Abstract
Background
Congenital myasthenic syndrome (CMS) due to mutations in GMPPB has recently been reported confirming the importance of glycosylation for the integrity of neuromuscular transmission.
Methods
Review of case notes of patients with mutations in GMPPB to identify the associated clinical, neurophysiological, pathological and laboratory features. In addition, serum creatine kinase (CK) levels within the Oxford CMS cohort were retrospectively analysed to assess its usefulness in the differential diagnosis of this new entity.
Results
All patients had prominent limb-girdle weakness with minimal or absent craniobulbar manifestations. Presentation was delayed beyond infancy with proximal muscle weakness and most patients recall poor performance in sports during childhood. Neurophysiology showed abnormal neuromuscular transmission only in the affected muscles and myopathic changes. Muscle biopsy showed dystrophic features and reduced α-dystroglycan glycosylation. In addition, myopathic changes were present on muscle MRI. CK was significantly increased in serum compared to other CMS subtypes. Patients were responsive to pyridostigimine alone or combined with 3,4-diaminopyridine and/or salbutamol.
Conclusions
Patients with GMPPB-CMS have phenotypic features aligned with CMS subtypes harbouring mutations within the early stages of the glycosylation pathway. Additional features shared with the dystroglycanopathies include myopathic features, raised CK levels and variable mild cognitive delay. This syndrome underlines that CMS can occur in the absence of classic myasthenic manifestations such as ptosis and ophthalmoplegia or facial weakness, and links myasthenic disorders with dystroglycanopathies. This report should facilitate the recognition of this disorder, which is likely to be underdiagnosed and can benefit from symptomatic treatment.
Introduction
Congenital myasthenic syndromes (CMS) are disorders caused by mutations in genes encoding proteins that are essential for maintaining the integrity of neuromuscular transmission.1 All syndromes share the clinical features of fatigable weakness, but age at onset, presenting symptoms, distribution of weakness and response to treatment vary depending on the molecular mechanism resulting from the genetic defect. At present, mutations in at least 21 different genes are known to cause CMS.2 Most of these genes encode proteins with a direct role in neuromuscular transmission, such as the acetylcholine receptor (AChR) subunits, and in the development or maintenance of the neuromuscular junction (NMJ) integrity such as DOK7.3 4 More recently, an unexpected relationship between defects in the glycosylation of proteins and CMS was found by using next-generation sequencing technology.5–7 Mutations in ALG2, ALG14,5 DPAGT16 and GFPT17 were shown to produce a CMS with a predominantly limb-girdle pattern of muscle weakness, and minimal facial and ocular involvement.
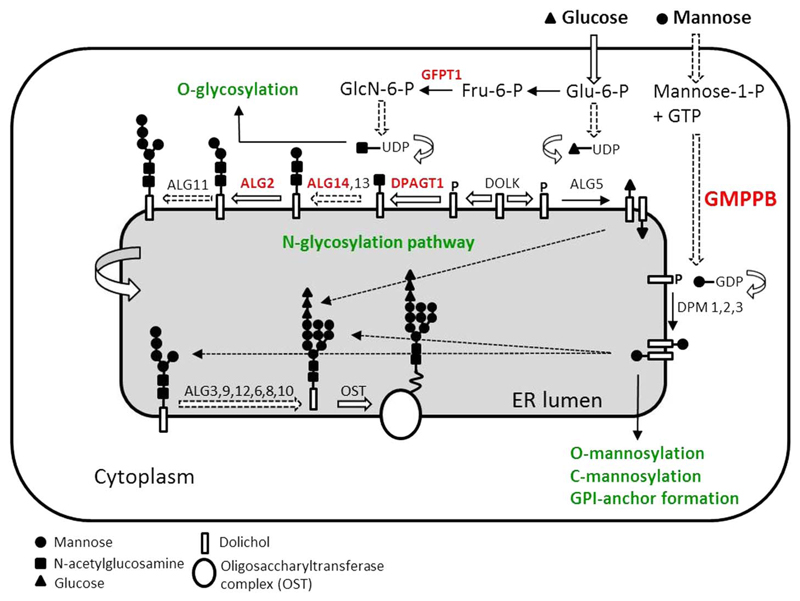
Glycosylation is a ubiquitous pathway defined by the attachment of sugar moieties to the polypeptide backbone (figure 1). Post-translational modification of proteins is crucial for protein folding, transport and functionality.8 In CMS, one suggested mechanism is through glycosylation of AChR subunits, which is required for the correct assembly of AChR pentamers and for efficient export to the cell surface;9 thus, abnormal glycosylation results in a reduction of AChRs at the muscle end plates and a reduced synaptic response to acetylcholine.7 Many other key NMJ proteins, such as MuSK, agrin, integrins and neural cell adhesion molecules (NCAM) are glycosylated10 and therefore, their abnormal glycosylation could also contribute to the pathogenic effect. It is not known why defects in this ubiquitous process result in a dysfunction mainly restricted to the NMJ.
Figure 1.
Schematic representation of the N-glycosylation pathway and CMS-causing genes (red colour). Initial steps of N-linked protein glycosylation take place in the ER. It starts with the assembly of the core glycan on the lipid dolichol, and follows the incorporation of sugar moieties by a series of glycosyltransferases, first on the cytoplasmic face of the ER and then in the ER lumen, once the product is flipped. The core glycan is then released from dolichol and transferred to asparagine residues of nascent proteins by the multimeric oligosaccharyl transferase complex (OST). GMPPB catalyses the formation of GDP-mannose from GTP and mannose-1-phospate; GFPT1 synthesises UDP-GlcNAc (Uridine diphosphate N-acetylglucosamine). While ALG2, ALG13/14 and DPAGT1 are glycosyltransferases implicated specifically in the N-linked glycosylation of proteins, GFPT1 and GMPPB are also involved in other glycosylation pathways (green). Of note, GMPPB is involved in the O-mannosylation pathway, which is required for the glycosylation of α-DG, and thus is of special relevance to the dystroglycanopathies. DOLK, dolichol kinase; DPM, dolichol-phosphate mannose synthase; Fru-6-P, fructose-6-phosphate; GlcN-6-P, glucosamine-6-phosphate; Glu-6-P, glucose-6-phosphate. CMS, congenital myasthenic syndrome; ER, endoplasmic reticulum.
GMPPB is a recently reported CMS-associated gene.11 GMPPB encodes GDP-mannose pyrophosphorylase B that catalyses the conversion of mannose-1-phosphate and GTP to GDP-mannose. GMPPB contributes to both the N-glycosylation and the O-mannosylation pathways (figure 1). Mutations within the O-mannosylation pathway were originally identified in patients with a form of muscular dystrophy within the spectrum of dystroglycanopathies.12 These disorders are characterised by reduction in α-dystroglycan glycosylation, which is the most well characterised and functionally relevant O-mannosylated protein. Patients with autosomal recessive mutations in GMPPB had reduced glycosylation of α-dystroglycan in skeletal muscle and variable degrees of clinical severity ranging from congenital muscular dystrophies with associated structural brain involvement to milder limb-girdle muscular dystrophy variants of late childhood onset.13 Recently, we showed that autosomal recessive mutations in GMPPB can also cause CMS and bridge myasthenic disorders with dystroglycanopathies.11 This increased the spectrum of disorders caused by GMPPB mutations and confirmed the importance of glycosylation for the integrity of neuromuscular transmission.
Here we describe in detail the clinical features and investigation results for eight patients with CMS due to mutations in GMPPB and investigate CMS syndrome-specific CK values within our cohort to confirm its usefulness in the differential diagnosis.
Methods
Next-generation sequencing and Sanger screening identified the underlying genetic mutations in GMPPB in eight individuals from six different kinships (table 1) with similar clinical features and a decremental response of compound muscle action potentials to repetitive nerve stimulation, as described previously.11 Cases 9–11, present in the report by Belaya et al,11 who have a muscular dystrophy dystroglycanopathy due to mutations in GMPPB but no clear defect in neuromuscular transmission are not included. Here we reviewed the case notes of these patients with GMPPB-CMS in order to produce a detailed clinical picture to allow improved recognition of this condition. We also retrospectively obtained the serum creatine kinase (CK) values in the Oxford CMS cohort to confirm that measuring CK can be useful in differentiating GMPPB-CMS from other CMS subtypes (using the local upper limit of normal range 200 IU/L).
Table 1. Clinical features of patients with GMPPB CMS.
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | Case 8 | |
|---|---|---|---|---|---|---|---|---|
| Mutation, DNA | c.79G>C c.859C>T |
c.781C>T c.130-3C>G |
c.79G>C c.760G>A |
c.79G>C c.907C>T |
c.559C>T c.578T>C |
c.308C>T homozyg |
c.308C>T homozyg |
c.308C>T homozyg |
| Mutation, protein | p.Asp27His p. Arg287Trp |
p.Arg261Cys Splicing |
p.Asp27His p.Val254Met |
p.Asp27His p.Leu303Phe |
p.Gln187* p.Ile193Thr |
p.Pro103Leu | p.Pro103Leu | p.Pro103Leu |
| Gender | F | F | F | F | F | F | F | M |
| Age (year) | ||||||||
| Presentation | 24 | 15 | 20 | 25 | 1.5 | 16 | 22 | 31 |
| Current | 48 | 68 | 28 | 44 | 18 | 43 | 45 | 35 |
| Presenting symptoms | Limited march tolerance (following influenza) | Unable to run. | Unable to get up from the floor and lift weights. | Unable to climb up ramps. | Acute episode of weakness | Difficulty in climbing stairs, cramps. | Difficulty in climbing stairs, cramps. | Difficulty in climbing stairs, cramps. |
| Ptosis | No | No | No | Mild, not fatigable* | No | Mild | No | No |
| Ophthalmoplegia | No | No | No | No | No | Mild | No | No |
| Facial weakness | No | No | No | No | No | No | No | No |
| Bulbar weakness | No | No | No | No | No | Mild | No | No |
| Neck weakness | Mild | No | Mild | Mild | Moderate | No | No | No |
| Proximal weakness | ||||||||
| UL | Moderate | Moderate | Moderate | Moderate | Moderate | Mild | Mild | No |
| LL | Severe | Severe | Moderate | Moderate | Severe | Moderate | Moderate | Mild |
| Distal weakness | ||||||||
| UL | No | No | No | No | Mild | No | No | No |
| LL | No | No | No | No | Mild | No | No | No |
| Axial weakness | Moderate | Moderate | No | Mild | Severe | Severe | Severe | No |
| Respiratory weakness | No | No | No | No | No | No | No | No |
| Cognitive impairment/learning difficulties | No | No | Mild | No | No | No | No | No |
| Other features | Cramps | Calves hypertrophy | Cataracts Cramps | Cramps | Cramps | Cramps | ||
| Treatment | P, D, S | P, S | P | P, S | P | P | P | P |
Ptosis appeared at age 26 years after developing thyrotoxicosis and receiving treatment for it.
D, 3,4-DAP; GMPPB CMS, GMPPB congenital myasthenic syndrome; LL, lower limbs; P, pyridostigmine; S, salbutamol; UL, upper limbs.
Patients
Patients 1–5 are white European originating from the UK. Patients 6–8 belong to the same kinship and originate from Iran. Consent for publication of data was obtained from all patients. Ethics approval for analysis of DNA and tissue samples was obtained (OXREC B: 04.OXB.017 and Oxfordshire REC C 09/H0606/74).
Results
Clinical features
Clinical presentation
Pregnancy and early-life features were uneventful in all patients except in case 4 with congenital hip dislocation and resulting delayed motor milestones. None of the patients were ventilated as infants or had feeding difficulties. The median age of onset was 22 years (range 1.5–50 years). Case 1 presented at age 24 years with limited walking tolerance following an influenza illness. Previously her gait was unrestricted. Case 2 was unable to run during her teens and had lumbar hyperlordosis from her 20s. Case 3 was unable to get up off the floor and had difficulty lifting heavy objects at age 20 years. She also had decreased walking tolerance and had to rest after 5 min of walking. Case 4 had difficulty climbing-up ramps and walking long distances from her mid-20s. Case 5 presented at 18 months with an acute episode of generalised weakness and persistently raised CK. Subsequently, she remained clumsier and slow at school compared to peers. Cases 6–8 presented with gait disturbance (waddling), difficulty climbing stairs and muscle cramps at the ages of 16, 22 and 31 years, respectively. Of note, even though most patients had onset in adult life, they recall being slower than peers at school and not good at sports.
Pattern of weakness at assessment
All patients had a predominantly limb-girdle pattern of muscle weakness, which was more severe in the lower than in the upper limbs, with moderate to severe impairment and marked waddling gait in some cases. Mild distal weakness was present only in cases 5 and 8. Facial and eye movements were normal in all patients, and only case 6 had mild fatigable ptosis. Two patients reported of occasional double vision (cases 2 and 6); however, no ophthalmoparesis was elicited during examination. Neck muscles were mild to moderately weak in four patients without manifest flexor/extensor preponderance. Axial weakness was present in six patients with moderate to severe lumbar hyperlordosis in five. Mild swallowing difficulties, in the absence of speech abnormalities, were reported by case 7. Only case 2 suffered from shortness of breath on exertion, although there was a background history of asthma. None of the patients have required respiratory support.
Additional clinical features
Episodes of muscle aches and cramps were reported (case 3, 5 and 6–8), mainly in the calves, which were activity-induced such as with walking. Cramps were occasional but variable in frequency depending on the period, and usually subsided by extending the affected muscle. Case 4 had hypertrophic calves and thighs on physical examination. Case 5 was diagnosed with cataracts at age 9. Contractures were found only in case 5 with tightness of Achilles tendons bilaterally. Unlike other myasthenic syndromes, most of the patients did not have easily demonstrable short-term fatigability of muscle strength. Case 3 had mild learning difficulties in school. None of the patients had known cardiac involvement.
Course of disease
The long-term course of disease was generally progressive with gradual deterioration in muscle strength and mobility over time. In addition, there were significant deteriorations during intercurrent illnesses. Case 1 currently walks only for short distances with assistance and requires physical assistance in terms of activities of daily living and self-care. Case 2, with onset in her teens, has been unable to climb stairs during the past decade, and has difficulties in standing from a seated position. Although she could lift loads during her adult life, she developed significant proximal arm weakness in her fifth decade. Case 3 is currently stable on treatment, and mainly finds it difficult to hold heavy objects and walk long distances. Case 4 had a waddling gait that became more pronounced over time, with a more recent requirement for a walking stick. Case 5 gradually deteriorated during childhood and adolescence with proximal weakness and mobility problems. Prior to treatment, she had limited walk tolerance, and on a bad day she could only walk around the house and used a wheelchair for distances. Cases 6–8 still walk independently although they have had progressive deterioration in their gait over the years. Fluctuations of muscle strength over the course of days and weeks were reported in cases 1–5. These were commonly related to intercurrent viral infections, although these also occurred following no particular precipitant (case 1 and 5). Cases 3, 4 and 6 reported significant worsening of symptoms with menstruation. Case 6 also reported worsening of symptoms during pregnancy.
Response to treatment
All patients treated with pyridostigmine had a positive response to treatment. The effect was notable in cases 1, 3, 5, 6, 7 and 8, but mild in cases 2 and 4. Case 3 increased her endurance so that she can walk up to 10 min without fatigue and is able to manage stairs at home independently. She recalls omitting her medication by accident on a few occasions and being much weaker afterwards. For Case 4, walking improved and she stopped using her walking stick. Her proximal strength in the upper and lower limbs is now graded Medical Research Council (MRC 5) from a previous MRC score of 4. Case 5 reported improvement in her muscle strength and walking speed, with less fatigability and reduced wheelchair use. After pyridostigimine, Case 6 improved her long distance walking, and improvements of ptosis and dysphagia were noted. Cases 1 reported additional benefit from 3,4-diaminopyridine (3,4 DAP) (30 mg/day) and salbutamol (0.1 mg/kg/day), and cases 2 and 4 reported benefit from additional salbutamol (0.05–0.2 mg/kg/day).
Investigations
Neurophysiology
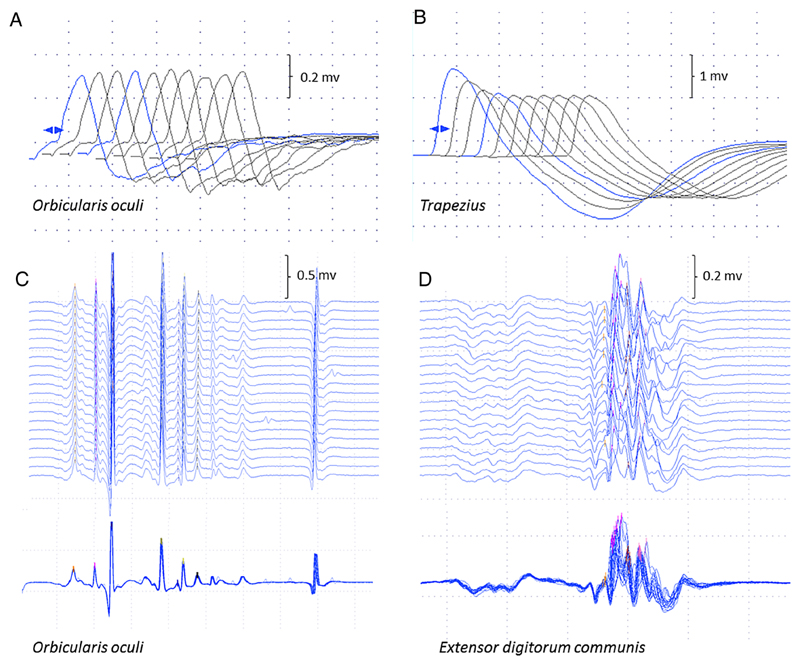
All patients had findings consistent with abnormal neuromuscular transmission on electrophysiological testing (table 2). Of note, repetitive nerve stimulation (RNS) and single fibre EMG (SFEMG) abnormalities were present only in proximal muscles, while testing of distal and facial muscles was normal in 4/4 cases tested, in keeping with the clinical absence of facial and distal involvement (figure 2). Repetitive discharges were not observed in any case. Concentric needle EMG examination of affected muscles showed mild to moderate myopathic changes in 7/7 patients tested. Of note, case 1 had 70% increment in compound motor action potential (CMAP) amplitude following voluntary contraction and high frequency stimulation. Mild post-exercise facilitation was also seen in cases 2, 3 and 6.
Table 2. Clinical investigations of patients with GMPPB CMS.
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | Case 8 | |
|---|---|---|---|---|---|---|---|---|
| RNS* Muscle Decrement (%) |
Anconeus (42%) | Anconeus (14%) | ADM (<10%) Anconeus (50%) |
ADM (<10%) Trapezius (30%) |
EDC (37%) FCU (17%) Nasalis (4%) OO (5%) Trapezius (25%) |
APB (<10%) Nasalis (<10%) Trapezius (20%) |
– | – |
| SFEMG | EDC Abnormal |
EDC Abnormal |
OO Normal EDC Abnormal |
EDC Abnormal |
OO Normal EDC Abnormal |
– | – | – |
| Cardiac studies | – | Normal EKG | – | Normal EKG | Normal echo | – | – | – |
| Myopathic EMG | Yes | Yes | Yes | Yes | Yes | Yes | – | – |
| CK (Normal≤200)† | 2800 | 418 | 1600 | 2668 | 4807 | 701 | – | – |
| Biopsy | ||||||||
| Muscle | Quadriceps | – | Quadriceps | Quadriceps | Quadriceps | – | – | – |
| Age | 27 | – | 25 | 35 | 10 | – | – | – |
| Features | Dystrophic | – | Dystrophic | Dystrophic | Dystrophic | – | – | – |
| α-DG | – | – | Reduced | Reduced | Reduced | – | – | – |
| Muscle MRI | ||||||||
| Age (year) | – | 66 | – | 42 | 8 | – | – | – |
| Result | – | Abnormal | – | Abnormal | Normal | – | – | – |
A decrement of more than 10% in CMAP amplitude indicates a defect in neuromuscular transmission.
The specific reference range for CK in serum depends on individual laboratories, but values greater than 200 are in general considered to be abnormal.
ADM, abductor digiti minimi; APB, abductor pollicis brevis; CK, creatine kinase; CMAP, compound motor action potential; EDC, extensor digitorum communis; FCU, flexor carpi ulnaris; OO, orbicularis oculi.
Figure 2.
Neurophysiological studies. Repetitive nerve stimulation in case 5 was normal in orbicularis oculi (A) compared to 25% decrement in trapezius (B) The stimulated SFEMG study of orbicularis oculi (C) was normal (mean consecutive difference 16.2 μs; 62% of the upper limit of normal) compared to the examination of extensor digitorum communis (D), which showed instability of the neuromuscular junction (mean consecutive difference 54.7 μs; 210% of the upper limit of normal), as seen in the superimposed traces. SFEMG, single fibre EMG.
Muscle biopsy
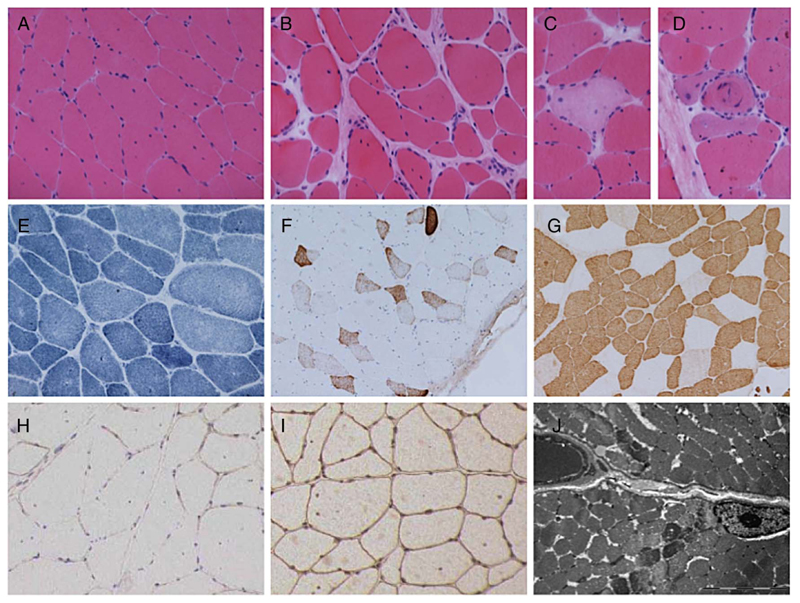
Cases 1, 3, 4 and 5 underwent muscle biopsy (table 2). All cases showed dystrophic features and reduced labelling for α-dystroglycan when immunohistochemical staining was performed. Secondary reduction of laminin-α2 chain on immunoblotting was noted in cases 3 and 4. Cases 3 and 5 showed sparse inflammation and focal upregulation of major histocompatibility complex I (MCH-I) complex, mostly within regenerating fibres. Tubular aggregates, which have previously been described in some forms of CMS due to glycosylation pathway defects, were not found in any of the samples. The muscle biopsy from case 3 is described in detail in figure 3A–I.
Figure 3.
Muscle biopsy. Muscle biopsy from quadriceps femoris in case 3 at age 25 years, showing variation in fibre size with occasional atrophic fibres and increase in internal nucleation with H&E staining (A). There is a focal increase in endomysial collagen (B), occasional necrotic fibres (C) and regenerating fibres (D). NADH staining shows mild disturbance of the internal architecture in a number of fibres (E). Numerous fibres express neonatal myosin (F). There is a pattern of slow fibre predominance with these being of generally small size (G). Immunohistochemical staining shows reduced labelling for α-dystroglycan (H) compared to β-dystroglycan (I). Ultrastructural examination shows lack of accumulation of granular material, rimmed vacuoles or tubular aggregates (J). Range of fibre size A-I=30–120 μm; bar in J=5 μm. NADH, nicotinamide adenine dinucleotide hydrogen.
Muscle MRI
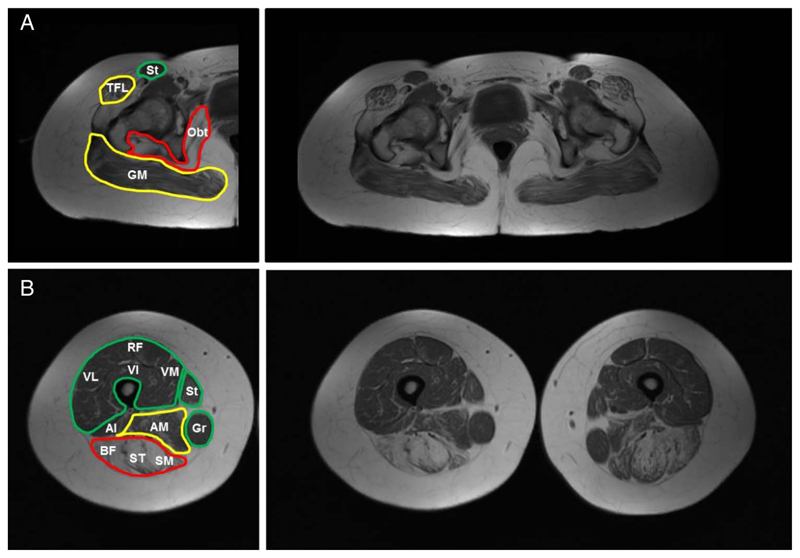
Muscle MRI from cases 2 and 4, at age 66 and 42 years, respectively, showed extensive changes in T1-weighted sequences in pelvis and thighs, with predominant involvement of the posterior compartment of the thighs and relative sparing of certain muscle groups (figure 4A, B). Case 2 had MRI of the calves that showed additional involvement.11 Case 5 had a normal muscle MRI at age 10 years.
Figure 4.
Muscle MRI. Individual muscles are labelled on the left side of the figure and coloured according to severity into spared/mild (green), moderate (yellow) and severe (red); complete T1 weighted sequences are shown on the right side. In the pelvis, there is prominent involvement of the gluteal muscles, tensor fasciae latae and obturator internus muscles (A). In the thighs, there is prominent involvement of the hamstrings and adductors and relative sparing of the anterior thigh musculature, sartorius and gracilis muscles (B). Al, adductor longus; AM, adductor magnus; BF, biceps femoris; GM, gluteus maximus; Gr, Gracillis; Obt, obturator internus; RF, rectus femoris; SM, semimembranosus; ST, semitendinosus; St, sartorious; TFL tensor fascia latae; VI, vastus intermedius; VL, vastus lateralis: VM, vastus medialis.
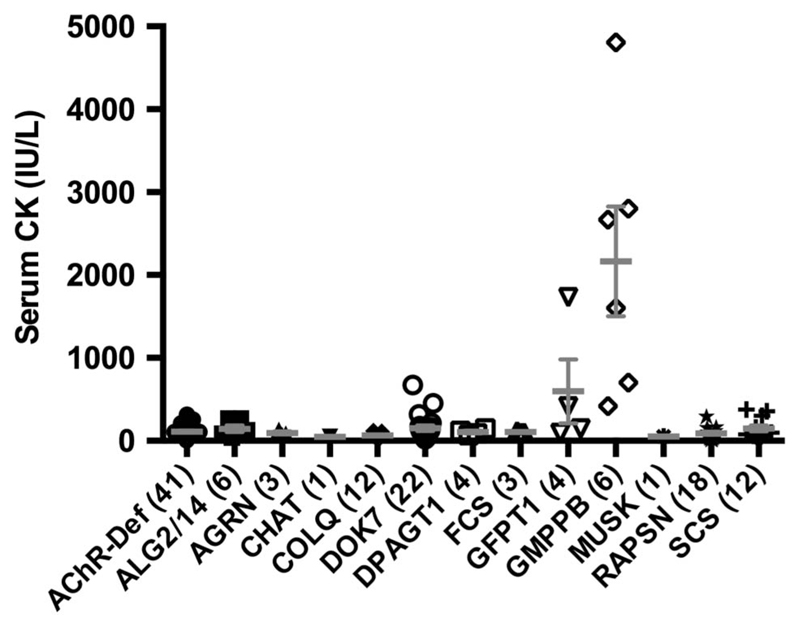
Serum CK
We reviewed the serum CK values of 133 available patients with CMS (figure 5). All patients with GMPPB-CMS tested had raised CK levels in serum (2350±660 UI/L; range 418–4807 UI/L). The mean serum CK value of patients with GMPPB-CMS was 10.74 times above the upper limit of normal and was significantly higher than in any other CMS subtype (one-way ANOVA; p<0.001, Dunnett’s multiple comparison test). Occasionally, patients with DOK7-CMS, Slow Channel syndrome and GFPT1-CMS had modestly raised CK values.
Figure 5.
CMS subtype-specific serum creatine kinase (CK) levels. The figure shows individual serum CK values for each CMS subtype together with mean±SEM for each CMS subtype. Patients with GMPPB-CMS had greater CK values in serum than any other CMS subtype (one-way ANOVA; p<0.001, Dunnett’s multiple comparison test). Numbers of patients included from each CMS category are displayed in brackets. The local upper limit of normal range 200 was IU/L. Mean±SD values are provided as supplementary data (see online supplementary table S1). ANOVA, analysis of variance; CMS, congenital myasthenic syndrome; SEM, standard error of the mean.
Discussion
We describe the clinical and complementary features of a new CMS subtype due to mutations in GMPPB, and show that high serum CK values are a key marker for differential diagnosis from other CMS. GMPPB-CMS shares the features of fatigable muscle weakness, significant decrement on RNS and a positive response to cholinesterase inhibitors, which is typical for the majority of patients with CMS. However, there are features that may help differentiate it from the more common CMS subtypes: (1) a later clinical onset; (2) presentation with proximal muscle weakness and, unlike many CMS, with sparing of ocular, bulbar and respiratory muscles; and (3) the presence of additional clinical features such as raised CK, dystrophic changes on muscle biopsy, activity-induced muscle cramps and variable association with cognitive impairment and cataracts.
Most, though not all of the more common forms of CMS, present earlier in life and have characteristic features such as severe ophthalmoplegia (AChR-mutations), and respiratory crises (RAPSN-CMS, CHAT-CMS and fast channel syndrome) and positive response to pyridostigmine. DOK7-CMS can have a late presentation with proximal weakness,14 but lacks the positive response to pyridostigmine and the dystrophic changes on muscle biopsy. Slow channel syndrome15 and DOK7-CMS16 can have a myopathic component with non-specific changes on muscle biopsy (such as increased variation in fibre size and corelike areas) and mildly raised CK levels, but these are rarely above five times the upper limit of normality. Cramps can also be seen in patients with DOK7-CMS, and may worsen on treatment with ß2-adrenergic agonists.
CMS due to other glycosylation pathway defects (GFPT1,7 DPAGT117 and ALG2/145) have clinical similarities to GMPPB-CMS, including presentation, pattern of weakness and response to treatment. However, GMPPB-CMS has a greater degree of myopathic involvement as shown by the higher CK levels and dystrophic changes on muscle biopsy. This is likely due to the additional role of GMPPB in the O-mannosylation pathway and glycosylation of α-dystroglycan. GFPT1, DPAGT1 and ALG2-CMS can have tubular aggregates on muscle biopsy.18 These have not been detected in GMPPB-CMS although electron microscopy, which is a more sensitive method to detect tubular aggregates, was only performed in one case. GFPT1-CMS can display modestly raised CK values,19 but in our experience these are not as high as seen in GMPPB-CMS. Extensive abnormalities in muscle MRI have also been seen in GFPT1, DPAGT1 and ALG2/14-CMS, but not consistently in the more common CMS subtypes; however, some patients with slow channel syndrome, and DOK7 and RAPSN mutations can show a wide range of fatty infiltration.20 It is, therefore, understandable that most of these disorders were classified previously as non-specific myopathies. Cases 3 and 5 were initially misdiagnosed as autoimmune myopathies and treated with immunosuppression without improvement. Serum CK levels are significantly higher in patients with GMPPB-CMS and therefore this will be helpful in the differential diagnosis. Biochemical studies of transferring glycosylation on serum samples have been found to be abnormal in only a few of our patients with DPAGT1 mutations,17 but could be abnormal in GMPPB-CMS. However, we believe that a combination of decrement of CMAP with repetitive nerve stimulation on EMG with measurement of serum CK will give a simpler and better diagnostic pointer.
We have not seen heart abnormalities in three patients with GMPPB-CMS undergoing cardiac studies. However, a small study showed mild cardiac abnormalities in patients with CMS with mutations in GFPT1 and DPAGT1, including abnormal electrocardiography (repolarisation and deep S waves or marked ventricular hypertrophy by voltage criteria), myocardial fibrosis, diastolic dysfunction and impaired energetics by cardiovascular magnetic resonance despite normal systolic function.21
Mutations in GMPPB underlie a wide clinical spectrum. At the severe end of the spectrum, mutations in GMPPB lead to CMD with eye and brain abnormalities (Walker-Warburg syndrome and muscle-eye-brain disease), and congenital LGMD and CMD with mental retardation.13 At the mild end of the spectrum, GMPPB mutations cause adult onset LGMD22 and CMS,11 with mild or absent intellectual disability and isolated rhabdomyolysis.22 In the middle, there are patients with onset during childhood with variable degrees of weakness and cognitive involvement.23 We have not observed a clear genotype/phenotype correlation and thus what drives the striking differences in the phenotype remains to be determined.
All patiens harbouring GMPPB mutations present with consistent muscle abnormalities. In skeletal muscle, the dystroglycan complex works as a link between the extracellular matrix and the cytoskeleton, providing structural integrity in muscle tissue.24 Post-translational glycosylation of α-dystroglycan is essential for its function and binding to extracellular ligands.25 A reduction in the glycosylation levels disrupts molecular interactions and contributes to sarcolemma instability and eventual muscle damage. In many cases, GMPPB mutations do not lead to abnormal neuromuscular transmission.11 Several patients with GMPPB-CMS had mild facilitation in RNS that could indicate additional presynaptic dysfunction. The clinical deterioration seen in GMPPB-CMS during intercurrent illnesses is also of interest. This is a classic feature of CMS, but clearly not unique to CMS as it is also seen in dystroglycanopathies due to FKTN mutations26 and other gene defects (F Muntoni, personal observation). The pathogenesis underlying this phenomenon is unknown.
In conclusion, we describe the clinical and complementary features of a new CMS subtype due to mutations in the glycosylation gene GMPPB. Patients with GMPPB-CMS have a comparable phenotype to other CMS subtypes with mutations affecting the early stages of the glycosylation pathway. These harbour additional features, shared with the dystroglycanopathies, include dystrophic changes on muscle biopsy and raised CK levels. We find that this CMS occurs in the absence of classic myasthenic manifestations, such as ptosis, ophthalmoplegia or facial weakness, and therefore is likely to be under-diagnosed. We would suggest performing RNS on weak muscles in patients with the phenotype we describe (or in patients in whom GMPPB mutations have been detected) to determine if there is involvement of neuromuscular transmission. This report adds to the expanding clinical spectrum of GMPPB mutations and should facilitate the recognition of a subset of this genetic disorder, which can be treated symptomatically.
Supplementary Material
Acknowledgements
The authors gratefully acknowledge the UK National Specialised Commissioning Team funding of the Diagnostic and Advisory service for CMS in Oxford. The support by the National Institute for Health Research Biomedical Research Centre at Great Ormond Street Hospital for Children NHS Foundation Trust and University College London Hospitals is also gratefully acknowledged.
Funding Medical Research Council (grant number MR/M006824/1).
Footnotes
Contributors PMRC was involved in the literature search, manuscript design and preparation. KB, SM, MS and WWL contributed to the genetic studies. RK and MP performed the neurophysiological studies. JLH characterised the muscle biopsy. KB, MEF, RP, TJW, AS, MP, HL, FM and JP were involved in the patients’ care. All authors contributed to the critical review and approval of the final manuscript. DB participated in the design of the study, had full access to all data in the study and had final responsibility for the decision to submit for publication.
Competing interests KB is a fellow of the Wellcome Trust-funded OXION: Ion Channels and Disease Initiative. JLH is supported by Myositis UK. FM has served on scientific advisory boards for Acceleron Pharma, Genzyme, AVI BioPharma, the Debiopharma Group, GlaxoSmithKline, Prosensa, Servier, Summit and Santhera Pharmaceutical. He receives research support from Trophos and Biomarin, PTC, Roche, Sarepta and Pfizer. He is a board advisor for Pfizer. JP is partly funded by highly specialised services to run a National congenital myasthenia service and a neuromyelitis optica service. She has received support for scientific meetings and honorariums for advisory work from Merck Serono, Biogen Idec, Novartis, Teva, Chugai Pharma, MedImmune, Alexion and Bayer Schering, and unrestricted grants from Merck Serono, Novartis, Biogen Idec, Teva, Genzyme and MS Society. Her hospital Trust receives funds for her role as clinical lead for the RSS, and she has received grants from the MS Society and Guthie Jackson Foundation for unrelated research studies. She is a board member for the charitable European MS foundation ‘The Charcot Foundation’ and on the steering committee for a European collaborative MS imaging group ‘MAGNIMS’. David Beeson holds MRC Programme Grant MR/M006824/1.
Provenance and peer review Not commissioned; externally peer reviewed.
References
- 1.Palace J, Beeson D. The congenital myasthenic syndromes. J Neuroimmunol. 2008;201–202:2–5. doi: 10.1016/j.jneuroim.2008.05.030. [DOI] [PubMed] [Google Scholar]
- 2.Rodríguez Cruz PM, Palace J, Beeson D. Inherited disorders of the neuromuscular junction: an update. J Neurol. 2014;261:2234–43. doi: 10.1007/s00415-014-7520-7. [DOI] [PubMed] [Google Scholar]
- 3.Beeson D, Higuchi O, Palace J, et al. Dok-7 mutations underlie a neuromuscular junction synaptopathy. Science. 2006;313:1975–8. doi: 10.1126/science.1130837. [DOI] [PubMed] [Google Scholar]
- 4.Ohno K, Engel AG, Shen XM, et al. Rapsyn mutations in humans cause endplate acetylcholine-receptor deficiency and myasthenic syndrome. Am J Hum Genet. 2002;70:875–85. doi: 10.1086/339465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cossins J, Belaya K, Hicks D, et al. Congenital myasthenic syndromes due to mutations in ALG2 and ALG14. Brain. 2013;136:944–56. doi: 10.1093/brain/awt010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belaya K, Finlayson S, Slater CR, et al. Mutations in DPAGT1 cause a limb-girdle congenital myasthenic syndrome with tubular aggregates. Am J Hum Genet. 2012;91:193–201. doi: 10.1016/j.ajhg.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zoltowska K, Webster R, Finlayson S, et al. Mutations in GFPT1 that underlie limb-girdle congenital myasthenic syndrome result in reduced cell-surface expression of muscle AChR. Hum Mol Genet. 2013;22:2905–13. doi: 10.1093/hmg/ddt145. [DOI] [PubMed] [Google Scholar]
- 8.Moremen KW, Tiemeyer M, Nairn AV. Vertebrate protein glycosylation: diversity, synthesis and function. Nat Rev Mol Cell Biol. 2012;13:448–62. doi: 10.1038/nrm3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gehle VM, Walcott EC, Nishizaki T, et al. N-glycosylation at the conserved sites ensures the expression of properly folded functional ACh receptors. Brain Res Mol Brain Res. 1997;45:219–29. doi: 10.1016/s0169-328x(96)00256-2. [DOI] [PubMed] [Google Scholar]
- 10.Martin PT. Glycobiology of the neuromuscular junction. J Neurocytol. 2003;32:915–29. doi: 10.1023/B:NEUR.0000020632.41508.83. [DOI] [PubMed] [Google Scholar]
- 11.Belaya K, Rodriguez Cruz PM, Liu W. Mutations in GMPPB cause congenital myashtenic syndrome and bridge myasthenic disorders with dystroglycanopathies. Brain. 2015;138:2493–504. doi: 10.1093/brain/awv185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muntoni F, Torelli S, Wells DJ, et al. Muscular dystrophies due to glycosylation defects: diagnosis and therapeutic strategies. Curr Opin Neurol. 2011;24:437–42. doi: 10.1097/WCO.0b013e32834a95e3. [DOI] [PubMed] [Google Scholar]
- 13.Carss KJ, Stevens E, Foley AR, et al. Mutations in GDP-mannose pyrophosphorylase B cause congenital and limb-girdle muscular dystrophies associated with hypoglycosylation of α-dystroglycan. Am J Hum Genet. 2013;93:29–41. doi: 10.1016/j.ajhg.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palace J, Lashley D, Newsom-davis J, et al. Clinical features of the DOK7 neuromuscular junction synaptopathy. Brain. 2007;130:1507–15. doi: 10.1093/brain/awm072. [DOI] [PubMed] [Google Scholar]
- 15.Chaouch A, Müller JS, Guergueltcheva V, et al. A retrospective clinical study of the treatment of slow-channel congenital myasthenic syndrome. J Neurol. 2012;259:474–81. doi: 10.1007/s00415-011-6204-9. [DOI] [PubMed] [Google Scholar]
- 16.Klein A, Pitt MC, McHugh JC, et al. DOK7 congenital myasthenic syndrome in childhood: early diagnostic clues in 23 children. Neuromuscul Disord. 2013;23:883–91. doi: 10.1016/j.nmd.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 17.Finlayson S, Palace J, Belaya K, et al. Clinical features of congenital myasthenic syndrome due to mutations in DPAGT1. J Neurol Neurosurg Psychiatry. 2013;84:1119–25. doi: 10.1136/jnnp-2012-304716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schiaffino S. Tubular aggregates in skeletal muscle: just a special type of protein aggregates? Neuromuscul Disord. 2012;22:199–207. doi: 10.1016/j.nmd.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 19.Guergueltcheva V, Müller JS, Dusl M, et al. Congenital myasthenic syndrome with tubular aggregates caused by GFPT1 mutations. J Neurol. 2012;259:838–50. doi: 10.1007/s00415-011-6262-z. [DOI] [PubMed] [Google Scholar]
- 20.Finlayson S, Morrow JM, Rodriguez Cruz PM, et al. Muscle MRI in congenital myasthenic syndromes. Muscle Nerve. 2016 Jan 20; doi: 10.1002/mus.25035. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lewis AJM, Finlayson S, Mahmod M, et al. A novel cardiac phenotype in patients with GFPT1 or DPAGT1 mutations. Exp Clin Cardiol. 2014;20:3139–45. [Google Scholar]
- 22.Cabrera-Serrano M, Ghaoui R, Ravenscroft G, et al. Expanding the phenotype of GMPPB mutations. Brain. 2015;138:836–44. doi: 10.1093/brain/awv013. [DOI] [PubMed] [Google Scholar]
- 23.DianaX BG, Neil E, Wiggs E, et al. Intrafamilial variability in GMPPB-associated dystroglycanopathy: broadening the phenotype. Neurology. 2015;84:1495–7. doi: 10.1212/WNL.0000000000001440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ibraghimov-Beskrovnaya O, Ervasti JM, Leveille CJ, et al. Primary structure of dystrophin-associated glycoproteins linking dystrophin to the extracellular matrix. Nature. 1992;355:696–702. doi: 10.1038/355696a0. [DOI] [PubMed] [Google Scholar]
- 25.Michele DE, Barresi R, Kanagawa M, et al. Post-translational disruption of dystroglycan—ligand interactions in congenital muscular dystrophies. Nature. 2002;418:417–22. doi: 10.1038/nature00837. [DOI] [PubMed] [Google Scholar]
- 26.Murakami T, Ishigaki K, Shirakawa S, et al. Severe muscle damage following viral infection in patients with Fukuyama congenital muscular dystrophy. Brain Dev. 2012;34:293–7. doi: 10.1016/j.braindev.2011.06.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.