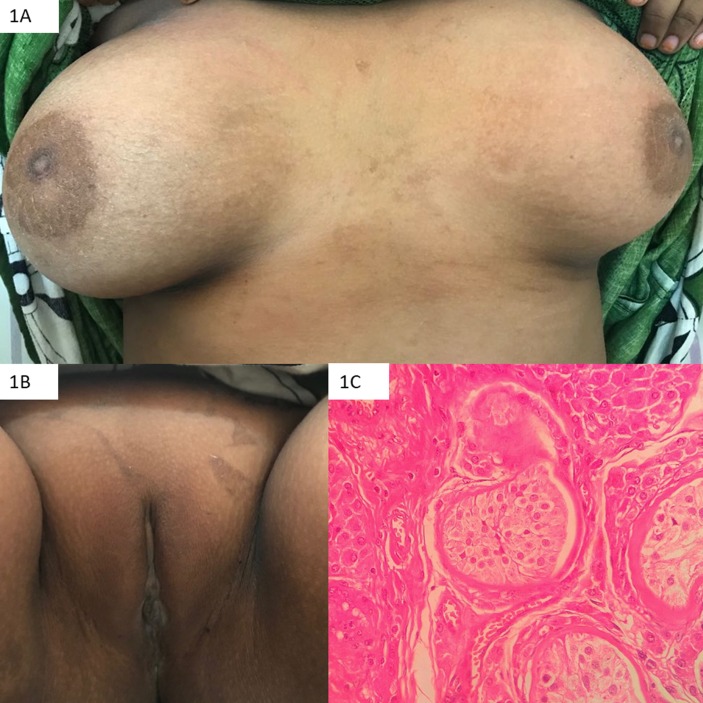
Patient weighed 55 kg and measured 156.4 cm (10th–25th centile on revised Indian academy of pediatrics (IAP) growth chart for girls and <3rd centile on revised IAP growth chart for boys). Mid-parental height for males was 163.9 cm and that for females was 150.9 cm. Upper segment to lower segment ratio was 0.85 with arm span height difference of 7.5 cm. Blood pressure was 110/70 mm Hg. Sexual maturity rating revealed stage 5 of breast development and normal female external genitalia with two separate openings in the introitus (no clitoromegaly) with normal anogenital ratio (0.45). Pubic hair (P1), axillary hair and body hair were absent (figure 1A,B). Gonads were palpable over the respective superficial inguinal canals.
Figure 1.
Photograph depicting spontaneous Tanner stage 5 breast development (A), absent pubic hair (B) and histopathology showing a testis with Leydig cell hyperplasia and seminiferous tubules predominantly containing Sertoli cells (C).
Investigations
Patient was evaluated with serum gonadotropins and total testosterone. Serum gonadotropins were elevated but serum total testosterone was low which was confirmed on repeated testing in multiple samples. Serum oestradiol was 34.64 pg/mL.
Ultrasound pelvis revealed no female internal genitalia and confirmed the testes-like structures in bilateral superficial inguinal rings. Further hormonal evaluation revealed undetectable androstenedione and very low dehydroepiandrosterone sulfate (DHEAS) with elevated 17-OH progesterone. Serum progesterone was high whereas serum antimullerian hormone (AMH) was slightly higher. Serum 08:00 hours cortisol was normal with inadequate response to adrenocorticotropic hormone (ACTH) stimulation. Serum potassium and plasma renin activity were normal (table 1).
Table 1.
Hormonal levels in blood and their comparison with normal reference ranges for age-matched males
| Observed value | Reference range | |
| Follicle stimulating hormone (mIU/mL) | 45.4 | 1.2–15.8 |
| Luteinising hormone (mIU/mL) | 53.2 | 1.3–9.6 |
| Total testosterone (ng/dL) | 27, 9, 36 | 280–1100 |
| Oestradiol (pg/mL) | 34.64 | 10–40 |
| Androstenedione (ng/mL) | <0.3 | 0.4–1.5 |
| Dehydroepiandrosterone sulfate (μg/dL) | 15.5 | 61.2–493 |
| 17α-OH progesterone (ng/mL) | 13.67 | 0.32–1.47 |
| Progesterone (ng/mL) | 4.5 | <0.2 |
| 8:00 hours cortisol (μg/dL) | 11 | 7–25 |
| ACTH stimulated cortisol (μg/dL) | 13.1 | >18 |
| Anti-mullerian hormone (ng/mL) | 21 | 0.7–19 |
| Potassium (mEq/L) | 4.1 | 3.5–5 |
| Plasma renin activity (ng/mL/hour) | 2.1 | 0.6–4.3 |
Karyotype was 46XY. Clinical exome analysis revealed homozygous c.1345C>T (p.Arg449Cys) variation in exon 8 of CYP17A1 which was confirmed with Sanger sequencing. This variant has not been reported in the 1000 genomes and has a minor allele frequency of 0.002% and 0.045% in the Exome Aggregation Consortium database. The in silico predictions of the variant are probably damaging by Polymorphism Phenotyping v2 and damaging by Sorting Intolerant from Tolerant, likelihood ratio test and Mutation Taster2. The reference codon is conserved across mammals. No significant variations were found in genes coding for p450 oxidoreductase, cytochrome b5 or androgen receptor.
Treatment and outcome
The patient underwent bilateral gonadectomy which was consistent with testes having Leydig cell hyperplasia (figure 1C). Genital examination under general anaesthesia revealed short, blind vagina. There were no perioperative complications. The patient was initiated on estradiol valerate (2 mg/day). Six months later, the patient was advised regular self vaginal dilatation.
Discussion
We report a case of CYP17A1 deficiency which was initially confused with complete androgen insensitivity syndrome (CAIS) due to a history of spontaneous breast development which was Tanner stage 5.1 3 This observation in our case suggests that the history of spontaneous breast development, even until stage 5, should not negate the possibility of CYP17A1 deficiency. She also had detectable serum oestradiol levels which might be responsible for spontaneous breast development. However, this finding remains unexplainable in CYP17A1 deficiency especially with very low DHEAS and undetectable androstenedione as seen in our patient.3
In fact, due to a history of spontaneous breast development, in conjunction with absent P1, she was suspected to have CAIS. Surprisingly, her serum total testosterone (9 ng/dL, 28 ng/dL, 37 ng/dL) levels were documented to be low at multiple times which suggested against CAIS. Complete gonadal dysgenesis was unlikely in view of palpable gonads and absent mullerian structures. Hence, she was suspected to have either testosterone biosynthetic defect or CAIS with acquired Leydig cell damage. Testing for testosterone precursors revealed elevated progesterone, mildly elevated 17-OH progesterone with very low DHEAS and undetectable androstenedione which suggested a likely defect in 17α hydroxylase/17,20 lyase (2,3). Although normotension, normokalemia and normal plasma renin activity favoured isolated 17,20 lyase deficiency, higher elevation of progesterone than 17-OH progesterone and inadequate cortisol response to ACTH also suggested the possibility of concomitant mild 17α hydroxylase deficiency.3 P450 oxidoreductase deficiency or cytochrome b5 mutations may lead to functional defects in combined 17α hydroxylase/17,20 lyase or 17,20 lyase alone respectively. Hence, mutations in these may produce similar biochemical findings and hence, were considered in the differential diagnosis.4
To differentiate among these causes and obtain definitive genetic diagnosis, clinical exome sequencing was ordered which revealed most likely pathogenic variation in CYP17A1. Previously few cases of 17α hydroxylase deficiency have been reported from India.5–9 However, there is only one report of two siblings with proven CYP17A1 mutation.9 Ours is the second report of genetically proven CYP17A1 deficiency from India. Previously R449C mutation in exon 8 has been reported in two patients from China but in compound heterozygous state with Y329K and R96Q.10 Ours is the first case in the world’s literature with homozygous R449C variation. Pathogenicity of Arg449Cys variation in CYP17A1 is not yet proven. In addition to the electrostatic charges at Arg-347, and Arg-358, that at Arg-449 is also essential for binding of the enzyme with cytochrome b5 with CYP17A1 for its lyase activity.11 12 Mutations at these sites are associated with preserved 17α hydroxylase activity but significantly reduced 17, 20 lyase activity.11 12 Change of a polar amino acid (Arginine) to a non-polar one (Cysteine) may alter the electrostatic charges. Though, no data on the enzyme activity of R449C mutation in CYP17A1 enzyme activities for different base changes at codon 449 have been reported. p.Arg449Ala is characterised by very low lyase activity but near normal hydroxylase activity whereas p.Arg449Lys mutation had 72% hydroxylase activity and 26% lyase activity.12 An activity profile similar to p.Arg449Lys mutation might have led to the combined deficiency but with no hypertension in our patient.
Learning points.
Evaluation of a patient with 46XY disorder of sex development presenting with primary amenorrhoea is often a great challenge, especially to reach the definitive diagnosis.
Spontaneous breast development, though extremely rare, may occur in patients with 17α hydroxylase/17,20 lyase (CYP17A1) deficiency and its presence should not negate the diagnosis of CYP17A1 deficiency.
We report the first case in the world’s literature of likely pathogenic homozygous R449C variation in CYP17A1.
Footnotes
Contributors: Patient was evaluated by VS, SA and CS. RR did the bilateral orchidectomy. VS drafted the manuscript; all others critically reviewed and approved the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Marsh CA, Grimstad FW. Primary amenorrhea: diagnosis and management. Obstet Gynecol Surv 2014;69:603–12. 10.1097/OGX.0000000000000111 [DOI] [PubMed] [Google Scholar]
- 2.Sarathi V. Approach to a child with disorder of sex development : Vasudev AS, Shah NK, Algorithms in pediatrics. 1st edn New Delhi: Jaypee Brothers, 2017:549–55. [Google Scholar]
- 3.Achermann JC, Hughes IA. et al. Disorders of sex development : Melmed S, Polonsky KS, Larsen PR, Kronenberg HM, Williams textbook of endocrinology. Philadelphia: Elsevier Saunders, 2011:900–2. [Google Scholar]
- 4.Auchus RJ. Steroid 17-hydroxylase and 17,20-lyase deficiencies, genetic and pharmacologic. J Steroid Biochem Mol Biol 2017;165:71–8. 10.1016/j.jsbmb.2016.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sukumar S, Uppula P, Kumar S, et al. Female phenotype with male karyotype: a clinical enigma. BMJ Case Rep 2017;2017:bcr-2016-219082 10.1136/bcr-2016-219082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kota SK, Modi K, Jha R, et al. 17-α-Hydroxylase deficiency: An unusual case with primary amenorrhea and hypertension. Indian J Endocrinol Metab 2011;15:127–9. 10.4103/2230-8210.81945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sathya A, Ganesan R, Kumar A, et al. Congenital adrenal hyperplasia masquerading as periodic paralysis in an adolescent girl. Singapore Med J 2012;53:e148–9. [PubMed] [Google Scholar]
- 8.Philip J, Anjali , Thomas N, et al. 17-Alpha hydroxylase deficiency: an unusual cause of secondary amenorrhoea. Aust N Z J Obstet Gynaecol 2004;44:477–8. 10.1111/j.1479-828X.2004.00275.x [DOI] [PubMed] [Google Scholar]
- 9.Roy A, Bhattacharjee R, Goswami S, et al. 17-α-Hydroxylase deficiency due to p.r362c mutation in two sisters from INDIA. AACE Clin Case Rep 2017;3:e322–5. 10.4158/EP161551.CR [DOI] [Google Scholar]
- 10.Tian Q, Yao F, Zhang Y, et al. Molecular study of five Chinese patients with 46XX partial 17a-hydroxylase/17,20-lyase deficiency. Gynecol Endocrinol 2012;28:234–8. 10.3109/09513590.2011.593665 [DOI] [PubMed] [Google Scholar]
- 11.Peng HM, Liu J, Forsberg SE, et al. Catalytically relevant electrostatic interactions of cytochrome P450c17 (CYP17A1) and cytochrome b5. J Biol Chem 2014;289:33838–49. 10.1074/jbc.M114.608919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee-Robichaud P, Akhtar ME, Wright JN, et al. The cationic charges on Arg347, Arg358 and Arg449 of human cytochrome P450c17 (CYP17) are essential for the enzyme’s cytochrome b5-dependent acyl-carbon cleavage activities. J Steroid Biochem Mol Biol 2004;92:119–30. 10.1016/j.jsbmb.2004.07.005 [DOI] [PubMed] [Google Scholar]