Abstract
BACKGROUND:
Non-daily smokers (NDS) who smoke on some but not all days are a growing subset of United States (US) tobacco users. Racial/ethnic minorities are more likely to be NDS. African American NDS have strikingly high levels of nicotine and carcinogen exposure, making treatment of this high risk group a priority.
METHODS:
The current study is one of three ongoing federally-funded clinical trials of NDS and, to our knowledge the only RCT focused on racial/ethnic minority NDS. The design has been guided by input from Patient and Stakeholder Advisory Panels who helped develop the research questions, design the intervention, and select the outcomes. The objective is to compare the effectiveness of smoking cessation counseling alone (C) or smoking cessation counseling plus participant’s choice of nicotine replacement therapy (NRT; C+NRT) for African American NDS. Two-hundred seventy-eight African American NDS will be randomized in a 2:1 fashion to C+NRT or C. All participants receive five sessions of smoking cessation counseling; those randomized to C+NRT receive their choice of nicotine gum, patch, and/or lozenge. Treatment in both groups lasts for 12 weeks. We hypothesize that C+NRT will be more effective than C on the primary outcome of biochemically-confirmed abstinence from smoking at week 12. Secondary aims will compare C+NRT and C on patient- and provider-desired outcomes including abstinence from smoking at week 26, change in biochemically-verified nicotine and carcinogen exposure, days abstinent, and treatment process measures (e.g., NRT use and side effects). Predictors of abstinence will also be explored.
DISCUSSION:
Findings will illuminate effective treatment options for African American NDS and contribute to development of evidence-based guidelines for treating the 8.9 million US adult NDS for whom no guidelines currently exist.
Keywords: Non-daily smokers, African American, smoking cessation, nicotine replacement therapy, smoking cessation behavioral counseling
1. Introduction
Non-daily smokers (NDS) report smoking on some but not all days and constitute 8.9 million adults or 24.3% of all adult smokers in the US [1]. Prevalence of NDS has increased almost 30% in the last decade. NDS are at risk for the same health consequences of smoking as daily smokers (DS) [2, 3], are nicotine dependent [4], and want to quit smoking [5], yet they are overlooked by health care providers and largely have been excluded from clinical trials. As a result, there is an almost complete paucity of information on effective treatments for this growing subgroup of smokers whose smoking patterns challenge traditional nicotine dependence treatment paradigms [5, 6]. NDS average 15–20 smoking days per month and routinely abstain for periods of 5–10 days without experiencing significant withdrawal or craving [7, 8], contributing to perceptions that NDS do not identify as smokers, are uninterested in quitting, are not addicted enough to warrant the use of smoking cessation medications, and concern that, if nicotine replacement therapy (NRT) is used, the amount of nicotine delivered could exceed the levels achieved through smoking [4, 6, 8]. These perceptions are not supported by the literature [6, 9, 10]; nonetheless, NDS are less likely than DS to be asked about their smoking status or advised to quit by a physician, have low utilization of smoking cessation pharmacotherapy or behavioral counseling, and experience difficulty in quitting; 73–82% of NDS who attempt to quit resume smoking within 90 days [5].
Racial/ethnic minorities are more likely to be NDS compared to non-Hispanic Whites (Whites) yet they bear a disproportionate burden of smoking-related diseases [11–13]. African Americans, in particular, have higher cardiovascular and cancer-disease risk at lower levels of smoking, including non-daily smoking [14, 15]. Concentrations of NNAL, a metabolite of the potent lung carcinogen NNK, and cotinine, the primary metabolite of nicotine, are three to six times higher in African American NDS compared to White and Hispanic/Latino NDS [16, 17]. This difference persists after adjustment for racial/ethnic variations in smoking level and nicotine metabolism, suggesting that African American NDS incur substantial risk from their intermittent pattern of smoking. African American NDS are also more likely than White and Hispanic/Latino NDS to have made a quit attempt in the past year and to intend to quit in the next 30 days [7], making the treatment of this high risk group a priority.
To date, only two small pilot studies have examined treatment of NDS [18, 19]. The current study is one of three ongoing federally-funded (NIH, PCORI) smoking cessation randomized clinical trials (RCT) examining effective treatments for NDS and the only RCT focused exclusively on racial/ethnic minority NDS. The 3-year study is comparing the effectiveness of smoking cessation counseling alone or smoking cessation counseling in combination with participant’s choice of NRT (i.e., nicotine patch, gum, lozenge) on abstinence in African American NDS interested in quitting. The primary aim is to test the hypothesis that 1) African American NDS randomized to C+NRT will have significantly higher biochemically verified smoking abstinence at 12 weeks (end of treatment) than African American NDS randomized to C. Secondary aims are: (2) test the hypothesis that African American NDS randomized to C+NRT will have significantly higher biochemically verified smoking abstinence at 26 weeks (end of follow-up) than African American NDS randomized to C, (3) test the hypothesis that AA NDS randomized to C+NRT will demonstrate significantly greater reductions in nicotine intake and carcinogen exposure and significantly more days abstinent, while experiencing no differences in side effects as participants randomized to C, and (4) identify predictors of successful quitting among African American NDS. Due to the exploratory nature of this aim, no specific hypotheses are proposed for this aim. This paper describes the study design, enrollment, and baseline characteristics of participants in the trial.
2. Methods
2.1. Study design.
The study is an unblinded and open-label RCT of 278 African American NDS randomized to receive either 5 sessions of smoking cessation counseling in combination with 12 weeks of their choice of nicotine patch, gum, or lozenge (C+NRT) or 5 sessions of smoking cessation counseling alone (C). A 2:1 randomization schema was used; for every one patient randomized to C, two were randomized to C+NRT. The schedule of enrollment, intervention, and assessment activities is displayed in Table 1. The primary outcome is biologically-confirmed 30-day point prevalence abstinence (PPA) from smoking at week 12. All study visits will be completed at the University of Kansas Medical Center or Swope Health Central, a Federally Qualified Health Center that serves a predominately African American clientele. Study procedures are approved and monitored by the University of Kansas Medical Center (KUMC) IRB (#00001602).
Table 1.
Schedule of enrollment, intervention, and assessment activities
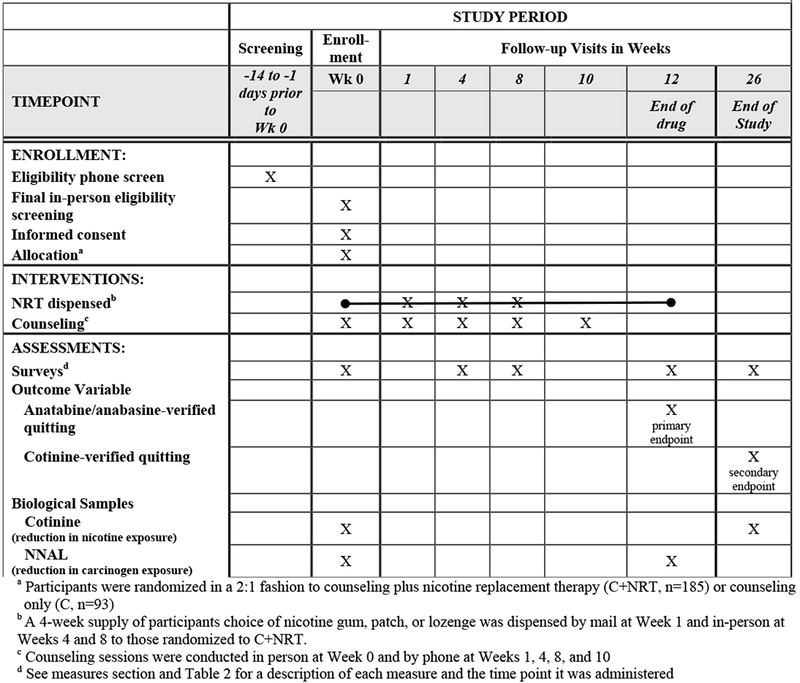
|
2.1. Patient and stakeholder engagement and study design rationale.
The design of this study has been guided by input from Patient and Stakeholder Advisory Panels comprised of nine African American NDS, two physicians serving a predominately African American patient population, and two experts from Optum (formerly Alere), one of the nation’s largest provider of cessation quit line services (http://map.naquitline.org/reports/administration/). The panels helped develop the research questions, design the intervention, and select the outcomes. For example, participants receive a choice of nicotine patch, gum, or lozenge because these are the medications that were preferred by our Patient and Stakeholder Advisory Panels. A counseling only control group was chosen because the providers on our Stakeholder Advisory Panel felt uncertain about whether pharmacotherapy conferred any benefits beyond those provided by counseling alone. This is consistent with literature suggesting that NDS, who are more likely to smoke because of the behavioral components of nicotine addiction (e.g., stimuli/cues, positive reinforcing effects) rather than to maintain blood nicotine levels, might benefit more from behavioral counseling focused on management of smoking cues/triggers than pharmacological approaches that replace nicotine in order to reduce withdrawal and craving [4, 18–20]. While our primary outcome, biochemically-confirmed abstinence from smoking, is the scientific ‘gold standard,’ our Patient and Stakeholder Advisory Panels noted other important outcomes of interest. The Patient Advisory Panel was particularly interested in harm reduction outcomes, including reductions in nicotine intake and carcinogen exposure and number of days abstinent. The Stakeholder Advisory Panel was interested in these harm reduction outcomes in addition to side effects and use of NRT in the intervention group.
The panels reviewed and approved the counseling protocol, quit smoking guide, NRT dosing criteria, recruitment materials, and surveys prior to initiation of the clinical trial. Ongoing input from the Patient Advisory Panel helps guide recruitment and retention strategies, interpretation of findings, and dissemination plans, particularly for reaching a broader, non-academic audience.
2.2. Recruitment.
Recruitment started in May 2015 and ended in May 2017. Final 6-month follow-up will be completed in January 2018. Participants are recruited through clinic- and community-based efforts, including fliers, physician letters, in-clinic recruitment, radio, television, and social media ads, and word-of- mouth referrals from current and former participants.
2.3. Eligibility.
Eligible participants are non-Hispanic African American adults (≥ 18 years) who had smoked at least 100 lifetime cigarettes and met criteria for NDS, defined as smoking cigarettes on 4–27 of the last 30 days and smoking at the current non-daily rate for ≥ 3 months [21]. Individuals are excluded if they are a daily user of non-cigarette tobacco products, have engaged in a pharmacotherapy-assisted quit attempt in the last 30 days, or are uninterested in quitting smoking, taking NRT, refraining from the use of electronic cigarettes or smoking cessation pharmacotherapy, and completing study-related requirements. Individuals with medical contraindications to NRT, including being pregnant or breastfeeding and having a cardiovascular event (i.e., heart attack, angina, arrhythmia, chest pain) in the past 30 days are also excluded; women must be willing to use birth control to avoid pregnancy while taking NRT. Use of non-cigarette tobacco products (e.g., cigarillos, little cigars) are common among NDS, [8, 22, 23] ; users of these products are included in the study as long as they are not daily users (i.e., ≥ 28 days in the past 30) of non-cigarette tobacco products.
2.4. Screening and Consent.
Interested individuals contact us by telephone and are screened for eligibility by study staff. Those who are provisionally eligible after the phone screening are scheduled for final, in-person eligibility screening, which consists of pregnancy testing and collection of urine for baseline assessment of nicotine biomarkers. Individuals who are eligible following final, in-person screening participate in a consenting interview conducted by study staff. Those providing written informed consent are enrolled into the study, randomized, and immediately participate in baseline (Week 0) activities (described below).
2.5. Intervention
2.5.1. Counseling (C and C+NRT).
Participants receive five smoking cessation counseling sessions in person at Weeks 0 (baseline) and by phone at Weeks 1, 4, 8, and 10. The counseling protocol is evidence-based [24–26], individualized and culturally-specific. It was developed by our team over 20 years in collaboration with an African American community advisory board and African American former study participants [27] and has been found to be superior for African American smokers in head-to-head comparisons with other counseling approaches (i.e., motivational interviewing, brief advice) [28, 29]. The goals of counseling are to increase knowledge, including information particularly relevant to African Americans, develop individualized behavioral and cognitive skills related to cessation, and provide intra-treatment social support. For the current study, counseling is tailored to meet the needs of NDS and, therefore, focuses heavily on managing smoking cues, triggers, and the acute positive reinforcing effects of smoking in addition to dealing with nicotine withdrawal and craving. Skills include strategies to address smoking triggers (e.g., avoidance of social situations that trigger smoking, coping responses to address withdrawal/craving), reduce cue-smoking contingencies (e.g., alcohol, marijuana), manage stress and negative affect, and increase self-efficacy. Our approach is intentionally flexible to match the needs of each participant over the course of behavior change. Counseling goes hand-in-hand with a 40-page culturally-targeted quit smoking guide, used in our previous trials with African American smokers [28, 30, 31]. For the purposes of rapport and continuity of care, participants meet with the same counselor each session. Counseling is delivered by our experienced staffs who are certified tobacco treatment specialists, have extensive experience treating African American smokers within clinical trials, and are active members of the community. The baseline session lasts 30 minutes, on average, with follow-up sessions averaging 15 minutes. Counseling fidelity is monitored by a licensed psychologist during bimonthly counseling supervision meetings.
2.5.2. Written materials (C and C+NRT).
Participants receive a 40-page stop smoking guide at baseline. The guide has been designed to go hand-in-hand with the counseling and includes information on the health consequences of tobacco use, benefits of quitting, strategies to promote abstinence such as making a quit plan, obtaining social support, identifying reasons for smoking and activities that could take the place of smoking, strategies for dealing with urges, managing withdrawal and craving, coping with a lapse, and relapse prevention. The guide for participants randomized to C+NRT includes an additional section on NRT that addresses choosing the right NRT, adjusting the dose, and managing side effects.
2.5.3. Nicotine replacement therapy (C+NRT).
Participants randomized to C+NRT are given a choice of nicotine gum, patch, or lozenge. To facilitate choice, participants are instructed on the proper use of gum, patch, and lozenge at baseline (Week 0) and sent home with a 3-day trial of each. Participants select their NRT during the Week 1 counseling phone call and a 4-week supply is immediately shipped. The target quit day for both groups is set at 2-weeks post-baseline to allow time for participants in both arms to properly prepare for their quit day and for participants in C+NRT to select, receive, and begin using their NRT. Refills of NRT are provided at Weeks 4 and 8. NRT treatment lasts 12 weeks.
Dosing NRT for NDS.
Current NRT dosing recommendations do not fully capture the needs of NDS whose natural smoking patterns include days of abstinence and variability in the amount of tobacco consumed on the days that they do smoke [24, 32]. To address these unique challenges, 30-day Timeline Follow Back (TLFB) methods are used to dose medication. Participants are asked to recall the amount and type of tobacco consumed each day for the past 30 days [33, 34]. NRT is dosed based on the number of tobacco products used on the highest smoking day using an algorithm that assumes that African Americans extract ~30% more nicotine per cigarette smoked, have higher blood cotinine levels at lower levels of smoking [35, 36], and have been under dosed on NRT in previous studies that used standard dosing guidelines [28, 37].
For participants selecting nicotine patch, our algorithm results in an initial recommendation of 7 mg patch for those smoking ≤ 5 tobacco products on their highest smoking day, 14 mg patch and ad lib nicotine gum or lozenge for those smoking 6–10 tobacco products, and 21 mg patch and ad lib nicotine gum or lozenge for those smoking ≥ 11 tobacco products. For participants selecting gum or lozenge, we use the general recommendation of 1 piece every 3–4 hours for those smoking ≤ 5 tobacco products on their highest smoking day, 1 piece every 2–3 hours for those smoking 6–10 tobacco products, and 1 piece every 1–2 hours for those smoking ≥ 11 tobacco products on their highest smoking day. Gum and lozenge are provided in 4 mg formulation. Participants who smoke 3–7 days of the week are encouraged to use NRT daily, even on non-smoking days. Those smoking 1–2 days per week are encouraged to use NRT only on the days that they smoked; however, those smoking 1–2 days per week but who report craving or withdrawal on non-smoking days are encouraged to use NRT daily. Response to NRT is monitored at Weeks 4 and 8; dosing recommendations and/or the medication(s) being used are modified, as needed, based on response to therapy and participant choice.
2.6. Retention.
Study staff contact participants one week prior to each study visit via phone, text, email and postcards. For any missed session, participants receive up to 6 additional contacts to facilitate rescheduling. Participants are compensated $40 at Weeks 0 and 12 and $20 at Weeks 1, 4, 8, 10, and 26 for their time. Remuneration is based on visit attendance and not on smoking status. Participants are eligible to receive an additional $60 for referring up to 3 friends who were eligible and enrolled in the study.
2.7. Outcomes Measures
2.7.1. Smoking abstinence.
The primary outcome is anabasine- and anatabine-verified 30-day PPA from smoking, defined as no tobacco products for the previous 30 days at Week 12. Anabasine and anatabine are tobacco alkaloids that represent the gold standard for confirming abstinence when cotinine measurements are invalid (i.e., detectable levels of cotinine reflect NRT use, smoking, or both) [38]. The recommended cut-off of 2 ng/ml will be used to differentiate smokers from non-smokers (considered a non-smoker if both alkaloids are < 2 ng/ml) [38]. The secondary endpoint is cotinine-verified 30-day PPA from smoking at Week 26. The recommended cut-off of 50 ng/ml will be used to differentiate smokers from non-smokers [39]. The longer 30-day PPA time frame is being used because the more customary 7-day PPA time frame could reflect normal variations in smoking and abstinence for NDS.
2.7.2. Nicotine exposure.
Nicotine exposure is assessed via urinary cotinine levels measured at Weeks 0 and 26 [40].
2.7.3. Carcinogen exposure.
Carcinogen exposure is assessed via urinary levels of the NNK metabolite 4-(methynitrosamino) -1-(3) pyridyle-1-butanol[NNAL] at Weeks 0 and 12. NNK is a tobacco-specific nitrosamine that is widely considered to be one of the most important causative agents in the development of lung cancer [41]. NNK is metabolized into NNAL, which can be measured in the urine and is used to indicate tobacco-specific exposure to this potent lung carcinogen.
2.7.4. Days abstinent.
Participants are asked to recall the number of cigarettes, hand rolled cigarettes, little cigars, cigarillos, full size cigars, blunts, pipes, bidis, smokeless tobacco, and hookah used for each of the past 30 days at Weeks 4, 8, and 12 [33, 34]. The total number of tobacco free days will be summarized from this data.
2.7.5. Treatment-related side effects.
Participants are asked about symptoms commonly associated with quitting smoking and/or NRT (e.g., nausea, trouble sleeping, skin or mouth irritation) at Weeks 0, 4, 8, and 12 using a standardized symptoms checklist [42–45].
2.8. Covariates
Little is known about African American NDS, therefore, an extensive survey battery is being used to adequately characterize and describe demographic characteristics, smoking patterns and behaviors, and comorbid conditions among this population and to examine how these factors impact abstinence.
2.8.1. Demographics.
Baseline demographic measures include participant age, gender, marital/partner status, employment and housing status, educational level, health care coverage [46], income [47], perceived health [48], height, weight, and body mass index [49].
2.8.2. Smoking, tobacco use, and quitting history.
Baseline assessment of smoking history includes amount of cigarettes smoked (days smoked in last 30 and number of cigarettes on days smoked), type of cigarette smoked (menthol versus non-menthol), use of non-cigarette tobacco products [33, 34], NDS type [i.e., formerly a daily smoker (converted) or always a NDS (native)] [8, 50], age when started smoking regularly [51], reasons for smoking some but not all days [21, 52], identity as a smoker [8], smoking triggers [21, 52], time to the first cigarette of the day (a marker of nicotine dependence) [53], dependence motives [54], nicotine withdrawal [55], and craving [56].
2.8.3. Social influences on smoking.
Baseline assessment of social influences on smoking includes the extent to which one smokes socially or alone [20], number of five best friends or family who smoke, number of smokers in the home, and partner smoking status [57].
2.8.4. Quitting history.
Baseline assessment of quitting history includes number of 24 hour quit attempts in the past year, length of the longest quit attempt, and previous use of behavioral and/or pharmacological quitting aids [5, 51].
2.8.5. Comorbid mental health and substance use.
Baseline assessment of comorbid mental health and substance use includes symptoms of depression [58], alcohol misuse [59], and past 30 day use of marijuana and prescription pain relievers (mostly opioids) [60].
2.8.6. Treatment process.
The number of counseling sessions completed will be summarized for participants in both groups. For those in C+NRT, data on the medication(s) dispensed is captured at Weeks 1, 4, and 8 and participants are asked about the amount of NRT used each day for the past 30 days at Weeks 4, 8, and 12.
2.9. Data analysis
2.9.1. Sample size justification.
The primary outcome is biochemically-confirmed 30-day PPA at Week 12. Because this is the first smoking cessation intervention study with AA NDS, our sample size estimates are based on data from randomized trials of AA low level DS [24, 31, 61, 62]. Based on these studies we postulate a 10% PPA rate in the C group and a 25% PPA in the C+NRT group. The current sample size of 93 in C and 185 in C+NRT provides 91% power to detect a difference in cessation with a type I error rate of 0.05 using a two-sample, two-tailed Chi-square test. If the difference between treatment and control groups is smaller than hypothesized (e.g., 10% abstinence in the control group versus 22% abstinence in the treatment group), we will have 78% power to detect these differences with a sample size of 278.
2.9.2. Missing Data.
All primary analyses on smoking cessation will be conducted using intent-to-treat and will code those lost to follow-up as smokers per the Russell Standard [63]. Subsequently we will evaluate the missing data pattern. If there is a differential loss based upon group, multiple imputation techniques will also be used. All 278 participants will be included in analyses of smoking outcomes. We also will look at completers only.
2.9.3. Statistical analyses for Aim 1: Smoking abstinence.
The chi-square test will be used to compare the verified abstinence rates at Week 12 (primary endpoint) and Week 26 (secondary endpoint) between C and C+NRT. For our primary comparison, those lost to follow-up will be considered as smokers. We also will look at completers only and will utilize multiple imputation techniques to ensure valid comparisons between the C and C+NRT if the loss to follow-up is related to treatment. Secondarily, we will utilize Generalize Estimating Equation methodology to longitudinally compare verified abstinence over time.
2.9.4. Statistical analyses for Aim 2: Nicotine and carcinogen exposure, days abstinent, and treatment-related side effects.
We will utilize linear mixed models to longitudinally compare nicotine (Week 0 to 26) and carcinogen exposure (Week 0 to 12) between C and C+NRT and then subsequently examine the effects of the covariates (Table 2) using a best subsets selection method for both nicotine and carcinogen exposure independently. A generalized linear mixed model approach will be used to model days abstinent, controlling for number of days in study, to assess the effect of treatment on this endpoint. We will compare the prevalence of any side effect as well as each specific side effect, from baseline to week 12, between the two groups using the chi-square test. If there is a global difference, we will examine, within the NRT group, if type of NRT impacts the side effect profile. Within the NRT group, we will descriptively summarize the number and percent of subjects who choose each type of NRT. Subsequently, we will describe and descriptively compare the percent of subjects within each of these subgroups who use the NRT of choice during the treatment period (i.e., percent of patients within the patch, gum, and lozenge groups who used the medication) and the relationship between adherence to NRT and initial NRT choice.
Table 2.
Measures at Each Time Pointa and Covariates to be Examined as Predictors of Outcomes
| Eligibility | Baseline Week 0 | Week 1 | Week 4 | Week 8 | Week 10 | Week 12 | Week 26 | |
|---|---|---|---|---|---|---|---|---|
| Smoking Abstinence Outcomes | ||||||||
| Anatabine/anabasine (primary) | x | |||||||
| Cotinine (secondary) | x | |||||||
| Patient- and Provider-Centered Outcomes | ||||||||
| Reduction in nicotine exposure (cotinine) | x | x | ||||||
| Reduction in carcinogen exposure (NNAL) | x | x | ||||||
| Days abstinent | x | x | x | x | ||||
| Treatment-related side effects | x | x | x | x | ||||
| Demographics | ||||||||
| Demographics (e.g., age, gender, education, income) | x | x | ||||||
| Perceived health | x | |||||||
| Body mass index | x | |||||||
| Smoking and Tobacco Use History | ||||||||
| Past 30 day use of all tobacco products | x | x | x | x | x | |||
| Menthol/Non-Menthol | x | |||||||
| Age first used cigarettes | x | |||||||
| Identity as smoker | x | |||||||
| Reasons for non-daily smoking | x | |||||||
| Non-daily type (i.e., native, converted) | x | |||||||
| Triggers for smoking | x | |||||||
| Social smoking | x | |||||||
| Withdrawal (Minnesota Withdrawal Questionnaire) | x | x | ||||||
| Craving (Questionnaire of Brief Smoking Urges) | x | x | ||||||
| Nicotine dependence (Time to First Cigarette) | x | |||||||
| Dependence motives (Wisconsin Inventory of Smoking Dependence Motives) | x | |||||||
| Social Influences on Smoking | ||||||||
| Smoking status of partner/spouse | x | |||||||
| Number of smokers in household | x | |||||||
| Home smoking restrictions | x | |||||||
| Number of friends/family who smoke | x | |||||||
| Quitting History | ||||||||
| Past year quitting (# of times and longest period) | x | |||||||
| Use of pharmacological and behavioral aids to quit | x | |||||||
| Alcohol, Other Drugs, and Depressive Symptoms | ||||||||
| Depressive symptoms (Patient Health Questionnaire-2) | x | |||||||
| Alcohol use (AUDIT-C) | x | x | x | x | x | |||
| Marijuana use | x | x | x | x | x | |||
| Use of prescription pain relievers | x | |||||||
| Treatment Process | ||||||||
| Counseling completion | x | x | x | x | x | |||
| NRT use (C+NRT only) | x | x | x | x | ||||
Assessments were completed in-person at Weeks 0, 4, 8, 12, and 26 and by phone at Weeks 1 and10.
2.9.5. Statistical analysis for Aim 3: Identify predictors of abstinence.
Assuming the expected treatment effect, we will descriptively examine the cessation rate within the C+NRT group by choice of NRT. Within the C+NRT treatment group, we will examine the effect of C+NRT use over the first 12 weeks on verified cessation using logistic regression. We will then examine the effects of the covariates on this endpoint by utilizing best subsets selection techniques. Our goal is to identify a final model utilizing the smallest subset of covariates that describes cessation over the length of the study. Our analysis for this study will be limited to main effect predictors of abstinence due to the 2:1 randomization schema and the hypothesized cessation rates by group (i.e., limited number of quitters), which make it implausible to examine the interaction of factors with treatment.
2.9.6. Analyses for the current paper.
In this paper we provide descriptive summaries of baseline (Week 0) demographic, smoking, and comorbid mental health and substance use characteristics using frequencies and percentages for categorical variables and means and standard deviations for quantitative variables. Future manuscripts will present findings on primary and secondary outcomes.
3. Results
3.1. Recruitment flow.
The flow of participants into the study is shown in Figure 1. Six-hundred and twenty-five African Americans initially identified as NDS out of 2,528 African American smokers (24.7%) who were pre-screened as part of enrollment for this study and another ongoing study of African American daily smokers (data not shown). Of these, 63 (10.0%) were excluded after more in-depth screening indicated that they did not meet study criteria for non-daily smoking (smoked cigarettes on 4–27 days per month for ≥ 3 months). In total, 22.2% (562/2,528) of African American smokers who were screened met our criteria for non-daily smoking. Of these, 553 met the other phone screen eligibility criteria but 249 (45.1%) did not keep their appointment to complete final, in-person, eligibility screening. Of the 304 who completed in-person eligibility screening, 279 were randomized, 185 to C+NRT and 94 to C. A protocol violation (i.e., randomized not according to code) resulted in one participant being removed, bringing the final sample size to 185 C+NRT and 93 C.
Figure 1.
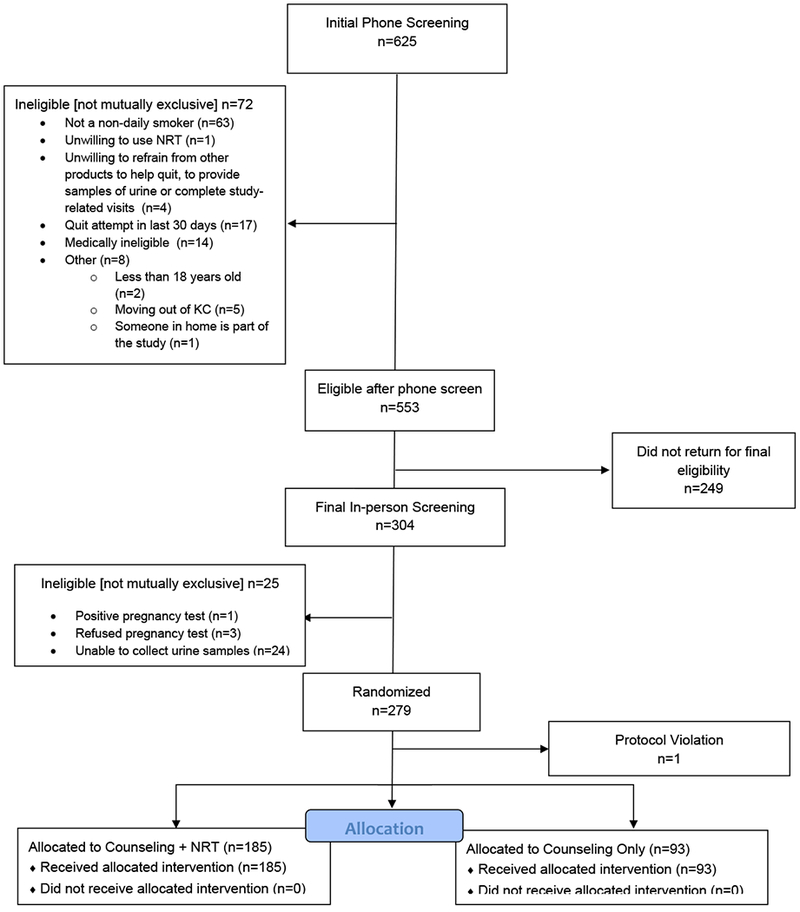
Study Flow Daigram.
3.2. Baseline characteristics.
Baseline characteristics overall and by study group are displayed in Table 3. Per CONSORT guidelines for the reporting of RCT, baseline differences between groups are not statistically compared [64] but, as can be seen, groups are comparable on most factors. A nearly equal proportion of men and women enrolled. The majority of participants are of lower socioeconomic status, as indicated by income, poverty level, employment status, and home ownership, and of good to fair perceived health. Participants smoke cigarettes an average of 10.6 (3.9) days per month, use 4.7 (3.5) cigarettes on the days smoked, and have been NDS for an average of 7.0 (9.9) years. The majority are former DS and indicate trying to cut back or quit as one of the primary reasons for smoking non-daily. Participants have tried to quit an average of 6.8 times (SD=24.7) in the past year; 56.5% have used quitting aids. Other non-cigarette tobacco products are used by about one-third of participants; although use is infrequent (1.9 products used an average of 4.7 days per month). Almost half smoke their first cigarette of the day after 30 minutes of waking, indicating less nicotine dependence, and have withdrawal and craving scores in the low to moderate range. They are more likely to smoke because of primary (e.g., to relieve withdrawal, craving, and because of tolerance) versus secondary (e.g., social cues/contexts, weight control, mood enhancement) dependence motives. One-third smoke mainly with others versus alone and most have a social network comprised of smokers. Use of other substances is high; nearly 50% meet criteria for alcohol misuse, 28.4% used marijuana in the past month, and almost 40% used prescription pain relievers in the past month. Finally, almost one-quarter screened positive for depressive symptoms.
Table 3.
Baseline participant characteristics
| Range for Scale Scores | Summary Statistics | ||||
|---|---|---|---|---|---|
| C+NRT (n=185) | Counseling (n=93) | All (n=278) | |||
| DEMOGRAPHIC CHARACTERISTICS | |||||
| Age, mean(SD) | 48.6 (11.6) | 49.0 (11.8) | 48.7 (11.7) | ||
| Gender, n (%) | |||||
| Female | 94 (50.8) | 47 (50.5) | 141 (50.7) | ||
| Male | 91 (49.2) | 46 (49.5) | 137 (49.3) | ||
| Cohabitation Status, n (%) | |||||
| Married or living with a partner | 48 (26.0) | 17 (18.3) | 65 (23.4) | ||
| Employment Status, n (%) | |||||
| Employed full-time | 45 (24.3) | 29 (31.2) | 74 (26.6) | ||
| Employed part-time or seasonally | 37 (20.0) | 20 (21.5) | 57 (20.5) | ||
| Not currently employed | 63 (34.1) | 34 (36.6) | 97 (34.9) | ||
| Retired | 27 (14.6) | 4 (4.3) | 31 (11.2) | ||
| Student/Homemaker | 13 (7.0) | 6 (6.5) | 19 (6.8) | ||
| Education Level, n (%) | |||||
| Less than high school (HS) graduate | 27 (14.6) | 13 (14.0) | 40 (14.4) | ||
| HS graduate or HS equivalent (GED) | 53 (28.7) | 23 (24.7) | 76 (27.3) | ||
| Some college or tech school | 75 (40.5) | 45 (48.4) | 120 (43.2) | ||
| College graduate or higher | 30 (16.2) | 12 (12.9) | 42 (15.1) | ||
| Health Insurance that Pays for Most Medical Care, n (%) | |||||
| No | 39 (21.1) | 26 (28.0) | 65 (23.4) | ||
| Yes | 146 (78.9) | 67 (72.0) | 213 (76.6) | ||
| Household Income in Thousands, mean (SD) | 26.9 (24.3) | 28.8 (31.7) | 27.5 (2.7) | ||
| Refused/Don’t Know | 18 (9.7) | 14 (15.1) | 32 (11.5) | ||
| Number of people in household, including self, mean (SD) | 2.3 (1.6) | 2.5 (1.7) | 2.4 (1.7) | ||
| Poverty level, n (%) | |||||
| Missing | 18 (9.7) | 14 (15.1) | 32 (11.5) | ||
| ≤100 | 79 (42.7) | 38 (40.9) | 117 (42.1) | ||
| 101–200 | 41 (22.2) | 20 (21.5) | 61 (21.9) | ||
| 201–250 | 17 (9.2) | 8 (8.6) | 25 (9.0) | ||
| 251–300 | 8 (4.3) | 4 (4.3) | 12 (4.3) | ||
| 301–400 | 10 (5.4) | 2 (2.2) | 12 (4.3) | ||
| > 400% | 12 (6.4) | 7 (7.5) | 19 (6.8) | ||
| Housing, n (%) | |||||
| Own a home | 25 (13.5) | 7 (7.5) | 32 (11.5) | ||
| Rent or stay with others | 160 (86.5) | 86 (92.5) | 246 (88.5) | ||
| Perceived Health, n (%) | |||||
| Good, Fair, or Poor | 142 (76.8) | 71 (76.3) | 213 (76.6) | ||
| Very good/Excellent | 43 (23.2) | 22 (23.7) | 65 (23.4) | ||
| Height in inches, mean(SD) | 67.6 (3.9) | 67.1 (3.6) | 67.4 (3.8) | ||
| Weight in pounds, mean(SD) | 203.5 (57.7) | 202.0 (58.4) | 203.0 (57.8) | ||
| BMI, mean(SD) | 31.3 (8.7) | 31.7 (9.8) | 31.5 (9.1) | ||
| TOBACCO USE CHARACTERISTICS | |||||
| Age when you started smoking regularly, mean (SD) | 21.0 (8.2) | 22.5 (9.9) | 21.5 (8.8) | ||
| Days smoked cigarettes in last 30, mean (SD) | 10.6 (3.9) | 10.4 (3.9) | 10.6 (3.9) | ||
| Number of cigarettes used on days smoked, mean (SD) | 4.8 (3.6) | 4.4 (3.2) | 4.7 (3.5) | ||
| Non-daily smoker type, n (%) | |||||
| Native (i.e., always been a non-daily smoker) | 55 (29.7) | 24 (25.8) | 79 (28.4) | ||
| Converted (i.e., formerly a daily smoker) | 130 (70.3) | 69 (74.2) | 199 (71.6) | ||
| Length of time as a non-daily smokers in years, mean (SD) | 6.9 (9.7) | 7.1 (10.4) | 7.0 (9.9) | ||
| Length of time as a non-daily smoker by category, n (%) | |||||
| 3–6 months | 26 (14.1) | 7 (7.5) | 33 (11.9) | ||
| 7–11 months | 28 (15.1) | 11 (11.8) | 39 (14.0) | ||
| 12 months or greater | 131 (70.8) | 75 (80.7) | 206 (74.1) | ||
| Reasons for smoking some but not all days, n (%) [check all that apply] | |||||
| Health | 96 (51.9) | 41 (44.1) | 137 (49.3) | ||
| Trying to cut back or quit | 158 (85.4) | 74 (80.0) | 232 (83.5) | ||
| Cost/can’t afford to smoke more | 84 (45.4) | 51 (54.8) | 135 (48.6) | ||
| Don’t have strong cravings or urges to smoke more | 113 (61.1) | 49 (52.7) | 162 (58.3) | ||
| Have control over smoking; can chose when and when not to smoke | 107 (57.8) | 47 (50.5) | 154 (55.4) | ||
| Only smoke in certain situations | 154 (83.2) | 81 (87.1) | 235 (84.5) | ||
| Because those around you disapprove | 67 (36.2) | 34 (36.6) | 101 (36.3) | ||
| Use of other tobacco products in the past 30 days, n (%) yesa | 58 (31.3) | 36 (38.7) | 94 (33.8) | ||
| Number of days used other tobacco products | 4.9 (4.5) | 4.4 (4.6) | 4.7 (4.5) | ||
| Average amount used per day | 2.1 (1.6) | 1.6 (1.0) | 1.9 (1.5) | ||
| Menthol smoker, n (%) | |||||
| Non-Menthol | 35 (18.9) | 14 (15.1) | 49 (17.6) | ||
| Menthol | 150 (81.1) | 79 (85.0) | 229 (82.4) | ||
| Nicotine Dependence, “How soon after waking do you first smoke,” n (%) | |||||
| After 30 minutes | 100 (54.1) | 51 (54.8) | 151 (54.3) | ||
| Within 30 minutes | 85 (46.0) | 42 (45.2) | 127 (45.7) | ||
| Withdrawal, mean(SD) | 0–32 | 10.3 (7.0) | 11.7 (7.5) | 10.8 (7.2) | |
| Craving, mean(SD) | 10–70 | 24.5 (15.2) | 25.7 (14.9) | 24.9 (15.1) | |
| Dependence Motives (WISDM Brief)b, mean (SD) | |||||
| Total | 11–77 | 32.6 (12.3) | 33.3 (12.5) | 32.8 (12.4) | |
| Primary dependence motives | 1–7 | 3.2 (1.4) | 3.2 (1.4) | 3.2 (1.4) | |
| Secondary dependence motives | 1–7 | 2.8 (1.1) | 2.9 (1.1) | 2.9 (1.1) | |
| SOCIAL INFLUENCES ON SMOKING | |||||
| Social Smoking, “In the past 30 days, did you smoke…” n (%) | |||||
| Mainly when with others | 53 (28.7) | 22 (23.7) | 75 (27.0) | ||
| Mainly when alone | 65 (35.1) | 38 (40.9) | 103 (37.1) | ||
| As often alone as with others | 67 (36.2) | 33 (35.5) | 100 (36.0) | ||
| Number of your five best friends smoke, mean(SD) | 0–5 | 3.0 (1.6) | 2.9 (1.7) | 3.0 (1.6) | |
| Number of smokers in the home (including self), mean(SD) | 1.9 (7.2) | 1.8 (3.1) | 1.9 (6.1) | ||
| Partner smoking status, n (%) | |||||
| No partner/spouse | 79 (42.7) | 46 (49.5) | 125 (45.0) | ||
| Partner/spouse is a non-smoker | 42 (22.7) | 22 (23.7) | 64 (23.0) | ||
| Partner/spouse is a smoker | 64 (34.6) | 25 (26.9) | 89 (32.0) | ||
| QUITTING HISTORY | |||||
| Number of 24 hour quit attempts in past year, mean (SD) | 7.3 (28.2) | 5.7 (15.6) | 6.8 (24.7) | ||
| Longest quit attempt in the past year, mean (SD) in months | 1.1 (1.7) | 1.3 (1.8) | 1.1 (1.7) | ||
| Use of quitting aids (i.e., behavioral or pharmacological), n (%) ever | 103 (55.7) | 54 (58.1) | 157 (56.5) | ||
| DEPRESSIVE SYMPTOMOLOGY, ALCOHOL, MARIJUANA, AND PRESCRIPTION PAIN USE | |||||
| Depressive symptoms (PHQ-2 ≥ 3), n (%) | 0–6 | 39 (21.1) | 23 (24.7) | 62 (22.3) | |
| Alcohol misuse (AUDIT-C ≥ 3 for women and ≥ 4 for men), n (%) | 91(49.2) | 47 (50.5) | 138 (49.6) | ||
| Marijuana, n (%) of participants who used in the past 30 days | 56 (30.3) | 23 (24.7) | 79 (28.4) | ||
| Refused | 0 (0.0) | 1 (1.1) | 1 (0.4) | ||
| Prescription pain relievers, n (%) of participants who used in the past 30 days | 75 (40.5) | 33 (35.5) | 108 (38.9) | ||
Daily users of non-cigarette other tobacco products, not including electronic cigarettes, were excluded at eligibility screening
Primary dependence motives include automaticity, craving, loss of control, and tolerance. Secondary dependence motives include affective enhancement, affiliative attachment, cognitive enhancement, cue exposure, social/environmental goads, taste, and weight control. Total dependence motives are a sum of all 11 subscales.
4. Discussion
A number of observations from the current study are worth noting to inform future cessation intervention studies with NDS. Nearly one-quarter of individuals screened met our NDS criteria. This is consistent with national prevalence data and reinforces the need for ongoing treatment studies with this growing subpopulation of smokers [1]. However, almost half of NDS who met our initial screening criteria did not keep their in-person appointment, possibly reflecting lower identity as a smoker and ambivalence about quitting smoking among this group [65]. As a result, we screened 2,528 people in order to randomize 278 NDS. We conservatively estimated accruing 20 participants per month into the study but our actual accrual rate was slower, averaging 11–12 participants per month. The large numbers of participants needing to be screened to meet recruitment goals (i.e., percent screened to enrolled ratio was 10.9% in the current study) and potentially slow accrual are factors that should be considered when designing and implementing cessation trials with NDS.
Characteristics of the current sample influence how abstinence is operationalized among NDS, as well as targets for intervention. Participants smoked an average of 10 of the last 30 days, which is within the range reported in other NDS studies [7, 8]. Cessation trials commonly use self-reported 7-day PPA from smoking as the standard for taking samples for biochemical verification of smoking status [39]. However, because NDS often abstain for seven days or more as part of their regular smoking pattern [7, 8, 12], longer periods of PPA should be used to confirm a NDS as quit; we used 30-day PPA in the current study. Similarly, one-third of NDS in the current study used non-cigarette tobacco products in the past 30 days. Use of non-cigarette tobacco products has grown exponentially in the US over the last two decades [66, 67] and is higher among NDS than DS [22]. Investigators working with NDS must decide if the goal is to achieve abstinence from all tobacco products or only cigarettes. All tobacco products are associated with harmful health effects [68, 69 , 70–73] and, therefore, we opted to achieve abstinence from all tobacco products, although it is a more conservative approach that may result in fewer quitters [74]. Alternatively, investigators could exclude poly-tobacco users but this limits generalizability to the larger population of NDS. The high rates of alcohol, marijuana, and prescription pain reliever use in this study are also striking but reflect growing national trends among smokers of all levels [3, 8, 75–78]. Other substance use often serves as triggers/cues to smoke, are commonly used in conjunction with tobacco to enhance the experience [79, 80] and may make quitting tobacco more difficult [75]. Our examination of these factors as predictors of abstinence will provide important information about how depression and co-use of alcohol, marijuana, and prescription pain relievers increase or decrease the odds of cessation among NDS; however, future studies with NDS should consider these factors as important targets for intervention.
Finally, the use of pharmacotherapy for NDS is an area of ongoing debate. NDS are currently conceptualized as ‘peak seekers’ who smoke because of external cues or acute positive reinforcing effects of nicotine (e.g., enhanced cognitive function, improved mood) and, therefore, behavioral counseling focused on management of external stimuli may be ideally suited to address the primary cessation needs of these smokers [81]. This may not be true for African American NDS. African American non-daily smokers take in more cotinine per cigarette and have nicotine blood levels in the range of White daily smokers [16, 17], suggesting that African American NDS may be in need of nicotine replacement to succeed in quitting. NDS in this study noted more primary versus secondary dependence motives. Primary dependence motives reflect traditional reasons for smoking (i.e., to relieve withdrawal, craving, and replace nicotine) [54, 82, 83], supporting our hypothesis that NRT may lead to higher rates of quitting in this population relative to counseling alone. However, periods of routine abstinence and within-smoker variability in the amount of tobacco consumed on smoking days make the dosing of NRT complicated. Nicotine patch provides steady nicotine throughout the day at levels that might exceed what some NDS get from tobacco and, therefore, might be considered inappropriate for NDS. Ad libitum nicotine gum and lozenge provide nicotine replacement only when needed and may be better suited to the natural smoking patterns of NDS; however compliance with ad libitum products is an issue [28, 37]. For these reasons, we provide a choice of nicotine patch, gum, and lozenge among participants randomized to C+NRT and the opportunity to switch medications in order to better manage side effects, withdrawal, craving, or compliance issues. Within the C+NRT group we will examine the cessation rate by choice of NRT as well as how type of NRT impacts side effects and medication use. These data will inform the debate about how to dose NRT for NDS.
5. Summary
Non-daily smoking is an increasing pattern of tobacco consumption in the US [1], yet a paucity of information exists on effective treatments for this growing subgroup of smokers. The current study will answer important questions about the comparative effectiveness of counseling only versus counseling in combination with NRT for smoking abstinence in African American NDS. Regardless of whether our hypothesis that counseling plus NRT will be more effective than counseling alone is supported, the current study addresses concerns among providers about lack of evidence-based recommendations, particularly related to use of smoking cessation pharmacotherapy, and concerns among NDS who indicate a strong desire to quit through use of behavioral and pharmacological strategies, and will inform treatment-guidelines for NDS.
Table 4:
Reasons for Ineligibility
| Reason | n |
|---|---|
| Ineligible based on initial phone screening (n=72/625) | 72 |
| < 18 years of age | 2 |
| Daily user of tobacco products (>27 days in last 30) | 14 |
| Smoked cigarettes and/or little cigars on <4 or >27 days in last 30 | 23 |
| Non-daily smoker for < 3 months (only among non-daily smokers) | 18 |
| Smoked < 100 cigarettes/little cigars in lifetime (i.e., non-smoker) | 8 |
| Unwilling to use choice of nicotine patch, gum, or lozenge as part of the study for the next 3 months | 1 |
| Unwilling to refrain from use of other smoking cessation pharmacotherapies, including e-cigarettes for the next 6 months | 1 |
| Unwilling to provide urine for biomarker analyses | 2 |
| Unable to make study-related visits | 1 |
| Another household member enrolled in study | 1 |
| Moving out of area in next 6 months | 5 |
| Pharmacotherapy- or electronic cigarette-assisted quit attempt in the past 30 days | 17 |
| Medical ineligibility | |
| Hospitalized for heart attack or irregular heart beat in past 30 days | 2 |
| Experienced angina or heart-related chest pain in past 30 days | 5 |
| Pregnant, planning to get pregnant, or breastfeeding (females only) | 2 |
| Unwilling to use birth control methods to avoid pregnancy (pre-menopausal or unsterilized females only) | 1 |
| Did not return for final, in-person eligibility screening (n=249/553) | 249 |
| Ineligible based on final in-person eligibility screening (n=25/304) | 25 |
| Unable to provide enough urine for biomarker analyses | 24 |
| Medical ineligibility | |
| Refused or positive pregnancy test (only among pre-menopausal or unsterilized females) | 4 |
FUNDING AND ACKNOWLEDGMENTS
Research reported in this publication was supported funded through a Patient-Centered Outcomes Research Institute (PCORI) Award (AD-1310–08709) and by a CTSA grant from NCATS awarded to the University of Kansas Medical Center for Frontiers: The Heartland Institute for Clinical and Translational Research # UL1TR000001. The statements in this publication are solely the responsibility of the authors and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute (PCORI), its Board of Governors or Methodology Committee.
Footnotes
TRIAL REGISTRATION NUMBER: ClinicalTrials.gov: NCT02244918
TRIAL STATUS
The final participant was enrolled in May 2017. The active treatment and follow-up phases are ongoing, with the final week 26 follow-up visit scheduled for January 2018. Outcomes data have not been examined and will not be available until December 2018.
COMPETING INTERESTS
None to report
Contributor Information
Nicole L. Nollen, Department of Preventive Medicine and Public Health, University of Kansas School of Medicine, Kansas City, Kansas
Lisa Sanderson Cox, Department of Preventive Medicine and Public Health, University of Kansas School of Medicine, Kansas City, Kansas, lcox@kumc.edu.
Matthew S. Mayo, Department of Biostatistics, University of Kansas School of Medicine, Kansas City, Kansas, mmayo@kumc.edu
Edward F. Ellerbeck, Department of Preventive Medicine and Public Health, University of Kansas School of Medicine, Kansas City, Kansas, eellerbe@kumc.edu
Sheshadri Madhusudhana, Department of Hematology/Oncology, Truman Medical Center, Kansas City, Missouri, sheshadri.madhusudhana@tmcmed.org.
Jasjit S. Ahluwalia, Department of Behavioral and Social Sciences, Brown University, Providence, Rhode Island, jasjit_ahluwalia@brown.edu
REFERENCES
- 1.Jamal A, et al. , Current Cigarette Smoking Among Adults - United States, 2005–2015. MMWR Morb Mortal Wkly Rep, 2016. 65(44): p. 1205–1211. [DOI] [PubMed] [Google Scholar]
- 2.Luoto R, Uutela A, and Puska P, Occasional smoking increases total and cardiovascular mortality among men. Nicotine Tob Res, 2000. 2(2): p. 133–9. [DOI] [PubMed] [Google Scholar]
- 3.Schane RE, Ling PM, and Glantz SA, Health effects of light and intermittent smoking: a review. Circulation. 121(13): p. 1518–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shiffman S, et al. , Tobacco dependence among intermittent smokers. Nicotine Tob Res, 2012. 14(11): p. 1372–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tindle HA and Shiffman S, Smoking cessation behavior among intermittent smokers versus daily smokers. Am J Public Health, 2011. 101(7): p. e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tong EK, et al. , Nondaily smokers should be asked and advised to quit. Am J Prev Med, 2006. 30(1): p. 23–30. [DOI] [PubMed] [Google Scholar]
- 7.Scheuermann TS, et al. , Intent to quit, quit attempts, and perceived health risk reduction among African American, Latino, and White nondaily and daily smokers in the United States. Ethn Health, 2017: p. 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shiffman S, et al. , Characteristics and smoking patterns of intermittent smokers. Exp Clin Psychopharmacol, 2012. 20(4): p. 264–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khariwala S, et al. , Do Health Care Providers Differentiate between Daily and Nondaily Smokers when Counseling for Smoking Cessation? Analysis by Race/Ethnicity. Journal of Family Medicine, 2015. 2(6): p. 1041–1048. [Google Scholar]
- 10.Sacks R, et al. , Exploring the next frontier for tobacco control: Nondaily smoking among New York City adults. J Environ Public Health, 2012. 2012: p. 145861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trinidad DR, et al. , A nationwide analysis of US racial/ethnic disparities in smoking behaviors, smoking cessation, and cessation-related factors. Am J Public Health, 2011. 101(4): p. 699–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trinidad DR, et al. , Intermittent and light daily smoking across racial/ethnic groups in the United States. Nicotine Tob Res, 2009. 11(2): p. 203–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.American Cancer Society, Cancer Facts & Figures for African Americans 2013–2014. 2013, Atlanta, GA: American Cancer Society. [Google Scholar]
- 14.American Cancer Society, Cancer Facts & Figures for African Americans, 2016–2018. 2017, American Cancer Society: Atlanta, GA. [Google Scholar]
- 15.Haiman CA, et al. , Ethnic and racial differences in the smoking-related risk of lung cancer. N Engl J Med, 2006. 354(4): p. 333–42. [DOI] [PubMed] [Google Scholar]
- 16.Khariwala SS, et al. , Cotinine and tobacco-specific carcinogen exposure among nondaily smokers in a multiethnic sample. Nicotine Tob Res, 2014. 16(5): p. 600–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shiffman S, Dunbar MS, and Benowitz NL, A comparison of nicotine biomarkers and smoking patterns in daily and nondaily smokers. Cancer Epidemiol Biomarkers Prev, 2014. 23(7): p. 1264–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berg CJ and Schauer GL, Results of a Feasibility and Acceptability Trial of an Online Smoking Cessation Program Targeting Young Adult Nondaily Smokers. Journal of Environmental and Public Health, 2012. 2012: p. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schane RE, Prochaska JJ, and Glantz SA, Counseling nondaily smokers about secondhand smoke as a cessation message: a pilot randomized trial. Nicotine Tob Res, 2013. 15(2): p. 334–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scheuermann TS, et al. , Smoking dependence across the levels of cigarette smoking in a multiethnic sample. Addict Behav, 2015. 43: p. 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shiffman S, et al. , Smoking patterns and stimulus control in intermittent and daily smokers. PLoS One, 2014. 9(3): p. e89911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McGrath DS, et al. , Polytobacco use in non-daily smokers: an issue requiring greater attention. Prev Med, 2011. 53(4–5): p. 353–4. [DOI] [PubMed] [Google Scholar]
- 23.Nollen NL, et al. , Adult Cigarette Smokers at Highest Risk for Concurrent Alternative Tobacco Product Use Among a Racially/Ethnically and Socioeconomically Diverse Sample. Nicotine Tob Res, 2016. 18(4): p. 386–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fiore M, et al. , Treating Tobacco Use and Dependence Clinical Practice Guideline: 2008 Update. 2008, Washington, DC: U.S. Department of Health and Human Services. [Google Scholar]
- 25.Perkins KA, Conklin CA, and Levine MD, Cognitive-behavioral therapy for smoking cessation: a practical guidebook to the most effective treatments. 2008: Taylor & Francis. [Google Scholar]
- 26.Webb Hooper M, et al. , Randomized Controlled Trial of Group-Based Culturally Specific Cognitive Behavioral Therapy Among African American Smokers. Nicotine Tob Res, 2017. 19(3): p. 333–341. [DOI] [PubMed] [Google Scholar]
- 27.Harris KJ, et al. , Addressing cultural sensitivity in a smoking cessation intervention: Development of the Kick it at Swope project. Journal of Community Psycholoy, 2001. 29(4): p. 447–458. [Google Scholar]
- 28.Ahluwalia JS, et al. , The effects of nicotine gum and counseling among African American light smokers: a 2 × 2 factorial design. Addiction, 2006. 101(6): p. 883–91. [DOI] [PubMed] [Google Scholar]
- 29.Catley D, et al. , A Randomized Trial of Motivational Interviewing: Cessation Induction Among Smokers With Low Desire to Quit. Am J Prev Med, 2016. 50(5): p. 573–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nollen NL, et al. , A clinical trial to examine disparities in quitting between African-American and White adult smokers: Design, accrual, and baseline characteristics. Contemp Clin Trials, 2016. 47: p. 12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cox LS, et al. , Bupropion for smoking cessation in African American light smokers: a randomized controlled trial. J Natl Cancer Inst, 2012. 104(4): p. 290–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hurt RD, et al. , Treating tobacco dependence in a medical setting. CA Cancer J Clin, 2009. 59(5): p. 314–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harris KJ, et al. , Timeline follow-back versus global self-reports of tobacco smoking: a comparison of findings with nondaily smokers. Psychol Addict Behav, 2009. 23(2): p. 368–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brown RA, et al. , Reliability and validity of a smoking timeline follow-back interview. Psychology of Addictive Behavior, 1998. 12(2): p. 101–112. [Google Scholar]
- 35.Benowitz NL, Hukkanen J, and Jacob P 3rd, Nicotine chemistry, metabolism, kinetics and biomarkers. Handb Exp Pharmacol, 2009(192): p. 29–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perez-Stable EJ, et al. , Nicotine metabolism and intake in black and white smokers. Jama, 1998. 280(2): p. 152–6. [DOI] [PubMed] [Google Scholar]
- 37.Okuyemi KS, et al. , Predictors of adherence to nicotine gum and counseling among African-American light smokers. J Gen Intern Med, 2010. 25(9): p. 969–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jacob P 3rd, et al. , Anabasine and anatabine as biomarkers for tobacco use during nicotine replacement therapy. Cancer Epidemiol Biomarkers Prev, 2002. 11(12): p. 1668–73. [PubMed] [Google Scholar]
- 39.Benowitz N, et al. , Biochemical verification of tobacco use and cessation. Nicotine Tob Res, 2002. 4(2): p. 149–59. [DOI] [PubMed] [Google Scholar]
- 40.Benowitz NL, et al. , Estimation of nicotine dose after low-level exposure using plasma and urine nicotine metabolites. Cancer Epidemiol Biomarkers Prev, 2010. 19(5): p. 1160–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hecht SS, Human urinary carcinogen metabolites: biomarkers for investigating tobacco and cancer. Carcinogenesis, 2002. 23(6): p. 907–22. [DOI] [PubMed] [Google Scholar]
- 42.Jorenby DE, et al. , Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. Jama, 2006. 296(1): p. 56–63. [DOI] [PubMed] [Google Scholar]
- 43.Nollen NL, et al. , A pilot clinical trial of varenicline for smoking cessation in black smokers. Nicotine Tob Res, 2011. 13(9): p. 868–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schnoll RA, et al. , Nicotine metabolic rate predicts successful smoking cessation with transdermal nicotine: a validation study. Pharmacol Biochem Behav, 2009. 92(1): p. 6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schnoll RA, et al. , Effectiveness of extended-duration transdermal nicotine therapy: a randomized trial. Ann Intern Med, 2010. 152(3): p. 144–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dorsey R and Graham G, New HHS data standards for race, ethnicity, sex, primary language, and disability status. JAMA, 2011. 306(21): p. 2378–9. [DOI] [PubMed] [Google Scholar]
- 47.Centers for Disease Control and Prevention, Behavioral Risk Factor Surveillance System Survey Questionnaire. 2012, U.S. Department of Health and Human Services,Centers for Disease Control and Prevention: Atlanta, Georgia. [Google Scholar]
- 48.Idler EL, Russell LB, and Davis D, Survival, functional limitations, and self-rated health in the NHANES I Epidemiologic Follow-up Study, 1992. First National Health and Nutrition Examination Survey. Am J Epidemiol, 2000. 152(9): p. 874–83. [DOI] [PubMed] [Google Scholar]
- 49.Lohman T, Roche A, and Martorell R, Anthropometric standardization reference manual. 1988, Champaign, IL: Human Kinectic Books. [Google Scholar]
- 50.Scheuermann TS, et al. , Correlates of Converted and Native Nondaily Smoking. Nicotine Tob Res, 2015. 17(9): p. 1112–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Al-Delaimy WK, et al. , Technical Report on Analytic Methods and Approaches Used in the 2008 California Tobacco Survey Analysis. Vol 1: Data Collection Methodology. 2009, University of California, San Diego: La Jolla, CA. [Google Scholar]
- 52.Scheuermann TS, et al. , A qualitative exploration of smoking influencs and quit attempts among nondaily smokers. Health Behavior and Poliy Review, 2014. 1(3): p. 1172–182. [Google Scholar]
- 53.Fagerstrom K, et al. , The Fagerstrom Test for Nicotine Dependence as a predictor of smoking abstinence: a pooled analysis of varenicline clinical trial data. Nicotine Tob Res, 2012. 14(12): p. 1467–73. [DOI] [PubMed] [Google Scholar]
- 54.Vajer P, et al. , Psychometric properties and construct validity of the brief Wisconsin inventory of smoking dependence motives in an Internet-based sample of treatment-seeking Hungarian smokers. Nicotine Tob Res, 2011. 13(4): p. 273–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hughes JR, Effects of abstinence from tobacco: valid symptoms and time course. Nicotine Tob Res, 2007. 9(3): p. 315–27. [DOI] [PubMed] [Google Scholar]
- 56.Tiffany ST and Wray JM, The clinical significance of drug craving. Ann N Y Acad Sci, 2012. 1248: p. 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Centers for Disease Control and Prevention (CDC) and National Center for Health Statistics (NCHS), National Health and Nutrition Examination Survey. 2012, U.S. Department of Health and Human Services, Centers for Disease Control and Prevention: Hyattsville, MD. [Google Scholar]
- 58.Kroenke K, Spitzer RL, and Williams JB, The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care, 2003. 41(11): p. 1284–92. [DOI] [PubMed] [Google Scholar]
- 59.Frank D, et al. , Effectiveness of the AUDIT-C as a screening test for alcohol misuse in three race/ethnic groups. J Gen Intern Med, 2008. 23(6): p. 781–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Center for Behavioral Health Statistics and Quality, 2014 National Survey on Drug Use and Health: Detailed Tables. 2015, Substance Abuse and Mental Health Services Administration: Rockville, MD. [Google Scholar]
- 61.Gariti P, et al. , Comparing smoking treatment programs for lighter smokers with and without a history of heavier smoking. Journal of Substance Abuse Treatment, 2009. 37(3): p. 247–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stead LF, et al. , Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev, 2012. 11: p. CD000146. [DOI] [PubMed] [Google Scholar]
- 63.West R, et al. , Outcome criteria in smoking cessation trials: proposal for a common standard. Addiction, 2005. 100(3): p. 299–303. [DOI] [PubMed] [Google Scholar]
- 64.CONSORT 2010 Explanation and Elaboration: updated guidelines for reporting parallel group randomised trials. BMJ, 2011. 343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pulvers K, et al. , Classifying a smoker scale in adult daily and nondaily smokers. Nicotine Tob Res, 2014. 16(5): p. 591–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kasza KA, et al. , Tobacco-Product Use by Adults and Youths in the United States in 2013 and 2014. N Engl J Med, 2017. 376(4): p. 342–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee YO, et al. , Multiple tobacco product use among adults in the United States: cigarettes, cigars, electronic cigarettes, hookah, smokeless tobacco, and snus. Prev Med, 2014. 62: p. 14–9. [DOI] [PubMed] [Google Scholar]
- 68.Cheng T, Chemical evaluation of electronic cigarettes. Tob Control, 2014. 23 Suppl 2: p. ii11–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Djordjevic MV and Doran KA, Nicotine content and delivery across tobacco products. Handb Exp Pharmacol, 2009(192): p. 61–82. [DOI] [PubMed] [Google Scholar]
- 70.Federal Trade Commission, Tar, nicotine, and carbon monoxide of the smoke of 1294 varieties of domestic cigarettes for the year 1998. 2000, Federal Trade Commission Report: Washington, D.C. [Google Scholar]
- 71.Goniewicz ML, et al. , Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control, 2014. 23(2): p. 133–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Janbaz KH, et al. , Risk for oral cancer from smokeless tobacco. Contemp Oncol (Pozn), 2014. 18(3): p. 160–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shihadeh A, et al. , Toxicant content, physical properties and biological activity of waterpipe tobacco smoke and its tobacco-free alternatives. Tobacco Control, 2015. 24(Suppl 1): p. i22–i30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Popova L and Ling PM, Alternative tobacco product use and smoking cessation: a national study. Am J Public Health, 2013. 103(5): p. 923–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Harrison EL, Desai RA, and McKee SA, Nondaily smoking and alcohol use, hazardous drinking, and alcohol diagnoses among young adults: findings from the NESARC. Alcohol Clin Exp Res, 2008. 32(12): p. 2081–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Montgomery L, Marijuana and tobacco use and co-use among African Americans: results from the 2013, National Survey on Drug Use and Health. Addict Behav, 2015. 51: p. 18–23. [DOI] [PubMed] [Google Scholar]
- 77.Schauer GL, et al. , Assessing the overlap between tobacco and marijuana: Trends in patterns of co-use of tobacco and marijuana in adults from 2003–2012. Addict Behav, 2015. 49: p. 26–32. [DOI] [PubMed] [Google Scholar]
- 78.Yoon JH, Lane SD, and Weaver MF, Opioid Analgesics and Nicotine: More Than Blowing Smoke. J Pain Palliat Care Pharmacother, 2015. 29(3): p. 281–9. [DOI] [PubMed] [Google Scholar]
- 79.Schauer GL, Rosenberry ZR, and Peters EN, Marijuana and tobacco co-administration in blunts, spliffs, and mulled cigarettes: A systematic literature review. Addict Behav, 2017. 64: p. 200–211. [DOI] [PubMed] [Google Scholar]
- 80.Schauer GL, et al. , Differences in the relationship of marijuana and tobacco by frequency of use: A qualitative study with adults aged 18–34 years. Psychol Addict Behav, 2016. 30(3): p. 406–14. [DOI] [PubMed] [Google Scholar]
- 81.Russell MA, Cigarette smoking: natural history of a dependence disorder. Br J Med Psychol, 1971. 44(1): p. 1–16. [DOI] [PubMed] [Google Scholar]
- 82.Piper ME, et al. , A multiple motives approach to tobacco dependence: the Wisconsin Inventory of Smoking Dependence Motives (WISDM-68). J Consult Clin Psychol, 2004. 72(2): p. 139–54. [DOI] [PubMed] [Google Scholar]
- 83.Allen AM, et al. , Gender Differences in Smoking Behavior and Dependence Motives Among Daily and Nondaily Smokers. Nicotine Tob Res, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]


