Abstract
Dopamine (DA) is a potent neuromodulator known to influence glutamatergic transmission in striatal medium spiny neurons (MSNs). It acts on D1- and D2-like DA receptors that are expressed on two distinct subpopulations. MSNs projecting to the sub-stantia nigra express D1 receptors (D1Rs), while those projecting to the lateral globus pallidus express D2 receptors (D2Rs). D1R signalling in particular can increase excitatory transmission through varied protein kinase A-dependent, cell-autonomous pathways. Mechanisms by which D1R signalling could increase excitatory transmission in D2R-bearing MSNs have been relatively less explored. Herein, the possibility is considered that D1R agonists increase levels of soluble factors that subsequently influence N-methyl-D-aspartate (NMDA)-stimulated calcium flux in D2R neurons. This study focuses on matrix metalloproteinases (MMPs) and MMP-generated integrin binding ligands, important soluble effectors of glutamatergic transmission that may be elevated in the setting of excess DA. It was observed that DA and a D1R agonist, SKF81297, increase MMP activity in extracts from striatal slices, as determined by cleavage of the substrate β-dystroglycan. Using mice engineered to express the calcium indicator GCaMP3 in striatopallidal D2R-bearing neurons, it was also observed that SKF81297 pretreatment of slices (60 min) potentiates NMDA-stimulated calcium increases in this subpopulation. Effects are diminished by pretreatment with an antagonist of MMP activity or an inhibitor of integrin-dependent signalling. Together, results suggest that DA signalling can increase excitatory transmission in D2R neurons through an MMP-dependent mechanism. Future studies may be warranted to determine whether D1R-stimulated MMP-dependent processes contribute to behaviours in which increased activity in striatopallidal MSNs plays a role.
Keywords: calcium, MMPs, mouse, MSN, NMDA, striatum
Introduction
Neuromodulators and dopamine (DA) in particular play an important role in working memory, attention and appetitive reward (for review, see Surmeier et al., 2010; Tritsch & Sabatini, 2012). Although receptors for DA are present in varied cell populations including pyramidal neurons of the cerebral cortex, medium spiny neurons (MSNs) of the striatum express relatively high levels (Tritsch & Sabatini, 2012). Striatal MSNs fall into one of two equally sized groups that are characterized by a predominance of D1 or D2 DA receptor expression and associated efferent pathways (Gerfen et al., 1990). While there are data to suggest that the two pathways can have opposing effects on select aspects of motor behaviour, recent work suggests that activity in both pathways may be increased with drug of abuse-associated plasticity as well as behaviours including decision-making and movement initiation (Koch et al., 2000; Bock et al., 2013; Cui et al., 2013). Consistent with coordinated activation of distinct DA receptor-bearing cell subpopulations, D1- and D2-like DA receptors play complementary roles in prefrontal cortex-associated emotional memory (Lauzon et al., 2009).
Dopamine exerts its behavioural effects through the modulation of neurotransmission, and it does this through several non-mutually exclusive mechanisms (Greengard, 2001). Because D1 DA receptors are coupled to Gs/olf-associated G-protein-coupled signalling pathways while D2 DA receptors are instead coupled to Gi, many studies examining the potential of DA to increase glutamatergic transmission focus on D1-dependent, cell-autonomous, effects. For example, D1 DA receptor activation has been linked to protein kinase A (PKA)-dependent modulation of voltage-gated ion channels and PKA-mediated phosphorylation of GluA1 glutamate receptor subunits (Surmeier et al., 1995; Tritsch & Sabatini, 2012). Though DA and D1 agonists also have the potential to generate soluble effectors of enhanced excitatory transmission, their potential to increase glutamate-stimulated responses in D2 MSNs has been relatively less explored.
Matrix metalloproteinases (MMPs) represent a family of zinc-dependent secreted endopeptidases that are expressed and/or activated in the setting of enhanced glutamatergic or dopaminergic transmission (Nagy et al., 2006; Meighan et al., 2007; Tian et al., 2007; Conant et al., 2010; Dziembowska et al., 2012). MMPs play a role in hippocampal and striatal learning and memory (Meighan et al., 2006; Nagy et al., 2006; Mizoguchi et al., 2008; Wiera et al., 2013; Smith et al., 2014), and their activity has been linked to structural changes in dendritic spines (Wang et al., 2008; Szepesi et al., 2013; Smith et al., 2014). Importantly, MMPs can act on synaptically localized substrates to generate soluble integrin-binding ligands that may in turn influence N-methyl-D-aspartate (NMDA)-stimulated calcium increases through downstream effects on the phosphorylation and function of GluNs, GluAs and/or ion channel subunits (Davis et al., 2002a; Wildering et al., 2002; Bernard-Trifilo et al., 2005; Chen et al., 2010; Lonskaya et al., 2013).
In the present study, mice engineered to show calcium-activated fluorescence in D2 DA receptor-bearing cells were used in order to explore the ability of DA and D1 agonists to increase NMDA-stimulated intracellular calcium flux in this cell type. Because DA may directly and potently enhance GluN-mediated calcium flux in D1 but not D2 MSNs (Higley & Sabatini, 2010; Tritsch & Sabatini, 2012), indirect potentiation by secondarily elevated soluble effectors is less likely to be masked in this latter subpopulation. Using acute striatal slices from these animals, specific mechanisms by which DA and a D1 DA receptor agonist can influence NMDA-stimulated glutamate responses in D2 DA receptor-bearing cells were also investigated. These studies focused on MMPs and integrin-dependent signalling.
Materials and methods
Chemicals
Studies employed commercially available chemicals as follows: DA (Sigma Aldrich; H8502); SKF81297 (Tocris Bioscience, Minneapolis, MN, USA; Cat#1447); GM6001 (Tocris Bioscience; Cat#2983); RGDS peptide (Tocris Bioscience; Cat#3498). DA was freshly prepared just prior to use. SKF81297, a DA D1-like receptor agonist, was prepared in water at stock concentration of 1 mM. GM6001, a broad-spectrum inhibitor of MMP activity including those implicated in striatal and hippocampal plasticity (Meighan et al., 2006; Nagy et al., 2006; Smith et al., 2014), was dissolved in dimethyl sulphoxide (DMSO) at a stock concentration of 10 mM. An RGDS peptide, a concentration-dependent antagonist of integrin binding by ligands with an RGD motif (Legler et al., 2001), was dissolved in phosphate-buffered saline (PBS) at stock concentration of 50 mM. Stock solutions were stored at −20 °C.
D2R-GCaMP3 mice
Cre-dependent GCaMP3 mice (Zariwala et al., 2012) were purchased from the Jackson Laboratory (Jackson Laboratory Stock # 014538). These animals were bred with mice that express Cre recombinase under the control of the D2R promoter (Gong et al., 2007). The presence of Cre and GCaMP3 in pups from this breeding strategy was confirmed using automated genotyping (Transnetyx, Cordova, TN, USA). All animal experimental procedures were performed in accordance with the guidelines of the Georgetown University Animal Care and use Committee.
Cortical astrocyte cultures
For astrocyte cultures, meninges were removed and brain tissue was dissociated as previously described (Conant et al., 1998). Cells were then plated onto 12-well plates at a density of 1.5 × 105/well. Astrocytes were maintained in Eagle’s minimal essential medium (Thermo-Fisher, Frederick, MD, USA) supplemented with 10% foetal bovine serum, 100 U/mL penicillin and streptomycin. Medium was changed weekly, and cultures were stored in a humidified 5% CO2 and 95% O2 incubator at 37 °C.
Slice preparation and treatments
Following death, the brain was removed and rapidly placed in ice-cold slicing solution comprising (in mM): NaCl, 85; NaHCO3, 25; KCl, 2.5; NaH2PO4, 1; glucose, 25; sucrose, 75; MgCl2, 4; CaCl2, 1; osmolarity 325 mosmol/L; saturated with 95% O2 and 5% CO2. Coronal slices of striatum (250 μm) from D2R-GCaMP3 mice were acutely prepared from animals ranging in age from postnatal 18 to 21. Slices were subsequently transferred to artificial cerebrospinal fluid (aCSF) comprising (in mM): NaCl, 124; KCl, 4.5; MgCl2, 1; dextrose, 10; CaCl2, 2.0; NaH2PO4, 1.24; NaHCO3, 26; osmolarity 325 mosmol/L; saturated with 95% O2 and 5% CO2; and incubated at 33 °C in a water bath for 30 min and subsequently stored at room temperature for an additional 40 min. The slices were then treated with 50 μM DA or 10 μM SKF81297 (diluted 1 : 100 in aCSF) and incubated in a cell incubator at 33 °C for 60 min. For the MMP inhibitor and integrin antagonist treatments, GM6001 was diluted to 25 μM from a 10 mM stock solution in DMSO and pre-applied for 10 min, as shown in Fig. 4a. Additionally, RGDS was diluted to 500 μM from 50 mM in PBS and added 30 min prior to SKF81297, as shown in Fig. 5a. Imaging experiments were performed between 1 and 4 h after brain dissection.
Fig. 4.
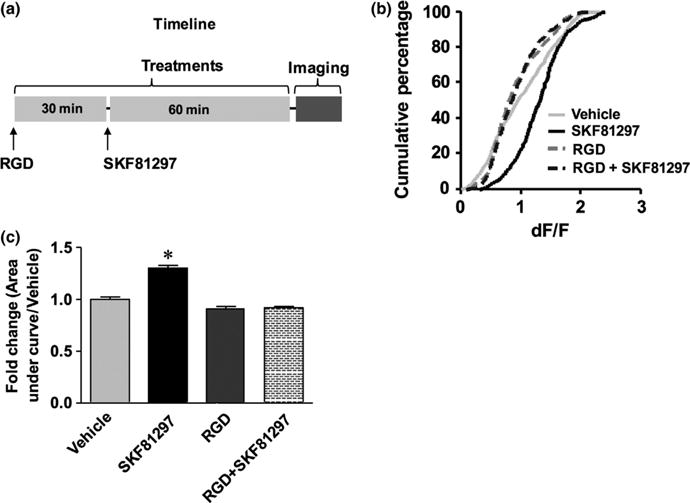
An RGD peptide integrin antagonist reduces the N-methyl-D-aspartate (NMDA)-stimulated calcium response in SKF81297-treated striatal slices. The treatment timeline is shown in (a), cumulative percentage plots are shown in (b), and area under the curve data in (c) (vehicle n = 554 cells from 11 fields; SKF81297 n = 476 cells from 11 fields; RGDS n = 673 cells from 14 fields; and RGDS + SKF81297 n = 603 cells from 14 fields). Ordinary one-way ANOVA showed a statistically significant difference across all groups (F3,2301 = 87.27, P < 0.0001). Post hoc testing with Tukey’s method revealed statistically significant differences between vehicle and SKF81297 (q2301 = 15.22, *P < 0.0001), and between SKF81297 and RGD + SKF81297 (q2301 = 19.95, *P < 0.0001). The difference between RGD and RGD + SKF81297 is not significant (q2301 = 0.1468, P > 0.05). Data were derived from vehicle and treated slices from each of three separate mice.
Fig. 5.
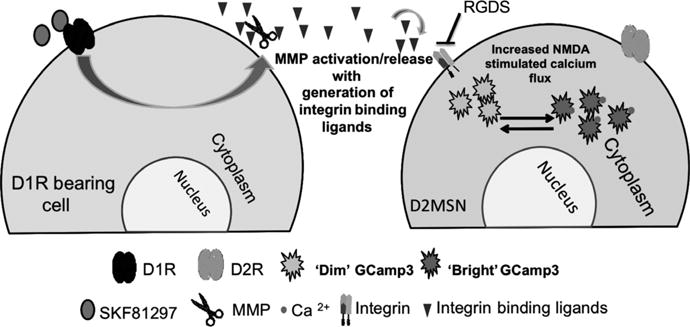
Hypothetical model of SKF81297 action in the striatum. SKF81297 acts on D1-like dopamine (DA) receptors to increase matrix metalloproteinase (MMP) release and/or activation with subsequent generation of integrin-binding ligands. Increased integrin signalling leads to enhanced calcium flux following exposure to N-methyl-D-aspartate (NMDA). Enhanced calcium flux could follow from increased entry through GluNs, in that subunit phosphorylation and receptor function are modulated by integrin-dependent signalling cascades (Bernard-Trifilo et al., 2005). Enhanced flux could also follow from secondary neuromodulatory mechanisms, including effects on ion channels, by which integrin engagement may stimulate an increase in intracellular free calcium (Wu et al., 1998; Wildering et al., 2002).
Calcium imaging recording and imaging analysis
Imaging from 250-μm coronal striatal slices was performed using an upright microscope (E600FN; Nikon, Tokyo, Japan) equipped with Nomarski optics and a 20 × water-immersion objective lens (aperture 0.050). During imaging, slices were maintained in aCSF at room temperature (22–24 °C). Recording was performed using a 16-bit Rolera-XR FAST 1394 camera (QImaging, Surrey, BC, Canada). Two-hundred and fifty frames (280 ms interval between frames) were captured for a total of 70 s. Slices were excited with 488 nm light to visualize neurons. For chemical stimulation, NMDA (40 μM; Sigma) was dissolved in normal aCSF and applied locally through a Y-tubing device as previously described (Partridge et al., 2014). Images were analysed from NMDA-stimulated calcium detection experiments with ImageJ software (NIH, Bethesda, MD, USA) to plot the fluorescence intensity of multiple user-defined regions of interest (ROIs) over time. To correct for baseline fluorescence, these values were divided by the average light intensity of the first 10 frames. The intensity vs. time monitor plugin in IMAGE J was used to visualize responses in individual ROIs. These regions were analysed in each captured frame, and changes in fluorescence intensity (dF/F) as a function of time were computed for each ROI with ClampFit (Molecular Devices LLC, Sunnyvale, CA, USA).
Western blot
Western blot was performed on lysates as previously described (Conant et al., 2011b). In brief, lysates from striatal slices as well as primary cells were prepared via the addition of lysis buffer containing (in mM): Tris–HCl, 50, pH 7.5; NaCl, 150; 0.1% sodium dodecyl sulphate; 1% NP-40; 0.5% sodium deoxycholate; phenylmethylsulphonyl fluoride, 0.2; dithiothreitol, 0.5; 1 × pro-tease and phosphatase inhibitor cocktail (Thermo Scientific, Wal-tham, MA, USA; #78440). The mixture was placed into a microfuge tube, sonicated for 10 s, kept on ice for 20 min, and then spun at 14 500 g for 15 min at 4 °C in a microcentrifuge. Protein concentrations in the lysates were quantified with the bicinchoninic acid assay (BCA assay). Equal quantities of protein were mixed with loading buffer containing β-mercaptoethanol and heated for 5 min at 95 °C. After resolution through gel electrophoresis using 4–20% precast gels (Bio-Rad, Hercules, CA, USA; Cat#456-1094), and transfer to nitrocellulose, blots were probed with antibodies to β-dystroglycan (Leica Biosystems, Nussloch, Germany; NCL-β-DG, 1 : 750 dilution) or GAPDH (R&D Systems, Minneapolis, MN, USA; PPS014, 1 : 1000 dilution). Molecular weights were inferred by comparison to prestained markers (BioRad). Following exposure of membranes to western-lighting plus-ECL (PerkinElmer, Waltham, MA, USA; 203-73391) and film, bands were quantified with IMAGE J software.
Statistics
Statistical analyses were performed with GraphPad Prism 5 (GraphPad Software, La Jolla, CA, USA). Data are shown as mean ± SEM. Student’s t-test was used for two group comparisons including imaging analysis of DA and vehicle-treated calcium flux in MSNs. For comparison of more than two groups, one-way ANOVA followed by Tukey post hoc testing was used to determine significance.
Results
DA pretreatment of striatal slices increases subsequent NMDA-stimulated calcium flux in D2 MSNs
To investigate the effects of DA on NMDA-stimulated calcium flux in striatal D2 MSNs, striatal slices were prepared from Cre-dependent reporter mice that express the genetically encoded Ca2+ sensor protein GCaMP3 in D2 MSNs (Partridge et al., 2014). Because significant MMP-dependent effects were expected to take minutes rather than seconds to emerge, and because select psychostimulants and anti-depressants can cause a lasting increase in DA (Peris & Zahniser, 1987; Nomikos et al., 1989), the slices were pretreated for 60 min. As shown in Fig. 1, following bath application of DA to the striatal slice (Fig. 1a), NMDA was added to the striatum (Fig. 1b). Fluorescent signal was recorded prior to and following NMDA treatment with average trace values superimposed (Fig. 1c). DA induced a right shift of dF/F as depicted in cumulative percentage plots (Fig. 1d). Quantitative data show fold increase in average area under the curve (Fig. 1e), and from this it can be appreciated that DA pretreatment stimulated a significant increase in the subsequent NMDA-stimulated response.
Fig. 1.
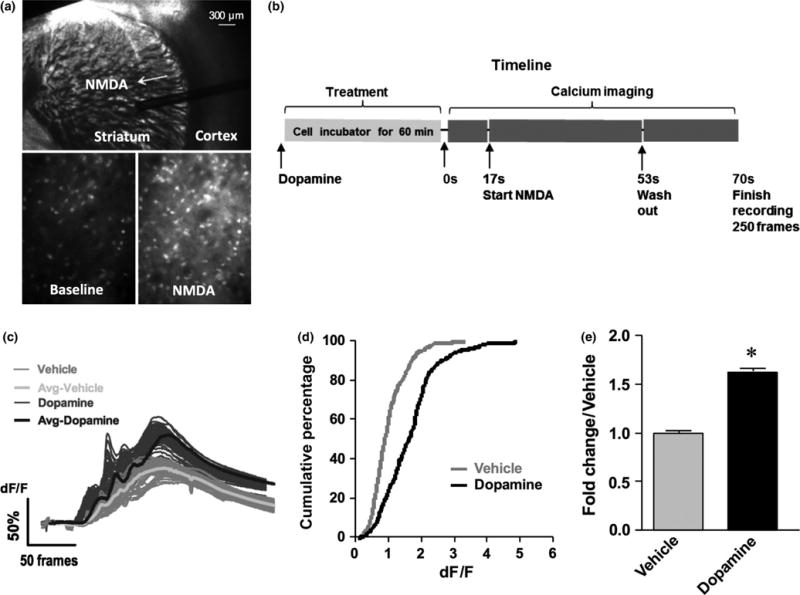
Pretreatment of slices with dopamine (DA) increases the N-methyl-D-aspartate (NMDA)-stimulated calcium response in D2 medium spiny neurons (MSNs). A representative image of a coronal striatal slice at postnatal day 18 is shown in (a). The arrow indicates placement of tubing for local NMDA application. Below left (a) is a representative image of the fluorescent signal in unstimulated D2 MSN GCaMP3-positive cells (baseline), and below right (a) is a representative image of fluorescence following NMDA application (NMDA). A graphic timeline of drug treatment and calcium imaging is shown in (b). Traces of the change in fluorescence as a function of time and stimulated by 40 μM NMDA in local regions of interest (ROIs) is shown in (c). Time is represented as frame number. Individual traces with averages superimposed are shown from 312 vehicle pre-treated cells from six separate field recordings, and 477 DA-pre-treated cells from nine separate field recordings. Cumulative percentage plots for calcium dF/F in vehicle and DA groups are shown in (d). Quantification of area under the curve data is shown in (e). Data represent fold change from vehicle, and the difference between vehicle and DA is significant (two-tailed t-test, t757 = 12.41, *P < 0.0001).
DA and a D1R-selective agonist, SKF81297, increase MMP activity
Activation of DA receptors may increase levels of soluble effectors of excitatory neurotransmission, including MMPs, with the potential to influence excitability in D2 MSNs. To evaluate this possibility, DA and a D1 agonist (SKF81297) were tested for their potential to stimulate cleavage of a known MMP substrate, β-dystroglycan (Michaluk et al., 2007; Court et al., 2011). These studies focused on striatal slices and cultured astrocytes. The latter express D1 receptors (Miyazaki et al., 2004), and have the potential to release MMPs such as MMP-2 that can modulate synaptic plasticity (Smith et al., 2014). As shown, DA and SKF81297 increased β-dystroglycan cleavage in striatal slices (Fig. 2a and b). In addition, increased β-dystroglycan cleavage was observed in DA-treated astrocytes (Fig. 2c).
Fig. 2.
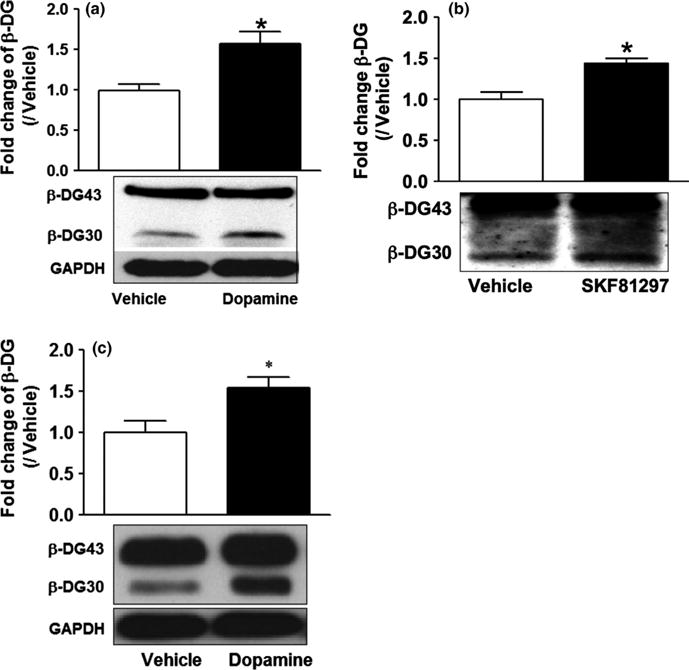
Dopamine (DA) and SKF81297 stimulate an increase in matrix metalloproteinase (MMP) activity. DA and SKF81297 stimulate increased cleavage of β-dystroglycan in striatal slices (a and b). Densitometric data shown in (a) are from four vehicle and five DA-treated slice lysates, and the difference is significant (two-tailed t-test, t7 = 3.259, *P = 0.0139). Densitometric data shown in (b) are from three vehicle and four SKF81297-treated slice lysates, and the difference is significant (two-tailed t-test, t5 = 4.22, *P = 0.0083). DA (60 min) also simulates increased β-dystroglycan cleavage in cultured astrocytes (c). Densitometric results represent the average fold increase from four vehicle and four DA-treated astrocyte culture lysates. The difference between β-DG30 in vehicle and DA-treated lysates was significant (two-tailed t-test, t6 = 2.87, *P = 0.0284). GAPDH is shown as a loading control.
SKF81297 increases the NMDA-stimulated calcium response in D2 MSNs; involvement of MMP activity
MMP activity has been linked to increased neuronal excitation and glutamatergic signalling. Depending on the system, MMP-generated integrin-binding ligands have the potential to stimulate changes in GluN and GluA subunit phosphorylation and overall receptor function. To determine whether SKF81297 could stimulate MMP-dependent increases in D2 MSN NMDA-stimulated calcium flux, this compound was tested in the presence and absence of GM6001, a broad-spectrum MMP inhibitor. The experimental time course (Fig. 3a), cumulative percentage data (Fig. 3b) and average area under the response curve (Fig. 3c) are shown. It was observed that pretreatment of slices with GM6001 had a small but significant effect on NMDA-stimulated calcium flux in control slices, which is consistent with the rapid NMDA-stimulated neuromodulatory effects of these enzymes (Meighan et al., 2007; Wojtowicz & Mozrzymas, 2014). Importantly, it was also observed that GM6001 blocked potentiation of the NMDA-stimulated response by SKF81297.
Fig. 3.
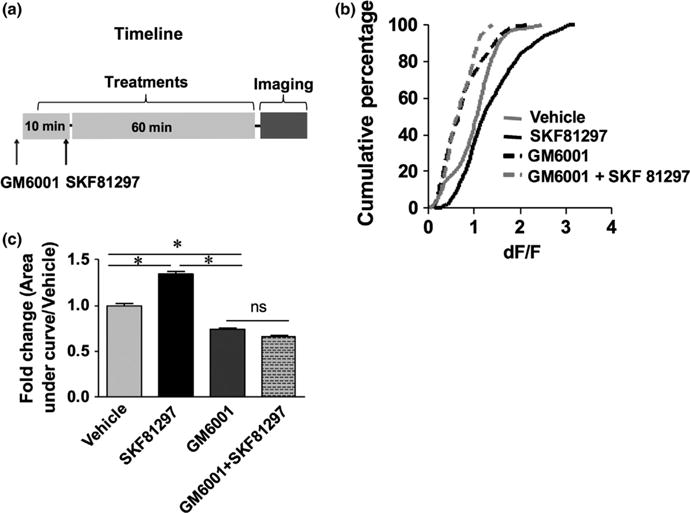
Pretreatment of slices with SKF81297 increases N-methyl-D-aspartate (NMDA)-stimulated calcium flux in D2 medium spiny neurons (MSNs), and a broad spectrum matrix metalloproteinase (MMP) inhibitor reduces the vehicle and SKF-stimulated response. The experimental timeline is shown in (a), cumulative percentage plots are shown in (b), and quantification of area under the curve data is shown in (c) (vehicle n = 493 cells from 11 separate fields; SKF81297 n = 704 cells from 12 fields; GM6001 n = 499 cells from 12 fields; and GM6001 + SKF81297 n = 389 cells from 11 fields). Ordinary one-way ANOVA showed a statistically significant difference across all groups (F3,2082 = 214.4, P < 0.0001). Post hoc testing with Tukey’s method revealed significant differences between vehicle and SKF81297 (q2082 = 16.50, *P < 0.0001), and between SKF81297 and GM6001 + SKF81297 (q2082 = 30.48, *P < 0.0001). The difference between vehicle and GM6001 is smaller but also significant (q2082 = 11.62, *P < 0.0001). The difference between GM6001 and GM6001 + SKF81297 is not significant (q2082 = 3.250, P > 0.05). Data were derived from vehicle and treated slices from each of three separate mice.
RGDS reduces the NMDA-stimulated calcium response in SKF81297-treated MSNs
MMPs are thought to enhance glutamatergic signalling in the hippocampus through their ability to generate integrin-binding ligands (Nagy et al., 2006; Meighan et al., 2007; Lonskaya et al., 2013; Sonderegger & Matsumoto-Miyai, 2014). To determine whether increased integrin signalling might contribute to the SKF81297-potentiated NMDA response in striatal neurons, next an integrin antagonist (RGDS) was tested for its potential to inhibit the same. RGD-containing peptides can interfere with the binding of β1 and β3 integrin ligands containing the RGD-binding motif. Both β1 and β3 integrins are important to neuronal circuit formation, with β1 of particular importance to Hebbian-dependent forms of plasticity (Huang et al., 2006; McGeachie et al., 2012). Slices were pretreated with 500 μM RGDS 30 min prior to SKF81297 as indicated in Fig. 4a. This relatively long pretreatment was to allow the peptide to better penetrate the slice. Though nanomolar concentrations of RGD peptides can have paradoxical effects on integrin signalling (Legler et al., 2001), a 500 μM concentration inhibits activation of RGD-binding β1 and β3 integrins (Legler et al., 2001), as well as MMP-dependent effects on hippocampal long-term potentiation (LTP; Staubli et al., 1998; Nagy et al., 2006). Concentrations in this range (200–500 μM) inhibit other forms of integrin-dependent plasticity as well (Rohrbough et al., 2000; Hernandez et al., 2001; LeBaron et al., 2003). Cumulative percentage data and average area under the response curve as a function of treatment group are shown (Fig. 4b and c). While RGDS pretreatment had a non-significant effect on the vehicle-stimulated response, it blocked potentiation by SKF81297. An overall schematic of the hypothetical mechanism by which SKF81297 stimulates an integrin-dependent increase in NMDA-stimulated calcium flux in D2 MSNs is shown in Fig. 5.
Discussion
Dopamine has been shown to increase glutamatergic neurotransmission through a variety of non-mutually exclusive mechanisms including D1-like receptor-dependent activation of PKA with subsequent changes in intrinsic membrane excitability and synaptic effects including GluA subunit phosphorylation (Tritsch & Sabatini, 2012). DA can also modulate GluN function in a developmentally specific manner (Tong & Gibb, 2008). Consistent with its ability to potently modulate neurotransmission, DA is known to play a role in the control of motor activity and in cognitive processes including decision-making (Tritsch & Sabatini, 2012). Increased DA signalling has also been implicated in addiction to drugs of abuse, including cocaine and methamphetamine (Sorg & Kalivas, 1991; Mizoguchi et al., 2008).
In addition to its potential to activate cell-autonomous PKA-dependent changes that could substantially contribute to the effect of DA on select behavioural outcomes, DA and D1R agonists have been shown to increase both the expression and activity of specific transmembrane and soluble proteinases (Ito et al., 2007; Iwakura et al., 2011). Nawa and colleagues showed that DA and a D1R agonist could stimulate increased MMP and A Disintegrin And Metallo-proteinase (ADAM) activity, as well as increased MMP-dependent transmembrane protein shedding (Iwakura et al., 2011) in striatal cultures. Drugs of abuse that elevate DA levels also increase MMP-2 and/or -9 in rodent frontal cortex and striatum (Liu et al., 2008; Conant et al., 2011a). These changes are of potential interest in that MMP activity may be critical to plasticity-related endpoints in varied brain regions. Chemical MMP inhibitors block LTP in hippocampal slices, and these inhibitors also block methamphetamine-associated conditioned place preference (Nagy et al., 2006; Meighan et al., 2007; Mizoguchi et al., 2008; Conant et al., 2010). Of additional relevance to a role for MMPs as effectors of DA-stimulated plasticity in particular, mice deficient in the MMP activator tissue plasminogen activator show reduced potentiation by cAMP analogues and D1/D5 agonists (Huang et al., 1996). In more recent work, it has been shown that MMP-2 and -9 are involved in synaptic changes occurring in the striatum of cocaine-extinguished and - reinstated animals (Smith et al., 2014). In particular, the number and/or size of postsynaptic processes for glutamatergic synapses is increased with cocaine-related plasticity, a finding consistent with accumulating evidence that glutamatergic signalling becomes critical in the addicted state (Doyle et al., 2014; Gipson & Kalivas, 2014).
In the present study, it was sought to determine whether DA could modulate NMDA-stimulated calcium flux in a subpopulation of MSNs. It was also sought to interrogate a specific molecular pathway involving D1R activation and MMP-generated integrin-binding ligands. A unique mouse model was taken advantage of, in which the calcium indicator GCaMP3 is expressed in D2R-bearing neurons.
It was found that DA and the D1R agonist SKF81297 can potentiate NMDA-stimulated calcium flux in D2R MSNs. An associated increase in MMP activity was observed, as inferred by cleavage of the MMP substrate β-dystroglycan (Michaluk et al., 2007). Consistent with a causative role for increased MMP activity, it was also observed that D1 agonist potentiation of the D2R MSN response is MMP dependent.
In studies of excitatory transmission in the hippocampus, it has been shown that MMP-dependent endpoints including LTP are integrin receptor dependent (Nagy et al., 2006; Meighan et al., 2007). RGD-binding integrins and RGD-binding β1 integrins in particular are thought to be critical to MMP-induced changes in spine size and the amplitude of hippocampal GluA mini excitatory postsynaptic currents (Wang et al., 2008). Integrin signalling in the striatum has also been implicated in cocaine-primed reinstatement (Wiggins et al., 2011). At the molecular level, integrin signalling modulates GluN subunit composition (Chavis & Westbrook, 2001), as well as surface diffusion (Michaluk et al., 2009). Integrin agonists also stimulate tyrosine phosphorylation of GluN2A and GluN2B in addition to enhancement of GluN-mediated synaptic responses (Bernard-Trifilo et al., 2005). Of interest, MMP-generated integrin-binding ligands also have the potential to increase membrane excitability through their ability to regulate ion channels (Davis et al., 2002b; Wildering et al., 2002; Wojtowicz & Mozrzymas, 2014). Therefore an RGD-binding integrin antagonist was examined for its potential to inhibit D1 agonist-stimulated NMDA responses in D2R MSNs. As shown in Fig. 4, it was observed that pretreatment of slices with the RGD-binding integrin antagonist RGDS reduced the D1 agonist-stimulated NMDA responses in D2R MSNs.
At a superficial level, a DA-associated increase in NMDA-stimulated calcium flux in D2R neurons is at odds with published reports that employ methods to specifically assess the GluN-mediated component of calcium entry. For example, in sophisticated studies using methods to eliminate confounds secondary to altered presynaptic glutamate release, it was shown that D2R signalling could inhibit both GluN and R-type voltage-gated calcium channel-mediated calcium entry in striatopallidal neurons (Higley & Sabatini, 2010). Related work has shown that cocaine can reduce calcium levels in D2R-expressing neurons by approximately 10%, and that this effect can last for at least 30 min (Luo et al., 2011). It should be appreciated, however, that the current results are observed in a complex system in which the NMDA-stimulated response in D2R MSNs was examined 60 min after bath application of DA or SKF81297. In addition, given that the endpoint was NMDA-stimulated calcium flux as opposed to stimulated flux through GluNs in particular, both direct and indirect effects of receptor activation need to be considered. NMDA can act on synaptic and extrasynaptic receptors to stimulate calcium flux, which may in turn stimulate secondary events that further elevate internal calcium. MMPs could influence direct and/or secondary events. For example, relatively direct effects of MMP activity on GluN function are supported by studies in which integrin-binding ligands cause rapid (5 min) tyrosine phosphorylation of GluN subunits and a β1 integrin-dependent increase in GluN-mediated synaptic responses in Bernard-Trifilo et al. (2005). In terms of indirect effects, MMP activity has been implicated in long-term changes in membrane excitability (Wojtowicz & Mozrzymas, 2014), and integrin signalling has the potential to influence voltage-gated ion channels (Davis et al., 2002b; Wildering et al., 2002) as well as GluA phosphorylation (Lonskaya et al., 2013). In addition, integrin-like kinase contributes to cocaine sensitization, and its silencing prevents sensitization-related serine-845 phosphorylation of GluA1 (Chen et al., 2010). Thus, while DA may directly act on D2Rs to reduce serine phosphorylation of GluA1 (Kreitzer & Malenka, 2008; Gardoni & Bellone, 2015), indirect effects mediated through nearby D1R-expressing cells could have an opposing influence on this endpoint.
An additional caveat to the current study is that it does not identify the cell type(s) through which DA and SKF81297 induce increased metalloproteinase activity and/or integrin-binding ligands within striatal slices. In previous work, it has been suggested that MMP-dependent cleavage of synaptic cell adhesion molecules (CAMs) releases soluble fragments that bind to previously unengaged integrins (Lonskaya et al., 2013). MMPs are expressed by varied D1R-positive striatal cell types, including MSNs, inhibitory neurons and glia. CAM substrates are expressed by varied cell types as well. Moreover, while the current discussion has been focused on soluble MMPs that are known to cleave β-dystroglycan and that have been implicated in cocaine-associated striatal plasticity, it should be noted that GM-6001 also inhibits transmembrane metalloproteinases with the potential to release integrin-binding ligands. Despite caveats relating to cell type, MMP and substrate specificity that underlie observed effects, it is worth highlighting that SKF81297 had a significant effect on isolated astrocytes. These cells are increasingly recognized as potential contributors to the multipartite synapse (Dityatev & Rusakov, 2011), and astrocyte-derived MMPs include an MMP that has been implicated in striatal spine generation, MMP-2 (Smith et al., 2014).
Studies presented herein demonstrated that a D1 agonist mimicked DA-dependent effects on endpoints that were examined. While this is consistent with a role for D1Rs in DA-stimulated effects, future studies with a D1R antagonist might be useful to test a potential contribution from D2Rs.
It should also be noted that the current studies involved a relatively lasting exposure to μM concentrations of DA and SKF81297. Lasting increases in striatal DA levels have been reported following injection of cocaine (Peris & Zahniser, 1987), and injection of the anti-depressant bupropion can increase DA levels for at least 2 h (Nomikos et al., 1989). In addition, though select reward-associated stimuli evoke striatal DA transients having nM concentrations (Roitman et al., 2008), μM concentrations have been observed following self-administration of cocaine in a primate model (Bradberry et al., 2000). Micromolar DA levels are also observed with in vitro rodent studies focused on amphetamine (Jones et al., 1998; Schmitz et al., 2001), and with an in vivo paradigm utilizing a combination of amphetamine and an autoreceptor antagonist (Park et al., 2011). It has also been suggested that μM concentrations of DA may be relevant to drug abuse in that, as compared with nM levels, μM levels of DA may best stimulate relatively sustained cAMP generation from D1Rs within the early endosome (Kotowski et al., 2011). It is therefore tempting to speculate that pathologically elevated DA levels may stimulate prolonged cAMP generation to increase the duration of enhanced glutamate responsiveness.
It is also acknowledged that DA metabolism can generate increased levels of reactive oxygen species through a variety of mechanisms (Meiser et al., 2013). And while prolonged exposure of cells to high concentrations of DA can stimulate cellular injury, this typically occurs with doses and time points greater than those used here in Clement et al. (2002).
Despite limitations including those discussed above, the potential for DA to enhance NMDA-stimulated calcium flux in both D1 and D2 MSNs is of interest. Many studies have shown that D1 and D2 MSNs have opposing effects on overall motor output, and thus it has been suggested that select behavioural endpoints are achieved through activation of one subpopulation and concurrent deactivation of the other. It is known, however, that under select circumstances both subpopulations can undergo LTP (Shen et al., 2008). And while MMP activity is important to several maladaptive correlates of addiction (Brown et al., 2007; Mizoguchi et al., 2007; Smith et al., 2014), as is direct pathway activation in particular (Lobo et al., 2010; Bock et al., 2013; Smith et al., 2013), recent work suggests that potentiation of glutamatergic inputs to D2 MSNs is observed in mice that show resilience towards compulsive cocaine seeking (Bock et al., 2013).
Of additional interest are studies focused on action initiation and D2 MSN activity. Activity in both direct and indirect pathways is observed with this endpoint (Cui et al., 2013). It has been suggested that co-activation of both pathways may promote desired motor programs through D1R neuronal projections while simultaneously inhibiting unwanted motor behaviours through D2R neuronal projections (Cui et al., 2013). The overall possibility that coactivation is a means of fine tuning an overall behavioural response is intriguing, and future studies will be necessary to better define the molecular mechanisms that facilitate such.
In summary, the current data suggest that MMP activity enhances NMDA-stimulated calcium responses in D2 MSNs. Future studies to determine whether MMPs play a role in adaptive D2-mediated behaviours, such as resilience towards compulsive drug use (Bock et al., 2013), may be warranted. Future studies may also be warranted to examine the potential for DA to stimulate specific MMP and integrin-dependent changes in D1 MSNs and/or lasting structural changes in both D1 and D2 cell types.
Acknowledgments
The authors would like to acknowledge funding from the Von Matsch professorship for neurological diseases and the National Institutes of Health (NS083410). The authors also acknowledge support from CONACyT of the Mexican Government (#381291 to A.S.R.).
Abbreviations
- aCSF
artificial cerebrospinal fluid
- CAMs
cell adhesion molecules
- DA
dopamine
- DMSO
dimethylsulphoxide
- LTP
long-term potentiation
- MMPs
matrix metalloproteinases
- MSNs
medium spiny neurons
- NMDA
N-methyl-D-aspartate
- PBS
phosphate-buffered saline
- PKA
protein kinase A
- ROI
region of interest
Footnotes
Conflicts of interest
The authors declare no conflict of interest.
References
- Bernard-Trifilo JA, Kramar EA, Torp R, Lin CY, Pineda EA, Lynch G, Gall CM. Integrin signaling cascades are operational in adult hippocampal synapses and modulate NMDA receptor physiology. J Neurochem. 2005;93:834–849. doi: 10.1111/j.1471-4159.2005.03062.x. [DOI] [PubMed] [Google Scholar]
- Bock R, Shin JH, Kaplan AR, Dobi A, Markey E, Kramer PF, Gremel CM, Christensen CH, Adrover MF, Alvarez VA. Strengthening the accumbal indirect pathway promotes resilience to compulsive cocaine use. Nat Neurosci. 2013;16:632–638. doi: 10.1038/nn.3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradberry CW, Barrett-Larimore RL, Jatlow P, Rubino SR. Impact of self-administered cocaine and cocaine cues on extracellular dopamine in mesolimbic and sensorimotor striatum in rhesus monkeys. J Neurosci. 2000;20:3874–3883. doi: 10.1523/JNEUROSCI.20-10-03874.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TE, Forquer MR, Cocking DL, Jansen HT, Harding JW, Sorg BA. Role of matrix metalloproteinases in the acquisition and reconsolidation of cocaine-induced conditioned place preference. Learn Memory. 2007;14:214–223. doi: 10.1101/lm.476207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavis P, Westbrook G. Integrins mediate functional pre- and postsynaptic maturation at a hippocampal synapse. Nature. 2001;411:317–321. doi: 10.1038/35077101. [DOI] [PubMed] [Google Scholar]
- Chen Q, Zhu X, Zhang Y, Wetsel WC, Lee TH, Zhang X. Integrin-linked kinase is involved in cocaine sensitization by regulating PSD-95 and synapsin I expression and GluR1 Ser845 phosphorylation. J Mol Neurosci. 2010;40:284–294. doi: 10.1007/s12031-009-9218-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement MV, Long LH, Ramalingam J, Halliwell B. The cytotoxicity of dopamine may be an artefact of cell culture. J Neurochem. 2002;81:414–421. doi: 10.1046/j.1471-4159.2002.00802.x. [DOI] [PubMed] [Google Scholar]
- Conant K, Garzino-Demo A, Nath A, McArthur JC, Halliday W, Power C, Gallo RC, Major EO. Induction of monocyte chemoattractant protein-1 in HIV-1 Tat-stimulated astrocytes and elevation in AIDS dementia. Proc Natl Acad Sci USA. 1998;95:3117–3121. doi: 10.1073/pnas.95.6.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conant K, Wang Y, Szklarczyk A, Dudak A, Mattson MP, Lim ST. Matrix metalloproteinase-dependent shedding of intercellular adhesion molecule-5 occurs with long-term potentiation. Neuroscience. 2010;166:508–521. doi: 10.1016/j.neuroscience.2009.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conant K, Lonskaya I, Szklarczyk A, Krall C, Steiner J, Maguire-Zeiss K, Lim ST. Methamphetamine-associated cleavage of the synaptic adhesion molecule intercellular adhesion molecule-5. J Neurochem. 2011a;118:521–532. doi: 10.1111/j.1471-4159.2010.07153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conant K, Lonskaya I, Szklarczyk A, Krall C, Steiner J, Maguire-Zeiss K, Lim ST. Methamphetamine-associated cleavage of the synaptic adhesion molecule intercellular adhesion molecule-5. J Neurochem. 2011b;118:521–532. doi: 10.1111/j.1471-4159.2010.07153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Court FA, Zambroni D, Pavoni E, Colombelli C, Baragli C, Figlia G, Sorokin L, Ching W, Salzer JL, Wrabetz L, Feltri ML. MMP2-9 cleavage of dystroglycan alters the size and molecular composition of Schwann cell domains. J Neurosci. 2011;31:12208–12217. doi: 10.1523/JNEUROSCI.0141-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui G, Jun SB, Jin X, Pham MD, Vogel SS, Lovinger DM, Costa RM. Concurrent activation of striatal direct and indirect pathways during action initiation. Nature. 2013;494:238–242. doi: 10.1038/nature11846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MJ, Wu X, Nurkiewicz TR, Kawasaki J, Gui P, Hill MA, Wilson E. Regulation of ion channels by integrins. Cell Biochem Biophys. 2002a;36:41–66. doi: 10.1385/CBB:36:1:41. [DOI] [PubMed] [Google Scholar]
- Davis MJ, Wu X, Nurkiewicz TR, Kawasaki J, Gui PC, Hill MA, Wilson E. Regulation of ion channels by integrins. Cell Biochem Biophys. 2002b;36:41–66. doi: 10.1385/CBB:36:1:41. [DOI] [PubMed] [Google Scholar]
- Dityatev A, Rusakov DA. Molecular signals of plasticity at the tetrapartite synapse. Curr Opin Neurobiol. 2011;21:353–359. doi: 10.1016/j.conb.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle SE, Ramoa C, Garber G, Newman J, Toor Z, Lynch WJ. A shift in the role of glutamatergic signaling in the nucleus accumbens core with the development of an addicted phenotype. Biol Psychiatry. 2014;76:810–815. doi: 10.1016/j.biopsych.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziembowska M, Milek J, Janusz A, Rejmak E, Romanowska E, Gorkiewicz T, Tiron A, Bramham CR, Kaczmarek L. Activity-dependent local translation of matrix metalloproteinase-9. J Neurosci. 2012;32:14538–14547. doi: 10.1523/JNEUROSCI.6028-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardoni F, Bellone C. Modulation of the glutamatergic transmission by Dopamine: a focus on Parkinson, Huntington and Addiction diseases. Fronti Cell Neurosci. 2015;9:25. doi: 10.3389/fncel.2015.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ, Jr, Sibley DR. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990;250:1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- Gipson CD, Kalivas PW. More cocaine-more glutamate-more addiction. Biol Psychiatry. 2014;76:765–766. doi: 10.1016/j.biopsych.2014.08.010. [DOI] [PubMed] [Google Scholar]
- Gong S, Doughty M, Harbaugh CR, Cummins A, Hatten ME, Heintz N, Gerfen CR. Targeting Cre recombinase to specific neuron populations with bacterial artificial chromosome constructs. J Neurosci. 2007;27:9817–9823. doi: 10.1523/JNEUROSCI.2707-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greengard P. The neurobiology of slow synaptic transmission. Science. 2001;294:1024–1030. doi: 10.1126/science.294.5544.1024. [DOI] [PubMed] [Google Scholar]
- Hernandez RV, Garza JM, Graves ME, Martinez JL, Jr, LeBaron RG. The process of reducing CA1 long-term potentiation by the integrin peptide, GRGDSP, occurs within the first few minutes following theta-burst stimulation. Biol Bull. 2001;201:236–237. doi: 10.2307/1543341. [DOI] [PubMed] [Google Scholar]
- Higley MJ, Sabatini BL. Competitive regulation of synaptic Ca2+ influx by D2 dopamine and A2A adenosine receptors. Nat Neurosci. 2010;13:958–966. doi: 10.1038/nn.2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YY, Bach ME, Lipp HP, Zhuo M, Wolfer DP, Hawkins RD, Schoonjans L, Kandel ER, Godfraind JM, Mulligan R, Collen D, Carmeliet P. Mice lacking the gene encoding tissue-type plasminogen activator show a selective interference with late-phase long-term potentiation in both Schaffer collateral and mossy fiber pathways. Proc Natl Acad Sci USA. 1996;93:8699–8704. doi: 10.1073/pnas.93.16.8699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z, Shimazu K, Woo NH, Zang K, Muller U, Lu B, Reichardt LF. Distinct roles of the beta 1-class integrins at the developing and the mature hippocampal excitatory synapse. J Neurosci. 2006;26:11208–11219. doi: 10.1523/JNEUROSCI.3526-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, Nagai T, Mizoguchi H, Sato K, Hayase M, Otsuka N, Fukakusa A, Kumagai N, Kim HC, Nabeshima T, Takuma K, Yamada K. Activation of post-synaptic dopamine D(1) receptors promotes the release of tissue plasminogen activator in the nucleus accumbens via PKA signaling. J Neurochem. 2007;103:2589–2596. doi: 10.1111/j.1471-4159.2007.04946.x. [DOI] [PubMed] [Google Scholar]
- Iwakura Y, Wang R, Abe Y, Piao YS, Shishido Y, Higashiyama S, Takei N, Nawa H. Dopamine-dependent ectodomain shedding and release of epidermal growth factor in developing striatum: target-derived neurotrophic signaling (Part 2) J Neurochem. 2011;118:57–68. doi: 10.1111/j.1471-4159.2011.07295.x. [DOI] [PubMed] [Google Scholar]
- Jones SR, Gainetdinov RR, Wightman RM, Caron MG. Mechanisms of amphetamine action revealed in mice lacking the dopamine transporter. J Neurosci. 1998;18:1979–1986. doi: 10.1523/JNEUROSCI.18-06-01979.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch M, Schmid A, Schnitzler HU. Role of nucleus accumbens dopamine D1 and D2 receptors in instrumental and Pavlovian paradigms of conditioned reward. Psychopharmacology. 2000;152:67–73. doi: 10.1007/s002130000505. [DOI] [PubMed] [Google Scholar]
- Kotowski SJ, Hopf FW, Seif T, Bonci A, von Zastrow M. Endocytosis promotes rapid dopaminergic signaling. Neuron. 2011;71:278–290. doi: 10.1016/j.neuron.2011.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC. Striatal plasticity and basal ganglia circuit function. Neuron. 2008;60:543–554. doi: 10.1016/j.neuron.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauzon NM, Bishop SF, Laviolette SR. Dopamine D1 versus D4 receptors differentially modulate the encoding of salient versus non-salient emotional information in the medial prefrontal cortex. J Neurosci. 2009;29:4836–4845. doi: 10.1523/JNEUROSCI.0178-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBaron RG, Hernandez RV, Orfila JE, Martinez JL., Jr An integrin binding peptide reduces rat CA1 hippocampal long-term potentiation during the first few minutes following theta burst stimulation. Neurosci Lett. 2003;339:199–202. doi: 10.1016/s0304-3940(03)00037-5. [DOI] [PubMed] [Google Scholar]
- Legler DF, Wiedle G, Ross FP, Imhof BA. Superactivation of integrin alphavbeta3 by low antagonist concentrations. J Cell Sci. 2001;114:1545–1553. doi: 10.1242/jcs.114.8.1545. [DOI] [PubMed] [Google Scholar]
- Liu Y, Brown S, Shaikh J, Fishback JA, Matsumoto RR. Relationship between methamphetamine exposure and matrix metalloproteinase 9 expression. NeuroReport. 2008;19:1407–1409. doi: 10.1097/WNR.0b013e32830dd606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo MK, Covington HE, 3rd, Chaudhury D, Friedman AK, Sun H, Damez-Werno D, Dietz DM, Zaman S, Koo JW, Kennedy PJ, Mouzon E, Mogri M, Neve RL, Deisseroth K, Han MH, Nestler EJ. Cell type-specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science. 2010;330:385–390. doi: 10.1126/science.1188472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonskaya I, Partridge J, Lalchandani RR, Chung A, Lee T, Vicini S, Hoe HS, Lim ST, Conant K. Soluble ICAM-5, a product of activity dependent proteolysis, increases mEPSC frequency and dendritic expression of GluA1. PLoS One. 2013;8:e69136. doi: 10.1371/journal.pone.0069136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Z, Volkow ND, Heintz N, Pan Y, Du C. Acute cocaine induces fast activation of D1 receptor and progressive deactivation of D2 receptor striatal neurons: in vivo optical microprobe [Ca2+]i imaging. J Neurosci. 2011;31:13180–13190. doi: 10.1523/JNEUROSCI.2369-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeachie AB, Skrzypiec AE, Cingolani LA, Letellier M, Pawlak R, Goda Y. beta3 integrin is dispensable for conditioned fear and hebbian forms of plasticity in the hippocampus. Eur J Neurosci. 2012;36:2461–2469. doi: 10.1111/j.1460-9568.2012.08163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meighan SE, Meighan PC, Choudhury P, Davis CJ, Olson ML, Zornes PA, Wright JW, Harding JW. Effects of extracellular matrix-degrading proteases matrix metalloproteinases 3 and 9 on spatial learning and synaptic plasticity. J Neurochem. 2006;96:1227–1241. doi: 10.1111/j.1471-4159.2005.03565.x. [DOI] [PubMed] [Google Scholar]
- Meighan PC, Meighan SE, Davis CJ, Wright JW, Harding JW. Effects of matrix metalloproteinase inhibition on short- and long-term plasticity of schaffer collateral/CA1 synapses. J Neurochem. 2007;102:2085–2096. doi: 10.1111/j.1471-4159.2007.04682.x. [DOI] [PubMed] [Google Scholar]
- Meiser J, Weindl D, Hiller K. Complexity of dopamine metabolism. Cell Commun Signal. 2013;11:34. doi: 10.1186/1478-811X-11-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaluk P, Kolodziej L, Mioduszewska B, Wilczynski GM, Dzwonek J, Jaworski J, Gorecki DC, Ottersen OP, Kaczmarek L. Beta-dystroglycan as a target for MMP-9, in response to enhanced neuronal activity. J Biol Chem. 2007;282:16036–16041. doi: 10.1074/jbc.M700641200. [DOI] [PubMed] [Google Scholar]
- Michaluk P, Mikasova L, Groc L, Frischknecht R, Choquet D, Kaczmarek L. Matrix metalloproteinase-9 controls NMDA receptor surface diffusion through integrin beta1 signaling. J Neurosci. 2009;29:6007–6012. doi: 10.1523/JNEUROSCI.5346-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki I, Asanuma M, Diaz-Corrales FJ, Miyoshi K, Ogawa N. Direct evidence for expression of dopamine receptors in astrocytes from basal ganglia. Brain Res. 2004;1029:120–123. doi: 10.1016/j.brainres.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Mizoguchi H, Yamada K, Mouri A, Niwa M, Mizuno T, Noda Y, Nitta A, Itohara S, Banno Y, Nabeshima T. Role of matrix metalloproteinase and tissue inhibitor of MMP in methamphetamine-induced behavioral sensitization and reward: implications for dopamine receptor down-regulation and dopamine release. J Neurochem. 2007;102:1548–1560. doi: 10.1111/j.1471-4159.2007.04623.x. [DOI] [PubMed] [Google Scholar]
- Mizoguchi H, Yamada K, Nabeshima T. Neuropsychotoxicity of abused drugs: involvement of matrix metalloproteinase-2 and -9 and tissue inhibitor of matrix metalloproteinase-2 in methamphetamine-induced behavioral sensitization and reward in rodents. J Pharmacol Sci. 2008;106:9–14. doi: 10.1254/jphs.fm0070139. [DOI] [PubMed] [Google Scholar]
- Nagy V, Bozdagi O, Matynia A, Balcerzyk M, Okulski P, Dzwonek J, Costa RM, Silva AJ, Kaczmarek L, Huntley GW. Matrix metalloproteinase-9 is required for hippocampal late-phase long-term potentiation and memory. J Neurosci. 2006;26:1923–1934. doi: 10.1523/JNEUROSCI.4359-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomikos GG, Damsma G, Wenkstern D, Fibiger HC. Acute effects of bupropion on extracellular dopamine concentrations in rat striatum and nucleus accumbens studied by in vivo microdialysis. Neuropsychopharmacology. 1989;2:273–279. doi: 10.1016/0893-133x(89)90031-6. [DOI] [PubMed] [Google Scholar]
- Park J, Takmakov P, Wightman RM. In vivo comparison of norepinephrine and dopamine release in rat brain by simultaneous measurements with fast-scan cyclic voltammetry. J Neurochem. 2011;119:932–944. doi: 10.1111/j.1471-4159.2011.07494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge JG, Lewin AE, Yasko JR, Vicini S. Contrasting actions of group I metabotropic glutamate receptors in distinct mouse striatal neurones. J Physiol. 2014;592:2721–2733. doi: 10.1113/jphysiol.2014.272773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peris J, Zahniser NR. One injection of cocaine produces a long-lasting increase in [3H]-dopamine release. Pharmacol Biochem Be. 1987;27:533–535. doi: 10.1016/0091-3057(87)90361-3. [DOI] [PubMed] [Google Scholar]
- Rohrbough J, Grotewiel MS, Davis RL, Broadie K. Integrin-mediated regulation of synaptic morphology, transmission, and plasticity. J Neurosci. 2000;20:6868–6878. doi: 10.1523/JNEUROSCI.20-18-06868.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitman MF, Wheeler RA, Wightman RM, Carelli RM. Real-time chemical responses in the nucleus accumbens differentiate rewarding and aversive stimuli. Nat Neurosci. 2008;11:1376–1377. doi: 10.1038/nn.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz Y, Lee CJ, Schmauss C, Gonon F, Sulzer D. Amphetamine distorts stimulation-dependent dopamine overflow: effects on D2 autoreceptors, transporters, and synaptic vesicle stores. J Neurosci. 2001;21:5916–5924. doi: 10.1523/JNEUROSCI.21-16-05916.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Flajolet M, Greengard P, Surmeier DJ. Dichotomous dopaminergic control of striatal synaptic plasticity. Science. 2008;321:848–851. doi: 10.1126/science.1160575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RJ, Lobo MK, Spencer S, Kalivas PW. Cocaine-induced adaptations in D1 and D2 accumbens projection neurons (a dichotomy not necessarily synonymous with direct and indirect pathways) Curr Opin Neurobiol. 2013;23:546–552. doi: 10.1016/j.conb.2013.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AC, Kupchik YM, Scofield MD, Gipson CD, Wiggins A, Thomas CA, Kalivas PW. Synaptic plasticity mediating cocaine relapse requires matrix metalloproteinases. Nat Neurosci. 2014;17:1655–1657. doi: 10.1038/nn.3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonderegger P, Matsumoto-Miyai K. Activity-controlled proteolytic cleavage at the synapse. Trends Neurosci. 2014;37:413–423. doi: 10.1016/j.tins.2014.05.007. [DOI] [PubMed] [Google Scholar]
- Sorg BA, Kalivas PW. Effects of cocaine and footshock stress on extracellular dopamine levels in the ventral striatum. Brain Res. 1991;559:29–36. doi: 10.1016/0006-8993(91)90283-2. [DOI] [PubMed] [Google Scholar]
- Staubli U, Chun D, Lynch G. Time-dependent reversal of long-term potentiation by an integrin antagonist. J Neurosci. 1998;18:3460–3469. doi: 10.1523/JNEUROSCI.18-09-03460.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier DJ, Bargas J, Hemmings HC, Jr, Nairn AC, Greengard P. Modulation of calcium currents by a D1 dopaminergic protein kinase/phosphatase cascade in rat neostriatal neurons. Neuron. 1995;14:385–397. doi: 10.1016/0896-6273(95)90294-5. [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Shen W, Day M, Gertler T, Chan S, Tian X, Plotkin JL. The role of dopamine in modulating the structure and function of striatal circuits. Prog Brain Res. 2010;183:149–167. doi: 10.1016/S0079-6123(10)83008-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szepesi Z, Bijata M, Ruszczycki B, Kaczmarek L, Wlodarczyk J. Matrix metalloproteinases regulate the formation of dendritic spine head protrusions during chemically induced long-term potentiation. PLoS One. 2013;8:e63314. doi: 10.1371/journal.pone.0063314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L, Stefanidakis M, Ning L, Van Lint P, Nyman-Huttunen H, Libert C, Itohara S, Mishina M, Rauvala H, Gahmberg CG. Activation of NMDA receptors promotes dendritic spine development through MMP-mediated ICAM-5 cleavage. J Cell Biol. 2007;178:687–700. doi: 10.1083/jcb.200612097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong H, Gibb AJ. Dopamine D1 receptor inhibition of NMDA receptor currents mediated by tyrosine kinase-dependent receptor trafficking in neonatal rat striatum. J Physiol. 2008;586:4693–4707. doi: 10.1113/jphysiol.2008.158931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritsch NX, Sabatini BL. Dopaminergic modulation of synaptic transmission in cortex and striatum. Neuron. 2012;76:33–50. doi: 10.1016/j.neuron.2012.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XB, Bozdagi O, Nikitczuk JS, Zhai ZW, Zhou Q, Huntley GW. Extracellular proteolysis by matrix metalloproteinase-9 drives dendritic spine enlargement and long-term potentiation coordinately. Proc Natl Acad Sci USA. 2008;105:19520–19525. doi: 10.1073/pnas.0807248105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiera G, Wozniak G, Bajor M, Kaczmarek L, Mozrzymas JW. Maintenance of long-term potentiation in hippocampal mossy fiber-CA3 pathway requires fine-tuned MMP-9 proteolytic activity. Hippocampus. 2013;23:529–543. doi: 10.1002/hipo.22112. [DOI] [PubMed] [Google Scholar]
- Wiggins A, Smith RJ, Shen HW, Kalivas PW. Integrins modulate relapse to cocaine-seeking. J Neurosci. 2011;31:16177–16184. doi: 10.1523/JNEUROSCI.3816-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildering WC, Hermann PM, Bulloch AG. Rapid neuromodulatory actions of integrin ligands. J Neurosci. 2002;22:2419–2426. doi: 10.1523/JNEUROSCI.22-07-02419.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtowicz T, Mozrzymas JW. Matrix metalloprotease activity shapes the magnitude of EPSPs and spike plasticity within the hippocampal CA3 network. Hippocampus. 2014;24:135–153. doi: 10.1002/hipo.22205. [DOI] [PubMed] [Google Scholar]
- Wu X, Mogford JE, Platts SH, Davis GE, Meininger GA, Davis MJ. Modulation of calcium current in arteriolar smooth muscle by alphav beta3 and alpha5 beta1 integrin ligands. J Cell Biol. 1998;143:241–252. doi: 10.1083/jcb.143.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zariwala HA, Borghuis BG, Hoogland TM, Madisen L, Tian L, De Zeeuw CI, Zeng H, Looger LL, Svoboda K, Chen TW. A Cre-dependent GCaMP3 reporter mouse for neuronal imaging in vivo. J Neurosci. 2012;32:3131–3141. doi: 10.1523/JNEUROSCI.4469-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]


