Abstract
Introduction
Pain management can be challenging following bariatric surgery, and patients with obesity tend to increase opioid use after undergoing surgery. This report quantifies marijuana (MJ) use and its relationship to pain and other surgery-related outcomes in a population from a state that has legalized MJ.
Methods
Data were collected for consecutive patients undergoing weight reduction surgeries between May 1, 2014 and July 31, 2015. Demographics, preoperative comorbidities, medications, and perioperative opioid use were analyzed. The primary outcome evaluated was inpatient opioid pain medication use quantified using natural log morphine equivalents. Secondary outcomes included percentage of total body weight loss after three months, postoperative complications, and changes in medical comorbidities.
Results
A total of 434 patients, among whom 36 (8.3%) reported MJ use, comprised the study population. Perioperative opioid requirements were significantly higher in the MJ-user group (natural log morphine equivalents of 3.92 vs 3.52, p = 0.0015) despite lower subjective pain scores (3.70 vs 4.24, p = 0.07). MJ use did not affect percentage of 90-day total body weight loss, development of postoperative complications, or improvement in medical comorbidities.
Conclusion
Perioperative opioid use was significantly higher in the MJ-user group despite lower subjective pain scores. The difference in opioid requirements suggests an interaction between MJ use and opioid tolerance or pain threshold. The percentage of total body weight loss, improvement in medical comorbidity, and incidence of postoperative complications at 90-day follow-up were not affected by MJ use in this cohort analysis.
INTRODUCTION
It is a challenge to optimize pain management and weight loss outcomes following bariatric surgery. Preoperative opioid use is associated with increased costs and health care utilization and poorer outcomes following nonemergent or elective abdominal surgeries.1,2 In the bariatric surgical population, rates of opioid use during the year following surgery (excluding the 30-day postoperative period) increase substantially among those who use opioids chronically or intermittently before their surgery.3,4 In light of the US opioid epidemic, understanding and managing this issue is an urgent challenge.5
Patients who undergo bariatric surgery may be especially vulnerable to substance abuse. Their predisposition to obesity and increased use of opioids or other substances such as marijuana (MJ) are mediated by overlapping gene environment interactions.6 The pathways associated with substance-use disorders vary by substance and are not uniform. Additionally, psychological “symptom substitution” theories suggest that when patients who undergo bariatric surgery are no longer able to consume food compulsively, alternative substance abuse behaviors emerge.7, 8 From a biological perspective, dopamine-based reward pathways are activated after consuming both highly palatable foods and drugs of abuse,9 although individual drug activation pathways may differ. Anatomical changes to the gut after bariatric surgery, particularly following Roux-en-Y gastric bypass (RYGB), accelerate alcohol absorption and lengthen elimination times.10 The most common reasons for substance use cited by inpatients undergoing bariatric surgery are addiction transfer (83%), “psychological problems” (75%), and stronger effects/faster onset (58%); these reasons point to the complex interaction of biopsychosocial factors associated with addiction onset after surgery.11 Consequently, understanding MJ’s role in the complex interaction between weight, food, and other substances of abuse following bariatric surgery is critical.
MJ’s effects on substance-use disorders, pain management, and other bariatric surgery-specific outcomes, including weight loss, comorbidity changes, and surgical adverse events, are not known. MJ is simultaneously recognized as a drug of abuse, a potential therapeutic agent, and a recreational substance.12 Commonly used forms of the drug have multiple active metabolites with various pharmacologic effects. Delta-9-tetrahydrocannabinol (THC) is the most active ingredient and is known to trigger the endogenous cannabinoid system. Two primary receptors, cannabinoid-1 (CB-1) and cannabinoid-2, have been identified. CB-1 receptors, located centrally in the nervous system, cause well-recognized psychotropic and euphoric effects. CB-1 receptors also can be found in adipose tissue, the liver, and skeletal muscle and are partial regulators of various metabolic functions.13 Cannabinoid-2 receptors primarily are located peripherally in immune cells.14 The endogenous cannabinoid system has been implicated in pathways regulating energy homeostasis, appetite, inflammation, and pain.15–17
CB-1-receptor activation in hypothalamic nuclei may increase caloric intake. Activation in the neurons of the mesolimbic system increases the desire for food. These receptors also are found in peripheral adipose tissue, and their activation stimulates lipogenesis.18 Animal models of obesity demonstrate that increased, long-lasting activation of CB-1 receptors of the endocannabinoid system results in obesity maintenance.18 Dysregulation of the endocannabinoid system is thought to lead to visceral obesity and contribute to diabetes.19, 20
Medical MJ use has been approved primarily for treatment of chronic pain, spasticity in multiple sclerosis, and for nausea or loss of appetite for patients receiving chemotherapy to treat cancer.21–23 Early investigational use of cannabinoids in the treatment of refractory pediatric epilepsy is ongoing; however, there currently is no accepted indication for use in surgical patients.24 Furthermore, when compared with opioid analgesia for acute pain, MJ is inferior to opioids primarily because of known central nervous system adverse effects.25 To date, to our knowledge only one case report has been published that demonstrates the successful use of THC extract to control protracted nausea and vomiting in a bariatric patient.26 Increased postoperative pain and requirements for opioid rescue analgesia in MJ users were reported in a Jamaican study.27 The lack of empiric data and concern for inferior weight loss and possible risk for substance-use disorders has prompted some practitioners to question whether MJ use should be a contraindication to bariatric surgery.28 This study’s primary hypothesis was that MJ use was unlikely to affect initial outcomes following laparoscopic bariatric surgeries. The secondary hypothesis was that side effects of MJ use such as increased appetite would adversely affect initial weight loss.
METHODS
This review analyzed MJ use in a cohort of 434 consecutive patients undergoing primary bariatric operations (RYGB, sleeve gastrectomy, or gastric banding) between May 1, 2014 and July 31, 2015. During the study period, 517 bariatric operations were performed in a Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program-accredited weight-loss surgery center after institutional review board approval. Eighty-three patients were excluded from this analysis for various reasons (Figure 1). Exclusion criteria included prior bariatric surgery, non-metabolic surgery, surgical revision of any type, and incomplete chart data. Of the 434 patients included, 197 (45.4%) were treated with laparoscopic RYGB and 212 (48.8%) were treated with laparoscopic sleeve gastrectomy. The remaining 25 patients (5.8%) underwent other laparoscopic interventions such as gastric banding, laparoscopic cholecystectomy, or diagnostic laparoscopy. Open procedures were not evaluated in this cohort analysis. Technical specifics for RYGB include a 120-cm antecolic-antegastric roux limb, a 50-cm biliopancreatic limb, and a 30-cc gastric pouch. Sleeve gastrectomy was performed over a 36Fr bougie. All procedures involved a completion endoscopy as a leak test to ensure appropriate and secure reconstruction.
Figure 1.
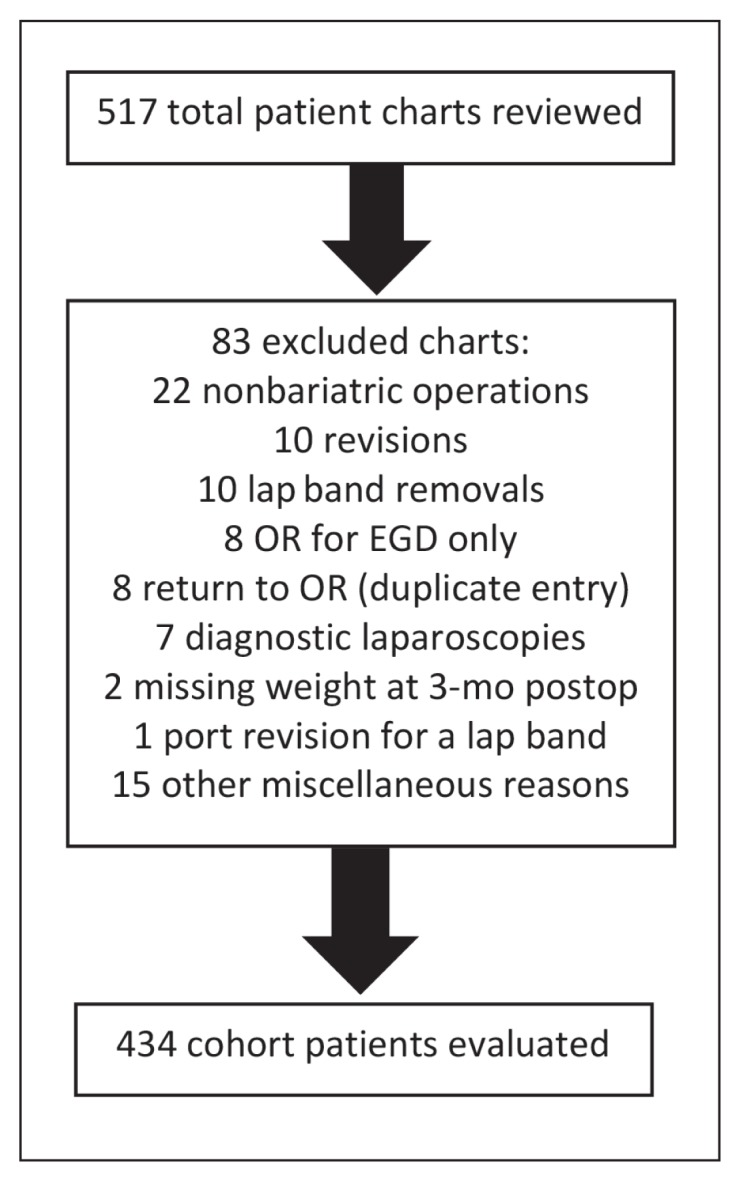
Study cohort patient chart exclusions by category.
EGD = esophagogastroduodenoscopy; lap = laparoscopic; OR = Operating Room; postop = postoperation.
Standardized pain management included 30-mg intravenous ketorolac every 8 hours for 24 hours if no chronic kidney disease, bleeding complications, or other contraindication existed. All patients received 1-g intravenous acetaminophen before surgery and 1–2 doses of 1-g intravenous acetaminophen after surgery. Liquid pain medication was offered immediately after surgery; however, most patients used intravenous morphine sulfate or hydromorphone as needed during the early postoperative period (initial 12 hours). All patients were offered heating pads and ice packs and were prescribed a 5-mg hydrocodone/325-mg acetaminophen per 240-mL solution elixir. Patients who could not tolerate the elixir were prescribed oxycodone tablets 5 mg to 15 mg every 4 hours as needed for pain.
Each patient was prospectively questioned about cannabis use during routine preoperative visits. Patients were asked if they considered themselves to be a regular user (at least 1 use per month). We chose to not subcategorize the route or type of administration (inhaled, edible, hashish, etc) because of limited statistical power to evaluate the association of MJ use subtype with outcomes of interest. Charts were reviewed to obtain data on demographics and preoperative comorbidities (Table 1). All patients received 3 standardized doses of ketoralac after surgery regardless of procedure type. No patient received oral nonsteroidal anti-inflammatory drugs during the immediate postoperative period (initial 30 days).
Table 1.
Preoperative demographics and comorbidities of study population
| Variable | Marijuana users (n = 36) | Nonusers (n = 395) | p value |
|---|---|---|---|
| Age, years, mean (SD) | 41.7 (13.1) | 48.8 (12) | 0.003a |
| Men, no. (%) | 9 (25.0) | 94 (23.7) | 1.0 |
| Preoperative weight, lbs, mean (SD) | 281.8 (49.9) | 278.5 (52.8) | 0.71 |
| LOS, days, mean (SD) | 1.97 (1.5) | 1.82 (1.9) | 0.59 |
| BMI, kg/m2, mean (SD) | 44.7 (6.1) | 45.1 (6.5) | 0.68 |
| Number of comorbidities, mean (SD) | 1.8 (1.3) | 2.5 (1.5) | 0.003a |
| Chronic pain present, no. (%)b | 18 (50) | 142 (35.8) | 0.13 |
| Tobacco use history, no. (%)b | 20 (55.6) | 152 (38.3) | 0.064 |
| CPAP with oxygen use, no. (%)b | 8 (22.2) | 123 (31) | 0.365 |
| Inhalers required, no. (%)b | 9 (25) | 117 (29.5) | 0.71 |
Statistically significant (p < 0.05).
Percentages correspond to within user/nonuser cohorts.
BMI = body mass index; CPAP = continuous positive airway pressure; LOS = length of stay; SD = standard deviation.
The primary study outcome was to quantify perioperative use of opioid pain medication using natural log morphine equivalents correlated with postoperative subjective pain scores (Table 2). Data regarding preoperative use of prescription opioids were not collected. Preoperative opioid use could be viewed as a proxy for burden of presurgical medical illness (severity of knee/hip arthritis, etc). Secondary study outcomes included initial (90-day) percentage of total body weight loss after 3 months, which is quantified in Table 3. We also examined a secondary hypothesis that MJ use would negatively influence weight loss because of increased postoperative appetite.
Table 2.
Perioperative opioid use and daily average pain score in study population
| Variable | Marijuana users (n = 36) | Nonusers (n = 395) | p value |
|---|---|---|---|
| LN total morphine unit equivalents, mean (SD) | 3.92 (0.67) | 3.52 (0.75) | 0.002a |
| Daily average pain score (0–10 scale), mean (SD) | 3.70 (1.3) | 4.24 (1.7) | 0.07 |
| Chronic pain diagnosis present, no (%) | 18 (50) | 142 (36) | 0.13 |
Statistically significant (p < 0.05).
LN = natural log; SD = standard deviation.
Table 3.
Univariate analysis of total body weight loss percentage
| Variable | Marijuana users (n = 36) | Nonusers (n = 395) | p value |
|---|---|---|---|
| Percentage total body weight loss, mean (SD) | −0.18 (0.06) | −0.18 (0.06) | 0.89 |
SD = standard deviation.
Surgical outcomes data included 30-day postoperative complications, Emergency Department (ED) visits, and readmissions (Table 4). Improvement in medical comorbidities was measured through analysis of pre- and postmedication dose requirements for treatment of hypertension, diabetes mellitus, psychiatric illness, and pulmonary disease.
Table 4.
30-day outcomes for marijuana users and nonusers after bariatric surgery
| Outcome variablea | Marijuana users (n = 35)b | Nonusers (n = 395) | p value |
|---|---|---|---|
| Surgical site infection, no. (%) | 1 (2.8) | 6 (1.5) | 0.55 |
| Readmissions, no. (%) | 3 (2.9) | 28 (7.1) | 0.69 |
| Postoperative Emergency Department visits, no. (%) | 6 (17.1) | 54 (13.7) | 0.57 |
A low incidence of pneumonia and urinary tract infection precluded analysis.
One patient died before 30-day follow-up analysis.
Continuous demographic variables were compared using Student’s t-tests, and categorical data were compared using Pearson χ2 tests between the MJ-user and nonuser groups. Continuous outcome variables were modeled using linear regression, and MJ use was entered as an independent variable along with other variables possibly associated with the outcome. Outcome variables were modeled using Poisson regression. All calculations were performed with R-3.3.2. Variables modeled included demographics; comorbidities; preoperative and postoperative weight; American Society of Anesthesiology class; oxygen dependence; continuous positive airway pressure use; preexisting chronic pain syndromes; history of tobacco use; and psychiatric diagnoses of depression, anxiety, and bipolar disorder. All postoperative weights were recorded at a 3-month postoperative visit (± 30 days). Medication data for the treatment of comorbidities that qualify patients for bariatric surgery were extracted from the pre- and posthospital medication reconciliation list. Perioperative opioid use was quantified by the pharmacy using natural log morphine equivalents. A logarithmic transformation was necessary to correct for the nonconstant variability of the morphine equivalent outcome data. Institutional standardized subjective pain scores (0–10 scale) were analyzed to quantify patient perception of pain levels.
RESULTS
The prevalence of regular MJ use in the study cohort was 8.3% (36/434). The MJ cohort was younger (41.7 years vs 48.8 years, p = 0.003) and healthier when comparing average number of comorbidities (1.8 vs. 2.5, p = 0.003) with the nonuser cohort (Table 1). The two cohorts were similar with respect to sex, American Society of Anesthesiology classification, and preoperative body mass index. The MJ-user cohort had a higher prevalence of chronic pain, but this was not statistically significant in comparison to the nonuser cohort (50% vs 36%, p = 0.13). There was a higher incidence of tobacco use in the MJ-user cohort (smoking with a 1+ pack/y history), which also was not significantly different between groups (56% vs 38%, p = 0.064). Although the MJ-user population had a lower recorded prevalence of home oxygen use, continuous positive airway pressure use, and use of maintenance inhalers, these data were not significant between groups. The MJ cohort had a lower prevalence of hypertension and diabetes. There was a high prevalence of the aforementioned psychiatric diagnoses in both populations.
A secondary multivariate analysis was performed, and no association between MJ use and weight loss was noted. Surgical outcome variables at 30 days were similar with respect to surgical site infection, 30-day readmission, and 30-day ED visits (2.8% vs 1.5%, p = 0.59; 2.9% vs 7.1%, p = 0.69; 17.1% vs 13.7%, p = 0.57, respectively; see Table 4).
Poisson regression was used to analyze the effect of MJ use on pre- and postoperative prescription requirements for the most common comorbidities affecting bariatric patients: Hypertension, type 2 diabetes mellitus, and psychiatric diagnoses. There were no statistically significant differences between preoperative and postoperative prescription requirements to treat hypertension or psychiatric conditions (p = 0.845 and p = 0.842, respectively). A clear trend was observed for lowering doses of diabetes medications, but this trend did not reach statistical significance (p = 0.0731).
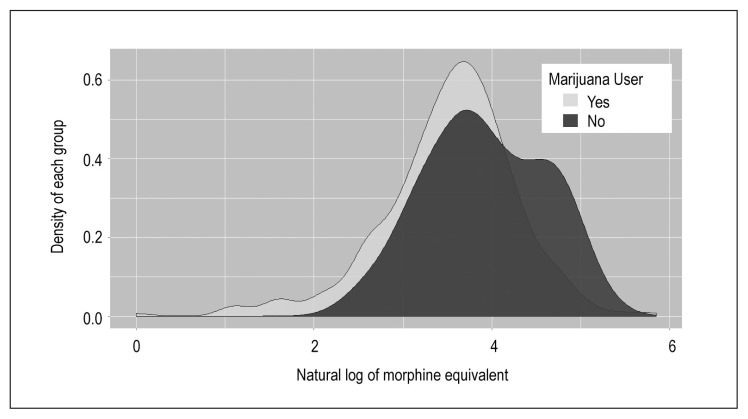
Pain scores were obtained 3 times daily during hospitalization. Our institution uses a standardized assessment in which 0 equals no pain and 10 equals maximum pain. These scores were then averaged to obtain a daily pain score (Table 2). There was a significant difference in total opioid use between groups; 47 mg for MJ users vs 37 mg for nonusers (natural log total morphine equivalents 3.92 vs 3.52, p = 0.0015; Figure 2). Despite higher opiate use, MJ users reported lower average pain scores (3.70 vs 4.24, p = 0.07), although this difference did not reach statistical significance.
Figure 2.
Distribution of natural log morphine equivalent between marijuana users and nonusers.
DISCUSSION
MJ remains an illegal substance according to federal law. However, as states legalize MJ, its use has increased for both recreational and medical purposes. Medical indications stem from limited published evidence and from data supporting the ways in which MJ and THC affect the endocannabinoid system. The primary hypothesis of this study, “Marijuana use is unlikely to affect initial outcomes following bariatric operations,” is supported by these data. The secondary hypothesis, “Marijuana use may adversely affect initial weight loss,” however, was refuted. We specified a low threshold with which to define MJ use (at least one use of any MJ preparation within 30 days of surgery). Urinary drug screening was not performed to validate or refute recent cannabis intake, which introduced potential for underreporting. Voluntary participation in the study could have been adversely affected if we mandated that patients undergo preoperative and postoperative urinary drug screening.
An unanticipated consequence of MJ use was discovered in this cohort analysis. Postoperative opioid analgesic use was statistically increased among MJ users vs nonusers. Jefferson et al’s 2013 study27 is the only publication in the medical literature to corroborate this finding. This finding is paradoxical because THC alters the endocannabinoid pathway to enhance antinociceptive effects rather than produce increased pain.29
In this study, preoperative opioid use was not quantified because there was no reliable way to confirm exact use patterns. This was a potential weakness because several patients entering the study with substantial opioid dependence could skew the results from a chronic-use perspective. Urinary drug screening could have detected such dependence but was not used in this cohort analysis. Studies on the long-term effects of MJ use and abuse demonstrate an MJ withdrawal syndrome that is characterized by a constellation of symptoms including irritability, weight loss, anorexia, insomnia, restlessness, and abdominal pain.30 Withdrawal occurs shortly after cessation and lasts 2 to 6 days on average.30 It is possible that patients with higher than average postoperative opioid use were experiencing a combination of intensifying pain stimuli and simultaneous MJ withdrawal. We did not have the capability to measure cannabinoid levels to validate withdrawal symptoms in the MJ-user cohort. Pain associated with MJ withdrawal may have contributed to the increased opioid requirement noted in our MJ-user data set. The increased perioperative opioid requirement also may reflect a decreased pain threshold in the MJ cohort, but this cannot be precisely determined because pain was a subjective variable in this study.
The secondary hypothesis that MJ use would adversely affect weight loss was rejected. This cohort analysis suggests that the combination of gastric restriction (provided by weight-loss surgery), a strict postoperative dietary program, and postoperative gastrointestinal hormonal changes ultimately led to weight loss not affected by presurgical MJ use. These findings also suggest that alterations in postoperative levels of glucagon-like peptide-1, peptide YY, and other gastrointestinal hormones following bariatric surgery31 are similar in nonuser and MJ-user cohorts. This analysis included only 90-day postoperative results. Longer-term data to assess contemporary use of MJ and whether such use is a risk factor for failed metabolic surgery clearly are needed. There were no differences between cohorts when the 90-day outcomes of surgical-site infections, readmissions, or ED visits were analyzed. Return to the ED was more frequent than expected and noted on our institutional semiannual reports supplied by the Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program. Quality improvement projects were undertaken, such as adding an early postoperative appointment, and ED-visit frequency returned to an acceptable rate.
The MJ-user population was younger and healthier than the nonuser group. A generational acceptance of MJ as a social norm likely explains why the prevalence of use in younger patients was higher. It is worth noting that a 2012 study demonstrated a decreased prevalence of diabetes in patients who use MJ.32
A limitation of this investigation was the lack of detail regarding route, quantity, and frequency of MJ use following the preoperative interviews. The study also had a small total number of regular MJ users, which limited the statistical power to detect differences between users and nonusers. Another study limitation was that preoperative opioid-use data were not included. It is interesting that the 8.3% prevalence of MJ use in this study in a state that recently legalized MJ for recreational use parallels the 9.5% use rate in national estimates.33
There was a single unexplained death in the user population, yielding a mortality rate of 1/36 (2.8%). This 55-year-old woman used MJ daily before surgery and dronabinol after surgery to treat her fibromyalgia. At the time of her reoperation, no leak or hemorrhage was identified. A pulmonary embolus was suspected, but an autopsy was not performed to prove that hypothesis. Because of the unclear cause of death, the role of cannabis and opioids remains speculative.
CONCLUSION
This report quantifies MJ use in a bariatric population residing in a state in which MJ use is legal. Prevalence of MJ use among participants approached the national average.33 Postoperative prescription opioid use using natural log morphine equivalents was significantly higher in the MJ-user group despite lower subjective pain scores. This difference in perioperative opioid requirements may suggest an MJ-withdrawal phenomenon, a cross-tolerance to opioids, or a decreased pain threshold in MJ users. As MJ becomes legal in more states, multicenter data will be required to further define withdrawal and cross-tolerance patterns. Further research is needed on the long-term risk for substance abuse among people who use MJ preoperatively, including use with co-administered opioids. The prevalence of MJ use in our population confirms that this topic warrants further study. Percentage of total body weight loss, improvement in medical comorbidity, and incidence of postoperative complications were not affected by perioperative MJ use in this cohort analysis. v
Acknowledgement
Brenda Moss Feinberg, ELS, provided editorial assistance.
Footnotes
Disclosure Statement
This study was approved by the Kaiser Permanente Colorado institutional review board and was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki. The study also was approved by the Sister of Clarity of Leavenworth Health-Front Range institutional review board, #201457. No funding sources were used in this study or in preparation of this manuscript. The author(s) have no conflicts of interest to disclose.
References
- 1.Cron DC, Englesbe MJ, Bolton CJ, et al. Preoperative opioid use is independently associated with increased costs and worse outcomes after major abdominal surgery. Ann Surg. 2017 Apr;265(4):695–701. doi: 10.1097/sla.0000000000001901. [DOI] [PubMed] [Google Scholar]
- 2.Waljee JF, Cron DC, Steiger RM, Zhong L, Englesbe MJ, Brummett CM. Effect of preoperative opioid exposure on healthcare utilization and expenditures following elective abdominal surgery. Ann Surg. 2017 Apr;265(4):715–21. doi: 10.1097/sla.0000000000002117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raebel MA, Newcomer SR, Reifler LM, et al. Chronic use of opioid medications before and after bariatric surgery. JAMA. 2013 Oct 2;310(13):1369–76. doi: 10.1001/jama.2013.278344. [DOI] [PubMed] [Google Scholar]
- 4.Raebel MA, Newcomer SR, Bayliss EA, et al. Chronic opioid use emerging after bariatric surgery. Pharmacoepidemiol Drug Saf. 2014 Dec;23(12):1247–57. doi: 10.1002/pds.3625. [DOI] [PubMed] [Google Scholar]
- 5.Bonnie RJ, Kesselheim AS, Clark DJ. Both urgency and balance needed in addressing opioid epidemic: A report from the national academies of sciences, engineering, and medicine. JAMA. 2017 Aug 1;318(5):423–4. doi: 10.1001/jama.2017.10046. DOI: https://10.1001/jama.2017.10046. [DOI] [PubMed] [Google Scholar]
- 6.Vanbuskirk KA, Potenza MN. The treatment of obesity and its co-occurrence with substance use disorders. J Addict Med. 2010 Mar;4(1):1–10. doi: 10.1097/ADM.0b013e3181ce38e7. DOI: https://10.1097/ADM.0b013e3181ce38e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lent MR, Swencionis C. Addictive personality and maladaptive eating behaviors in adults seeking bariatric surgery. Eat Behav. 2012 Jan;13(1):67–70. doi: 10.1016/j.eatbeh.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 8.Sogg S. Alcohol misuse after bariatric surgery: Epiphenomenon or “Oprah” phenomenon? Surg Obes Relat Dis. 2007 May-Jun;3(3):366–8. doi: 10.1016/j.soard.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 9.Pelchat ML. Food addiction in humans. J Nutr. 2009 Mar;139(3):620–2. doi: 10.3945/jn.108.097816. [DOI] [PubMed] [Google Scholar]
- 10.Hagedorn JC, Encarnacion B, Brat GA, Morton JM. Does gastric bypass alter alcohol metabolism? Surg Obes Relat Dis. 2007 Sep-Oct;3(5):543–8. doi: 10.1016/j.soard.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 11.Ivezaj V, Saules KK, Wiedemann AA. “I didn’t see this coming.” Why are postbariatric patients in substance abuse treatment? Patients’ perceptions of etiology and future recommendations. Obes Surg. 2012 Aug;22(8):1308–14. doi: 10.1007/s11695-012-0668-2. [DOI] [PubMed] [Google Scholar]
- 12.Chaudhry HJ, Hengerer AS, Snyder GB. Medical board expectations for physicians recommending marijuana. JAMA. 2016 Aug 9;316(6):577–8. doi: 10.1001/jama.2016.7741. [DOI] [PubMed] [Google Scholar]
- 13.Pagotto U, Marsicano G, Cota D, Lutz B, Pasquali R. The emerging role of the endocannabinoid system in endocrine regulation and energy balance. Endocr Rev. 2006 Feb;27(1):73–100. doi: 10.1210/er.2005-0009. [DOI] [PubMed] [Google Scholar]
- 14.Sachs J, McGlade E, Yurgelun-Todd D. Safety and toxicology of cannabinoids. Neurotherapeutics. 2015 Oct;12(4):735–46. doi: 10.1007/s13311-015-0380-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Battista N, Di Tommaso M, Bari M, Maccarrone M. The endocannabinoid system: An overview. Front Behav Neurosci. 2012 Mar 14;6:9. doi: 10.3389/fnbeh.2012.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mouslech Z, Valla V. Endocannabinoid system: An overview of its potential in current medical practice. Neuro Endocrinol Lett. 2009;30(2):153–79. [PubMed] [Google Scholar]
- 17.Atakan Z. Cannabis, a complex plant: Different compounds and different effects on individuals. Ther Adv Psychopharmacol. 2012 Dec;2(6):241–54. doi: 10.1177/2045125312457586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pagotto U, Vicennati V, Pasquali R. The endocannabinoid system and the treatment of obesity. Ann Med. 2005;37(4):270–5. doi: 10.1080/07853890510037419. [DOI] [PubMed] [Google Scholar]
- 19.Scherer T, Buettner C. The dysregulation of the endocannabinoid system in diabesity—a tricky problem. J Mol Med (Berl) 2009 Jul;87(7):663–8. doi: 10.1007/s00109-009-0459-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Engeli S. Dysregulation of the endocannabinoid system in obesity. J Neuroendocrinol. 2008 May;20(Suppl 1):110–5. doi: 10.1111/j.1365-2826.2008.01683.x. [DOI] [PubMed] [Google Scholar]
- 21.Alexander SP. Therapeutic potential of cannabis-related drugs. Prog Neuropsychopharmacol Biol Psychiatry. 2016 Jan 4;64:157–66. doi: 10.1016/j.pnpbp.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 22.Thompson AE. JAMA patient page. Medical marijuana. JAMA. 2015 Jun 23–30;313(24):2508. doi: 10.1001/jama.2015.6676. [DOI] [PubMed] [Google Scholar]
- 23.Farrimond JA, Mercier MS, Whalley BJ, Williams CM. Cannabis sativa and the endogenous cannabinoid system: Therapeutic potential for appetite regulation. Phytother Res. 2011 Feb;25(2):170–88. doi: 10.1002/ptr.3375. [DOI] [PubMed] [Google Scholar]
- 24.Hussain SA, Zhou R, Jacobson C, et al. Perceived efficacy of cannabidiol-enriched cannabis extracts for treatment of pediatric epilepsy: A potential role for infantile spasms and Lennox-Gastaut syndrome. Epilepsy Behav. 2015 Jun;47:138–41. doi: 10.1016/j.yebeh.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 25.Campbell FA, Tramer MR, Carroll D, Reynolds DJ, Moore RA, McQuay HJ. Are cannabinoids an effective and safe treatment option in the management of pain? A qualitative systematic review. BMJ. 2001 Jul 7;323(7303):13–6. doi: 10.1136/bmj.323.7303.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Merriman AR, Oliak DA. Use of medical marijuana for treatment of severe intractable nausea after laparoscopic Roux-en-Y gastric bypass surgery. Surg Obes Relat Dis. 2008 Jul-Aug;4(4):550–1. doi: 10.1016/j.soard.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 27.Jefferson DA, Harding HE, Cawich SO, Jackson-Gibson A. Postoperative analgesia in the Jamaican cannabis user. J Psychoactive Drugs. 2013 Jul-Aug;45(3):227–32. doi: 10.1080/02791072.2013.803644. [DOI] [PubMed] [Google Scholar]
- 28.Rummell CM, Heinberg LJ. Assessing marijuana use in bariatric surgery candidates: Should it be a contraindication? Obesity Surg. 2014 Oct;24(10):1764–70. doi: 10.1007/s11695-014-1315-x. [DOI] [PubMed] [Google Scholar]
- 29.Welch SP. Interaction of the cannabinoid and opioid systems in the modulation of nociception. Int Rev Psychiatry. 2009 Apr;21(2):143–51. doi: 10.1080/09540260902782794. [DOI] [PubMed] [Google Scholar]
- 30.Budney AJ, Hughes JR, Moore BA, Vandrey R. Review of the validity and significance of cannabis withdrawal syndrome. Am J Psychiatry. 2004 Nov;161(11):1967–77. doi: 10.1176/appi.ajp.161.11.1967. [DOI] [PubMed] [Google Scholar]
- 31.Ionut V, Burch M, Youdim A, Bergman RN. Gastrointestinal hormones and bariatric surgery-induced weight loss. Obesity (Silver Spring) 2013 Jun;21(6):1093–103. doi: 10.1002/oby.20364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rajavashisth TB, Shaheen M, Norris KC, et al. Decreased prevalence of diabetes in marijuana users: Cross-sectional data from the National Health and Nutrition Examination Survey (NHANES) III. BMJ Open. 2012 Feb 24;2:e000494. doi: 10.1136/bmjopen-2011-000494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hasin DS, Saha TD, Kerridge BT, et al. Prevalence of marijuana use disorders in the United States between 2001–2002 and 2012–2013. JAMA Psychiatry. 2015 Dec;72(12):1235–42. doi: 10.1001/jamapsychiatry.2015.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]