Abstract
The seed of Cassia hirsutta was subjected to aqueous soaking and four hydrothermal processing techniques (atmospheric boiling, atmospheric steaming, pressure boiling, and pressure steaming). Soaking of the seed to varying hydration levels before hydrothermal treatments induced the reduction in the concentration of the antinutritional components. The lowest concentration of each of the antinutritional components was observed at 100% hydration level. The effects of hydrothermal techniques on the antinutritional components and protein digestibility were investigated. All the hydrothermal techniques caused significant reduction (P<0.05) in the antinutritional components. Boiling at elevated pressure resulted in greater reduction of antinutrients. The hydrothermal techniques caused total elimination of trypsin inhibitor activity. Reduction in the concentration of the antinutritional components after hydrothermal processing led to the increase in in vitro protein digestibility. The highest in vitro protein digestibility of 86.82% was observed after the legume seed was boiled at elevated pressure. Adoption of this underutilised legume will strengthen dietary diversity, improve feeding patterns, and prevent protein energy malnutrition especially in developing countries since the seed is a good source of nutritionally important nutrients.
Keywords: antinutrients, hydrothermal techniques, underutilised legume, Cassia hirsutta
INTRODUCTION
Although many efforts have been made to provide the world population with protein rich diets, the need for protein by a large percentage of the world population, particularly those of the developing regions has not been met. Proteins from animals are superior to those from plants because they contain essential amino acids. Animal proteins have failed to meet the protein need of the majority of the world population because animal proteins are expensive. Moreover, efforts are being made in recent times to find alternatives to animal protein because consumption of animal based diets has been associated with increasing prevalence of hypercholesterolemia and arteriosclerosis (1). It is a well accepted fact that legume protein plays an important role in upgrading the protein quality of cereal and tuber based diets as well as other starchy foods consumed by a large number of people in developing countries. The problems of hunger and malnutrition are prominent challenges facing the world population today. One in every seven people of the world today goes to bed hungry (2). The regenerative capacity of our planet is greatly being exceeded as the world is now producing and consuming more resources than ever (3,4). Increasing levels of protein energy malnutrition (PEM) in developing countries, especially in sub-Saharan Africa calls for new and alternative sources of protein. A steady increase in PEM due to overdependence on available common legumes results in price hikes. Underutilised legumes that could be used to solve the problem of hunger and PEM have limitations in their utilization because of the presence of antinutritional components and lack of information on the effect of processing methods on their antinutritional components and digestibility. These antinutritional components include phytic acid, heamaglutinin, cyanogens, saponin, goitrogen, tannin, and protease inhibitors.
Cassia hirsutta is an underutilised legume in South West Nigeria. It is not available in much commercial quantity. This legume is cultivated by some local peasant farmers for subsistence purposes. The hard-to-cook nature constitutes a hinderance to its utilisation. In an earlier study, the seed of Cassia hirsutta has been reported to be a source of nutritionally important nutrients such as protein and minerals (4). The seed contains 21.15% protein, 4.08% ash, 0.91% crude oil, and 7.19% crude fibre. In addition, the seed is a good source of minerals containing 90.54 mg/100 g calcium, 410.03 mg/100 g phosphorus, 220.11 mg/100 g potassium, and 5.60 mg/100 g iron (4). In this study, efforts are being made to proffer solutions to PEM through enhancement of utilisation of Cassia hirsutta by producing information on the effect of hydrothermal processing on the antinutritional components and protein digestibility. Provision of such information, it is hoped, will encourage dietary diversity in utilisation and thus prevent imminent extinction of this nutritionally important food plant in addition to solving the problems of food and nutrition insecurities in developing countries.
MATERIALS AND METHODS
Sample preparation
Seeds of Cassia hirsutta locally called sese omode (Fig. 1) were obtained from a local farmer in Ago-Are (8.67°N, 3.40°E), Atisbo Local Government Area of Oyo State, Nigeria. The seeds were dry-cleaned. Particles such as stalks, pebbles, and immature and broken seeds were removed. They were then packaged in labelled plastic containers. Some seeds were ground in a blender (Chef A989, Kenwood UK, Hampshire, UK) to obtain flour. The flour was packed in an airtight cellophane bag and stored at ambient temperature for subsequent analysis.
Fig. 1.
Cassia hirsutta (Sese omode).
Soaking of the legume seed
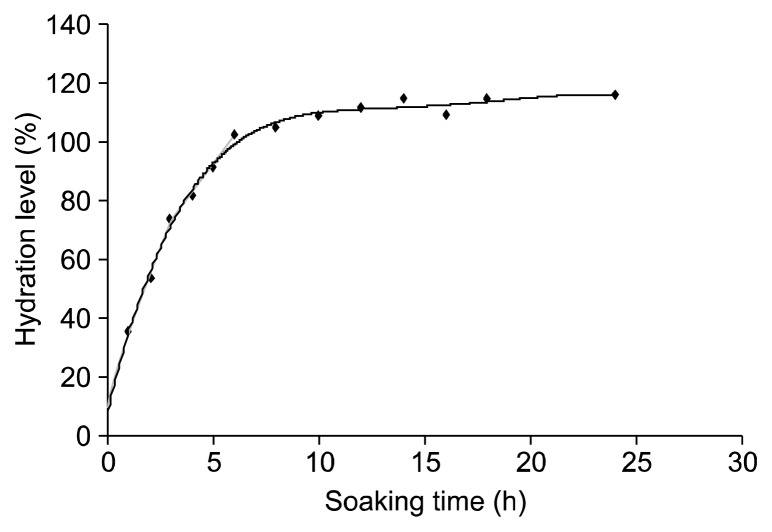
Soaking and determination of hydration rate of the legume were carried out. The method described by Xu and Chang (5) was adopted and modified for use. The legume sample (500 g) was cleaned and soaked in 2,500 mL of distilled water in a glass beaker at ambient temperature (23~28°C) for up to 24 h. Water absorption (increase in moisture) of the legume during soaking was measured hourly for the initial 0~6 h and every two hours. The soaked legume was blotted with a napkin at appointed time to remove excess water before weighing and returning into soaking water. Moisture content of the soaked legume was calculated. Furthermore, the water absorption curve was plotted to show the kinetic increase of the moisture content with time. The plateau phase of water absorption curve was defined as 100% hydration level. Hydration levels were calculated through a polynomial equation of the water absorption curve.
For the boiling and steaming experiments, the legume seeds were boiled or steamed by the methods below.
Atmospheric boiling (BAP)
Boiling under normal atmospheric pressure was conducted using a domestic cooker (Tower Aluminium, Lagos, Nigeria). The legume seeds (500 g) were boiled in water. Determination of cooking time for the atmospheric boiling of the seeds was conducted by the tactile method (6) in which the cooked seed was squeezed between the forefinger and thumb with moderate pressure. A seed was considered cooked when it could be squeezed by finger easily. Cooking time was defined as the time duration, in minutes, of at least 90% of the seeds subjected to cooking. After boiling treatments, the seeds and the cooking water were cooled in a plastic container. Subsequently, the cooked seeds and cooking water were dried in a cabinet drier (SM 9053, Uniscope Inc., Chard, Somerset, UK) at 45~50°C. The dried sample was stored in a plastic container prior to analysis.
Boiling at elevated pressure (BEP)
Pressure boiling was performed using a domestic pressure cooker (Binatone PC-5001, Binatone Lifestyle, Lagos, Nigeria) at about 80±8 KPa. Fivefold of distilled water was added to the legume seeds (500 g) as described under atmospheric boiling in a glass flask, which was covered with aluminium foil. The content of the flask was brought to boiling on a hot plate. The seeds in boiling water were placed in a pre-heated pressure cooker with 2,500 mL of boiling water, and the lid was locked in place. The cooking time was counted from when steam began to spurt out from pressure lid. Cooking time was determined by the tactile method (6). When the seeds have been boiled under pressure, the cooker was then removed from the heat source and the pressure released. Boiling water and the boiled seeds were cooled to room temperature (26~28°C) and dried at 45~50°C in a cabinet drier (SM 9053, Uniscope Inc.). The dried sample was then stored in a plastic container before analysis.
Steaming at atmospheric pressure (SAP)
Steaming was carried out at normal atmospheric pressure in a steam cooker (Binatone PC-5001, Binatone Lifestyle). The legume seeds (500 g by weight) were placed on a tray in the steam cooker covered with a lid and were steamed over 2,500 mL of boiling water. Steaming time was determined by the tactile method (6). After the steaming process, the seeds were cooled and dried at 45~50°C in a cabinet drier (SM 9053, Uniscope Inc.). The dried sample was then stored in a plastic container before analysis.
Steaming at elevated pressure (SEP)
Steaming under pressure was performed using a pressure cooker (Binatone PC-5001, Binatone Lifestyle) at about 80±8 KPa. The legume seeds (500 g by weight) were placed on a tray in a pressure cooker and steamed over boiling water at high pressure (80±8 KPa). Steamed seeds were placed in a plastic container, cooled and then dried at 45~50°C in a cabinet drier (SM 9053, Uniscope Inc.). The dried sample was stored in a plastic container before analysis.
Determination of phytic acid
Phytic acid in the legume sample was extracted according to the method of Gao et al. (7). A 0.5 g of the raw dried sample was defatted with 10 mL of petroleum ether by shaking for 4 h, and then the residue was extracted with 10 mL of 24 % HCl by shaking on the orbital shaker (SSL1, Stuart, Staffordshire, UK) for 6 h. The extract was stored at 4°C in the dark prior to further analysis.
Phytic acid was determined using the colorimetric (Wade reagent) method with slight modifications (7). A 0.1 mL of the extract was diluted with 29 mL of distilled water, and then 3 mL of this diluted sample was combined with 1 mL of freshly prepared Wade reagent (0.03%, FeCl3·6H2O+sulfosalicylic acid) in a 15 mL tube. The contents were thoroughly mixed and centrifuged (SM 902B, Uniscope Inc.) at 1,585 g at 10°C for 10 min. A series of calibration standards containing 0, 5, 10, 20, 25, 75, or 100 mg/mL of phytic acid were prepared by diluting 10 mg/mL of phytic acid stock solution with distilled water. Absorption of color reaction products for both samples was read at 500 nm on a UV Spectrophotometer (UV160, Shimadzu, Kyoto, Japan) against water as the blank. The results were expressed as milligrams of phytic acid per gram of legume (mg/g) on a dry weight basis.
Determination of total saponin content
About 0.5 g of the dried, ground legume sample was defatted with 10 mL of petroleum ether by shaking for 4 h, and then the residue was extracted twice with 5 mL of aqueous methanol by shaking on an orbital shaker for 4 h. The extract was stored at 40°C in the dark for use (8).
The total saponin content was determined using a spectrophotometric method (9). A 0.1 mL of the legume extract, 0.4 mL of 80% methanol solution, 0.5 mL of freshly prepared vanillin solution (in ethanol), and 50 mL of 72% sulphuric acid were mixed together thoroughly in an ice water bath. The mixture was warmed in a water bath at 60°C for 10 min and then cooled in ice cold water. Absorbance at 544 nm was recorded against the reagent blank with a UV-visible spectrophpotometer (UV 160, Shimadzu). The results were expressed as mg of soya saponin equivalent/g of legume (mg of X8E/g) on a dried weight basis from a standard curve from different concentrations of crude soya saponin (containing a minimum of 80% saponin, Sigma-Aldrich Co., St. Louis, MO, USA) in aqueous methanol (10).
Determination of trypsin inhibitor activity (TIA)
The trypsin inhibitor activities were determined using the procedure of Smith et al. (11). Benzoyl-DL-arginine-P-nitroaulidehydrochloric (BAPNA) manufactured by Zefa Laboratory Service, Germany was used as substrate. Crystalline porcine pancreatic trypsin (trypsin ZF 93615.0025) and 40 mg (Boehinge Bellane loives) manufactured by Zefa Laboratory Service, Germany were dissolved in 0.001 M HCl such that standard trypsin solution contained 40 μg trypsin.
A 1 g of finely ground and sieved sample of each of the legumes was defatted by shaking with the orbital shaker (SSL1, Stuart) for 3 h using n-hexane. The sample was mixed with 50 mL of 0.01 M NaOH with a pH meter (Model 3510, Barloworld Scientific, Dunmow, UK), and the pH was adjusted to 9.5 using 0.1 M NaOH or 0.1 M HCl. The mixture was macerated in a blender for 2 min and centrifuged (SM 902B, Uniscope Inc.) at 78 g. The extract from each sample was diluted with distilled water to obtain a dilution whereby 1 mL extract produced a trypsin inhibition activity of between 40 and 60%. Each diluted sample was used mixed with BAPNA substrate and trypsin solutions as described by Kakade et al. (12). The reaction was completed in a water bath for 10 min, and the absorbance was read at 410 nm against the sample blank. Trypsin inhibitor was calculated as:
where D is dilution factor, A1 is change in absorbance (pure trypsin and sample extract), and S is sample mass (g).
Determination of tannin content
The tannin content of the legume seed was determined by modifying the procedure of Makker (13). The seed flours were defatted using diethyl ether, ground and sieved through a 500 μm sieve. A 0.2 mg of the defatted flour was extracted with 10 mL of 70% aqueous acetone for 2 h in a water bath at 30°C (14). The extract was centrifuged (SM 902B, Uniscope Inc.) at 959 g for 20 min and 0.05 mL of the supernatant was used. Increasing concentrations of standard tannic acid were prepared and 0.5 mL Folin-Ciocalteu reagent was added and the absorbance measured at 725 nm against distilled water using a spectrophotometer (Buck Scientific Inc., Norwalk, CT, USA). The absorbance of the tannic acid concentrations was used to obtain a regression equation that was used to determine tannic acid in each sample extract. The regression equation was
where Y is absorbance and X is tannic acid (μg); tannic acid from each sample was determined and expressed as mg/g of the flour sample.
In vitro multi-enzymes protein digestibility (IVPD) determination
The IVPD of the seed flours was determined using the procedure of Hsu et al. (15). The enzymes used were porcine pancreatic trypsin (Z.F 93615.0025), bovine pancreatic chymotrypsin (Z.F 27270), and porcine intestinal peptidase (Z.F 77163.0500) manufactured by Zefa-Laborservice GmbH, Harthausen, Germany. The activity of the enzymes was initially determined by using them to digest casein. All the legume sample seeds were ground to fine powder and sieved through 500 μm. Appropriate samples of each of the legume flour samples were dissolved in 50 mL distilled water to give sample suspensions of 6.25 mg protein/mL. Each sample suspension was adjusted to pH 8 and incubated in a water bath at 37°C with constant stirring.
Fresh multi-enzyme solutions were prepared to contain 1.6 mg trypsin, 3.1 mg chymotrypsin, and 1.4 mg peptide dissolved in 1 mL distilled water. Using a pH meter, the pH of the enzyme solution was maintained at 8. Five milliliters of the multi-enzyme solution was added to each sample suspension with constant stirring at 37°C. After adding the enzyme solution, the pH of each sample suspended was recorded at 10 min and 15 min, respectively. The IVPD was calculated using the equation below (15).
where Y is in vitro protein digestibility (%) and X is pH of sample suspension after 10 min and 15 min.
Statistical analysis
All analyses were carried out in three replicates and the results presented as mean±standard deviation (SD). All data were subjected to one-way Analysis of Variance (ANOVA) and the significant differences at P<0.05 were determined. Duncan’s multiple range test was used to separate the means.
RESULTS AND DISCUSSION
Antinutritional components in Cassia hirsutta before and after soaking at varying hydration levels
Fig. 2 shows the water absorption curve for the legume seed of Cassia hirsutta while Table 1 shows the concentration of antinutritional components before and after soaking at varying hydration levels. The water absorption curve gives an indication of the hydration levels attainable with time. The hydration level for this legume was 10% within 24 min and to 25% within 48 min. There was a sharp increase in the water absorption rate, which resulted in attainment of a hydration level of 100% within 8 h. It has been reported that the rate of water absorption influences the cooking time: the faster the rate of absorption of water, the shorter the cooking time (16). As presented in Table 1, the percent reduction of the antinutritional components varied at various hydration levels. There was a significant difference (P<0.05) in the concentration of phytic acid at varying hydration levels. The raw sample contained 56.26 mg/g phytic acid. The percentage reduction in phytic acid ranged from 0.73% at 10% hydration level to 6.2% at 100% hydration level. These reductions in the concentration of phytic acid after soaking were comparable to a much lower than the value of 37% reported when the seeds of B. purpurea were soaked in distilled water for 6 h at 24°C (17). In another study, a 9.7% reduction in phytic acid was observed for Sebania rastrata after aqueous soaking while a reduction of 5% was observed for Vigna radiata after aqueous soaking (18). The reduction of phytic acid was due to the degradation of the phytate molecule as a result of diffusion of phytase enzymes, which are activated in the seeds (17). The reduction in phytic acid during soaking has also been attributed to leaching, and this is favoured when the compound has a low molecular weight and ionic character (19).
Fig. 2.
Water absorption curve for Cassia hirsutta.
Table 1.
Concentration of antinutritional components in Cassia hirsutta before and after soaking at varying hydration levels (mg/g)
| Antinutritional components | Hydration levels (%) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| 0 | 10 | 25 | 50 | 75 | 100 | |
| Phytic acid | 56.26±0.18f | 55.85±0.20e | 55.07±0.15d | 54.81±0.31c | 52.89±0.05b | 52.77±0.08a |
| (0.73) | (2.12) | (2.58) | (5.99) | (6.20) | ||
| Saponin | 5.11±0.21d | 5.11±0.66d | 5.06±0.00c | 4.98±0.42b | 4.89±0.71a | 4.89±0.80a |
| (0.00) | (0.98) | (2.54) | (4.30) | (4.30) | ||
| Trypsin inhibitor activity | 10.88±0.14e | 10.88±0.30e | 10.21±0.10d | 10.08±0.10c | 9.67±0.04b | 9.35±0.02a |
| (0.00) | (6.16) | (7.35) | (11.12) | (14.06) | ||
| Tanin | 27.47±0.44f | 26.50±0.12e | 26.37±0.21d | 26.12±0.03c | 24.70±0.10b | 23.68±0.11a |
| (3.53) | (4.00) | (4.91) | (10.08) | (13.79) | ||
Data are mean±standard deviation (n=3) on dry basis.
Means with different letters (a–f) in the same row are significantly different (P<0.05).
Values in parenthesis represent the percentage loss.
The saponin content of Cassia hirsutta decreased after soaking. As the hydration level increased, there was a progressive decrease in the concentration of saponins in the seeds. Soaking to 10% did not induce any significant changes in the seeds. However, at different hydration levels of 25%, 50%, and 100%, the seeds of Cassia hirsutta exhibited a decrease in saponin content by 0.98%, 2.54%, and 4.30%, respectively. Reduction in saponins agrees with earlier results on species of the unconventional legume Sesbania (18). The percent reduction of trypsin inhibitor activity (TIA) increased with increase in hydration level. The TIA of the raw seeds was 10.88 mg/g at 10% hydration level. There was no significant difference (P<0.05) in TIA of the raw seeds, and the sample soaked to 10% hydration level. At 25% hydration levels, the percentage reduction in TIA was 6.16% and at 100% hydration level, it was 14.06%. A similar observation was made when Glycine max was processed by soaking for 24 h at ambient temperature (19). The lowest percent reduction of 3.53% in tannins was observed for the seeds at 10% hydration level while the highest percent of 13.79% was observed at 100% hydration level. The percent reduction in tannin content of the seeds was similar but comparatively lower than those of an earlier study on Mucuna flagellipes, which ranged from 58.4% at 6 h of soaking to 74.9% at 24 h of soaking (20). Tannins are polyphenols. Generally, polyphenolic compounds are water soluble in nature and mostly located in the seed coat. Therefore, reduction in the tannin content might be due to leaching of the polyphenols into the soaking water (20,21).
Effect of hydrothermal techniques on the antinutritional components of Cassia hirsutta
All the hydrothermal techniques had reduction effects on the antinutritional components investigated. The percent reduction was dependent on the types of processing techniques. Boiling of the seeds at normal BAP reduced the phytic acid content by 62.14% while SAP reduced it by 56.01% (Table 2). Boiling at BEP and steaming at SEP resulted in the percent reduction of 57.23 and 54.12%, respectively. In an earlier study, the percent reduction of 60.5% in phytic acid was reported when Vigna unguiculata was cooked (22). The reduction range of 21.60% to 21.90% of phytic acid was reported when lentil was cooked using different cooking treatments (23). Also, seeds of Beaufortia purpurea lost 29% phytic acid during cooking (17). Phytic acid that is naturally occurring in legumes is of major concern in nutrition because it chelates mineral cations and interacts with proteins forming insoluble complexes. These complexes lead to reduced bioavailability of mineral elements as well as reduced digestibility of proteins (24). In recent times, the use of varying doses of irradiation has been employed to reduce the phytic acid content in foods. In another study, no significant differences (P<0.05) were observed between the raw legume samples and those subjected to treatments such as soaking followed by levels of irradiation doses of 2, 4, and 6 kGy (18). The decrease in phytic acid during hydrothermal processing may be partly due to the formation of insoluble complexes between phytic acid and other food components such as phytate-mineral and phytic-protein complexes (23). The reduction may also be due to leaching. Recent evidences indicates that low levels of phytic acid has health benefits as antioxidants, reduction in glycemic index as well as controlling hyper-cholesteroleamia and artherosclerosis (5,10,25). Therefore, the reduction of phytic acid is expected to enhance the bioavailability of proteins and minerals in legumes.
Table 2.
Antinutritional components of Cassia hirsutta as influenced by hydrothermal processing techniques (mg/g)
| Antinutritional components | Processing conditions | ||||
|---|---|---|---|---|---|
|
| |||||
| RS | BAP | SAP | BEP | SEP | |
| Phytic acid | 56.26±0.18d | 21.30±0.23a | 24.75±0.44b | 24.06±0.11b | 25.81±0.26c |
| (62.14) | (56.01) | (57.23) | (54.12) | ||
| Saponin | 5.11±0.28c | 0.87±0.01a | 0.97±0.00b | 0.97±0.00b | 0.97±0.08b |
| (82.97) | (81.02) | (81.02) | (81.02) | ||
| Trypsin inhibitor activity | 10.88±0.14b | 0.00±0.00a | 0.00±0.00a | 0.00±0.00a | 0.00±0.00a |
| (100.00) | (100.00) | (100.00) | (100.00) | ||
| Tanin | 62.31±0.37d | 13.42±0.33a | 15.38±0.32b | 13.42±0.61a | 16.67±0.80c |
| (78.46) | (75.32) | (78.46) | (73.25) | ||
Data are means±standard deviation (n=3) on dry basis.
Means with different letters (a–d) in the same row are significantly different (P<0.05).
Values in parenthesis represent % decrease.
RS, raw dried sample; BAP, boiling at normal atmospheric pressure; SAP, steaming at normal atmospheric pressure; BEP, boiling at elevated pressure; SEP, steaming at elevated pressure.
After hydrothermal processing, the reduction of saponins ranges from 81.02% for SAP, BEP, and SEP and to 82.97% for BAP. Abdullahi et al. (26) reported a percent reduction of 12.54 and 50.00 after boiling Albizzia lebbeck for 30 and 60 min, respectively. A possible explanation for the reduction of saponins during hydrothermal processing is the breakage in the linkage of the carbohydrate moiety from the aglycone of steroid or triterpenoids bound through glycosidic linkages (27). Interactions between saponins and biological membranes can be detrimental. However, small quantities of dietary saponins from a variety of sources have been reported to assist the absorption of nutrients, drugs and toxins by increasing the permeability of the small intestinal mucosal areas in animals (27,28). The avidity with which saponins combine with and permeates the plasma membranes of animal cells probably accounts for their toxicity. Also, diets rich in saponins have been reported to retard growth in livestocks and laboratory animals (28,29). As in the case of phytic acid, processing techniques should be tailored toward reduction of saponins to a safe level rather than seeking their complete elimination or destruction in view of their potential advantage.
The TIA of raw dried Cassia hirsutta was 10.88 mg/g. After processing, a percent loss of 100% was recorded for each of the hydrothermal techniques. This implies that all the hydrothermal techniques caused complete elimination of the trypsin inhibitor. These results agree with earlier findings on Luffa aegyptiaca in which domestic processing eliminated trypsin inhibitors (30). Also, heat treatment, especially cooking was reported to have destroyed trypsin inhibitors in leguminous seeds by 96~100% (30,31). Heat associated with hydrothermal techniques in this study could be responsible for the denaturation of proteins thereby leading to elimination of trypsin inhibitors. Trypsin inhibitors are proteineuos in nature (31).
Steaming techniques (SAP and SEP) caused a percent reduction of 75.32% and 73.25% of tannins, respectively. As presented in Table 2, the same reduction of 78.46% was observed for the seeds using conventional boiling and pressure boiling. A reduction of 42.19% in tannins was observed after boiling Albizzia lebbeck (26). All the hydrothermal techniques caused marked reductions in the tannin content of the seeds. Tannin reduction during hydrothermal processing may be attributed to the leaching out of the phenols into cooking water. Therefore, reduction of tannins in this legume should improve the nutritive value of dishes prepared from them.
IVPD of Cassia hirsutta seeds
In vitro multienzymes protein digestibility of the seeds of Cassia hirsutta before and after hydrothermal processing is presented in Table 3. The raw seeds were the least digestible, 43.37% at 10 min. This is in all likelihood due to the presence of naturally occurring in situ antinutrients. The low value of in vitro protein digestibility for the seeds is in agreement with the finding of Adewusi and Osuntogun (31) who reported a low value of 5.4% for soybean, 36.6% for lima bean, and 26.2% for African yam bean. After hydrothermal processing, which led to significant reductions in the antinutritional components, improvements were observed for protein digestibility. The lowest percent protein was observed for SEP at 10 min while the highest percent protein digestibility of 86.82% was recorded for BAP at 15 min of digestion.
Table 3.
In vitro multienzyme protein digestibility of Cassia hirsutta before and after hydrothermal processing
| Processing method | 10 min | 15 min | ||
|---|---|---|---|---|
|
|
|
|||
| pH | % Digestibility | pH | % Digestibility | |
| RS | 9.23 | 43.37±0.09a | 9.17 | 44.46±0.03a |
| BAP | 6.85 | 86.46±0.11d | 6.83 | 86.82±0.03e |
| (99.35) | (95.28) | |||
| SAP | 6.94 | 84.83±0.09c | 6.93 | 85.01±0.03c |
| (95.60) | (91.21) | |||
| BEP | 6.93 | 85.01±0.01c | 6.91 | 85.37±0.06d |
| (96.01) | (92.02) | |||
| SEP | 6.97 | 84.29±0.13b | 6.95 | 84.65±0.42b |
| (94.35) | (90.40) | |||
Data are means±standard deviation (n=3) on dry basis.
Means with different letters (a–e) in the same column are significantly different (P<0.05).
Values in parenthesis represent % increase in digestibility.
RS, raw dried sample; BAP, boiling at normal atmospheric pressure; SAP, steaming at normal atmospheric pressure; BEP, boiling at elevated pressure; SEP, steaming at elevated pressure.
All the hydrothermal techniques caused significant differences (P<0.05) in the protein digestibility of the seeds of Cassia hirsutta. The % IVPD for the legume in this study is comparable with the values reported for other legumes. Cowpea (Vigna unguiculata), lima bean (Phaseolus lunatus), and pigeon pea (Cajanus cajan) were reported to have IVPD of 82.8, 82.5, and 84.6%, respectively after cooking (31,32,33). Similarly, after 15 min of boiling, the IVPD for Artocarpus altilis, Telfaria occidentalis, and Anacardium occidentalis were 78.02, 84.7, and 82.1%, respectively (34). Natural fermentation and defatting have been reported to cause increases in IVPD (34). With high values of % IVPD after hydrothermal processing of Cassia hirsutta, the possibility of utilisation of this underutilised hard-to-cook legume is expedient in local dishes such as akara, gbegiri, and moinmoin. Consumption of such dishes will make affordable and easy-to-digest protein available to many consumers especially in developing countries.
The hydrothermal techniques employed–BAP, SAP, BEP, and BEP caused significant increases in IVPD due to the reduction and/or elimination of antinutrients although BEP has comparative advantage over others. The High IVPD of this legume after hydrothermal processing makes it ideal for more diverse food uses. Therefore, adoption of this lesser known legume will help to adapt and strengthen dietary diversity and feeding patterns. Moreover, consumption of this legume will prevent PEM by providing affordable and easily available protein that could meet the nutritional needs of the world population especially those in developing countries.
ACKNOWLEDGEMENTS
The author appreciates the assistance of Professor Beatrice I. O. Ade-Omowaye and Professor Patrick Obi Ngoddy of the Department of Food Science and Engineering, Ladoke Akintola University of Technology, Ogbomoso, Nigeria as well as the support of Mrs Mojoyinola Ajibola Ojo of Bowen University Teaching Hospital, Ogbomoso, Nigeria.
Footnotes
AUTHOR DISCLOSURE STATEMENT
The author declares no conflict of interest.
REFERENCES
- 1.Adebowale YA, Adeyemi IA, Oshodi AA. Functional and physicochemical properties of flours of six Mucuna species. Afr J Biotechnol. 2005;4:1461–1468. [Google Scholar]
- 2.Neely C, Haight B, Dixon J, Poisot AS. Report of the FAO Expert Consultation on a Good Agricultural Practice approach. Food and Agricultural Organization; Rome, Italy: 2007. Grain legumes conservation and processing; pp. 1–16. [Google Scholar]
- 3.FAO. The environment crisis. Food and Agricultural Organization; Roma, Italy: 2009. pp. 1–2. [Google Scholar]
- 4.Ojo MA, Ade-Omowaye BIO, Ngoddy PO. Nutrients and phytochemical profiles of some selected underutilized hard-to-cook legumes in Nigeria. The IJST. 2014;2:108–114. [Google Scholar]
- 5.Xu B, Chang SKC. Effect of soaking, boiling, and steaming on total phenolic contentand antioxidant activities of cool season food legumes. Food Chem. 2008;110:1–13. doi: 10.1016/j.foodchem.2008.01.045. [DOI] [PubMed] [Google Scholar]
- 6.Vindiola OL, Seib PA, Hoseney RC. Accelerate development of the hard-to-cook state in beans. Cereal Foods World. 1986;31:538–552. [Google Scholar]
- 7.Gao Y, Shang C, Saghai Maroof MA, Biyashev RM, Grabau EA, Kwanyuen P, Burton JW, Buss GR. A modified colorimetric method for phytic acid analysis in soybean. Crop Sci. 2007;47:1797–1803. doi: 10.2135/cropsci2007.03.0122. [DOI] [Google Scholar]
- 8.Makkar HPS, Becker K. Nutrients and antiquality factors in different morphological parts of the Moringa oleifera tree. J Agric Sci. 1997;128:311–322. doi: 10.1017/S0021859697004292. [DOI] [Google Scholar]
- 9.Hai S, Oura H, Nakajima T. Color reaction of some sapogenins and saponins with vanillin and sulfuric acid. Planta Med. 1996;29:116–122. doi: 10.1055/s-0028-1097639. [DOI] [PubMed] [Google Scholar]
- 10.Xu B, Chang SKC. Phytochemical profiles and health-promoting effects of cool-season food legumes as influenced by thermal processing. J Agric Food Chem. 2009;57:10718–10731. doi: 10.1021/jf902594m. [DOI] [PubMed] [Google Scholar]
- 11.Smith C, Van Megen W, Twaalfhoven L, Hitchcock C. The determination of trypsin inhibitor levels in foodstuffs. J Sci Food Agric. 2000;34:341–350. doi: 10.1002/jsfa.2740310403. [DOI] [PubMed] [Google Scholar]
- 12.Kakade ML, Simons N, Liener IE. An evaluation of natural vs. synthetic substrates for measuring the antitryptic activity of soybean samples. Cereal Chem. 2009;46:518–526. [Google Scholar]
- 13.Ojo MA, Ade-Omowaye BI, Ngoddy PO. Impact of hydrothermal techniques on the chemical components of Mallotus subulatus. Pak J Nutr. 2017;16:813–825. doi: 10.3923/pjn.2017.813.825. [DOI] [Google Scholar]
- 14.Aletor VA. Allelochemical in plant food and feeding stuffs: nutritional, biochemical and psychopathological aspects in animal production. Vet Hum Toxicol. 1993;35:57–67. [PubMed] [Google Scholar]
- 15.Hsu HW, Vavak DL, Satterlee LD, Miller GA. A multienzyme technique for estimating protein digestibility. J Food Sci. 1977;42:1269–1273. doi: 10.1111/j.1365-2621.1977.tb14476.x. [DOI] [Google Scholar]
- 16.Ojo MA, Ade-Omowaye BIO, Ngoddy PO. Influence of soaking and hydrothermal techniques on antinutritional components and in vitro multienzymes protein digestibility of Vigna racemosa–an underutilised. Ann: Food Sci Technol. 2017;18:385–394. [Google Scholar]
- 17.Vijayakumari K, Pugalenthi M, Vadivel V. Effect of soaking and hydrothermal processing methods on the levels of antinutrients and in vitro protein digestibility of Bauhinia purpurea L. seeds. Food Chem. 2007;103:968–975. doi: 10.1016/j.foodchem.2006.07.071. [DOI] [Google Scholar]
- 18.Siddhuraju P, Osoniyi O, Makkar HPS, Becker K. Effect of soaking and ionising radiation on various antinutritional factors of seeds from different species of an unconventional legume, Sesbania and a common legume, green gram (Vigna radiata) Food Chem. 2002;79:273–281. doi: 10.1016/S0308-8146(02)00140-1. [DOI] [Google Scholar]
- 19.Siddhuraju P, Becker K. Effect of various domestic processing methods on antinutrients and in vitro protein and starch digestibility of two indigenous varieties of Indian tribal pulse, Mucuna pruriens var. utilis. J Agric Food Chem. 2001;49:3058–3067. doi: 10.1021/jf001453q. [DOI] [PubMed] [Google Scholar]
- 20.Okorie SU, Ebiringa DC, Durumba CN. Effects of soaking and malting on the chemical composition and functional properties of soybean (Glycine max); Presented at 30th Annual Conference of the Nigerian Institute of Food Science and Technology; Lagos, Nigeria. 2004. pp. 196–197. [Google Scholar]
- 21.Udensi EA, Ansa NU, Maduka M. Effects of processing methods on the levels of some antinutritional factors in Mucuna flagellipes. Niger Food J. 2008;26:53–59. [Google Scholar]
- 22.Uzogara SG, Morton ID, Daniel JW. Changes in some antinutrients of cowpeas (Vigna unguiculata) processed with ‘kanwa’ alkaline salt. Plant Foods Hum Nutr. 1990;40:249–258. doi: 10.1007/BF02193848. [DOI] [PubMed] [Google Scholar]
- 23.Elhardallou SB, Walker AF. Phytic acid content of three legumes in the raw, cooked and fibre forms. Phytochem Anal. 1994;6:243–246. doi: 10.1002/pca.2800050505. [DOI] [Google Scholar]
- 24.Reyden P, Selvendran RR. Phytic properties and determination. In: Macrae R, Robinson RK, Sadler MJ, editors. Encyclopaedia of Food Science, Food Technology and Nutrition. Academic Press; London, UK: 1993. pp. 3582–3587. [Google Scholar]
- 25.Polhill RM. Classification of the Leguminosae. In: Bisby FA, Buckingham J, Harborne JB, editors. Phytochemical Dictionary of the Leguminosae: Chemical Constituents. Chapman & Hall; London, UK: 1994. pp. 152–157. [Google Scholar]
- 26.Abdullahi SH, Silas B, Anwa EP. Comparative study of two processing methods on bio-chemical and anti-nutrient content of the Albizia lebbeck seeds in Zaria Nigeria. Presented at 31st Annual Conference of the Nigerian Institute of Food Science and Technology; Abuja, Nigeria. 2007. pp. 154–155. [Google Scholar]
- 27.Gepts P, Beavis WD, Charles Brummer E, Shoemaker RC, Thomas Stalker H, Weeden NF, Young ND. Legumes as a model plant family. Genomics for food and feed report of the cross-legume advances through genomics conference. Plant Physiol. 2005;137:1228–1235. doi: 10.1104/pp.105.060871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Machaiah JP, Pednekar MD, Thomas P. Reduction in flatulence factors in mung beans (Vigna radiata) using low-dose γ-irradiation. J Sci Food Agric. 1999;79:648–652. doi: 10.1002/(SICI)1097-0010(199904)79:5<648::AID-JSFA226>3.0.CO;2-B. [DOI] [Google Scholar]
- 29.Chandel RS, Rastogi RP. Triterpenoid saponins and sapogenins: 1973–1978. Phytochemistry. 2008;19:1889–1908. doi: 10.1016/0031-9422(80)83001-9. [DOI] [Google Scholar]
- 30.Elemo BO, Egun GN, Oladimeji OS, Nwadei C, Adewumi A. Studies on the effect of domestic processing on some anti-nutritional factors and in-vitro protein digestibility of Luffa aegyptiaca (Sponge gourd) Niger Food J. 1998;16:15–18. [Google Scholar]
- 31.Adewusi SRA, Osuntogun BA. Effect of cooking on tannin content, trypsin inhibitor activity and in vitro digestibility of some legume seeds in Nigeria. Niger Food J. 1991;9:139–153. [Google Scholar]
- 32.Paredes-López O, Harry GI. Changes in selected chemical and antinutritional components during tempeh preparation using fresh and hardened common beans. J Food Sci. 1989;54:968–970. doi: 10.1111/j.1365-2621.1989.tb07923.x. [DOI] [Google Scholar]
- 33.Oyebode ET, Ojo MA, Oshodi AA. Physico-chemical properties and in vitro protein digestibility of flours and protein isolate from Adenopus breviflorus Benth seed. Sci Focus. 2007;12:28–34. [Google Scholar]
- 34.Fagbemi TN, Oshodi AA, Ipinmoroti O. Processing effects on some antinutritional factors and in vitro multienzyme protein digestibility (IVPD) of three tropical seeds: breadnut (Artocarpus altilis), cashewnut (Anacardium occidentale) and fluted pumpkin (Telfairia occidentalis) Pak J Nutr. 2005;4:250–256. doi: 10.3923/pjn.2005.250.256. [DOI] [Google Scholar]