Abstract
An amyloidosis is a group of diseases that occurs when amyloid proteins are deposited in tissues and organs. In this study, we demonstrated, at the first time, the presence of amyloid deposits in the kidneys of dystrophin-deficient mdx mice, which are widely used as an experimental model of Duchenne muscular dystrophy. We examined cases of renal amyloidosis in aged mdx mice using traditional methods for amyloid detection (Congo red and Thioflavin T), as well as a new fluorescent dye, disodium salt of 2,7-(1-amino-4-sulfo- 2-naphthylazo) fluorene (DSNAF). Using these different fluorescent dyes, we confirmed the amyloid structure of protein deposits in kidneys of aged mdx mice. Additionally, we found that fixation method has profound effects on downstream staining procedures, and demonstrated that the application of specific fixative, zinc-ethanolformaldehyde, instead of traditional neutral-buffered formalin allows reducing the background fluorescence. Also we illustrated the usefulness of novel fluorescent dye DSNAF for detection of amyloid deposits in mouse tissues. Taken together, our results indicate the association of amyloid formation with Duchenne muscular dystrophy and provides new opportunities for studying naturally occurring amyloidosis development in tissues also.
Key words: Amyloidosis, mdx mice, Congo red, Thioflavin T, 2, 7-(1-amino-4-sulfo-2- naphthylazo) fluorene (DSNAF)
Introduction
Amyloidosis comprises a group of more than 30 clinical disorders characterized by extracellular deposition of insoluble fibrillar pathological deposits of proteins (amyloids) in organs and tissues.1 The presence of amyloid in muscle biopsies of patients with different types of muscular dystrophy was previously demonstrated.2,3 However, no attempts have been made earlier to identify amyloid deposits in organs and tissues from the patients with Duchenne muscular dystrophy (DMD), the most common form of human hereditary myodystrophy. The mdx mice (acronym for “X-chromosome-linked muscular dystrophy”) are now widely used as the experimental model of DMD.4 In this regard, the characterization of amyloid formation in organs and tissues of mdx mice is essential to reveal the mechanism of this aggregation and its role in the manifestation of DMD. The aim of this study was to identify amyloid deposits in the kidneys of mdx mice using different fluorescent dyes.
Diagnosis of amyloidosis, systemic and local, is based on the specific binding of a number of dyes to fibrillar structures stabilized by cross-beta layers in the biopsy of the affected organ (heart, kidney, liver, subcutaneous adipose tissue, bone marrow).5 The current diagnostic standard for amyloidosis in clinical practice is histochemical staining with a diazo dye Congo red (CR).1,6 The dye incorporates regularly between amyloid fibrils acquiring the characteristic absorption spectra and birefringence in polarized light.1,7 Fluorescence microscopy (amyloid stained with CR fluoresce in the red spectral range) was used to identify minor amyloid aggregates undetectable by bright field microscopy.8 However, CR has a number of limitations including background staining (background fluorescence), low fluorescence intensity and false-positive staining.9 Therefore, other fluorescence methods are also used for amyloid detection in the tissues. An example is the benzothiazole dye, Thioflavin T (ThT), which is widely used for amyloid fibril detection in vitro.10 ThT gives a strong fluorescence signal (with excitation and emission maximum 450 and 482 nm, correspondingly) upon binding to cross-betasheet structures, which determines its relatively low specificity in tissues.11 Nevertheless, ThT is not always the method of choice for amyloid detection also. Some reasons for weak ThT fluorescence are: at basic pH ThT is hydroxylated and some amyloid fibrils only show modest ThT fluorescence, which depends on morphology, pH, and protein forming the fibril.10
Therefore, a complex of fluorescencebased methods should be applied to characterize tissue localization of amyloid deposits and interactions between proteins of extracellular matrix and fibrils more precisely. The development of new compounds to study, monitor and inhibit amyloid formation in vivo is also important. The problem is especially relevant for amyloidosis of unknown types and with unusual clinical manifestation (for example, on experimental animal models). Thus, in our study we used different fluorescent dyes for amyloid detection, including the traditional Congo red and Thioflavin T, as well as a new fluorescent dye, disodium salt of 2,7-(1-amino-4-sulfo-2-naphthylazo) fluorene (DSNAF).
Materials and Methods
Animals
The study was performed on 1.5-yearold male and female mdx mice carrying a mutation in DMD gene (n=8) (RRID: IMSR_HAR:1217). The aged (1.2-1.5-yearold) male and female C57BL/6J mice (n=5) were used as a negative control. Mice were housed four per cage and kept on a 12-h light, 12-h dark cycle; food, and water were available ad libitum. Animals were deeply anesthetized and sacrificed by cervical dislocation.
All procedures were approved by the local Ethics Committee of the Institute of Experimental Medicine (St. Petersburg, Russia). All reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA) if not indicated otherwise.
General histology
For histological analysis, tissue pieces were fixed using two methods. One group was fixed in 10% neutral-buffered formalin (NBF), the second one in a zinc-ethanolformalin fixative (ZEF), previously shown to be highly effective in immuno - histochemical staining.12 Briefly, the ZEF was prepared as follows: 1 g ZnCl2 was dissolved in mixture of 90 mL 96% ethanol and 10 mL concentrated (35-39%) formaldehyde (Vekton, St. Petersburg, Russia). After dehydration, tissue samples were embedded in paraffin. Sections 5 μm thick were cut using rotary microtome (RM 2125RT; Leica Microsystems GmbH, Wetzlar, Germany) and mounted on poly-Llysine- coated (Polysine™; Menzel-Gläser, Germany) glass slides.
Amyloid staining
ThT staining was performed as previously described.13 Briefly, after rehydration, the slides were immersed in ThT solution (1 g of ThT in 100 mL distilled water) for 20 min in the dark. For CR staining, slides were put in the staining solution (0.5 % solution in water) for 25 min.
We also used a new fluorescent dye, disodium salt of 2,7-(1-amino-4-sulfo-2- naphthylazo) fluorene (DSNAF), for detecting amyloid in mouse tissues. For the synthesis of 2,7-(1-amino-4-sulfo-2- naphthylazo) fluorene (DSNAF), 2,7-diaminofluorene (1 mol. eq.) and naphthionic acid (2 mol. Eq.) were used as the starting compounds. The synthesis was performed by diazotation of 2,7- diaminofluorene under argon followed by azo coupling with naphthionic acid. The reaction product was precipitated with sodium chloride in acidic conditions. The desired compound was purified by HPLC on reversed-phase column using acetonitrile gradient. The product was lyophilized. The validation of DSNAF was carried out by mass spectrometry.
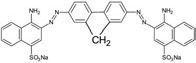 |
Structure of 2,7-(1-amino-4-sulfo-2- naphthylazo) fluorene disodium salt
According to fluorimetric assay, the Congo red analogue has an excitation maximum of 530 nm, emission maxima of 595 and 620 nm. Formation of complexes of this compound with fibrils of lysozyme, beta 2-microglobulin and insulin leads to significant increases in the fluorescence intensity as compared to that for DSNAF in presence of monomers of said proteins. Given the structural similarity of DSNAF to Congo red and the ability of the analogue to interact with fibrils of amyloidogenic proteins, we suggested to use the dye for detection of amyloid in tissues. For staining with disodium salt of DSNAF, the solution of this compound (0.035% or 0.2%) was applied to slides for 5 min.
In all experiments, sections were counterstained with alum hematoxylin. After staining the slides were rinsed quickly twice in distilled water, air dried and coverslipped with antifade mounting medium (Dako, Glostrup, Denmark). Images were acquired with a DM750 microscope (ICC50 camera, Leica C Plan 40 ×/0.65 [infinity]/0.17 Objective), and DM2500 fluorescent microscope (DFC420 camera, Leica 40× N Plan Microscope Objective) equipped with a fluorescence filter sets “A” BP340-380, “I3” BP450-490, and “N2.1” BP515-560 (Leica Microsystems GmbH) using standardized settings.
Results
Pathological features
In the kidneys of aged C57BL/6J mice, the amyloid deposits have never been detected using both bright-field and fluorescence microscopy (data not shown). In contrast, analysis of kidney samples of mdx mice stained with CR, ThT and DSNAF confirmed the pervasive presence of a particular type of protein aggregates, amyloid deposits, in the tissues of all examined mice. No variations were identified in amyloid distribution, i.e., amyloid deposits were uniquely localized in the area of renal corpuscles in CR (Figure 1 A-D), ThT (Figure 1E) and DSNAF (Figure 1 F-H) stained sections. Amyloid mass replaced most of the glomerulus and contained cavities – lumens of the destroyed capillaries and voids in place of degenerated cellular elements.
Figure 1.
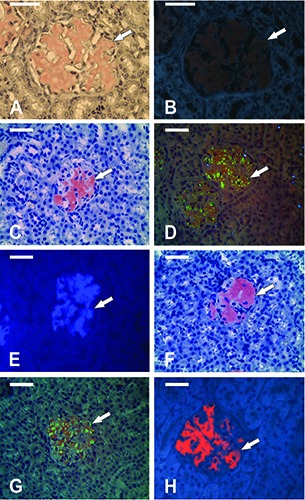
Amyloid deposits in kidney cortex of mdx mice. A-D) Congo red staining. E) Thioflavin T staining. F-H) Staining with disodium salt of 2,7-(1-amino-4-sulfo-2-naphthylazo) fluorene (DSNAF) in final concentration 0.2%. (F,G) and 0.035% (H). Counterstain with alum hematoxylin. A,B) Fixation in 10% neutral-buffered formalin. C-H) Fixation in zinc-ethanol-formaldehyde. Amyloid deposits (arrow) are localized in the area of renal corpuscles in Congo red (A-D), Thioflavin T (E) and DSNAF (F-H) stained sections. The amyloid structure of the deposits in kidneys of aged mdx mice is confirmed using bright-field (A,C,F) and polarized (D,G) light microscopy, and fluorescent microscopy (B,E,H) (excitation filter BP 340-380 nm). After fixation in zincethanol- formaldehyde and staining with DSNAF (F-H) amyloid deposits showed more saturated reddish-pink color in bright-field microscopy (F) and higher fluorescence without background in fluorescence microscopy (H) as compared to Congo red (A-D) and Thioflavin T (E) staining. Magnification: 40x. Scale bars: 50 m.
Nevertheless, the comparative analysis revealed a number of discrepancies between staining patterns depending on the stain used, as well as on the method of material fixation (NBF or ZEF).
Fixation procedure effect on CR staining result
When CR staining was applied to samples fixed in NBF, amyloid had a pinkish-orange color viewed in transmitted light (Figure 1A). These deposits show apple-green birefringence in polarized light, which allowed us to attribute aggregates to amyloid form. At the same time, a significant background nonspecific staining in the renal tubule area was observed (birefringence of CR was not detected in these regions). Amyloid deposits stained with CR after NBF fixation were characterized by the moderate fluorescence intensity in the red spectral range accompanied by strong background fluorescence (Figure 1B).
When ZEF was used as a fixative, amyloid attained a deeper pink color if bound with CR (as compared with NBF fixation). In this case, the cytoplasm of cells forming the renal tubules was characterized by a darker bluish-gray color, making the amyloid look more contrasting (Figure C). In polarized light, amyloid deposits were seen as reddish and bright green discrete aggregates localized in the renal corpuscles (Figure 1D). Nonspecific staining was observed in the surrounding tissue under both, transmitted and polarized light microscopy; however, it was significantly less pronounced compared with that after NBF fixation. Samples analyzed by fluorescence microscopy displayed a decrease in background fluorescence (data not shown).
After staining with ThT (ZEF fixation) amyloid deposits showed an intense blue fluorescence and looked like structures virtually uniform in fluorescence intensity. Nonspecific fluorescence was also observed (Figure 1E).
Disodium salt of 2,7-(1-amino-4- sulfo-2-naphthylazo) fluorene (DSNAF) can be successfully used for amyloid detection in mouse tissues
Amyloid deposits in the kidney samples stained with DSNAF (ZEF fixation) were characterized by a saturated reddish-pink color (Figure 1F). Pre-staining with hematoxylin followed by DSNAF staining resulted in a darker blue shade of the cytoplasm of renal tubule cells (as compared to CR staining), which increased visually the contrast of amyloid in bright-field microscopy (Figure 1F). Distinct dichroism in amyloid deposits staining (reddish and apple-green fluorescence) was observed by the polarized light analysis (Figure 1G). Background staining was not detected in both bright-field (Figure 1F) and polarized light (Figure 1G) microscopy.
As demonstrated by fluorescence microscopy, DSNAF dye bound to amyloid provided a high fluorescence yield in the red spectral range (600-650 nm), demonstrating the low level of background fluorescence (Figure 1H). The optimal level of fluorescence was achieved using 0.035% DSNAF solution. A more contrasting fluorescent imaging of amyloid deposits can be achieved by exciting autofluorescence of the renal tissue by short-wave light.
Discussion
The traditional approaches to the identification and quantification of amyloid fibrils are based on monitoring the changes in the absorption spectrum of the azo dye CR and in the emission spectrum of the benzothiazole dye ThT.1,6,10,11 However, these methods have a number of limitations including background staining (background fluorescence), low fluorescence intensity and false-positive staining.9,10 In our study we demonstrated that some of this limitations can be partially eliminated by use of specific fixative, ZEF. Instead of traditional NBF, ZEF using allows to reduce significantly the background fluorescence in case of CR staining.
We have also illustrated herein the application of novel fluorescent dye DSNAF for detection of amyloid deposits in mouse tissues. We demonstrated that the sensitivity and specificity of amyloid detection by DSNAF method is comparable with the Congo red staining. The undoubted advantages of DSNAF using are the stability of the resulting staining, the high fluorescence intensity of amyloid deposits and complete absence of background fluorescence. The verification of DSNAF for detecting amyloid in human tissues will provide a conclusion on the applicability of the developed staining method in clinical research practice and high-resolution fluorescent imaging.
Our data confirmed the amyloid structure of protein deposits in kidneys of aged mdx mice. Previous morphological investigation of mdx mouse kidneys identified diffuse hemorrhages in the cortex and the medulla, as well as narrowing of the space between the Bowman’s capsule and the capillary glomerulus of the renal corpuscle.14 Apparently, some of the detected abnormalities are associated with dystrophin gene mutation. It was demonstrated earlier that in normal rodent kidney tissues both full-size dystrophin and its short isoforms Dp71 and Dp140 are expressed. All three dystrophin isoforms, Dp427, Dp140, and Dp71 were found mainly in the basal epithelial cells of the renal tubuli.15,16 It is believed that these proteins are required for the maintenance of the normal structure of epithelial cell membranes and participate in the anchoring of transporter proteins and ion channels in the basal membrane.17 It seems plausible that a deficit or complete lack of dystrophin and its isoforms as a result of mutations in the corresponding gene can induce pathological changes in the tubular epithelium. However, this cannot explain the detected abnormalities in the structure of renal corpuscles, where no constitutive dystrophin expression was observed.15 We suggest that these abnormalities develop due to the accumulation of amyloid, the components of which originate from the blood serum.
The fact of amyloid formation in kidney of mdx mice is important, as the available clinical data clearly indicate renal failure in patients with DMD.18,19 Speculatively, the impaired renal function in patients with DMD may be the result of amyloid accumulation in renal corpuscles, where amyloid components are brought by serum, which is typical for systemic amyloidosis with predominating glomerular deposition.20 The cases of DMD associated with concurrent kidney pathology should be carefully analyzed for the presence of amyloid in kidney tissues. This would facilitate the selection of a more appropriate treatment strategy in diagnosed DMD and improve the patient prognosis.
Taken together, these results indicate the association of amyloid formation with DMD and provides new opportunities for studying naturally occurring amyloidosis development in tissues also.
Funding Statement
Funding: This research was supported by a grant from the President of the Russian Federation (MK-8111.2016.4).
References
- 1.Sipe JD, Benson MD, Buxbaum JN, Ikeda SI, Merlini G, Saraiva MJ, et al. Amyloid fibril proteins and amyloidosis: chemical identification and clinical classification International Society of Amyloidosis 2016 Nomenclature Guidelines. Amyloid 2016;23:209-13. [DOI] [PubMed] [Google Scholar]
- 2.Spuler S, Carl M, Zabojszcza J, Straub V, Bushby K, Moore SA, et al. Dysferlin-deficient muscular dystrophy features amyloidosis. Ann Neurol 2008;63:323-8. [DOI] [PubMed] [Google Scholar]
- 3.Milone M, Liewluck T, Winder TL, Pianosi PT. Amyloidosis and exercise intolerance in ANO5 muscular dystrophy. Neuromuscul Disord 2012; 22:13-5. [DOI] [PubMed] [Google Scholar]
- 4.Manning J, O'Malley D. What has the mdx mouse model of Duchenne muscular dystrophy contributed to our understanding of this disease? J Muscle Res Cell Motil 2015;36:155-67. [DOI] [PubMed] [Google Scholar]
- 5.Gillmore JD, Hawkins PN. Pathophysiology and treatment of systemic amyloidosis. Nat Rev Nephrol 2013;9:574-86. [DOI] [PubMed] [Google Scholar]
- 6.Real de Asúa D, Costa R, Galván JM, Filigheddu MT, Trujillo D, Cadiñanos J. Systemic AA amyloidosis: epidemiology, diagnosis, and management. Clinical Epidemiology 2014;6:369-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bennhold H. Eine spezifische amyloidfarbung mit Kongorot [Specific staining of amyloid with Congo red].[In German]. Munchener Medizinische Wochenschrifte 1922;69:1537-8. [Google Scholar]
- 8.Clement CG, Truong LD. An evaluation of Congo red fluorescence for the diagnosis of amyloidosis. Hum Pathol 2014;45:1766-72. [DOI] [PubMed] [Google Scholar]
- 9.Khurana R, UverskyVN Nielsen L, Fink A. Is Congo red an amyloid-specific dye? J Biol Chem 2001;276: 22715-21. [DOI] [PubMed] [Google Scholar]
- 10.Groenning M. Binding mode of Thioflavin T and other molecular probes in the context of amyloid fibrils-current status. J Chem Biol 2010;3:1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Picken MM, Herrera GA. Thioflavin T stain: An easier and more sensitive method for amyloid detection. Picken MM, Dogan A, Herrera GA, Editors. Amyloid and related disorders. Surgical pathology and clinical correlations. Springer Science; 2012; p. 187-9. [Google Scholar]
- 12.Korzhevskii DE, Sukchorukova EG, Kirik OV, Grigoriev IP. Immuno - histochemical demonstration of specific antigens in the human brain fixed in zinc-ethanol-formaldehyde. Eur J Histochem 2015;59:2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morimatsu M, Hirai S, Muramatsu A, Yoshikawa M. Senile degenerative brain lesions and dementia. J Am Geriatr Soc 1975;23:390-406. [DOI] [PubMed] [Google Scholar]
- 14.Oliveira DM, Santos AC, Bertassoli BM, Viana DC, Prado AAF, Assis Neto AC. Comparative study of the kidneys from dystrophic mice. J Morphol Sci 2013;30: 186-90. [Google Scholar]
- 15.Lumeng CN, Hauser M, Brown V, Chamberlain JS. Expression of the 71 kDa dystrophin isoform (Dp71) evaluated by gene targeting. Brain Res 1999;830:174-8. [DOI] [PubMed] [Google Scholar]
- 16.Lidov HG, Kunkel LM. Dystrophin and Dp140 in the adult rodent kidney. Lab Invest 1998;78:1543-51. [PubMed] [Google Scholar]
- 17.Haenggi T, Fritschy JM. Role of dystrophin and utrophin for assembly and function of the dystrophin glycoprotein complex in nonmuscle tissue. Cell Mol Life Sci 2006;63:1614-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsumura T, Saito T, Fujimura H, Sakoda S. Renal dysfunction is a frequent complication in patients with advanced stage of Duchenne muscular dystrophy. Rinsho Shinkeigaku 2012;52:211-7. [DOI] [PubMed] [Google Scholar]
- 19.Motoki T, Shimizu-Motohashi Y, Komaki H, Mori-Yoshimura M, Oya Y, Takeshita E, et al. Treatable renal failure found in non-ambulatory Duchenne muscular dystrophy patients. Neuromuscul Disord 2015;25:754-7. [DOI] [PubMed] [Google Scholar]
- 20.Dember LM. Amyloidosis-associated kidney disease. J Am Soc Nephrol 2006; 17:3458-71. [DOI] [PubMed] [Google Scholar]


