Abstract
We investigated the rapid initial response to wounding damage generated by straight cuts to the leaf lamina and midrib transversal cuts in mature aspen (Populus tremula) leaves that can occur upon herbivore feeding. Wound-induced volatile emission time-courses of 24 compounds were continuously monitored by a proton-transfer-reaction time-of-flight mass spectrometer (PTR-TOF-MS). After the mechanical wounding, an emission cascade was rapidly elicited, resulting in emissions of key stress volatiles methanol, acetaldehyde and volatiles of the lipoxygenase pathway, collectively constituting ca. 99% of the total emission. For the same wounding magnitude, midrib cuts lead to six-fold greater emissions of volatiles per mm2 of surface cut than lamina cuts during the first emission burst (shorter than seven minutes), and exhibited a particularly high methanol emission compared to the emissions of other volatiles. This evidence suggests that feeding by herbivores capable of consuming the leaf midrib can result in disproportionally greater volatile release than feeding by smaller herbivores incapable of biting through the major veins.
Keywords: abiotic stress, green volatiles, LOX products, mass spectrometry, methanol, proton-transfer-reaction
Introduction
Leaf wounding due to herbivory or by mechanical damage induces the emission of biogenic volatile organic compounds (BVOCs). As small damage as a few millimetre cut of leaf lamina generates a rapid release of free fatty acids from plant membranes triggering the lipoxygenase chain reaction and leading to emissions of volatile lipoxygenase (LOX) pathway products, mainly C6 aldehydes, alcohols and esters (Brilli et al. 2011; Dudareva et al. 2006). The rapid burst of volatile emission upon mechanical wounding lasts for several minutes until the emissions return to the pre-stress level (Portillo-Estrada et al. 2015). Thus, the LOX products repel or attract herbivores and their natural enemies during leaf feeding (Scala et al. 2013) and have antibacterial and antifungal properties for the open leaf wound (Kishimoto et al. 2008). The wound-induced volatile emissions are also of special interest because volatile LOX products and semi-volatile compounds such as jasmonic acid and methyl jasmonate trigger the expression of defence genes and synthesis of other volatile and non-volatile metabolites downstream of the signalling cascade (Scala et al. 2013). Moreover, the wound-related BVOCs can travel through the air to neighbouring plants and induce the emission of methyl salicylate and volatile terpenoids, which may have a protective role in coping with biotic and abiotic stresses (Heil 2014).
Under natural conditions plant leaves are exposed to attacks by a variety of herbivores. Whilst many insect herbivores avoid consumption of the leaf midrib and primarily feed on the intercostal leaf lamina, some insect herbivores exhibit plant defence sabotage behaviours as girdling, furrowing, and canal cutting before feeding on the leaf lamina in order to render it more palatable. Notodontid caterpillars create girdles that completely encircle stems, petioles, and rachises (Dussourd 2015; Ganong et al. 2012), and also cut furrows in leaf midribs or sever leaf petioles (Dussourd et al. 2016). Canal cutting by multiple lineages of lepidopteran larvae, beetles, and katydids (e.g. Scudderia furcata, Aulacophora nigripennis, Danaus plexippus) (Dussourd 2009; Dussourd 2017; Helmus & Dussourd 2005) consists on first biting and severing the midrib or side veins of a latex-secreting leaf to reduce the amount of latex, phloem sap, or resin canals flow downstream of the bite. Thus the area of the leaf affected has less resins, latex and phloem-located defences (e.g. toxins, antifeedants, and other sticky secretions) (Mescher 2012). Girdles to Populus deltoides stems, for example, have been observed to eliminate induced phenolic synthesis in an adjacent leaf (Arnold et al. 2004). Given that major veins are crucial for supplying leaves with water and thus, the leaves and their malfunctioning cannot be compensated by minor veins (Sack et al. 2004), damage of major vein might lead to disproportionately greater release of volatiles than damage of minor veins or leaf lamina. There is evidence that the release of stress volatiles from leaves attacked by large herbivores capable of cutting though and consuming leaf midrib and other major veins is greater than the emissions from leaves attacked by herbivores feeding on intercostal areas (Copolovici et al. 2017), although the effect of the severance of midrib alone has not been assessed. Moreover, Oedemasia leptinoides (Notodontidae) larvae transferred from their host tree (Carya illinoinensis) to Populus deltoides are capable of producing girdles in the petioles of this species (Ralph 2009), suggesting that the widely used model genus Populus might be used to analyse how petiole and midrib damage affects tree physiology (Dussourd 2017).
In this short communication, we present the quantitative and qualitative differences between the wound-induced BVOC emissions of leaf lamina and midrib in European aspen (Populus tremula L.) and demonstrate that midrib damage has a much greater influence on stress volatile release than damage of intercostal leaf areas.
Methods and Materials
Plant Material
We used Populus tremula mature leaves of similar age, 7th to 9th leaf from the shoot tip, in order to avoid the effect of leaf ontogeny on the wounding BVOC emission response (Portillo-Estrada et al. 2017). The leaves grew on root suckers (genetically identical) produced by trees naturally growing in the campus of the Estonian University of Life Sciences (Tartu, Estonia, 58.39° N, 26.70° E, elevation 41 m). The average ± SE leaf structural characteristics were: dry mass of 0.258 ± 0.015 g, water content of 59.9 ± 0.6 %, area of 40.8 ± 1.9 cm2, dry mass per unit area of 63.4 ± 2.2 g m−2, lamina thickness of 0.2066 ± 0.0028 mm, and midrib thickness of 1.198 ± 0.037 mm. Right after the harvest, the shoots were re-cut under water and kept at a room temperature of 22 °C under a 500 W halogen stab lamp (model J-118, Philips) providing a quantum flux density of ca. 350 μmol m−2 s−1 at the shoot level to acclimate the leaves to the measurement conditions.
Analytical Setup
Leaf photosynthetic activity, stomatal conductance and volatile emissions were measured online using a GFS-3000 gas exchange system (Walz GmbH, Effeltrich, Germany) combined with a proton-transfer-reaction time-of-flight mass spectrometer (PTR-TOF-MS) model 8000 (Ionicon Analytic GmbH, Innsbruck, Austria). The clip-on type leaf cuvette (3010-S of Walz GFS-3000) covered 8 cm2 of leaf surface, and the enclosed leaf area was illuminated with an LED array/PAM-fluorometer 3055-FL providing saturating light of 500 μmol m−2 s−1. The chamber was kept at a controlled constant temperature of 25 °C, and was operated at a flow rate of 750 μmol s−1. Ambient air with constant air humidity (16000 ppm H2O, approx. 60% relative humidity) and constant CO2 concentration of 400 μmol mol−1 purified by passing through a custom-made ozone trap and a charcoal filter was used. A constant flow of 74 μmol s−1 exiting the Walz gas exchange system outlet was used for PTR-TOF-MS measurements.
The PTR-TOF-MS instrument detected the volatile compounds in real time, averaging 31250 spectra (m/z 0-316) per second. The drift tube was operated at 600 V drift voltage, 2.3 mbar pressure, and 60 °C temperature. Details regarding the functioning of the PTR-TOF-MS instrument, the settings used, the analytical setup, and the data post-processing are provided in Portillo-Estrada et al. (2015), and the details on the calibration and the procedure of resolving multi-peaks in the spectra in Portillo-Estrada et al. (2018). The calibration of BVOCs concentrations was done with a gas mixture of pure standards for methanol, acetone, acetaldehyde, isoprene, hexenal, hexenol, and monoterpenes (using α-pinene). For the rest of BVOCs, their reaction rate constants were retrieved from the Supplementary material of Cappellin et al. (2012) or otherwise they were assumed to be 2×10−9 cm3 s−1. We analysed in detail the emissions of 24 protonated compounds (Table 1) corresponding to typical BVOCs that leaves emit constitutively and due to abiotic stress. This list includes constitutively produced isoprene, the enzymatic products of the lipoxygenase pathway (LOXs) that typically occur after plant tissue damage, lightweight oxygenated compounds (LOCs) and some large molecular size compounds traditionally considered to function as hormones.
Table 1.
Biogenic volatile organic compound (BVOC) blend emitted during seven minutes after straight cuts to the leaf lamina and transversal cuts to the leaf midrib of mature aspen (Populus tremula) leaves. The seven-minute integrated emission is expressed in picomol of compound per unit surface area (mm2) of open wound exposed after the cut (calculated from the leaf lamina thickness, midrib diameter, and cut length) or expressed in nanomol per unit cut length (mm). When average lamina and midrib cut BVOC emissions were significantly different (P < 0.05, Mann-Whitney U test), P-values were shown in bold.
| Compound | Molecular formula of the protonated compound | Protonated molecular mass of the parent ion | Average (± SE) volatile emission per wound surface (pmol mm−2) | Average (± SE) volatile emission per cut length (nmol mm−1) | ||||
|---|---|---|---|---|---|---|---|---|
| Lamina | Midrib | P-value | Lamina | Midrib | P-value | |||
| Lightweight oxygenated compounds | 1,230 ± 60 | 8,600 ± 500 | < 0.001 | 253 ± 14 | 2820 ± 170 | < 0.001 | ||
| Formaldehyde | CH3O+ | 31.018 | 3.03 ± 0.35 | 14.5 ± 2.7 | < 0.001 | 0.62 ± 0.07 | 4.9 ± 1.0 | < 0.001 |
| Methanol | CH5O+ | 33.034 | 407 ± 30 | 4,760 ± 280 | < 0.001 | 85 ± 7 | 1580 ± 110 | < 0.001 |
| Acetaldehyde | C2H5O+ | 45.034 | 770 ± 50 | 3,680 ± 340 | < 0.001 | 159 ± 12 | 1200 ± 100 | < 0.001 |
| Formic acid | CH3O2+ | 47.013 | 8.3 ± 1.0 | 13.2 ± 2.0 | 0.031 | 1.70 ± 0.20 | 4.3 ± 0.7 | < 0.001 |
| Ethanol | C2H7O+ | 47.049 | 9.8 ± 0.9 | 27.0 ± 4.2 | < 0.001 | 2.01 ± 0.19 | 12.7 ± 0.8 | < 0.001 |
| Acetone | C3H7O+ | 59.049 | 14.2 ± 1.2 | 40.1 ± 3.1 | < 0.001 | 2.92 ± 0.24 | 12.2 ± 1.0 | < 0.001 |
| Acetic acid | C2H5O2+ | 61.028 | 9.7 ± 0.8 | 27 ± 4.2 | < 0.001 | 2.01 ± 0.18 | 9.0 ± 1.4 | < 0.001 |
| Lipoxygenase pathway products | 1,760 ± 100 | 10,100 ± 1,000 | < 0.001 | 360 ± 21 | 3320 ± 300 | < 0.001 | ||
| Pentenal + pentenone | C5H9O+ | 85.065 | 14.4 ± 0.8 | 77 ± 9 | < 0.001 | 2.95 ± 0.17 | 25.1 ± 2.6 | < 0.001 |
| Pentanal + pentenol | C5H11O+ | 87.080 | 4.19 ± 0.34 | 14.6 ± 2.1 | < 0.001 | 0.86 ± 0.07 | 4.7 ± 0.6 | < 0.001 |
| Hexenal | C6H11O+ | 99.080 | 1600 ± 100 | 9,100 ± 900 | < 0.001 | 330 ± 20 | 2980 ± 270 | < 0.001 |
| Hexenol + hexanal | C6H13O+ | 101.096 | 139 ± 9 | 920 ± 90 | < 0.001 | 28.2 ± 2.1 | 307 ± 29 | < 0.001 |
| Hexanol | C6H15O+ | 103.112 | 2.13 ± 0.12 | 8.5 ± 0.7 | < 0.001 | 0.437 ± 0.024 | 2.81 ± 0.22 | < 0.001 |
| Hexenyl acetate | C8H15O2+ | 143.107 | 0.473 ± 0.034 | 1.85 ± 0.18 | < 0.001 | 0.098 ± 0.007 | 0.612 ± 0.060 | < 0.001 |
| Hexyl acetate | C8H17O2+ | 145.122 | 0.051 ± 0.007 | 0.336 ± 0.044 | < 0.001 | 0.0104 ± 0.0014 | 0.107 ± 0.014 | < 0.001 |
| Jasmonic acid | C12H19O3+ | 211.133 | 0.049 ± 0.012 | 0.039 ± 0.008 | 0.002 | 0.0103 ± 0.0026 | 0.0121 ± 0.0022 | < 0.001 |
| Methyl jasmonate | C13H21O3+ | 225.146 | 0.048 ± 0.008 | 0.107 ± 0.012 | 0.017 | 0.0099 ± 0.0016 | 0.0361 ± 0.0046 | < 0.001 |
| Benzenoids | 0.105 ± 0.015 | 0.259 ± 0.044 | < 0.001 | 0.0213 ± 0.0030 | 0.083 ± 0.013 | < 0.001 | ||
| Methyl benzoate | C8H9O2+ | 137.060 | 0.074 ± 0.011 | 0.089 ± 0.011 | 0.33 | 0.0151 ±0.0023 | 0.0312 ± 0.0043 | 0.004 |
| Methyl salicylate | C8H9O3+ | 153.055 | 0.058 ± 0.006 | 0.21 ± 0.05 | 0.006 | 0.0118 ± 0.0013 | 0.064 ± 0.015 | < 0.001 |
| Induced isoprenoids (isoprenoids other than isoprene) | 0.626 ± 0.045 | 1.65 ± 0.13 | < 0.001 | 0.129 ± 0.009 | 0.542 ± 0.042 | < 0.001 | ||
| Monoterpenes | C10H17+ | 137.133 | 0.367 ± 0.026 | 0.72 ± 0.09 | < 0.001 | 0.075 ± 0.005 | 0.237 ± 0.028 | < 0.001 |
| DMNT a | C11H19+ | 151.148 | 0.074 ± 0.008 | 0.25 ± 0.05 | 0.016 | 0.0152 ± 0.0017 | 0.081 ± 0.016 | 0.001 |
| Monoterpene alcohol | C10H19O+ | 155.143 | 0.139 ± 0.016 | 0.144 ± 0.006 | 0.46 | 0.0286 ± 0.0033 | 0.0467 ± 0.0028 | < 0.001 |
| Sesquiterpenes | C15H25+ | 205.195 | 0.075 ± 0.011 | 0.382 ± 0.048 | < 0.001 | 0.0152 ± 0.0023 | 0.128 ± 0.018 | < 0.001 |
| TMTT b | C16H27+ | 219.211 | 0.084 ± 0.015 | 0.182 ± 0.018 | < 0.001 | 0.0177 ± 0.0032 | 0.059 ± 0.005 | < 0.001 |
| BVOC sum | 2980 ± 150 | 18700 ± 1400 | < 0.001 | 610 ± 30 | 6150 ± 450 | < 0.001 | ||
(E)-4,8-dimethyl-1,3,7-nonatriene;
(E,E)-4,8,12-trimethyl-1,3,7,11-tridecatetraene.
Experimental Protocol
The measurement routine started with the empty cuvette (background) measurement during 5 minutes followed by careful insertion of the selected leaf in the cuvette. The leaf was stabilized under the measuring conditions until steady-state values of stomatal conductance (gs), net CO2 assimilation rate (A), and isoprene emission rate (m+/z = 69.070) were reached. This step usually lasted 30 to 40 minutes. Then the leaf was rapidly removed from the cuvette and a 7 mm cut was made with a razor blade either on leaf lamina avoiding leaf veins or transversally through the midrib. The midrib severance and lamina cuts were located distally 1/3 from the leaf base, where the site of midrib feeding is usually located (Delaney & Higley 2006). The leaf was immediately re-inserted into the cuvette, clamping exactly the same part of the leaf measured previously. Thereafter, the BVOC release was recorded during the following ten minutes. Finally, the leaf was removed and the empty cuvette background emissions were recorded again. A given leaf was only used once for the mechanical wounding procedure. A total of 35 lamina cuts and 15 midrib cuts were performed, altogether 50 leaves were used.
The wound-induced BVOC emission burst occurs right after the cuts are performed, but the actual BVOC measurements began a few seconds later when the leaf was re-clamped in the cuvette. Therefore, in order to integrate the whole burst peak, the actual BVOC emission data recorded after the re-clamping was modelled by a bi-Gaussian function as in Portillo-Estrada et al. (2015) and this function was extrapolated to the data missing period, that corresponded to < 10 % of the total BVOCs released during the emission burst. Control measurements, where leaves were removed from the leaf cuvette for a similar period than for the wounding experiments and then re-inserted, were done for several leaves. The control measurements resulted in a rapid stabilization of leaf water exchange, photosynthesis rate, and no wound-induced BVOC release.
The wound-induced BVOC emissions from both, lamina and midrib, were normalized by the surface of open wound generated (mm2) to allow a comparison between both types of tissue. Thus, we estimated the wound area of lamina cuts by approximating their area to a rectangle, i.e. by multiplying the lamina thickness by the cut length. In the case of midrib wounding, we considered also the area of the midrib section, that was approximated to a circle (where midrib thickness was taken as the circle diameter). Wound-induced BVOC emission burst data were used to estimate the integrated BVOC emission per wound surface during the first minutes after wounding (nmol mm−2) and the maximum BVOC emission rate per wound surface (pmol mm−2 s−1). In addition, for comparison with previous studies we reported the BVOC emissions also on the basis of mm of cut length.
Results
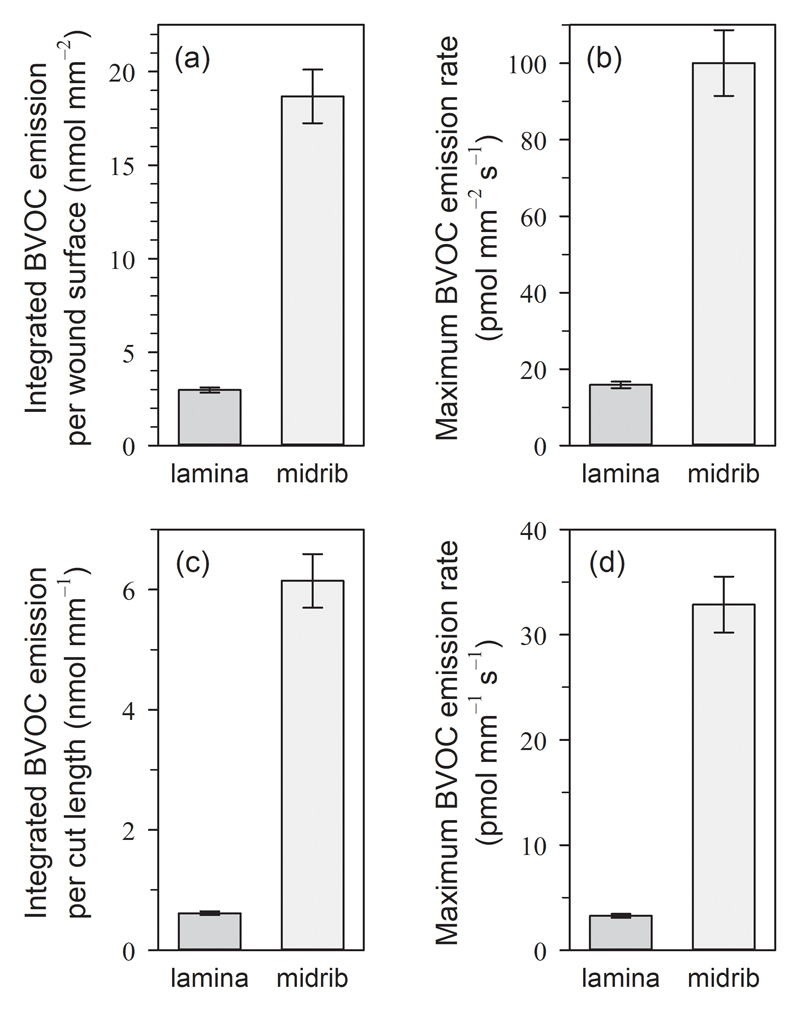
The midrib cuts resulted in much stronger BVOC emissions at a given wound surface (mm2) than lamina cuts for the total integrated BVOC emission burst (P < 0.001, Student’s t-test; Fig. 1a) as well as for the maximum BVOC emission rate recorded within the emission burst (peak emission): 17.9 ± 1.2 pmol mm−2 s−1 in lamina cuts and 99 ± 11 pmol mm−2 s−1 (P < 0.001; Fig. 1b). If considering only the cut length (mm), the difference between the total amounts of BVOCs released from both cut types became even higher (10-fold; P < 0.001): 0.610 ± 0.030 nmol mm−1 for lamina cuts and 6.15 ± 0.45 nmol mm−1 for midrib cuts (Fig. 1c); as well as the difference between maximum emission rates (Fig. 1d).
Figure 1.
Quantitative differences in the (a,c) total wound-induced BVOC blend emitted during seven minutes and (b,d) maximum emission rates after (dark grey) straight cuts to the leaf lamina and (light grey) transversal cuts across the midrib of mature leaves of aspen (Populus tremula). The values (average ± SE, n = 35 for lamina cuts and n = 15 for midrib cuts) refer to the (a,b) open wound surface exposed (in mm2) and (c,d) the wound length (in mm), calculated from the leaf lamina thickness, midrib diameter, and cut length. Note the different scales and units used in the y-axes.
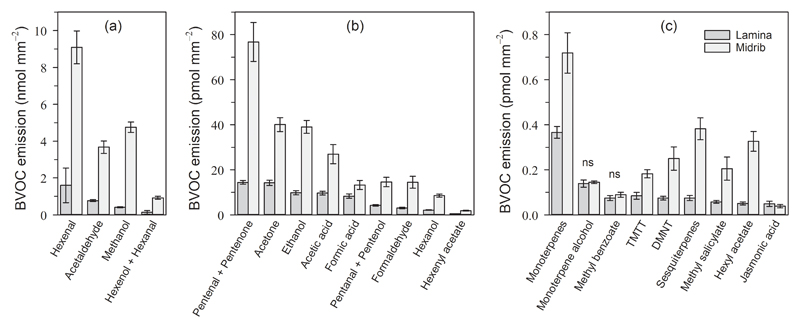
The lamina and midrib wound-induced volatile blends were composed of a wide spectrum of BVOCs (Table 1). In both cut types, the BVOC blend was dominated by the emission of hexenal (m+/z 99.080), acetaldehyde (m+/z 45.034) and methanol (m+/z 33.034) (Fig. 2a, Table 1), amounting together to 93.2 % of the total emission for lamina and 93.8 % for midrib cuts. As a general trend, the emission per wound surface of all BVOCs was significantly higher in midrib cuts compared to lamina cuts (Fig. 2, Table 1); with the exception of methyl benzoate (m+/z 137.160), monoterpene alcohol (m+/z 155.143), and jasmonic acid (m+/z 211.133) (Fig.2c, Table 1). Methanol emission was particularly high for midrib cuts (11.7 times higher than for lamina cuts; P < 0.001) while the emission of other LOCs and LOXs products were usually three- to six-fold higher for midrib cuts. The difference between lamina and midrib wound-induced BVOC emissions was generally higher when referring the emissions to cut length instead to wound surface area (Table 1).
Figure 2.
Quantitative differences in the wound-induced BVOC blend emitted during seven minutes after straight cuts to the leaf lamina (in dark grey) and transversal cuts across the midrib (in light grey) of mature leaves of aspen (Populus tremula). The values (average ± SE, n = 35 for lamina cuts and n = 15 for midrib cuts) refer to the open wound surface exposed (in mm2), calculated from the leaf lamina thickness, midrib diameter, and cut length. Note the different scales and units used in the y-axes. P-values after Mann-Whitney U tests between lamina and midrib cuts were < 0.05 in most of cases, otherwise they were considered non-significant “ns”. TMTT = (E,E)-4,8,12-trimethyl-1,3,7,11-tridecatetraene. DMNT = (E)-4,8-dimethyl-1,3,7-nonatriene. More information on the molecular formulae of the compounds, P-values, and exact emission values are provided in Table 1.
The mechanical wounding forced water loss; quantitatively assessed as the stomatal conductance for water vapour (gs). The increase in gs was remarkable after the wounding compared to the pre-wounding conditions, in particular, it was much higher for midrib cuts (21.1 ± 1.9 %) than for lamina cuts (12.4 ± 0.9 %, P < 0.001). However, we did not find evidence of correlation between the degree of water loss and the magnitude of BVOC emission rates. Leaf isoprene emission rate decreased by 10.5 ± 2.5 % (P < 0.001, paired t-test) when comparing emissions before and after lamina cuts and by 25.1 ± 4.2 % (P < 0.001, paired t-test) in the case of midrib cuts. The effect of midrib cuts on isoprene emission was greater than the effect of lamina cuts (P < 0.003, t-test).
Discussion
Although both mechanical wounding types applied in this experiment resulted in cellular damage, the sites of leaf damaged differ in structure and function and so do BVOC emissions, both qualitatively and quantitatively. A razor blade cut results in direct exposure of the broken cell walls and the cytoplasmic and vacuolar contents to oxidation by atmospheric air. It generates the conditions for triggering the action of lipoxygenase (LOX) enzymes and the synthesis and subsequent emission of LOX pathway products (Fall et al. 1999; Fall et al. 2001) as well as the emission of BVOCs contained in cell reservoirs. In addition, cutting through the leaf midrib also exposes the contents of plant vascular bundles (xylem and phloem) to the air. This can lead to release of specific vascular-contained volatiles, e.g. ethanol, that is produced in the roots in anoxic conditions and then transported to the leaves through the xylem. Likewise, the leaf midrib contains a high density of relatively small cells, among those collenchyma cells, that have thick cell walls, and lignified xylem cells. Therefore, the cross section cut of a leaf midrib exposes a higher amount of cell walls, and likely also plamamembranes per unit cut surface area than a cut through a leaf lamina that has larger and less densely packed cells. This can explain why the BVOC emissions related to membrane degradation and cell wall metabolism were higher in midrib cutting treatments. In particular, emissions of methanol were strongly enhanced. Methanol release occurs due to pectin demethylation and activation of pectin methylesterases with associated release of methanol as one of the first stress reactions (Pelloux et al. 2007). Furthermore, LOX products derived from the degradation of fatty acids of the cell membranes are characteristic stress volatiles emitted once there is membrane-level damage. However, methanol in cell walls can strongly accumulate in the leaf liquid phase (Niinemets et al. 2004), and part of the rapid methanol release might result from the release of methanol stored in the leaf liquid phase. Nevertheless, the water solubility of acetaldehyde (Henry’s law constant of 7.00 Pa m3 mol−1) is much smaller than of methanol (0.461 Pa m3 mol−1), thus the high emission level of acetaldehyde certainly reflects de novo synthesis upon wounding. In addition, the high degree of elicitation of BVOC emission from midrib cuts might reflect generally higher capacity for formation of reactive oxygen species in leaf veins, as demonstrated in several plant species (Beneloujaephajri et al. 2013).
The increased water loss upon leaf wounding, also observed by Brilli et al. (2011) and Portillo-Estrada et al. (2015), reflects the generation of a free liquid surface for evaporation that was particularly large for midrib cuts where vascular bundles were exposed to air. Moreover, the increased leaf transpiration rate can also indicate a disproportionately greater desiccation stress developing rapidly downstream of the cut with associated loss of turgor and impairment of leaf physiological functioning (Sack et al. 2004), so-called Ivanov effect (Moldau et al. 1993). In fact, the mechanical tissues in midribs are typically lignified (Niinemets 1999) and might not be that active in generating the stress volatile release, but once the midrib is cut, water availability rapidly decreases in lamina area downstream the cut and might generate a secondary stress to leaf tissues with concomitant release of volatiles. In the case of methanol, stomatal opening downstream the cut due to loss of turgor (Moldau et al. 1993) can generate a methanol burst even without de novo methanol synthesis (Niinemets & Reichstein 2003).
In an ecological context, the cuts to the lamina resemble the damage done by herbivore skeletonizers and hole feeders such as some moth and beetle larvae, whilst the midrib cuts resemble the damage performed by free feeders such as large-sized caterpillars (e.g. notodontids), beetles, and katydids. Although herbivory damage of major veins is relatively less common compared to lamina feeding, it does occasionally occur, implying that it is important to gain mechanistic insight into differences in volatile release from veins and intercostal mesophyll tissues. This study demonstrated the rapid BVOC release in response to wounding of both lamina and midrib and highlights the much higher release of volatiles per wound surface in cuts through the midrib, especially regarding methanol emission.
Acknowledgements
We gratefully acknowledge the help of Dr. Taras Kazantsev during the data collection. This work was supported by the Estonian Ministry of Science and Education [institutional grant IUT-8-3], the European Commission through the European Regional Fund [Center of Excellence EcolChange], the European Research Council [advanced grant 322603, SIP-VOL+] and the European Social Fund ESF [MJD 438]. Further support was provided by the Methusalem funding of the Flemish Community through the Research Council of the University of Antwerp as well by the Flemish Science Foundation (FWO, Brussels).
References
- Arnold T, Appel H, Patel V, Stocum E, Kavalier A, Schultz J. Carbohydrate translocation determines the phenolic content of Populus foliage: a test of the sink-source model of plant defense. New Phytologist. 2004;164:157–164. doi: 10.1111/j.1469-8137.2004.01157.x. [DOI] [PubMed] [Google Scholar]
- Beneloujaephajri E, Costa A, L’Haridon F, Métraux J-P, Binda M. Production of reactive oxygen species and wound-induced resistance in Arabidopsis thaliana against Botrytis cinerea are preceded and depend on a burst of calcium. BMC Plant Biology. 2013;13:160. doi: 10.1186/1471-2229-13-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brilli F, Ruuskanen TM, Schnitzhofer R, Müller M, Breitenlechner M, Bittner V, Wohlfahrt G, Loreto F, Hansel A. Detection of plant volatiles after leaf wounding and darkening by proton transfer reaction "time-of-flight" mass spectrometry (PTR-TOF) PloS ONE. 2011;6:e20419. doi: 10.1371/journal.pone.0020419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappellin L, Karl T, Probst M, Ismailova O, Winkler PM, Soukoulis C, Aprea E, Mark TD, Gasperi F, Biasioli F. On quantitative determination of volatile organic compound concentrations using proton transfer reaction time-of-flight mass spectrometry. Environmental Science & Technology. 2012;46:2283–2290. doi: 10.1021/es203985t. [DOI] [PubMed] [Google Scholar]
- Copolovici L, Pag A, Kännaste A, Bodescu A, Tomescu D, Copolovici D, Soran ML, Niinernets Ü. Disproportionate photosynthetic decline and inverse relationship between constitutive and induced volatile emissions upon feeding of Quercus robur leaves by large larvae of gypsy moth (Lymantria dispar) Environmental and Experimental Botany. 2017;138:184–192. doi: 10.1016/j.envexpbot.2017.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney KJ, Higley LG. An insect countermeasure impacts plant physiology: midrib vein cutting, defoliation and leaf photosynthesis. Plant Cell and Environment. 2006;29:1245–1258. doi: 10.1111/j.1365-3040.2006.01504.x. [DOI] [PubMed] [Google Scholar]
- Dudareva N, Negre F, Nagegowda DA, Orlova I. Plant volatiles: Recent advances and future perspectives. Critical Reviews in Plant Sciences. 2006;25:417–440. [Google Scholar]
- Dussourd DE. Do canal-cutting behaviours facilitate host-range expansion by insect herbivores? Biological Journal of the Linnean Society. 2009;96:715–731. [Google Scholar]
- Dussourd DE. Theroa zethus caterpillars use acid secretion of anti-predator gland to deactivate plant defense. PloS ONE. 2015;10 doi: 10.1371/journal.pone.0141924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dussourd DE. Behavioral sabotage of plant defenses by insect folivores. Annual Review of Entomology. 2017;62:15–34. doi: 10.1146/annurev-ento-031616-035030. [DOI] [PubMed] [Google Scholar]
- Dussourd DE, Peiffer M, Felton GW. Chew and spit: tree-feeding notodontid caterpillars anoint girdles with saliva. Arthropod-Plant Interactions. 2016;10:143–150. [Google Scholar]
- Fall R, Karl T, Hansel A, Jordan A, Lindinger W. Volatile organic compounds emitted after leaf wounding: on-line analysis by proton-transfer-reaction mass spectrometry. Journal of Geophysical Research-Atmospheres. 1999;104:15963–15974. [Google Scholar]
- Fall R, Karl T, Jordon A, Lindinger W. Biogenic C5 VOCs: release from leaves after freeze-thaw wounding and occurrence in air at a high mountain observatory. Atmospheric Environment. 2001;35:3905–3916. [Google Scholar]
- Ganong CN, Dussourd DE, Swanson JD. Girdling by notodontid caterpillars: distribution and occurrence. Arthropod-Plant Interactions. 2012;6:621–633. [Google Scholar]
- Heil M. Herbivore-induced plant volatiles: targets, perception and unanswered questions. New Phytologist. 2014;204:297–306. [Google Scholar]
- Helmus MR, Dussourd DE. Glues or poisons: which triggers vein cutting by monarch caterpillars? Chemoecology. 2005;15:45–49. [Google Scholar]
- Kishimoto K, Matsui K, Ozawa R, Takabayashi J. Direct fungicidal activities of C6-aldehydes are important constituents for defense responses in Arabidopsis against Botrytis cinerea. Phytochemistry. 2008;69:2127–2132. doi: 10.1016/j.phytochem.2008.04.023. [DOI] [PubMed] [Google Scholar]
- Mescher MC. Manipulation of plant phenotypes by insects and insect-borne pathogens. In: Hughes DP, Brodeur J, Thomas F, editors. Host manipulation by parasites. University of Oxford Press; Oxford, UK: 2012. pp. 73–92. [Google Scholar]
- Moldau H, Wong S-C, Osmond CB. Transient depression of photosynthesis in bean leaves during rapid water loss. Australian Journal of Plant Physiology. 1993;20:45–54. [Google Scholar]
- Niinemets Ü. Differences in chemical composition relative to functional differentiation between petioles and laminas of Fraxinus excelsior. Tree Physiology. 1999;19:39–45. doi: 10.1093/treephys/19.1.39. [DOI] [PubMed] [Google Scholar]
- Niinemets Ü, Loreto F, Reichstein M. Physiological and physicochemical controls on foliar volatile organic compound emissions. Trends in Plant Science. 2004;9:180–186. doi: 10.1016/j.tplants.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Niinemets Ü, Reichstein M. Controls on the emission of plant volatiles through stomata: a sensitivity analysis. Journal of Geophysical Research - Atmospheres. 2003;108:4211. [Google Scholar]
- Pelloux J, Rustérucci C, Mellerowicz EJ. New insights into pectin methylesterase structure and function. Trends in Plant Science. 2007;12:267–277. doi: 10.1016/j.tplants.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Portillo-Estrada M, Kazantsev T, Niinemets Ü. Fading of wound-induced volatile release during Populus tremula leaf expansion. Journal of Plant Research. 2017;130:157–165. doi: 10.1007/s10265-016-0880-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portillo-Estrada M, Kazantsev T, Talts E, Tosens T, Niinemets Ü. Emission timetable and quantitative patterns of wound-induced volatiles across different leaf damage treatments in aspen (Populus tremula) Journal of Chemical Ecology. 2015;41:1105–1117. doi: 10.1007/s10886-015-0646-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portillo-Estrada M, Zenone T, Arriga N, Ceulemans R. Contribution of volatile organic compound fluxes to the ecosystem carbon budget of a poplar short-rotation plantation. Global Change Biology Bioenergy. 2018 doi: 10.1111/gcbb.12506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph SG. Studying Populus defenses against insect herbivores in the post-genomic era. Critical Reviews in Plant Sciences. 2009;28:335–345. [Google Scholar]
- Sack L, Streeter CM, Holbrook NM. Hydraulic analysis of water flow through leaves of sugar maple and red oak. Plant Physiology. 2004;134:1824–1833. doi: 10.1104/pp.103.031203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scala A, Allmann S, Mirabella R, Haring MA, Schuurink RC. Green leaf volatiles: a plant's multifunctional weapon against herbivores and pathogens. International Journal of Molecular Sciences. 2013;14:17781–17811. doi: 10.3390/ijms140917781. [DOI] [PMC free article] [PubMed] [Google Scholar]