Abstract
BACKGROUND
Variation in an individual’s genetic status can impact the development of pancreatic ductal adenocarcinoma; however, the m ajority of familial pancreatic cancers (FPC) cannot yet be attributed to a specific inherited mutation. We present data suggesting a correlation between loss-of-function single nucleotide polymorphisms (SNPs) in an immune regulator gene, indoleamine-2,3-dioxygenase-2 (IDO2), and an increased risk of FPC.
STUDY DESIGN
Germline DNA from patients who underwent resection for pancreatic ductal adenocarcinoma (n = 79) was sequenced for the IDO2 SNPs R248W and Y359Stop. Genotypes resulting in inactivation of IDO2 (Y325X homozygous, R248W homozygous) were labeled as homozygous, and the other genotypes were grouped as wild-type or heterozygous. Genotype distributions of each SNP were analyzed for Hardy-Weinberg deviation. A genotype frequency set from the 1000 Genomes Project (n = 99) was used as a genetic control for genotype distribution comparisons.
RESULTS
A significant 2-fold increase in the overall prevalence of the Y359Stop homozygous genotype compared with the expected Hardy-Weinberg equilibrium was noted (p < 0.05). Familial pancreatic cancer was noted in 15 cases (19%) and comparison of the FPC cohort set to the genetic control set showed a 3-fold increase in Y359Stop homozygous rates (p = 0.054). Overall in our cohort, the homozygous genotype group was associated with increased risk of FPC (odds ratio 5.4; 95% CI 1.6 to 17.6; p < 0.01). Sex, age at diagnosis, and history of tobacco use were not found to be significantly associated with FPC.
CONCLUSIONS
Our preliminary data suggest a strong association between the IDO2 inactivating Y359Stop SNP and an increased risk of FPC when compared with the control group. Future studies will evaluate the value of IDO2 genotyping as a prognostic, early detection marker for pancreatic ductal adenocarcinoma and a predictive marker for novel immune checkpoint therapies.
Pancreatic ductal adenocarcinoma (PDA) will soon become the second leading cause of cancer-related deaths in the US.1 Although the majority of PDAs are sporadic, roughly 10% are classified as familial pancreatic cancer (FPC).2 Although different definitions of FPC are found in the literature, a common practice is to define the familial form of PDA as instances in which multiple family members (first-degree relatives) are afflicted with PDA.3,4
Thorough genomic and epidemiologic analyses of familial registries have identified FPC cases and have provided the field with important insights,2 yet only a subset of cases can be attributed to inherited mutations; single nucleotide polymorphisms (SNPs); or environmental elements4,5 (Tables 1 and 2). In fact, only a small portion of FPC cases are related to specific inherited syndromes, such as hereditary breast-ovarian cancer (eg BRCA2), Peutz-Jeghers syndrome, Lynch syndrome (or hereditary nonpolyposis colorectal carcinoma), familial adenomatous polyposis, ataxia-telangiectasia, hereditary pancreatitis, and familial atypical multiple mole melanoma (Table 1).7 The remaining FPC cases have not been attributed to a common driving inherited genetic alteration. It was recently reported that the frequent genetic drivers (ie Kras and TP53) of FPC are virtually identical to the drivers of sporadic PDA.3
Table 1.
Inherited Syndromes and Associated Lifetime Risk of Pancreatic Ductal Adenocarcinoma
| Inherited syndrome | Lifetime risk of pancreatic cancer |
|---|---|
| Hereditary breast and ovarian cancer syndrome, RR* | 3.5–5.9 |
| Peutz-Jeghers syndrome, % | 11–36 |
| Hereditary pancreatitis, % | 25–40 |
| Hereditary nonpolyposis colorectal cancer syndrome (Lynch syndrome), % | 3.7 |
| Familial atypical multiple mole melanoma, % | 17 |
| Familial adenomatous polyposis, % | 1.7 |
Table 2.
Inherited Syndromes, Related Genes, and Single Nucleotide Polymorphisms Associated with Familial Pancreatic Cancer
| Familial disorder | Genetic mutation |
|---|---|
| Hereditary breast and ovarian cancer syndrome | BRCA1, BRCA2, PALB2, ATM |
| Peutz-Jeghers syndrome | STK11/LKB1 |
| Hereditary pancreatitis | PRSS1, SPINK1 |
| Hereditary nonpolyposis colorectal cancer syndrome (Lynch syndrome) | Mismatch repair genes (HNPCC) |
| Familial atypical multiple mole melanoma | p16 (CDKN2A or MTS1) |
| Familial adenomatous polyposis | APC |
| 1q32.1 (NR5A2 or LRH-1) | rs3790844 (A>G), rs10919791 (G>A) |
| 5p15.33 (CLPTM1/TERT) | rs401681 (C>T) |
| 6q25.3 (FOXQ1) | rs9502893 (C>T) |
| 9p34.2 (ABO) | rs505922 (A>G) |
| 12p11 (BICD1) | rs708224 (A>G) |
| 13q22.1 (KLF5) | rs9543325 (C>T), rs9564966, (A>G) |
Together, these and other studies support the notion that unidentified genetic susceptibility alterations exist, along with interactions with the environment or host (eg the immune system) that cooperate to influence the unusual frequency of PDA found in certain families (ie FPC). Single nucleotide polymorphisms are subtle genetic alterations that are frequently found in the general population (>1%); inherited; and not classified as somatictumor mutations. Associations have been made with SNPs and FPC (Table 2); however, these associations cannot completely explain FPC susceptibility found in high-risk individuals, with the best odds ratios not reaching the influence of smoking as a risk factor (eg odds ratio < 2.0).8
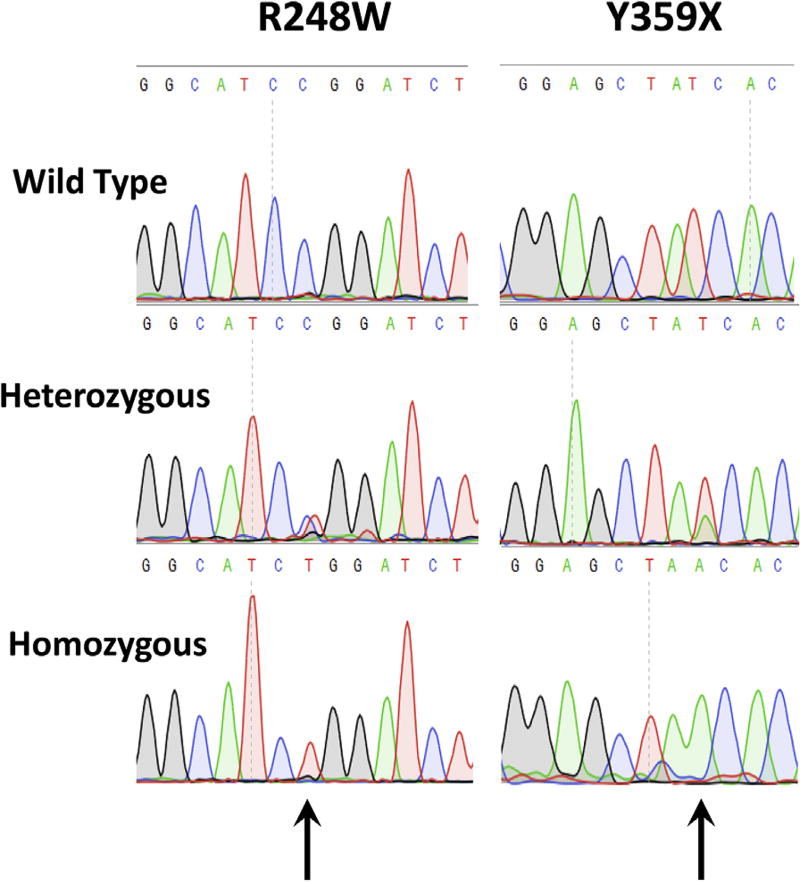
We previously carried out expression and genotype analysis of the indoleamine 2,3-dioxygenase-2 (IDO2) gene in sporadic PDA.9 The IDO2 gene is adjacent to and structurally similar to the IDO1 gene on chromosome 8p12.10 Functionally, IDO2 is also similar to its paralog, IDO1, in that it can catabolize tryptophan. Notably, several studies have shown that the IDO system (both the IDO1 and IDO2 genes) functions in restraining the activity of the immune system in its interactions with multiple tumor systems.11 Previous work has postulated that functional IDO2 enzymatic activity represses immune responses in a host, which in turn, facilitates PDA tumorigenesis. In theory, a functional IDO2 enzyme in tumor cells could aid PDA to avoid the immune system. Alternatively, Køllgaard and colleagues12 demonstrated that a functionally intact IDO2 enzyme could be presented to elicit an immune response compared with an inactive IDO2 protein. We previously discovered and described the presence of 2 loss-of-function polymorphisms within the coding region of the IDO2 gene, with a high prevalence in the general population.10 The 2 SNPs are the R248W polymorphism, defined as having a >90% reduction in IDO2 catalytic activity and the Y359STOP polymorphism, generating a premature stop codon, completely inactivating IDO2 activity10 (Fig. 1). Taking into account the importance of chronic inflammation on PDA pathogenesis and based on previous work showing IDO2’s role in immune regulation, we used our vast PDA clinical database (the Jefferson Pancreatic Tumor Registry) and patient population to determine whether the IDO2 genotype had any correlation to FPC susceptibility.
Figure 1.
Representative chromatograms of direct sequencing of patient constitutional genomic DNA showing the 3 possible sequences of homozygous, heterozygous, or wild-type sequence: R248W polymorphism (left) and Y359STOP (right).
MATERIALS AND METHODS
Patient population
The cohort used for this data set included 79 patients (approximately 130 normal and tumor tissue samples) diagnosed with PDA, who underwent primary pancreatic resection at the Thomas Jefferson University Hospital (TJUH) between August 2006 and February 2013. Patients in the study all had available tissue for DNA analysis. Medical history, preoperative laboratory tests, surgical and histologic findings, and oncologic follow-up data were recorded from the patients’ medical records. Cases in which the index patient had at least 1 first-degree relative with a history of PDA were considered FPC, and the rest of the cases were classified as sporadic PDA. We used the Jefferson Pancreatic Tumor Registry as a valuable resource to evaluate whether the indexed patient’s tumor was classified as FPC. The Jefferson Pancreatic Tumor Registry is IRB approved, and participating patients provided appropriate informed consent.
DNA sequencing of IDO2 polymorphisms
Genomic DNA from surgically resected pancreatic tissue specimens (normal and tumor tissues, n = 79 patients) was isolated using the DNAeasy Blood and Tissue Kit genomic DNA purification kit (Qiagen Inc). Polymerase chain reactions (PCR) were used to amplify exons containing the IDO2 coding region polymorphisms rs4503083 and rs10109853, based on previously validated primer sets (R248W (rs10109853) Forward Primer 5′-GAACATTCTATCCCCCGTTGC-3′; R248W (rs10109853) Reverse Primer 5′-TTACCTGAGAGTGGATCCCTAGCA-3′; Y359Stop (rs4503083) Forward Primer 5′-TCTTGTGCTCCCTCCAAAACA-3′; Y359Stop (rs4503083) Reverse Primer 5′-TGGTTTGGCTTCCCATGCTT-3′).10 The PCR reactions were performed in 25 µL reactions using 2 µL DNA, 0.5 U/µL Taq polymerase (USB), 2.5 µL 10× PCR buffer (USB), and 0.5 µL 10 mM dNTP Mix (Invitrogen). Conditions were set for 35 cycles at (95°C for 2 minutes, 95°C for 30 seconds, 55°C for 30 seconds, 72°C for 30 seconds, followed by an extension of 4 minutes at 72°C). Sequencing reactions included PCR purified products using DNA purification columns (Qiagen) and the forward primers for each PCR reaction. Each PCR reaction was separated by DNA electrophoretic separation on a 0.75% DNA agarose gel.13 Sequencing was then performed by capillary electrophoresis in the Sidney Kimmel Cancer Center DNA core facility at Thomas Jefferson University. Genotyping steps were blinded and annotated by number to clinical data and familial-sporadic patient status. The representative sequencing chromatograms were used to identify a wild-type, heterozygous, or homozygous IDO2 genotype (see Fig. 1). Genotypes considered as resulting in inactivation of the IDO2 enzyme (Y325Stop homozygous and R248W homozygous) were categorized as the homozygous group, and the other genotypes were grouped as wild-type or loss-of-function heterozygous. All genotypes provided in this study reflect the germline and were not from microdissected samples enriched with neoplastic cells.
Genetic distribution data from the 1000 Genomes Project. The 1000 Genomes Project is a global effort to map, through sequencing, human genetic variation across the globe (ie a global reference for human genetic variation).14 In brief, it contains genetic variation data of more than 2,500 subjects from around the world. The data were obtained through a planned sequencing of target populations and, as such, can be divided into specific geographical subsets. The CEU subset (Utah residents with northern and western European ancestry) was selected to serve as a genetic distribution control due to its closeness to the TJUH patient cohort in terms of ethnoracial distribution.
Statistical analysis
Genotype distributions of each polymorphism were analyzed for Hardy-Weinberg (HW) deviation using chi-square test and Fisher’s exact test. A genotype distribution set of Utah residents with northern and western ancestry available from the 1000 genomes project (CEU, n = 99) was used as a genetic control for distribution comparisons. Distribution comparisons were also performed using chi-square test and Fisher’s exact test. Age, sex, tobacco use, familial history positive for any type of cancer, R248W genotype, Y359Stop genotype, and IDO2 homozygous status were assessed individually for association with FPC. Correlative analysis was performed using Spearman’s test. Factors with p < 0.2 were subsequently included in a multivariate regression model for correlation with FPC. The model was further optimized by sequential exclusion of statistically nonrelevant factors (p ≥ 0.2) until achievement of a final optimal model fit (p ≤ 0.05). A p value ≤ 0.05 was considered statistically significant. Statistical analysis was performed using IBM SPSS, version 20 (IBM Corp).
RESULTS
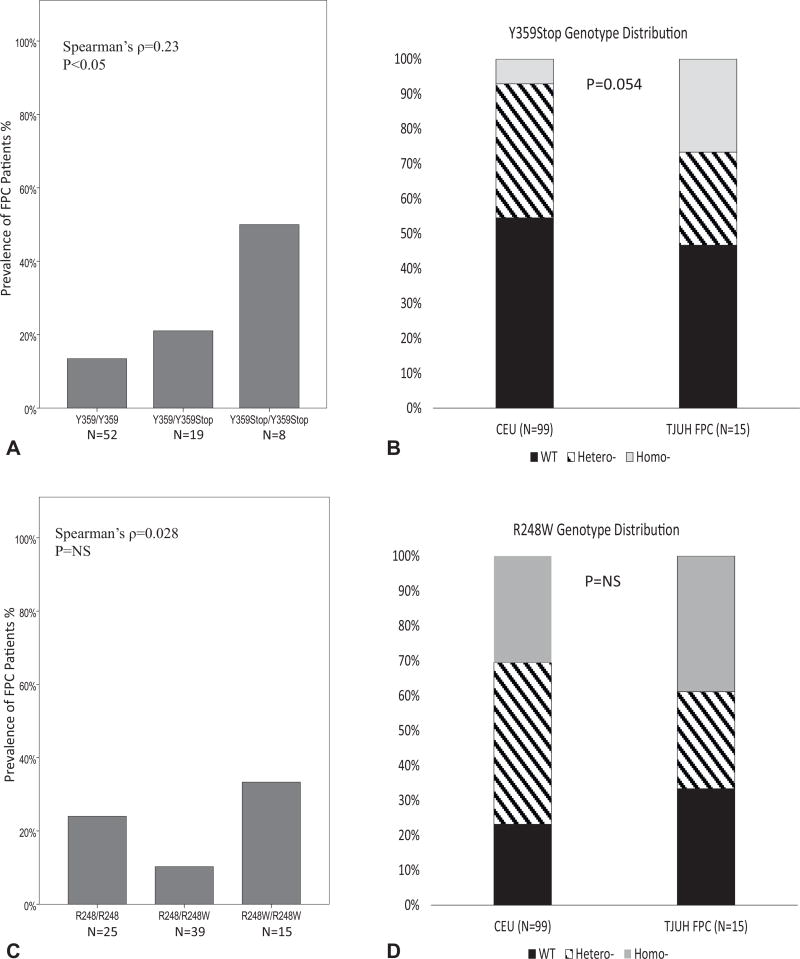
An increased frequency of the Y359Stop SNP but not the R248W SNP uncovered in FPC patients. Sanger sequencing revealed that 52 cases (66%) of the TJUH cohort had the wild-type Y359/Y359 genotype configuration, 19 (24%) had the Y359/Y359Stop configuration and 8 (10%) had the homozygous IDO2 inactive Y359Stop/Y359Stop configuration (see Fig. 1 for chromatograms for Sanger sequencing and Table 3 for distributions). From this analysis, a statistically significant deviation from the HW equilibrium was noted in the TJUH cohort (p < 0.05) with under-representation of the Y359/Y359Stop heterozygous genotype. Stratification of FPC and sporadic PDA did not reveal a significant HW disequilibrium with the chi-square test. However, Fisher’s exact test (employed due to the small size of the subsets) suggested a trend toward HW disequilibrium (p = 0.08). Allelic distributions between the FPC and CEU control cohorts were 50% vs 55% with a Y359/Y359 configuration, 22% vs 38% with a Y3598/Y359Stop configuration and 28% vs 7% with the completely inactive Y359Stop/Y359Stop configuration (Table 3). The frequency of Y359STOP alleles significantly correlated with increased rates of FPC compared with sporadic PDAs (Fig. 2A, p < 0.05), resulting in increased rates of FPC in Y359STOP heterozygous carriers and even higher rates in Y359STOP homozygous patients (50%). Furthermore, overall comparison of Y359Stop genotype distribution demonstrated a strong trend showing a greater representation of the Y359Stop/Y359Stop configuration in the FPC subset compared with the CEU normal control group (Fig. 2B, p = 0.054).
Table 3.
Overall Association of Indoleamine-2,3-Dioxygenase-2 Genotypes to Thomas Jefferson University vs Control Cohorts
| R248 (WT) | R248W (hetero-) |
R248W (homo-) |
Y359 (WT) | Y359Stop (hetero-) |
Y359Stop (homo-) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|
|||||||
| IDO2 genotype | n | % | n | % | n | % | n | % | n | % | n | % |
| TJUH cohort, n = 79 | 25 | 32 | 39 | 49 | 15 | 19 | 52 | 66 | 19 | 24 | 8 | 10 |
|
| ||||||||||||
| TJUH FPC cohort, n = 15 | 6 | 40 | 4 | 27 | 5 | 33 | 7 | 46 | 4 | 27 | 4 | 27 |
|
| ||||||||||||
| CEU control cohort, n = 99 | 26 | 27 | 44 | 44 | 29 | 29 | 54 | 55 | 38 | 38 | 7 | 7 |
CEU, Utah residents with northern and western European ancestry; FPC, familial pancreatic cancer; Hetero-, heterozygous; Homo-, homozygous; IDO2, indoleamine-2,3-dioxygenase-2; TJUH, Thomas Jefferson University Hospital; WT, wild-type.
Figure 2.
Functionally inactive indoleamine-2,3-dioxygenase-2 (IDO2) alleles are frequently found in familial pancreatic cancer (FPC) patients. (A) Prevalence of FPC in various Y359Stop genotypes (wild-type, heterozygous, and homozygous) in the entire Thomas Jefferson University Hospital (TJUH) cohort (FPCs and sporadic pancreatic ductal adenocarcinoma [PDA]). Spearman’s correlation test (ρ = 0.229, p < 0.05). (B) Y359Stop genotype distribution in FPC as compared with CEU (Utah residents with northern and western European ancestry) control cases. chi-square test, 2 × 3 comparison, p = 0.054. WT, wild type. (C) Rates of FPC in various R248W genotypes. Spearman’s correlation test (ρ = 0.028, p = NS) in the entire TJUH cohort (FPCs and sporadic PDA). (D) R248W genotype distribution in FPC compared with CEU control cases. Chi-square test, 2 × 3 comparison, p = NS.
By way of comparison, Sanger sequencing determined that 25 cases (32%) of the TJUH cohort had a R248/R248 genotype configuration, 39 (49%) had a R248/R248W configuration, and 15 (19%) had the homozygous R248W/R248W configuration. Overall and with stratification to FPC and sporadic PDA, this genotype distribution did not deviate from the HW equilibrium. Allelic distributions among the FPC and CEU control cohorts 33% vs 27% with an R248/R248 genotype configuration, 28% vs 44% with an R248/R248W configuration and 39% vs 29% with a homozygous R248W/R248W configuration (Fig, 2C, Table 3). Fisher’s exact test revealed no significant differences in genotype distribution between the TJUH cohort (or its sub-populations) and the CEU control group. The R248W polymorphism, although having slightly increased FPC rates with the homozygous configuration (39%) compared with the heterozygous and wild-type genotypes (33% and 28%, respectively), did not significantly correlate with FPC (Fig. 2D).
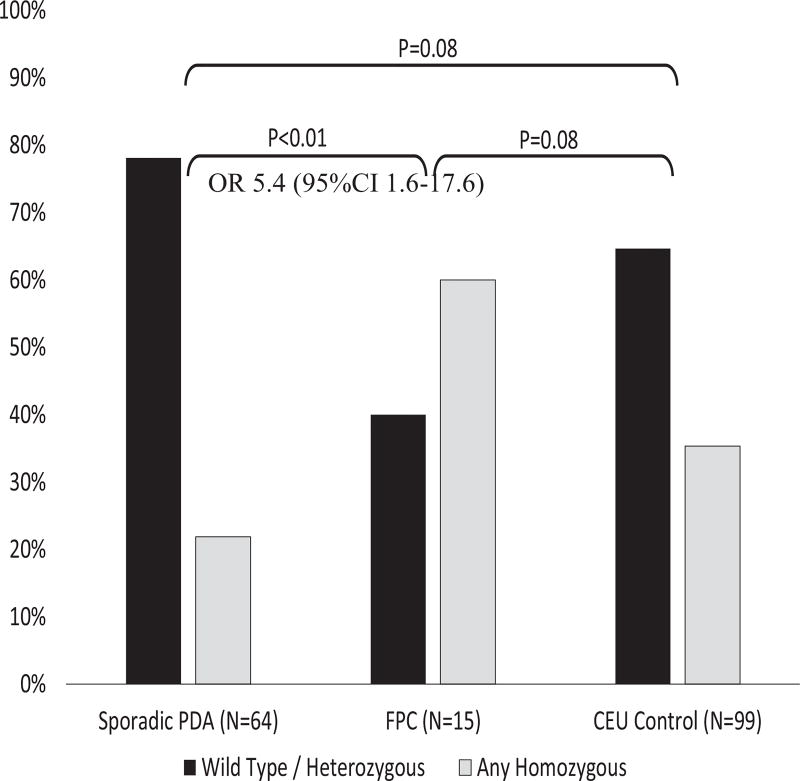
The combined homozygous group (Y359STOP and R248W) was strongly associated with FPC (Fig. 3), with an odds ratio of 5.4 (95% CI 1.6 to 17.6; p < 0.01) compared with sporadic PDA cases. Taken together, these data demonstrated that in our cohort the inactive IDO2 genotype (ie the homozygous group) correlated with individuals with FPC compared with the control cohort or patients with sporadic PDA (Fig. 3).9 A full bivariant distribution table is available (Table 4).
Figure 3.
Indoleamine-2,3-dioxygenase-2 (IDO2) genotype variations in familial pancreatic cancer (FPC) compared with sporadic pancreatic ductal adenocarcinoma (PDA) cases and CEU (Utah residents with northern and western European ancestry) control cases. Chi-square/Fisher’s exact comparisons. OR, odds ratio.
Table 4.
Bivariant Genotype Distribution of the Thomas Jefferson University Cohort (n = 79)
| Genotype | Y359/ Y359 |
Y359/ Y359Stop |
Y359Stop/ Y359Stop |
|---|---|---|---|
| R248/R248 | 15 | 6 | 4 |
| R248/R248W | 26 | 9 | 4 |
| R248W/R248W | 11 | 4 | 0 |
Evaluations of clinical risk factors for FPC were not significant in these cohorts. Age, sex, smoking, and familial cancer history were not associated with FPC. However, due to a large body of work linking smoking to PDA and increased risk for PDA in families with known PDA,15,16 we included smoking in 3 separate regression models (with Y359Stop, R248W, and the combined homozygous grouping). A regression model analyzing the interaction of the combined IDO2 homozygous status with smoking status showed the non-homozygous genotypes combined with non-active smoker status suggested an association with a decreased risk for FPC (nonsmoker/quit ≥15 years ago: relative risk 0.27; 95% CI 0.6 to 1.1; p = 0.07; quit <15 years ago: relative risk 0.17; 95% CI 0.2 to 1.5; p = 0.11).
DISCUSSION
Pancreatic ductal adenocarcinoma has an overall 5-year survival rate of 9%.17 To date, the molecular drivers (eg BRCA2 and PALB2) of PDA prevalence are only known for a small percentage of high-risk families2 (Tables 1 and 2). In this study, we evaluated the frequency of loss-of-function SNPs in the IDO2 gene in our institutional patient population that contained FPC. Although this was a small patient cohort, we found a high frequency of the inactive, homozygous IDO2 genotype in FPC patients (see Figs. 2 and 3).
These data are compelling, yet this study has a number of limitations. First, due to our limited numbers, we restricted our definition of FPC to cases in which the index patient had at least 1 first-degree relative with a history of PDA. It is possible that our results would be stronger if we included only patients with 2 or more affected family members. Second, increasing our numbers could dilute our positive signal in the FPC cohort. Third, additional molecular correlates from the patients would support our conclusions, including the knowledge of any predisposing genotypes (eg BRCA2 mutations) or immune signatures. Fourth, having more clinical data about the individuals genotyped in this study including their history of autoimmune disorders or pancreatitis could be informative. Finally, it would be interesting to see if in IDO2 (+/−) heterozygote germline genotyped patients there was evidence of selection for a loss of the wild-type and/or SNP allele in the tumors of these patients.
In theory, these data appear counterintuitive, as an inactive IDO2 host genotype has been predicted to produce an overactive immune system that would suppress PDA tumorigenesis.9,10 According to this theory, an inactive host IDO2 system (as indicated by homozygous loss of function SNPs), can enable a heightened, proinflammatory host environment that cooperates with Kras activation to induce PDA tumorigenesis.18 Simply put, an inactive host IDO2 genotype could contribute to a tumor-promoting, inflammatory environment.19 Yet, the data from this study might support an opposing theory of how the host immune (IDO) system can facilitate FPC tumorigenesis. Findings from Køllgaard and colleagues’12 work led them to postulate that individuals harboring the inactive homozygous Y359STOP host genotype are unable to mount a specific, IDO2-directed immune response. The investigators discovered that specific T cells primed against different HLA-A2-restricted peptides derived from the IDO2 protein were restricted to wild-type or heterozygous IDO2 genotyped individuals, and no T-cell responses were observed in individuals homozygous for the Y359STOP IDO2 alleles. Therefore, these data support the notion that to achieve an IDO2 specific T-cell response, an individual must have a functional IDO2 enzyme (ie genotyped IDO2 heterozygous or wild-type). A possible explanation for why the R238W does not elicit a comparable effect is that, although the R238W appears to interfere with substrate accessibility to the active site, the Y359Stop allele eliminates an essential histidine that, from studies of the IDO1 enzyme, has been shown to be essential for coordinating with the heme iron. Breaking this heme ironhistidine bond results in conformational changes that are thought to be responsible for enhanced proteosomal degradation of IDO1. The Y359Stop allele might not only abrogate activity, but could also lead to the elimination of the IDO2 protein itself, so that any non-enzymatic effects it might have are also eliminated.
In the scenario mentioned, high-risk individuals who are homozygous for the inactive IDO2 Y359Stop genotype might be unable to mount a proper immune response against PDA cells due to the lack of presentable IDO2 antigens. The evidence for these 2 opposing hypotheses highlights the dual-edged sword of the IDO2 system. That is, either dysregulated inflammation and/or an inactive immune response can facilitate the tumorigenesis process. Ongoing studies in both a mouse model for PDA tumorigenesis20,21 and human specimens are being performed to further investigate these countervailing hypotheses.
CONCLUSIONS
Other possible implications of this study relate to early detection and predictive biomarker strategies in the PDA field. Future studies will demonstrate whether high-risk individuals in FPC families, with unknown genetic drivers, should be IDO2 genotyped. In one scenario, these individuals could be identified for immunesuppressing therapies in an effort to modify an overactive host immune system facilitating PDA tumorigenesis. More realistic deliverables of this work are the immediate clinical implications for FPC patients with an inactive IDO2 genotype. These patients might be refractory to novel IDO inhibitor-based therapies, yet they might respond better to other immune checkpoint therapies (eg PD-1/PDL1 inhibitors).22 Larger-scale validation studies and future retrospective studies from immunotherapy-based clinical trials will be required to assess the prognostic and predictive value of IDO2 genotyping.
Acknowledgments
Support: Dr Nevler was supported by the Mary Halinski Fellowship (as part of the Mary Halinski Pancreatic Cancer Research Fund).
Support for this study: This study was supported in part by National Cancer Institute grant R01 CA191191 and Cancer Center Support Grant 5P30CA056036-17 (Shared Resource, Nucleic Acid Facility).
Abbreviations and Acronyms
- FPC
familial pancreatic cancer
- HW
Hardy-Weinberg
- IDO2
indoleamine 2, 3-dioxygenase-2
- PCR
polymerase chain reaction
- PDA
pancreatic ductal adenocarcinoma
- SNP
single nucleotide polymorphism
- TJUH
Thomas Jefferson University Hospital
Footnotes
Disclosure Information: Nothing to disclose.
Presented at the Southern Surgical Association 129th Annual Meeting, Hot Springs, VA, December 2017.
Author Contributions
Study conception and design: Nevler, Muller, Winter, TP Yeo, Lavu, CJ Yeo, Prendergast, Brody
Acquisition of data: Nevler, Cozzitorto, Goetz, Brody
References
- 1.Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–2921. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 2.Jones S, Hruban RH, Kamiyama M, et al. Exomic sequencing identifies PALB2 as a pancreatic cancer susceptibility gene. Science. 2009;324:217. doi: 10.1126/science.1171202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Norris AL, Roberts NJ, Jones S, et al. Familial and sporadic pancreatic cancer share the same molecular pathogenesis. Fam Cancer. 2015;14:95–103. doi: 10.1007/s10689-014-9755-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yeo TP, Hruban RH, Brody J, et al. Assessment of “gene-environment” interaction in cases of familial and sporadic pancreatic cancer. J Gastrointest Surg. 2009;13:1487–1494. doi: 10.1007/s11605-009-0923-6. [DOI] [PubMed] [Google Scholar]
- 5.Welinsky S, Lucas AL. Familial pancreatic cancer and the future of directed screening. Gut Liver. 2017;11:761–770. doi: 10.5009/gnl16414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klein AP. Genetic susceptibility to pancreatic cancer. Mol Carcinog. 2012;51:14–24. doi: 10.1002/mc.20855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petersen GM, Hruban RH. Familial pancreatic cancer: where are we in 2003? J Natl Cancer Inst. 2003;95:180–181. doi: 10.1093/jnci/95.3.180. [DOI] [PubMed] [Google Scholar]
- 8.Zhang M, Wang Z, Obazee O, et al. Three new pancreatic cancer susceptibility signals identified on chromosomes 1q32.1, 5p15.33 and 8q24.21. Oncotarget. 2016;7:66328–66343. doi: 10.18632/oncotarget.11041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Witkiewicz AK, Costantino CL, Metz R, et al. Genotyping and expression analysis of IDO2 in human pancreatic cancer: a novel, active target. J Am Coll Surg. 2009;208:781–787. doi: 10.1016/j.jamcollsurg.2008.12.018. discussion 787–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Metz R, Duhadaway JB, Kamasani U, et al. Novel tryptophan catabolic enzyme IDO2 is the preferred biochemical target of the antitumor indoleamine 2,3-dioxygenase inhibitory compound D-1-methyl-tryptophan. Cancer Res. 2007;67:7082–7087. doi: 10.1158/0008-5472.CAN-07-1872. [DOI] [PubMed] [Google Scholar]
- 11.Prendergast GC, Smith C, Thomas S, et al. Indoleamine 2,3-dioxygenase pathways of pathogenic inflammation and immune escape in cancer. Cancer Immunol Immunother. 2014;63:721–735. doi: 10.1007/s00262-014-1549-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Køllgaard T, Klausen TW, Idorn M, et al. Association of a functional Indoleamine 2,3-dioxygenase 2 genotype with specific immune responses. Oncoimmunology. 2012;1:441–447. doi: 10.4161/onci.19654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brody JR, Calhoun ES, Gallmeier E, et al. Ultra-fast high-resolution agarose electrophoresis of DNA and RNA using low-molarity conductive media. Biotechniques. 2004;37:598, 600, 602. doi: 10.2144/04374ST04. [DOI] [PubMed] [Google Scholar]
- 14.Genomes Project, C. Auton A, Brooks LD, et al. A global reference for human genetic variation. Nature. 2015;526:68–74. doi: 10.1038/nature15393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iodice S, Gandini S, Maisonneuve P, Lowenfels AB. Tobacco and the risk of pancreatic cancer: a review and meta-analysis. Langenbecks Arch Surg. 2008;393:535–545. doi: 10.1007/s00423-007-0266-2. [DOI] [PubMed] [Google Scholar]
- 16.Becker AE, Hernandez YG, Frucht H, Lucas AL. Pancreatic ductal adenocarcinoma: risk factors, screening, and early detection. World J Gastroenterol. 2014;20:11182–11198. doi: 10.3748/wjg.v20.i32.11182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pishvaian MJ, Brody JR. Therapeutic implications of molecular subtyping for pancreatic cancer. Oncology (Williston Park) 2017;31:159–166. [PubMed] [Google Scholar]
- 18.Kitajima S, Thummalapalli R, Barbie DA. Inflammation as a driver and vulnerability of KRAS mediated oncogenesis. Semin Cell Dev Biol. 2016;58:127–135. doi: 10.1016/j.semcdb.2016.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dai JJ, Jiang MJ, Wang XP, Tian L. Inflammation-related pancreatic carcinogenesis: mechanisms and clinical potentials in advances. Pancreas. 2017;46:973–985. doi: 10.1097/MPA.0000000000000886. [DOI] [PubMed] [Google Scholar]
- 20.Hingorani SR, Petricoin EF, Maitra A, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4:437–450. doi: 10.1016/s1535-6108(03)00309-x. [DOI] [PubMed] [Google Scholar]
- 21.Hingorani SR, Wang L, Multani AS, et al. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell. 2007;7:469–483. doi: 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 22.Feng M, Xiong G, Cao Z, et al. PD-1/PD-L1 and immunotherapy for pancreatic cancer. Cancer Lett. 2017;407:57–65. doi: 10.1016/j.canlet.2017.08.006. [DOI] [PubMed] [Google Scholar]