Abstract
A 75-year-old man with type 2 diabetes mellitus presented with complete loss of vision in his right eye and severe headaches for the past 24 hours. He had been treated for suspected giant cell arteritis (GCA) with high-dose corticosteroids which were being tapered to stop after an inconclusive right temporal artery biopsy and an erythrocyte sedimentation rate (ESR) value of 8. His current acute presentation, however, raised further concern for partially treated GCA and precipitated treatment with pulsed methylprednisolone. The patient, taking metformin, developed diabetic ketoacidosis and was transferred to the intensive care unit where a swollen, painful right eye with chemosis and complete ophthalmoplegia was subsequently revealed to be secondary to cavernous sinus thrombosis. Rhino-orbital skin necrosis with positive samples for the organism Rhizopus on eventual orbital exenteration revealed angioinvasive fungal infection, mucormycosis, to be the cause. We discuss here the lessons learnt, and how best to treat a susceptible cohort within our ageing western population.
Keywords: neurosurgery, infectious diseases, vasculitis, neuroimaging, contraindications and precautions
Background
Giant cell arteritis (GCA) is the most common primary medium-to-large vessel vasculitis in an ageing western population.1 Associated significant morbidity, including irreversible visual loss, means prompt treatment with glucocorticoids is the mainstay of treatment.2 The gold standard investigation remains a temporal artery biopsy; however, variable sensitivity and false-negative rates for the test complicate interpretation. In an increasingly elderly population, coexisting diseases worsened by long-term immunosuppression including diabetes mellitus present a risk for high-dose steroid treatment.3 Our patient presented with a rare angioinvasive fungal infection caused by the organism Rhizopus following high-dose steroid therapy for suspected GCA. Its intracranial spread resulted in cerebral venous sinus thrombosis, subarachnoid haemorrhage (SAH) and hydrocephalus, eventually proving fatal. Lessons learnt from it are important for an ageing global population, and therefore we discuss here the likely pathophysiology and recommended treatment options, as well as the key role of a specialist multidisciplinary team.
Case presentation
A 75-year-old man with type 2 diabetes mellitus (T2DM) was admitted to hospital with a 24-hour history of complete loss of vision in his right eye and severe right-sided headaches.
He had suffered from generalised headaches for the past 1 year, and 1 month before this admission was seen in the emergency eye clinic with a week’s history of worsening pressure on the right side of his head and gums. On examination, he had temporal tenderness with a pulsatile right temporal artery, and visual acuities with pin holes were 6/9 on the right and 6/6 on the left. Differential diagnoses included GCA and a course of oral prednisolone 60 mg once a day was started in addition to organising a right temporal artery biopsy (TAB).
The biopsy performed a week later was reported as non-diagnostic, and serum inflammatory markers were unremarkable (as shown underneath). With persistent right-sided headaches by the time of repeat follow-up, a plan was made to wean his steroids by 10 mg each week and for the general practitioner to monitor with a referral to rheumatology.
On his subsequent acute admission to his local hospital 3 weeks later, examination revealed a right-sided ptosis, miosis and a relative afferent pupillary defect. The fundi could not be visualised because of pain, and the patient had a tender scalp, with an otherwise unremarkable examination.
A CT scan of the head (CTH) showed no acute pathology, and the new visual loss and limited examination secondary to a painful eye prompted treatment with pulsed methylprednisolone for suspected GCA. The patient, taking metformin for his T2DM, developed an acute kidney injury and diabetic ketoacidosis over the next few hours, requiring intensive care unit (ICU) admission and underwent a CT angiogram (CTA) and CT venogram (CTV) of the head to identify other causes of his now painful, swollen right eye with chemosis, periorbital swelling and ophthalmoplegia. These suggested no evidence of a cavernous sinus thrombosis (CST) but a possible right-sided carotid–cavernous fistula, and he was therefore transferred to the regional neurosurgical unit for further investigation and management.
Neurosurgical admission
On admission to the neurosurgical department, the patient had a very swollen right eye with complete ophthalmoplegia, and a fixed pupil with no perception of light. However, he had an opaque, white sclera, no obvious orbital bruit and a Glasgow Coma Scale (GCS) score of 15.
His CTA and CTV showed a dilated right superior ophthalmic vein (as described underneath), with no venous thrombosis or a fistula. There was crowding of the orbital apex, however, and so an urgent ophthalmology opinion was sought again for a suspected infective or granulomatous process.
Investigations
At local hospital:
Bloods:
C-reactive protein (CRP) <5, ESR 8, thyroid function tests (TFTs) normal, autoimmune screen for Antineutrophil cytoplasmic antibodies (ANCA), rheumatoid factor (RF) and anti -glomerular basement membrane (anti-GBM) antibodies all negative.
Imaging:
Initial CTH on acute admission showed small vessel disease and a focal area of subcortical white matter low attenuation as the only positive findings.
CTA and CTV head showed a dilated right superior ophthalmic vein (figure 1, red arrow), with no venous thrombosis or a fistula. There was crowding of the orbital apex.
Figure 1.
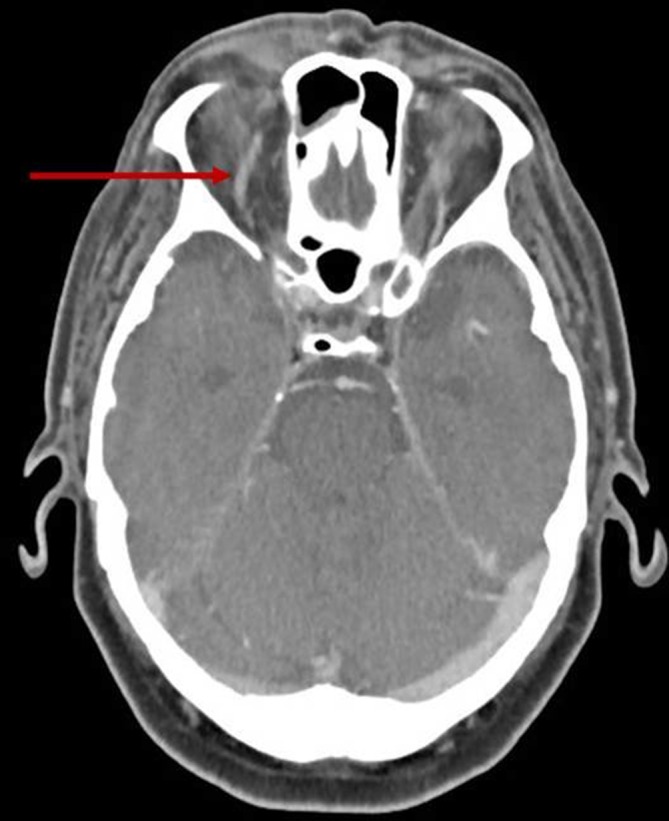
CT venogram of the head in the local hospital showing a dilated right superior ophthalmic vein (red arrow).
Pathology:
Right TAB (in outpatients, previous month): non-diagnostic.
At neurosurgical unit:
Imaging:
Repeat CTV head, 4 days after the 1st, showed new, bilateral CST (figure 2, red oval).
Repeat CTH on dropping GCS: diffuse SAH with hydrocephalus (figure 3).
Subsequent repeat CTA head during ICU stay revealed a new right cerebellar infarct and a severe irregular narrowing of the right superior cerebellar artery (SCA), consistent with angioinvasion, infective vasculitis and subsequent infarction from a fungal infection.
Figure 2.
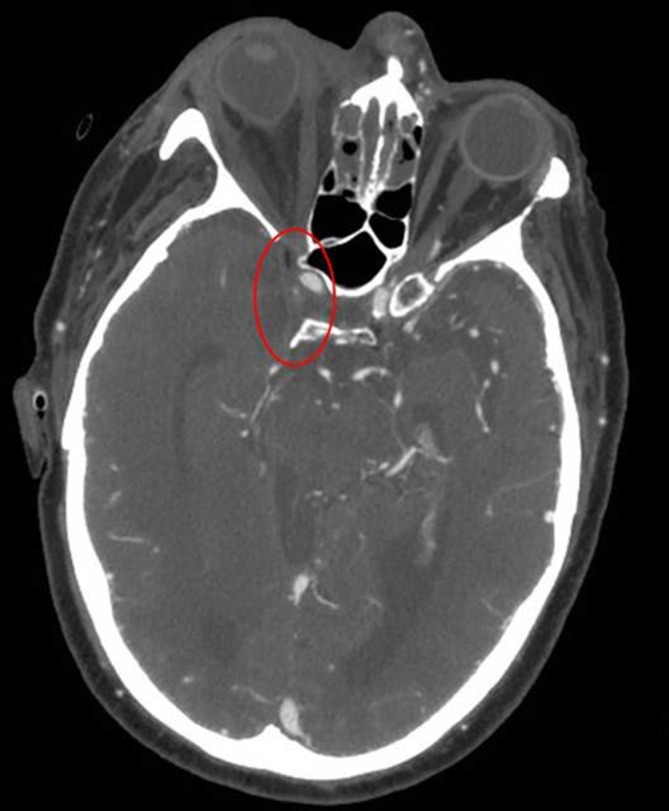
Repeat CT venogram of the head, 4 days after the first, showing absent filling of right cavernous sinus (red oval) and minimal filling on the left.
Figure 3.
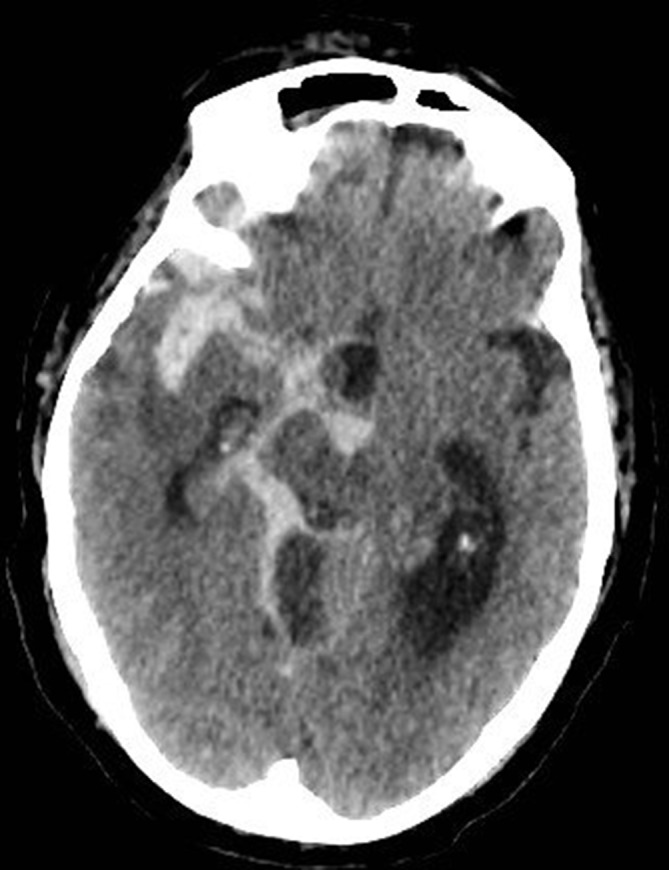
Extensive subarachnoid haemorrhage and hydrocephalus, following commencement of therapeutic-dose heparin for cavernous sinus thrombosis.
Pathology/microbiology:
Right cheek swab from ward grew Herpes Simplex Virus type 1 (HSV-1).
Right eye swab grew Rhizopus, confirming diagnosis of mucormycosis.
Theatre samples during right orbital exenteration, and debridement of the sinuses as well as nearby frontomaxillary skin grew Klebsiella, Candida and methicillin-sensitive Staphylococcus aureus from bone and maxillary sinus tissue.
Differential diagnosis
GCA with right-sided periorbital cellulitis.
Right carotid–cavernous fistula (though classically direct fistulae present with chemosis and an ocular/cranial bruit—the patient had neither on initial assessment).
Right CST with skin necrosis secondary to angioinvasive fungal infection (subsequently found to be bilateral CST, resulting from mucormycosis infection).
Treatment
The patient was initially started on intravenous ceftriaxone and vancomycin locally for possible periorbital cellulitis. Within 12 hours of transfer to the neurosurgical unit, he was also noted to have a rash in the right V1 and V2 distributions, with medial rhino-orbital skin necrosis.
Swabs from the right eye and cheek were sent for microbiology analysis, and the patient was started on empirical treatment with intravenous meropenem, clindamycin, amphotericin B and aciclovir for suspected mucormycosis and/or superimposed varicella-zoster virus and bacterial infection. We weaned his steroid dosing aggressively to a stop within 4 days, while maintaining a variable-rate insulin infusion for glycaemic control.
Following the repeat CTV showing signs of bilateral CST, the patient started treatment with therapeutic-dose intravenous heparin.
Later that evening, his GCS score dropped to 6 (E1V2M3), and a CTH revealed diffuse SAH with hydrocephalus (figure 3). The patient was intubated and transferred to theatre for an emergency external ventricular drain and admitted to the ICU subsequently. His SAH was thought to be mycotic or secondary to vasculitis, precipitated after anticoagulation for the CST. Repeat CTA of the head revealed a new right cerebellar infarct and a severe irregular narrowing of the right SCA, consistent with angioinvasion, infective vasculitis and subsequent infarction from a fungal infection.
The patient’s eye swab had grown Rhizopus, and therefore a diagnosis of mucormycosis was confirmed. In an attempt to contain the disease, the patient underwent right orbital exenteration and aggressive debridement of the mucosa, ethmoids and nearby frontomaxillary skin. Intraoperative findings confirmed infective, friable tissues with nasal and paranasal sinus involvement (figure 4).
Figure 4.
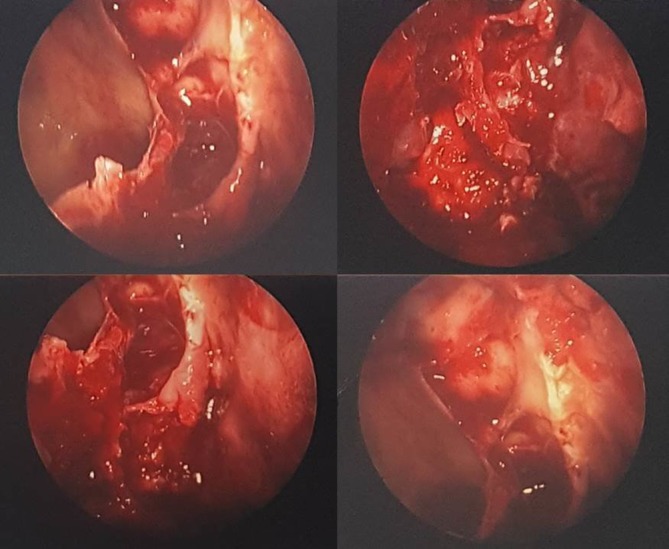
Endoscopic views of the right middle meatal antrostomy during initial sinus washout and tissue debridement.
Outcome and follow-up
Despite radical debridement of infected tissue and appropriate antifungal treatment, the patient’s neurological status off sedation remained poor, and further multiple territory intracranial infarcts were demonstrated on repeat CTH (figure 5). Following discussion with the family, palliative treatment was instituted, and the patient passed away within 12 hours.
Figure 5.
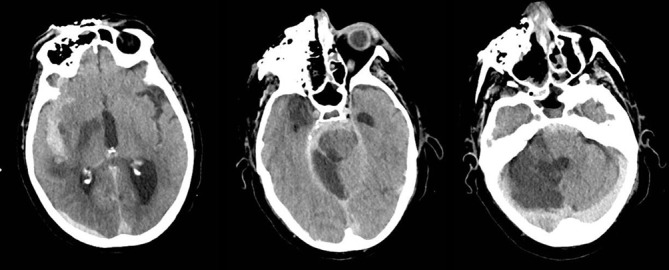
Multiple territory established infarcts, diffuse subarachnoid haemorrhage and hydrocephalus.
Discussion
Mucormycosis is a rare angioinvasive fungal infection with an associated mortality over 50%.4 Caused by organisms in the order Mucorales, the most common genera found in human infections are Rhizopus, Mucor and Rhizomucor.4 5
The disease is thought to affect around 1–2 individuals per million, with common risk factors being diabetes mellitus, especially in the context of ketoacidosis, and treatment with glucocorticoids.4–10 This is particularly true for patients with its most common clinical presentation of rhino-orbitocerebral infection, where around 70% of cases are found to have diabetes and most of them are acidotic at presentation.6 11 Causative organisms have a ketone reductase enzyme which enables better growth in an acidotic, high-glucose environment.12 Additionally, patients have a poorer phagocytic response and endothelial dysfunction secondary to the hyperosmolar metabolic acidosis which allows fungal hyphae to invade the surrounding vasculature, leading to infarction and necrosis of the underlying tissue.
The infection often starts by inhalation of fungal spores. As the infection spreads through the nasal mucosa, into paranasal sinuses and orbit, its extension into nearby vasculature can lead to involvement of the cavernous sinus, resulting in thrombosis and ophthalmoplegia.
Best current management includes treatment for underlying risk factors (for instance, poorly controlled diabetes mellitus and acidosis), antifungal first-line therapy with liposomal amphotericin B (providing reduced renal toxicity) and aggressive surgical debridement of necrotic infected tissues.13 Our patient had maximal possible treatment, however, mortality with intracranial vascular invasion remains high, with a reported range between 50% and 80%.14
National Institute for Health and Care Excellence guidelines for suspected GCA include early commencement of high-dose oral prednisolone and reassessing response within 48 hours, while awaiting specialist review.15 Empirical glucocorticoid therapy in either high doses or for long periods, however, in at-risk patients with suspected GCA carries a significant morbidity.3 Although untreated GCA carries the risk of severe visual morbidity, alternative diagnoses must be considered, particularly infection in susceptible individuals, such as the elderly and those with poorly controlled diabetes. Where GCA is suspected, a biopsy-proven diagnosis is prudent before (or soon after) commencing corticosteroid therapy.
TAB have a reported false-negative rate of between 5% and 9%, with GCA often presenting as skip lesions on histopathological analysis.16 For sufficient diagnostic yield, a minimum tissue biopsy length of 1.5 cm is therefore recommended.17 In individuals with a negative or equivocal result, a contralateral TAB may be warranted.1 Additionally, those with a negative biopsy should have alternative diagnoses sought and treated, with rapid glucocorticoid tapering within 2 weeks.18
In our patient, it is likely that the primary diagnosis was sinus and orbital fungal infection on the background of poorly controlled diabetes. However, high-dose steroids and the subsequent ketoacidosis may have precipitated the fulminant nature of the disease process. A negative TAB should always raise the suspicion of alternative diagnoses and high-dose corticosteroids should be used with caution in this setting.
Similar cases reported in the literature have included rhino-orbitocerebral mucormycosis in patients with diabetic ketoacidosis following corticosteroid therapy for idiopathic thrombocytopenic purpura and systemic lupus erythematosus.19 20 International case registries for rare, aggressive fungal infections like mucormycosis are one such effort towards furthering our understanding of this condition and optimising its treatment.
Learning points.
Giant cell arteritis remains a common vasculitis among an ageing western population, with significant associated morbidity including irreversible visual loss.
However, in patients with stable vision and prone to complications due to existing comorbidities, a biopsy-proven diagnosis may be worth establishing prior to long-term high-dose corticosteroid therapy.
Rare infections like mucormycosis are much more common in susceptible patient cohorts, including inpatients with diabetes mellitus in an acidotic state.
In these individuals, a high index of suspicion is needed for early diagnosis, and prompt aggressive treatment with amphotericin B and surgical debridement of infected tissue is recommended for disease control.
Additionally, periodic follow-up with serial imaging during treatment is often beneficial.
Footnotes
Contributors: HB authored the manuscript. GZ and CH revised it for key content. CH was also the senior clinician, with all authors contributing to the patient’s care as well as draft revisions prior to final submission.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Next of kin consent obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Gonzalez-Gay MA. The diagnosis and management of patients with giant cell arteritis. J Rheumatol 2005;32:1186–8. [PubMed] [Google Scholar]
- 2.Fraser JA, Weyand CM, Newman NJ, et al. The treatment of giant cell arteritis. Rev Neurol Dis 2008;5:140–52. [PMC free article] [PubMed] [Google Scholar]
- 3.Nesher G, Sonnenblick M, Friedlander Y. Analysis of steroid related complications and mortality in temporal arteritis: a 15-year survey of 43 patients. J Rheumatol 1994;21:1283–6. [PubMed] [Google Scholar]
- 4.Roden MM, Zaoutis TE, Buchanan WL, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis 2005;41:634–53. 10.1086/432579 [DOI] [PubMed] [Google Scholar]
- 5.Petrikkos G, Skiada A, Drogari-Apiranthitou M. Epidemiology of mucormycosis in Europe. Clin Microbiol Infect 2014;20:67–73. 10.1111/1469-0691.12563 [DOI] [PubMed] [Google Scholar]
- 6.McNulty JS. Rhinocerebral mucormycosis: predisposing factors. Laryngoscope 1982;92(10 Pt 1):1140. [PubMed] [Google Scholar]
- 7.Adam RD, Hunter G, DiTomasso J, et al. Mucormycosis: emerging prominence of cutaneous infections. Clin Infect Dis 1994;19:67–76. 10.1093/clinids/19.1.67 [DOI] [PubMed] [Google Scholar]
- 8.Petrikkos G, Skiada A, Lortholary O, et al. Epidemiology and clinical manifestations of mucormycosis. Clin Infect Dis 2012;54 Suppl 1:S23–S34. 10.1093/cid/cir866 [DOI] [PubMed] [Google Scholar]
- 9.Kontoyiannis DP, Wessel VC, Bodey GP, et al. Zygomycosis in the 1990s in a tertiary-care cancer center. Clin Infect Dis 2000;30:851–6. 10.1086/313803 [DOI] [PubMed] [Google Scholar]
- 10.Ibrahim AS, Spellberg B, Walsh TJ, et al. Pathogenesis of mucormycosis. Clin Infect Dis 2012;54:S16–22. 10.1093/cid/cir865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spellberg B, Edwards J, Ibrahim A. Novel perspectives on mucormycosis: pathophysiology, presentation, and management. Clin Microbiol Rev 2005;18:556–69. 10.1128/CMR.18.3.556-569.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gale GR, Welch AM. Studies of opportunistic fungi. I. Inhibition of Rhizopus oryzae by human serum. Am J Med Sci 1961;241:604. [PubMed] [Google Scholar]
- 13.Farmakiotis D, Kontoyiannis DP. Mucormycoses. Infect Dis Clin North Am 2016;30:143–63. 10.1016/j.idc.2015.10.011 [DOI] [PubMed] [Google Scholar]
- 14.Scheckenbach K, Cornely O, Hoffmann TK, et al. Emerging therapeutic options in fulminant invasive rhinocerebral mucormycosis. Auris Nasus Larynx 2010;37:322–8. 10.1016/j.anl.2009.09.001 [DOI] [PubMed] [Google Scholar]
- 15.National Institute of Clinical Excellence. Giant cell arteritis: management. 2017. https://cks.nice.org.uk/giant-cell-arteritis#!scenario.
- 16.Davies C, Frost B, Eshan O, et al. Temporal artery biopsy… who needs one? Postgrad Med J 2006;82:476–8. 10.1136/pgmj.2005.043646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taylor-Gjevre R, Vo M, Shukla D, et al. Temporal artery biopsy for giant cell arteritis. J Rheumatol 2005;32:1279–82. [PubMed] [Google Scholar]
- 18.Dasgupta B, Borg FA, Hassan N, et al. BSR and BHPR guidelines for the management of giant cell arteritis. Rheumatology 2010;49:1594–7. 10.1093/rheumatology/keq039a [DOI] [PubMed] [Google Scholar]
- 19.Haliloglu NU, Yesilirmak Z, Erden A, et al. Rhino-orbito-cerebral mucormycosis: report of two cases and review of the literature. Dentomaxillofac Radiol 2008;37:161–6. 10.1259/dmfr/14698002 [DOI] [PubMed] [Google Scholar]
- 20.Toumi A, Larbi Ammari F, Loussaief C, et al. Rhino-orbito-cerebral mucormycosis: five cases. Med Mal Infect 2012;42:591–8. 10.1016/j.medmal.2012.10.001 [DOI] [PubMed] [Google Scholar]


