Abstract
Campylobacter fetus (C. fetus) is a rare condition and mostly seen in elderly or immunocompromised patients. We present the first case of C. fetus spondylodiscitis in a virologically suppressed HIV seropositive patient with low back pain. MRI was performed and showed spondylodiscitis of the L4–L5 region. Empirical antibiotic therapy with flucloxacillin was started after blood cultures were drawn and an image-guided disc biopsy was performed. Blood cultures remained negative. The anaerobic culture of the puncture biopsy of the disc revealed presence of C. fetus after which the antibiotic treatment was switched to ceftriaxone. Guided by the susceptibility results, the therapy was switched to ciprofloxacin orally for 6 weeks after which the patient made full clinical, biochemical and radiographic recovery. Since no other immune-deficient conditions were noted, it is important to highlight that patients with HIV infection with restored CD4 counts and complete virological suppression can still be susceptible for infections caused by rare pathogens. Low back pain should raise suspicion for these conditions and should be examined properly.
Keywords: bone and joint Infections, Hiv / Aids
Background
This case illustrates that a trivial symptom as low back pain should raise suspicion for severe conditions like spondylodiscitis in HIV seropositive patients, even though CD4 counts are restored. Campylobacter fetus (C. fetus) should be considered as potentialcausative organism, especially in patients with gram-negative bacteraemia. A puncture biopsy should be performed to confirm the diagnosis.
Case presentation
A 53-year-old patient was admitted to the hospital because of several weeks of increasing low back pain without radiation. He was known to be HIV seropositive for the past 11 years and currently treated with triple antiretroviral therapy consisting of dolutegravir, abacavir and lamivudine, resulting in enduring virological suppression and restoration of cellular immunity. Five months before presentation, he had a CD4 count of 580 cells/µL without detectable plasma HIV RNA. The CD4 nadir before start of therapy 7 years earlier was 284 cells/µL. In addition, his medical history included a basocellular skin carcinoma 12 years earlier with complete remission after surgery. At presentation, there were no systemic symptoms: no fever, no weight loss, no night sweats. He reported no change in behaviour or a decrease in therapy adherence.
Physical examination of the patient revealed pain on vertebral percussion L4–L5 without radiation or loss of motor or sensory function.
Investigations
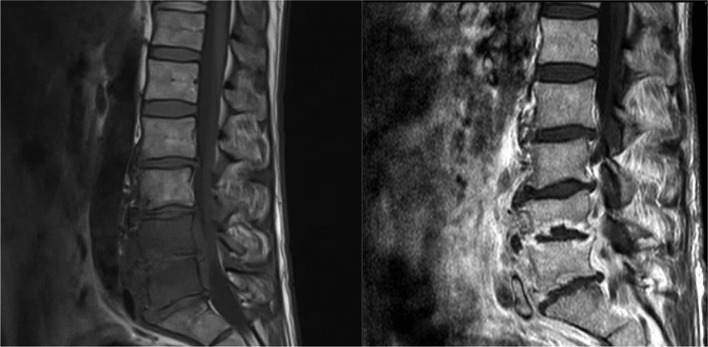
A laboratory examination showed normocytic anaemia (haemoglobin 12.4 g/dL), normal leucocyte count and differential count, elevated creatinine levels of 1.25 mg/dL with normal lactate dehydrogenase and liver function tests. There was an elevated C-reactive protein of 100 mg/L and an elevated blood sedimentation rate (33 mm/hour). The HIV plasma viral load was undetectable, while the CD4 count at presentation was 270 cells/µL, most probably caused by the inflammatory reaction. A subsequent MRI of the lumbar spine revealed diffuse oedema in vertebrae L4 and L5, hyperintensity of the intervertebral disc spaces on inversion images and inflammation of the surrounding tissue with small abscesses in the psoas muscles, suggesting spondylodiscitis of the L4–L5 region with paravertebral abscess (figure 1). Two sets of blood cultures were obtained at the same time. A puncture biopsy of the intervertebral discus L4–L5 was performed to obtain material for microbiological culture.
Figure 1.
MRI lumbar spine, T1 Fast spin T1 FSE sagittal view (left) and gadolinium contrast image (right) showing diffuse oedema in vertebrae L4 and L5 and inflammation of the surrounding tissue with small abscesses in the psoas muscles.
Treatment
After the biopsy, empirical antimicrobial treatment was initiated with flucloxacillin 1 g six times daily. The blood cultures later revealed Staphylococcus epidermidis in one aerobic and one anaerobic bottle of four culture bottles drawn at the same time, interpreted as contamination. The aerobic culture became positive after 27 hours, the anaerobic bottle became positive after 18 hours. The anaerobic culture of the discus, however, revealed presence of C. fetus species. The aerobic culture remained negative. There was no growth of mycobacteria or fungi. The empirical treatment was switched to ceftriaxone 2 g intravenously once daily. There was no need for neurosurgical abscess drainage or debridement. The subsequent drug susceptibility tests, performed in the Belgian reference laboratory with the Mueller-Hinton broth blood agar disk diffusion assay, showed a low minimum inhibitory concentration for ampicillin, amoxicillin–clavulanic acid, erythromycin, tetracycline and ciprofloxacin (table 1). After obtaining these results, treatment was switched to oral ciprofloxacin 500 mg twice daily for 6 weeks.
Table 1.
Minimal inhibitory concentration (MIC) values of the Campylobacter fetus strain
| Antimicrobial tested | MIC (µg/mL) |
| Ampicillin | 1.0 |
| Amoxicillin/clavulanic acid | 0.38 |
| Tetracycline | 0.25 |
| Ciprofloxacin | 0.125 |
| Erythromycin | 0.75 |
| Gentamicin | 0.25 |
Outcome and follow-up
The patient made full clinical recovery without any residual symptoms. MRI was performed 1 month after diagnosis and showed regression of the previous findings. Three months later during routine HIV follow-up, the blood results showed an undetectable viral load with CD4 counts back to 540 cells/µL.
Discussion
Spondylodiscitis due to C. fetus is rare. A literature search resulted in only six reports of C. fetus spondylodiscitis.1–6 C. fetus is a gram-negative, microaerophilic, curved rod with three subspecies that cause infections in humans, C. fetus subsp. fetus, C. fetus subsp. venerealis and C. fetus subsp. testudinum. C. fetus subsp. fetus is the most commonly detected species of the genus Campylobacter to cause infections in humans and Campylobacter bacteraemia. Patients with high age (median age of 78 years in the cohort of Gazaigne et al) and underlying disease, such as cardiovascular disease, diabetes or an immune-deficient condition, have a higher risk for Campylobacter bacteraemia.7 However, in the other case reports, median age was 61 years (range 37–91 years), and no underlying condition was reported in any of the cases. The main source of C. fetus infections is thought to be animal products from cattle or sheep.8 The portal of entry is believed to be the gastrointestinal tract,8 but the urinary tract is also described as a source of infection.3 In our case, no gastrointestinal symptoms were apparent which does not exclude a subclinical enteritis with subsequent haematogenic spread. There is a possible role of person-to-person transmission among men who have sex with men.9
C. fetus has a preference for endovascular surfaces, leading to vascular pathology as mycotic aneurysms and endocarditis. The vascular tropism of C. fetus has been linked to a surface layer protein with high affinity for the endothelium and to the production of a local pro-coagulant that promotes thrombus formation.10 It is important to highlight the need for blood cultures and evaluation for a potential cardiovascular focus when bacteraemia caused by C. fetus is diagnosed. Other possible manifestations are neurological infections,4 cellulitis, osteomyelitis and arthritis.8
Spondylodiscitis is most commonly detected with MRI. Patients with C. fetus bacteraemia and low back pain should undergo MRI examination. In accordance with the IDSA guidelines11 an image-guided aspirate biopsy was performed, and we started empiric antibiotic therapy with flucloxacillin targeting gram-positive organisms since methicillin-sensitive Staphylococcus aureus is the most frequent pathogen to cause spondylodiscitis in our population. Antibiotic therapy was switched to ceftriaxone when the anaerobic culture of the discus biopsy yielded C. fetus. The treatment was further streamlined following the results of the susceptibility testing while also considering tissue penetration. In the other case reports, antibiotic treatment was also selected based on sensitivity results since therapeutic management is not standardised due to the rarity of the disease.12 The organism is generally susceptible to ampicillin, cephalosporins, aminoglycosides and carbapenems. Fluoroquinolone resistance is rare. The antibiotics used in the mentioned case reports were tetracyclines, quinolones, erythromycin and amoxicillin. Duration of treatment was guided by clinical signs, inflammatory parameters and improvement of image findings. Our patient was treated for 6 weeks, based on Infectious Diseases Society of America (IDSA) guidelines,11 and made full clinical, biochemical and radiographic recovery.
Although the presence of Campylobacter infections in patients with HIV infection is reduced since the introduction of antiretroviral therapy, there is still a higher incidence in comparison with the normal population.13 The patient in our case had a virological suppressed HIV infection and restored CD4 count, but this chronic condition made him more susceptible to infection with C. fetus. No other risk factors for an immunosuppressive state were noted.
Conclusion
Low back pain with elevated inflammatory markers in a patient with HIV infection should raise suspicion for an underlying infection. MRI should be performed to diagnose spondylodiscitis. Blood cultures and an aspirate biopsy are necessary to identify the pathogenic organism, including rare pathogens like C. fetus. Empirical antibiotic therapy should include covering of gram-negative bacteria and should be switched on microbiological results. Duration of treatment is guided by the clinical, biochemical and radiographic evaluation of the patient. In case of ongoing bacteraemia or unsuspected clinical progression, investigation should be performed to rule out cardiovascular sources.
Patient’s perspective.
I feel glad and happy to contribute to the increase in scientific insight. Although my condition is now much improved, I sincerely hope this report might help other people or doctors in coping with unforeseen circumstances!
Learning points.
When spondylodiscitis is diagnosed based on imaging, blood cultures should be drawn and a puncture biopsy is indicated when the causing agent cannot be identified by other less invasive means.
Antibiotic treatment of Campylobacter fetus infections should be guided by susceptibility results since there are no standardised treatment regimens.
HIV seropositive patients are still susceptible for infections caused by rare pathogens, even with restored CD4 count.
Footnotes
Contributors: DL: first author, wrote manuscript, managed patient care. MP: wrote and revised manuscript, managed patient care. JCHvdH: wrote and revised manuscript. PM: last Author, wrote and revised manuscript, managed patient care.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Mathieu E, Koeger AC, Rozenberg S, et al. Campylobacter spondylodiscitis and deficiency of cellular immunity. J Rheumatol 1991;18:1929–31. [PubMed] [Google Scholar]
- 2.Bachmeyer C, Grateau G, Sereni D, et al. [Campylobacter fetus spondylodiscitis]. Rev Rhum Mal Osteoartic 1992;59:77–9. [PubMed] [Google Scholar]
- 3.Yamashita K, Aoki Y, Hiroshima K, et al. Pyogenic vertebral osteomyelitis caused by Campylobacter fetus subspecies fetus. A case report. Spine 1999;24:582–4. 10.1097/00007632-199903150-00018 [DOI] [PubMed] [Google Scholar]
- 4.Ozeki T, Nokura K, Koga H, et al. [A case of meningoencephalitis and spondylodiscitis caused by Campylobacter fetus subsp. fetus infection]. Rinsho Shinkeigaku 2002;42:38–41. [PubMed] [Google Scholar]
- 5.Chaillon A, Baty G, Lauvin MA, et al. Campylobacter fetus subspecies fetus spondylodiscitis. J Med Microbiol 2010;59:1505–8. 10.1099/jmm.0.023382-0 [DOI] [PubMed] [Google Scholar]
- 6.Tanaka A, Takahashi J, Hirabayashi H, et al. A case of pyogenic spondylodiscitis caused by campylobacter fetus for which early diagnosis by magnetic resonance imaging was difficult. Asian Spine J 2012;6:274–8. 10.4184/asj.2012.6.4.274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gazaigne L, Legrand P, Renaud B, et al. Campylobacter fetus bloodstream infection: risk factors and clinical features. Eur J Clin Microbiol Infect Dis 2008;27:185–9. 10.1007/s10096-007-0415-0 [DOI] [PubMed] [Google Scholar]
- 8.Wagenaar JA, van Bergen MA, Blaser MJ, et al. Campylobacter fetus infections in humans: exposure and disease. Clin Infect Dis 2014;58:1579–86. 10.1093/cid/ciu085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marchand-Senécal X, Bekal S, Pilon PA, et al. Campylobacter fetus cluster among men who have sex with men, Montreal, Quebec, Canada, 2014-2016. Clin Infect Dis 2017;65:1751–3. 10.1093/cid/cix610 [DOI] [PubMed] [Google Scholar]
- 10.Morrison VA, Lloyd BK, Chia JK, et al. Cardiovascular and bacteremic manifestations of Campylobacter fetus infection: case report and review. Rev Infect Dis 1990;12:387–92. 10.1093/clinids/12.3.387 [DOI] [PubMed] [Google Scholar]
- 11.Berbari EF, Kanj SS, Kowalski TJ, et al. 2015 Infectious diseases society of america (idsa) clinical practice guidelines for the diagnosis and treatment of native vertebral osteomyelitis in adults. Clin Infect Dis 2015;61:e26–e46. 10.1093/cid/civ482 [DOI] [PubMed] [Google Scholar]
- 12.Tremblay C, Gaudreau C, Lorange M, et al. Epidemiology and antimicrobial susceptibilities of 111 Campylobacter fetus subsp. fetus strains isolated in Québec, Canada, from 1983 to 2000. J Clin Microbiol 2003;41:463–6. 10.1128/JCM.41.1.463-466.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Larsen IK, Gradel KO, Helms M, et al. Non-typhoidal Salmonella and Campylobacter infections among HIV-positive patients in Denmark. Scand J Infect Dis 2011;43:3–7. 10.3109/00365548.2010.517780 [DOI] [PubMed] [Google Scholar]