Abstract
Historically, vitamin D has been associated with the regulation of bone metabolism. However, increasing evidence demonstrates a strong association between vitamin D signaling and many biological processes that regulate immune responses. The discovery of the vitamin D receptor in multiple immune cell lineages, such as monocytes, dendritic cells, and activated T cells credits vitamin D with a novel role in modulating immunological functions and its subsequent role in the development or prevention of autoimmune diseases. In this review we, discuss five major areas in vitamin D biology of high immunological significance: (1) the metabolism of vitamin D; (2) the significance of vitamin D receptor polymorphisms in autoimmune diseases, such as multiple sclerosis, type 1 diabetes mellitus, and systemic lupus erythematosus; (3) vitamin D receptor transcriptional regulation of immune cell lineages, including Th1, Th17, Th2, regulatory T, and natural killer T cells; (4) the prevalence of vitamin D insufficiency/deficiency in patients with multiple sclerosis, type 1 diabetes mellitus, and systemic lupus erythematosus; and finally, (5) the therapeutic effects of vitamin D supplementation on disease severity and progression.
Keywords: Vitamin D, Autoimmunity, Multiple sclerosis, Type 1 diabetes mellitus, Systemic lupus erythematosus
Introduction
Vitamin D deficiency is an increasingly described phenomenon worldwide [1]. Compelling evidence from human disease associations and basic physiological studies demonstrated the significance of vitamin D deficiency in various physiological disorders including neuropathy [2], malignancy [3, 4], infertility [5], cardiovascular diseases [6, 7], kidney diseases [8], glucose metabolism [9], and immunological dysfunctions [10–13]. Vitamin D was first identified as a nutritional regimen for rickets in the early twentieth century and was broadly defined as a compound with curative effects on rickets. Chemically, vitamin D is the derivative of a steroid, 7-dehydrocholesterol, derived from cholesterol and is found in the sebaceous glands of the skin of animals. Upon exposure to sunlight, 7-dehydrocholesterol will absorb UVB light (~280 to 315 nm) and convert to precalciferol (also called previtamin D3) in the skin. Much of the precalciferol eventually is isomerized into cholecalciferol (also called vitamin D3) through thermal conversion [14]. Since sunlight is necessary for photosynthesis of previtamin D3 in human skin, vitamin D is also commonly called “sunshine vitamin” (Fig. 1). In addition to 7-dehydrocholesterol in animals, ergosterol is another commonly occurring steroid in plants that can be activated by irradiation to produce ergocalciferol (also called vitamin D2).
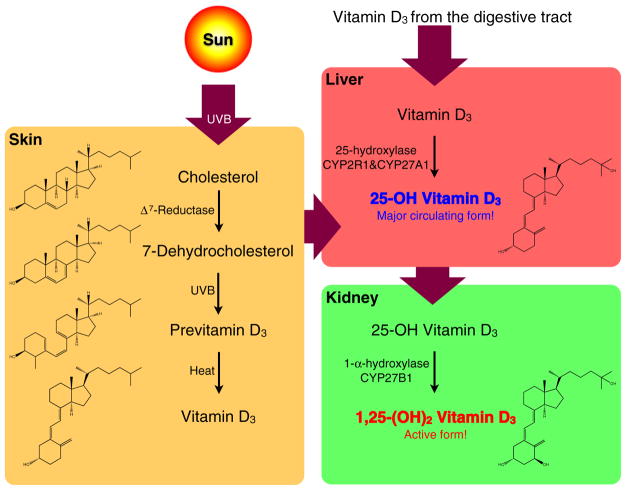
Fig. 1.
The metabolism of vitamin D. 7-Dehydrocholesterol, the derivative of cholesterol in the skin, can be converted to previtamin D3 via UVB irradiation from sunshine, and be thermally isomerized to vitamin D3. Both vitamin D3 spontaneously formed in the skin and absorbed from the digestive tract are further hydroxylated to a major circulating form, 25-OH vitamin D3, in the liver, and finally hydroxylated to a biologically active form, 1,25-(OH)2 vitamin D3, in the kidney
Both vitamin D3 formed in the skin and vitamin D3 absorbed from the digestive tract, travel to the liver, where they are hydroxylated at carbon 25 to form clacidiol (also called 25-hydroxy vitamin D3, abbreviated as 25(OH)D) by liver 25-hydroxylase, CYP2R1 and CYP27A1. 25(OH)D is the major circulating vitamin D metabolite and a reliable indicator of vitamin D status. Following the hydroxylation in liver, calcidiol is further hydroxylated by 1-α-hydroxylase, CYP27B1, in the proximal convoluted tubule cells of kidney, forming calcitriol (also called 1,25-dihydroxy vitamin D3, abbreviated as 1,25(OH)2D) which is considered the active form of vitamin D [15] (Fig. 1).
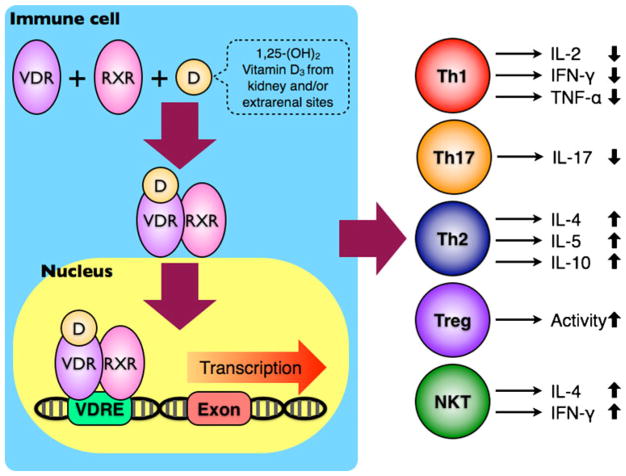
At the cellular level, 1,25(OH)2D interacts with nuclear vitamin D3 receptor (VDR), which belongs to the superfamily of nuclear hormone receptors, to modulate gene transcription. Ligand binding initiates a conformational change that increases the receptor’s affinity to the retinoid X receptor (RXR). Once the VDR-1,25(OH)2D complex is heterodimerized with RXR, this complex will bind to vitamin D3 response elements (VDREs) and recruit a number of nuclear coactivator or corepressor proteins. The transcription of genes for specific mRNA may be ultimately either enhanced or inhibited by this ligand-activated transcription factor [16] (Fig. 2).
Fig. 2.
The immunomodulatory effects of vitamin D on immune cells. After binding to VDR, the biologically active 1,25-(OH)2 vitamin D3 can induce a conformational change on VDR and increase its affinity to RXR. The VDR-RXR heterodimer becomes a transcriptional factor, interacts with VDREs in the promoter regions of different genes, and ultimately leads to functional changes in multiple immune cell lineages, including Th1, Th17, Th2, Treg, and NKT cells
Vitamin D3 Receptor (VDR) Polymorphism
In humans, the VDR locus is located at chromosome 12q13.1. The gene encoding VDR spans over 100 kb and contains nine exons and eight introns. Four polymorphisms, ApaI (rs7975232), BsmI (rs1544410), TaqI (rs731236), and FokI (rs10735810) have been identified in the VDR gene. ApaI, BsmI (in the intron between exon 8 and 9), and TaqI (in exon 9) are located at the 3′ end of the gene, whereas FokI (in exon 2) is located near the translation start codon. The ApaI (G/T substitution), BsmI (A/G substitution), and TaqI (T/C substitution) single nucleotide polymorphisms (SNPs) do not produce any structural change on the VDR protein, but they are in strong linkage disequilibrium (LD), which occurs when genetic variations at different loci are dependent of another non-random associations of alleles at different loci. [17].
The FokI (T/C substitution) polymorphism on the other hand, introduces a second start codon in the VDR gene and yields two potential initiation sites. Therefore, two protein variants can be produced from the two translation initiation sites: a longer protein with three additional amino acids (f allele) and a shorter version (F allele) [18]. The actual structural change on the VDR protein caused by the FokI polymorphism suggests a potential functional consequence. Colin et al. demonstrated that peripheral blood mononuclear cells from subjects with the FF VDR genotype had a lower ED50 of 1,25 (OH)2D when compared with the Ff VDR genotype in an in vitro growth inhibition study [19]. In addition, van Etten et al. reported that monocytes and dendritic cells from subjects with the FF genotype expressed more interleukin (IL)-12 and proliferated more significantly after phytohemagglutinin stimulation than those with the homozygous ff genotype [20].
In addition to the effects of the FokI VDR polymorphism on functions of immune cells, FokI has also been associated with vitamin D status (serum levels of 25(OH)D) in twins with multiple sclerosis (MS) [21]. This correlation was also reported in unrelated MS patients and showed that the F allele corresponded with lower serum levels of 25(OH)D (circulating form) in both MS patients and controls, while the F allele was associated with higher serum levels of 1,25 (OH)2D (active form) in MS patients [22]. The similar association between serum 25(OH)D and the FokI polymorphism was also reported in systemic lupus erythematosus (SLE). Patients carrying the FokI ff genotype had significantly higher serum 25(OH)D concentrations compared with patients carrying the FF genotype [23]. The relationship between FokI polymorphism and vitamin D status in patients with type 1 diabetes mellitus (T1DM) was not reported; however, multiple studies yielded controversial results on the association between the FokI VDR polymorphism and T1DM [24–32], whereas the association between the FokI polymorphism and autoimmunity was not observed in MS and SLE [22, 23, 33–35]. Furthermore, SNPs of the VDR gene, especially BsmI and TaqI, are closely linked with disease risk of MS, T1DM, and SLE among different populations [25, 30, 32, 34, 36–43]. Other studies found only weak or no association between these SNPs and autoimmune diseases [23, 26, 29, 31, 35, 44–48] (Table 1). Although the inconsistent results make it difficult to conclude the functional consequences of these VDR polymorphisms, the three SNPs in LD with other polymorphisms within the VDR gene may underlie a potential effect on vitamin D status and disease risk of autoimmunity.
Table 1.
Association between VDR gene polymorphisms and autoimmune diseases
| Author | Year | Disease subject | Healthy control | Polymorphism | Association | Ref. no. | |
|---|---|---|---|---|---|---|---|
| Multiple sclerosis | Cox et al. | 2012 | 726 | 604 | TaqI, FokI | Only weak association between TaqI and MS. | [48] |
| Sioka et al. | 2011 | 69 | 81 | BsmI, TaqI | No. | [47] | |
| Simon et al. | 2010 | 214 | 428 | ApaI, BsmI, TaqI, FokI, Cdx2 | No. But dietary intake of vitamin D was inversely related to MS risk in only the MS patients with the FokI ff genotype. | [35] | |
| Smolders et al. | 2009 | 212 | 289 | ApaI, TaqI | No. | [45] | |
| Smolders et al. | 2009 | 212 | 289 | FokI | No. But the F allele was associated with lower serum 25(OH)D levels in both MS patients and controls. However, the F-allele corresponded with higher 1,25 (OH)2D levels in MS patients. | [22] | |
| Tajouri et al. | 2005 | 104 | 104 | ApaI, TaqI, FokI | Yes. Only the frequency of the TaqI allele/genotype and the ApaI allele was different between MS patients and controls. | [34] | |
| Niino et al. | 2000 | 77 | 95 | ApaI | Yes. The ApaI A allele/AA genotype were more prevalent in MS patients than in controls. | [39] | |
| Fukazawa et al. | 1999 | 77 | 95 | BsmI | Yes. The BsmI b allele/bb genotype were more prevalent in MS patients than in controls. | [36] | |
| Type 1 diabetes mellitus | Mohammadnejad et al. | 2012 | 87 | 100 | ApaI, BsmI, TaqI, FokI | Yes. Only the frequency of the TaqI T allele/TT genotype was higher in controls compared to T1DM patients. | [32] |
| Gogas Yavuz et al. | 2011 | 117 | 134 | ApaI, BsmI, TaqI, FokI | No. | [31] | |
| Panierakis et al. | 2009 | 100 | 96 | ApaI, BsmI, TaqI, FokI | Yes. The ApaI A allele/AA genotype and the TaqI T allele/TT genotype were more frequent in T1DM patients, whereas the BsmI B allele/BB genotype and the FokI F allele/FF genotype were less frequent in T1DM. | [30] | |
| Shimada et al. | 2008 | 774 | 599 | BsmI | Yes. The BB genotype frequency was significantly higher in T1DM patients compared to controls. | [42] | |
| Lemos et al. | 2008 | 207 | 249 | ApaI, BsmI, TaqI, FokI | No. | [29] | |
| Capoluongo et al. | 2006 | 246 | 246 | BsmI, FokI | Yes. Only the frequency of the FokI ff genotype was higher in TIDM patients compared to controls. | [28] | |
| Audi et al. | 2004 | 89 | 116 | BsmI, FokI | Yes. The frequency of the FokI ff genotype was lower in T1DM patients compared to controls. | [27] | |
| Gyorffy et al. | 2002 | 107 | 103 | ApaI, BsmI, FokI, Tru9I | No. | [26] | |
| Fassbender et al. | 2002 | 75 | 57 | BsmI, TaqI, FokI | Yes. Only the frequency of the TaqI TT genotype was higher in T1DM patients than in controls. | [25] | |
| Taverna et al. | 2002 | 101 | 99 | TaqI | Yes. The frequency of the TT genotype was lower in T1DM patients compared to controls. | [41] | |
| Ban et al. | 2001 | 110 | 250 | Fok I | Yes. There was a higher prevalence of the F allele/FF genotype in T1DM patients compared to controls. | [24] | |
| Chang et al. | 2000 | 157 | 248 | ApaI, BsmI, TaqI | Yes. Only the allelic frequency of the BsmI B allele was higher in T1DM patients than in controls. | [37] | |
| Systemic lupus erythematosus | Luo et al. | 2012 | 337 | 239 | BsmI | Yes. The alleic frequency of the B allele, but not the frequency of the BB genotype, was higher in SLE patients compared to controls. | [43] |
| Monticielo et al. | 2012 | 195 | 201 | BsmI, FokI | No. But 25(OH)D concentrations were significantly higher in SLE patients carrying the FokI ff genotype compared with patients carrying the FF genotype. | [23] | |
| Abbasi et al. | 2010 | 60 | 45 | BsmI | No. | [46] | |
| Sakulpipatsin et al. | 2006 | 101 | 194 | BsmI | No. | [44] | |
| Huang et al. | 2002 | 52 | 90 | FokI | No. | [33] | |
| Huang et al. | 2002 | 47 | 90 | BsmI | Yes. The distribution of the B allele/BB genotype was increased in SLE patients. | [40] | |
| Ozaki et al. | 2000 | 58 | 87 | BsmI | Yes. The frequency of the BB genotype was significantly higher in SLE patients than in controls. | [38] |
Immunomodulatory Effects of Vitamin D
Although historically vitamin D has been associated with the regulation of bone metabolism, it is now evident that vitamin D is involved in many biological processes that regulate immune responses [49–52]. There has been increasing interest in possible immunomodulatory effects of vitamin D since VDR was first discovered in monocytes [53, 54] and subsequently in dendritic cells and activated T cells [55, 56]. In vitro studies showed that vitamin D inhibits pro-inflammatory activity of CD4+ Th1 cells and their production of cytokines such as IL-2, interferon (IFN)-γ, and tumor necrosis factor-α [57–59]. 1,25(OH)2D exerts an inhibitory effect on T cell proliferation, the expression of IL-2 [60, 61] and IFN-γ mRNA and protein in T cells [62], which could be caused by binding of the VDR–RXR heterodimer to the VDREs in the promoter regions of genes encoding IL-2 and IFN-γ [57]. In addition to its anti-inflammatory effects, vitamin D also promotes Th2 responses by enhancing IL-4, IL-5, and IL-10 production, thus skewing the T cell compartment from an inflammatory Th1 state to a more anti-inflammatory and regulated Th2 state [63]. Some studies reported that vitamin D could increase regulatory T (Treg) activity [64] and suppress Th17 responses [65]. Interestingly, vitamin D is not only required for the development of natural killer T (NKT) cells, but also increased IL-4 and IFN-γ production of NKT cells [66] (Fig. 2). Logically, these extensive effects of vitamin D on multiple immune cell lineages strongly suggest that vitamin D could play important roles in immune-mediated disorders in autoimmunity.
Vitamin D and Autoimmune Diseases
Multiple Sclerosis (MS)
MS is a chronic inflammatory disease characterized by immune-mediated damage of central nervous system [67]. The etiology of MS is not well understood but, like other autoimmune diseases, could be attributed to genetic predisposition and/or environmental factors [68, 69]. Epidemiologic evidence showed that MS has a geographical distribution: the prevalence of the disease is lower in equatorial regions and becomes higher with increasing latitudes [70]. This phenomenon could be caused by the lack of sunshine in high-latitude regions that is required for the cutaneous synthesis of vitamin D and suggests that vitamin D insufficiency could be a potential risk factor for MS.
Soilu-Hanninen et al. and Correale et al. demonstrated that the serum concentration of 25(OH)D and 1,25(OH)2D were lower in MS patients than those in controls [71, 72]. Seasonal variation of serum 25(OH)D levels were similar in MS patients and controls [73], but 25(OH)D levels were lower during MS relapses than in remission [71–73], suggesting that vitamin D could be involved in the regulation of the clinical disease progress and severity of MS. Multiple studies also demonstrated that most of the MS patients were deficient in vitamin D [74–76]. In particular, low serum 25 (OH)D levels were associated with high disability and relapse rate in MS patients [74, 75]; however, serum 1,25 (OH)2D, which is the biologically active form of vitamin D, was not directly associated with both disability and relapse rate [75]. Munger et al. and Kragt et al. reported that there was a strong inverse correlation between the level of serum 25(OH)D and MS risk [77, 78]; however, the correlation was only evident among whites but not blacks and Hispanics [77] (Table 2). The reason why MS patients generally have lower serum 25(OH)D levels than healthy controls could be a combination of insufficient vitamin D intakes and decreased outdoor activities caused by lifestyle changes associated with increasing disability. However, it is unknown whether the high prevalence of vitamin D deficiency found in MS patients is the cause or effect of the disease.
Table 2.
Serum vitamin D status in subjects with autoimmune diseases
| Author | Year | Disease subject | Healthy control | Result | Ref. no. | |
|---|---|---|---|---|---|---|
| Multiple sclerosis | Hiremath et al. | 2009 | 199 | / | Large numbers (84 %) of MS patients had insufficient levels (≤100 nmol/L) of 25(OH)D. | [76] |
| Correale et al. | 2009 | 132 | 60 | Serum 25(OH)D and 1,25(OH)2D levels were lower in MS patients. The levels were lower during MS relapses than remission. | [72] | |
| Kragt et al. | 2009 | 103 | 110 | Higher circulating levels of 25(OH)D were associated with a lower incidence of MS. | [78] | |
| Smolders et al. | 2008 | 267 | / | Low serum 25(OH)D levels were associated with high disability and relapse rate in MS patients. | [75] | |
| Soilu-Hanninen et al. | 2008 | 23 | 23 | Seasonal variation of 25(OH)D was similar in MS patients and controls, but 25(OH)D serum levels were lower during MS relapses than in remission. | [73] | |
| van der Mei et al. | 2007 | 136 | 272 | Vitamin D insufficiency was only observed in MS patients with higher disability but not in those with lower disability. | [74] | |
| Munger et al. | 2006 | 257 | 514 | High circulating levels of 25(OH)D were associated with a lower risk of MS among whites but not blacks and Hispanics. | [77] | |
| Soilu-Hanninen et al. | 2005 | 40 | 40 | Serum 25(OH)D concentrations were lower in MS patients from June to September. The levels were lower during MS relapses than remission. | [71] | |
| Type 1 diabetes mellitus | de Boer et al. | 2012 | 1193 | / | Low plasma concentrations of 25(OH)D and 24,25(OH)2D were associated with increased risk of microalbuminuria in T1DM. | [79] |
| Borkar et al. | 2010 | 50 | 50 | Plasma 25(OH)D levels were lower in T1DM children. Vitamin D deficiency was higher in T1DM children compared to controls. | [80] | |
| Singh et al. | 2009 | 40 | 41 | Serum 1,25(OH)2D levels were lower in T1DM patients compared to controls. | [81] | |
| Bener et al. | 2009 | 170 | 170 | Vitamin D deficiency was higher in T1DM children compared to non-diabetic. | [82] | |
| Littorin et al. | 2006 | 138 | 208 | Plasma 25(OH)D levels were lower in T1DM patients than in control subjects. The levels were lower in men than in women with T1DM. | [83] | |
| Systemic lupus erythematosus | Bogaczewicz et al. | 2012 | 49 | 49 | Serum 25(OH)D concentrations were lower in SLE patients only during the warm season. The cold season was found to be a risk factor for vitamin D deficiency. | [84] |
| Kim et al. | 2010 | 104 | 49 | Serum 25(OH)D levels were lower and vitamin D insufficiency was more common in SLE patients. Vitamin D levels had no relation with SLE severity. | [85] | |
| Amital et al. | 2010 | 378 | / | Vitamin D serum concentrations were found to be inversely related to SLE activity. | [86] | |
| Wright et al. | 2009 | 38 | 207 | Severe deficiency (≤10 ng/mL) of 25(OH)D was more frequent among subjects with SLE. 25(OH)D and 1,25(OH)2D were both lower in SLE. | [87] | |
| Borba et al. | 2009 | 36 | 26 | A high prevalence of 25(OH)D deficiency was observed in SLE patients. Low levels of 25(OH)D were present in patients in overt disease and negatively related to disease activity. | [88] | |
| Ruiz-Irastorza et al. | 2008 | 92 | / | Vitamin D insufficiency and deficiency were common in SLE patients. Vitamin D levels had no relation with SLE severity. | [89] |
Recent studies showed that vitamin D deficiency might increase the risk of MS [90]. As a result, detection of vitamin D deficiency and restoring vitamin D status to adequate levels may be considered as part of the clinical treatment of MS. In fact, vitamin D intervention has been implemented in several studies [91–93] (Table 3). However, these clinical trials were unable to provide definitive evidence to support the therapeutic effects of vitamin D intervention, mostly due to an extremely small number of MS patients recruited. Due to the lack of a double-blind, placebo-controlled, and randomized study with a large number of patients, the beneficial effects of oral vitamin D supplementation on MS progression need to be further investigated [97]. In addition to clinical effectiveness, the optimal dose and duration of vitamin D supplementation are not well defined and may vary between patients.
Table 3.
Vitamin D supplementation and autoimmune disease severity and progression
| Author | Year | Disease subject | Result | Ref. no. | |
|---|---|---|---|---|---|
| Multiple sclerosis | Wingerchuk et al. | 2005 | 13 | Four patients experienced a total of five clinical relapses during the 48 week study. | [93] |
| Achiron et al. | 2003 | 5 | One patient improved, but another one patient had an acute relapse. | [92] | |
| Nordvik et al. | 2000 | 16 | Supplementation of vitamin D in combination with other nutriments reduced the exacerbation rate and disability in MS. | [91] | |
| Type 1 diabetes mellitus | Li et al. | 2009 | 35 | 1-α(OH)D plus insulin therapy preserved pancreatic β-cell function in patients with latent autoimmune diabetes. | [94] |
| Pitocco et al. | 2006 | 70 | 1,25(OH)2D had a modest effect on residual pancreatic β-cell function. | [95] | |
| Systemic lupus erythematosus | Ruiz-Irastorza et al. | 2010 | 80 | Increasing 25(OH)D levels by oral vitamin D supplementation might have a beneficial effect on fatigue but not SLE activity. | [96] |
Type 1 Diabetes Mellitus (T1DM)
T1DM is an immune-mediated disease that is caused by autoimmune destruction of pancreatic β cells that produce insulin, leading to insulin deficiency [98]. Similar to other autoimmune diseases, environmental factors and/or genetic susceptibility could play roles in the onset and progression of T1DM [99]. Epidemiologic studies also showed that the incidence rate of T1DM was higher in geographical regions with lower UV exposure that resulted in less spontaneous vitamin D synthesis [100]. This inverse association of T1DM prevalence with UV radiation is consistent with that reported for MS, suggesting that the environmental UVexposure correlating with cutaneous synthesis of vitamin D could influence the development of multiple autoimmune disorders.
A birth-cohort study by Hypponen et al. demonstrated that dietary vitamin D supplementation is associated with reduced risk of T1DM [101]. The possibility that ensuring sufficient vitamin D could decrease the frequency of T1DM has raised an interest of further investigating nutritional vitamin D levels in T1DM patients. It has been reported that the plasma 25(OH)D levels were lower in T1DM patients than in control subjects among young adults and children [80, 82, 83]. Moreover, the prevalence of vitamin D deficiency/insufficiency was higher in T1DM children compared to controls [80, 82]. Interestingly, 25(OH)D levels were lower in male compared to female patients [83], suggesting that the gender difference in plasma vitamin D status might contribute to the high incidence of T1DM in males. In addition to plasma 25(OH)D, circulating 1,25(OH)2D levels were also lower in T1DM patients than in controls [81]. The low serum 1,25(OH)2D levels were indicative of tubulo-interstitial dysfunction in T1DM before persistent microalbuminuria. Interestingly, low concentrations of circulating 1,25(OH)2D were not associated with increased risk of microalbuminuria in T1DM [79] (Table 2). However, the circulating 1,25(OH)2D levels may not be equal to the local conversion of 25(OH)D into 1,25(OH)2D that results in beneficial effects.
Because of the strong correlation between vitamin D and T1DM, it has been suggested that vitamin D could prevent the damage, rescue the function of pancreatic β cells, and reduce the incidence of T1DM. Pitocco et al. and Li et al. both demonstrated that vitamin D had a protective effect on preserving β-cell function [94, 95], although the effect was not evident in the former study and vitamin D supplementation could only temporarily reduce the clinical insulin dose, which might be attributed to the low 1,25(OH)2D dose (0.25 μg on alternate days) administered in T1DM [95]. In Li’s study, the insulin plus vitamin D treatment (1-α-hydroxy vitamin D3; 0.5 μg per day) group developed no β-cell failure, while 27.8 % in the control group developed β-cell failure; however, the number of patients in this randomized controlled study is very limited (Table 3). Similar to MS, further clinical studies are necessary to determine the therapeutic value of vitamin D supplementation in T1DM.
Systemic Lupus Erythematosus (SLE)
SLE is a systemic autoimmune disease that can cause chronic inflammation and damage in multiple tissues and organs [102]. Environmental factors and genetic susceptibility are both responsible for the pathogenesis of SLE [103, 104]. One of such factors is vitamin D deficiency. SLE patients tend to have inadequate vitamin D since most of them are photosensitive to UV radiation and unable to expose themselves to sunlight [105]. The correlation between vitamin D deficiency/insufficiency and SLE has been documented in multiple studies. Ruiz-Irastorza et al. observed a high frequency of inadequate vitamin D status in SLE patients (75 % of them had insufficient vitamin D levels, serum 25 (OH)D<30 ng/ml, and 15 % of them had deficient vitamin D levels, serum 25(OH)D<10 ng/ml) [89]. Borba et al. reported the similar high prevalence of vitamin D insufficiency/deficiency in SLE; however, serum 25(OH)D levels were lower only in SLE patients with high disease activity, but not mild activity, than in controls [88]. In another study by Kim et al., serum 25(OH)D titers were significantly lower in SLE than in controls. Although only 16.3 % of SLE patients had vitamin D insufficiency (serum 25(OH)D <30 ng/ml), the risk of vitamin D insufficiency was 4.6-fold increased in SLE [85] (Table 2).
Several observational studies yield inconsistent results on the correlation between vitamin D levels and disease severity. Some studies suggested that vitamin D concentrations were inversely related to SLE activity [86–88], while others claimed that no such relation was observed [85, 89]. In addition to 25(OH)D, 1,25(OH)2D was also lower in SLE patients and the vitamin D deficiency was particularly associated with overweight SLE patients [87]. Similar to other autoimmune diseases, seasonal variation of vitamin D status has been observed in SLE patients. In Bogaczewicz’s study, the serum 25(OH)D levels in both SLE patients and controls during the cold season were lower compared to the warm season [84]. Interestingly, serum 25(OH)D concentrations were lower in SLE patients than in controls only during the warm season but not the cold season. Furthermore, the cold season was found to be a risk factor for vitamin D deficiency, mainly due to the lack of sunlight exposure and/or the decrease in outdoor activities during the cold season of the year. In addition, there was no connection between serum concentrations of IL-17 and vitamin D status, whereas serum IL-23 was lower in SLE patients with vitamin D deficiency.
Ruiz-Irastorza et al. evaluated the therapeutic effects of oral vitamin D supplementation on SLE in an observational study [96] (Table 3). After around 2 years of oral vitamin D treatment, mean 25(OH)D levels in all treated patients were increased. However, the majority (71 %) of the SLE patients still had insufficient levels of vitamin D. Although it appears that there was no improvement of SLE severity after oral vitamin D supplementation in this study, the effect of vitamin D intervention in SLE has not been extensively investigated, probably because there are too many confounding factors that could affect vitamin D metabolism in clinical medications used to treat SLE [106].
Conclusion
Vitamin D, in addition to its crucial role in bone metabolism, has been associated with multiple autoimmune diseases in several epidemiological studies. Due to its unique capability to bind to VDR and serve as a transcriptional factor, vitamin D can regulate gene expression and further exert its immunomodulatory effects on immune cells. It has been shown to inhibit Th17 cytokine production, enhance Treg activity, induce NKT cell functions, suppress Th1, and promote Th2 cytokine production, and thus skew T cells toward Th2 polarization. Furthermore, accumulating evidence suggest that VDR polymorphisms and serum vitamin D status are both closely associated with disease risk of MS, T1DM, and SLE. Therefore, impaired vitamin D signaling and/or inadequate vitamin D intake caused by genetic predisposition (e.g. VDR polymorphisms) and/or environmental factors (e.g. insufficient sunlight exposure in high-latitude regions or during the cold season) may contribute to the onset and progression of autoimmunity. Because of the high prevalence of vitamin D insufficiency/deficiency in patients with MS, T1DM, and SLE, vitamin D supplementation has been considered a prospective candidate for the treatment of such autoimmune diseases. However, due to the lack of larger randomized trials, more well-organized studies with methodological design are essential in order to further confirm the potential of vitamin D to prevent and ameliorate autoimmunity.
Take-Home Messages.
Vitamin D can be spontaneously synthesized from cutaneous cholesterol upon UVB exposure and has pleiotropic effects on the immune system.
Vitamin D, after metabolized into a biologically active form, 1,25(OH)2D, and bound to VDR/RXR, can initiate gene transcription and exert its immunomodulatory effects.
Both environmental trigger (insufficient sunshine exposure) and genetic factor (VDR polymorphism) could contribute a poor vitamin D status.
Vitamin D deficiency (low serum levels of 25(OH)D) is prevalent in multiple autoimmune diseases, e.g. MS, TIDM, and SLE.
Because the vitamin D status is highly associated with the risk of autoimmunity, vitamin D has been implicated in prevention and protection from autoimmune diseases.
Contributor Information
Chen-Yen Yang, Division of Rheumatology, Allergy and Clinical Immunology, University of California at Davis School of Medicine, 451 Health Sciences Drive, Suite 6510, Davis, CA 95616, USA.
Patrick S. C. Leung, Division of Rheumatology, Allergy and Clinical Immunology, University of California at Davis School of Medicine, 451 Health Sciences Drive, Suite 6510, Davis, CA 95616, USA
Iannis E. Adamopoulos, Division of Rheumatology, Allergy and Clinical Immunology, University of California at Davis School of Medicine, 451 Health Sciences Drive, Suite 6510, Davis, CA 95616, USA. Institute of Pediatric and Regenerative Medicine, Shriners Hospital for Northern California, Sacramento, CA, USA
M. Eric Gershwin, Division of Rheumatology, Allergy and Clinical Immunology, University of California at Davis School of Medicine, 451 Health Sciences Drive, Suite 6510, Davis, CA 95616, USA.
References
- 1.Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr. 2008;87:1080S–1086S. doi: 10.1093/ajcn/87.4.1080S. [DOI] [PubMed] [Google Scholar]
- 2.Agmon-Levin N, Kivity S, Tzioufas AG, et al. Low levels of vitamin-D are associated with neuropathy and lymphoma among patients with Sjogren’s syndrome. J Autoimmun. 2012;39:234–239. doi: 10.1016/j.jaut.2012.05.018. [DOI] [PubMed] [Google Scholar]
- 3.Giovannucci E, Liu Y, Rimm EB, et al. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J Natl Cancer Inst. 2006;98:451–459. doi: 10.1093/jnci/djj101. [DOI] [PubMed] [Google Scholar]
- 4.Giovannucci E. Vitamin D and cancer incidence in the Harvard cohorts. Ann Epidemiol. 2009;19:84–88. doi: 10.1016/j.annepidem.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 5.Twig G, Shina A, Amital H, Shoenfeld Y. Pathogenesis of infertility and recurrent pregnancy loss in thyroid autoimmunity. J Autoimmun. 2012;38:J275–J281. doi: 10.1016/j.jaut.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 6.Anderson JL, May HT, Horne BD, et al. Relation of vitamin D deficiency to cardiovascular risk factors, disease status, and incident events in a general healthcare population. Am J Cardiol. 2010;106:963–968. doi: 10.1016/j.amjcard.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 7.Sood A, Arora R. Vitamin D deficiency and its correlations with increased cardiovascular incidences. Am J Ther. 2010;17:e105–e109. doi: 10.1097/MJT.0b013e31819e9e88. [DOI] [PubMed] [Google Scholar]
- 8.Qazi RA, Martin KJ. Vitamin D in kidney disease: pathophysiology and the utility of treatment. Rheum Dis Clin N Am. 2012;38:115–123. doi: 10.1016/j.rdc.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 9.Pittas AG, Dawson-Hughes B. Vitamin D and diabetes. J Steroid Biochem Mol Biol. 2010;121:425–429. doi: 10.1016/j.jsbmb.2010.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cantorna MT, Mahon BD. D-hormone and the immune system. J Rheumatol Suppl. 2005;76:11–20. [PubMed] [Google Scholar]
- 11.Holick MF. Vitamin D: evolutionary, physiological and health perspectives. Curr Drug Targets. 2011;12:4–18. doi: 10.2174/138945011793591635. [DOI] [PubMed] [Google Scholar]
- 12.Arnson Y, Amital H, Agmon-Levin N, et al. Serum 25-OH vitamin D concentrations are linked with various clinical aspects in patients with systemic sclerosis: a retrospective cohort study and review of the literature. Autoimmun Rev. 2011;10:490–494. doi: 10.1016/j.autrev.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 13.Hajas A, Sandor J, Csathy L, et al. Vitamin D insufficiency in a large MCTD population. Autoimmun Rev. 2011;10:317–324. doi: 10.1016/j.autrev.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 14.Holick MF, MacLaughlin JA, Clark MB, et al. Photosynthesis of previtamin D3 in human skin and the physiologic consequences. Science. 1980;210:203–205. doi: 10.1126/science.6251551. [DOI] [PubMed] [Google Scholar]
- 15.Jones G, Strugnell SA, DeLuca HF. Current understanding of the molecular actions of vitamin D. Physiol Rev. 1998;78:1193–1231. doi: 10.1152/physrev.1998.78.4.1193. [DOI] [PubMed] [Google Scholar]
- 16.Haussler MR, Whitfield GK, Haussler CA, et al. The nuclear vitamin D receptor: biological and molecular regulatory properties revealed. J Bone Miner Res. 1998;13:325–349. doi: 10.1359/jbmr.1998.13.3.325. [DOI] [PubMed] [Google Scholar]
- 17.Smolders J, Peelen E, Thewissen M, et al. The relevance of vitamin D receptor gene polymorphisms for vitamin D research in multiple sclerosis. Autoimmun Rev. 2009;8:621–626. doi: 10.1016/j.autrev.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 18.Uitterlinden AG, Fang Y, Van Meurs JB, Pols HA, Van Leeuwen JP. Genetics and biology of vitamin D receptor polymorphisms. Gene. 2004;338:143–156. doi: 10.1016/j.gene.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 19.Colin EM, Weel AE, Uitterlinden AG, et al. Consequences of vitamin D receptor gene polymorphisms for growth inhibition of cultured human peripheral blood mononuclear cells by 1, 25-dihydroxyvitamin D3. Clin Endocrinol (Oxf) 2000;52:211–216. doi: 10.1046/j.1365-2265.2000.00909.x. [DOI] [PubMed] [Google Scholar]
- 20.van Etten E, Verlinden L, Giulietti A, et al. The vitamin D receptor gene FokI polymorphism: functional impact on the immune system. Eur J Immunol. 2007;37:395–405. doi: 10.1002/eji.200636043. [DOI] [PubMed] [Google Scholar]
- 21.Orton SM, Morris AP, Herrera BM, et al. Evidence for genetic regulation of vitamin D status in twins with multiple sclerosis. Am J Clin Nutr. 2008;88:441–447. doi: 10.1093/ajcn/88.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smolders J, Damoiseaux J, Menheere P, Tervaert JW, Hupperts R. Fok-I vitamin D receptor gene polymorphism (rs10735810) and vitamin D metabolism in multiple sclerosis. J Neuroimmunol. 2009;207:117–121. doi: 10.1016/j.jneuroim.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 23.Monticielo OA, Brenol JC, Chies JA, et al. The role of BsmI and FokI vitamin D receptor gene polymorphisms and serum 25-hydroxyvitamin D in Brazilian patients with systemic lupus erythematosus. Lupus. 2012;21:43–52. doi: 10.1177/0961203311421798. [DOI] [PubMed] [Google Scholar]
- 24.Ban Y, Taniyama M, Yanagawa T, et al. Vitamin D receptor initiation codon polymorphism influences genetic susceptibility to type 1 diabetes mellitus in the Japanese population. BMC Med Genet. 2001;2:7. doi: 10.1186/1471-2350-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fassbender WJ, Goertz B, Weismuller K, et al. VDR gene polymorphisms are overrepresented in german patients with type 1 diabetes compared to healthy controls without effect on biochemical parameters of bone metabolism. Horm Metab Res. 2002;34:330–337. doi: 10.1055/s-2002-33262. [DOI] [PubMed] [Google Scholar]
- 26.Gyorffy B, Vasarhelyi B, Krikovszky D, et al. Gender-specific association of vitamin D receptor polymorphism combinations with type 1 diabetes mellitus. Eur J Endocrinol. 2002;147:803–808. doi: 10.1530/eje.0.1470803. [DOI] [PubMed] [Google Scholar]
- 27.Audi L, Marti G, Esteban C, et al. VDR gene polymorphism at exon 2 start codon (FokI) may have influenced Type 1 diabetes mellitus susceptibility in two Spanish populations. Diabet Med. 2004;21:393–394. doi: 10.1111/j.1464-5491.2004.01126.x. [DOI] [PubMed] [Google Scholar]
- 28.Capoluongo E, Pitocco D, Concolino P, et al. Slight association between type 1 diabetes and “ff” VDR FokI genotype in patients from the Italian Lazio Region. Lack of association with diabetes complications. Clin Biochem. 2006;39:888–892. doi: 10.1016/j.clinbiochem.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 29.Lemos MC, Fagulha A, Coutinho E, et al. Lack of association of vitamin D receptor gene polymorphisms with susceptibility to type 1 diabetes mellitus in the Portuguese population. Hum Immunol. 2008;69:134–138. doi: 10.1016/j.humimm.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 30.Panierakis C, Goulielmos G, Mamoulakis D, Petraki E, Papavasiliou E, Galanakis E. Vitamin D receptor gene polymorphisms and susceptibility to type 1 diabetes in Crete, Greece. Clin Immunol. 2009;133:276–281. doi: 10.1016/j.clim.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 31.Gogas Yavuz D, Keskin L, Kiyici S, et al. Vitamin D receptor gene BsmI, FokI, ApaI, TaqI polymorphisms and bone mineral density in a group of Turkish type 1 diabetic patients. Acta Diabetol. 2011;48:329–336. doi: 10.1007/s00592-011-0284-y. [DOI] [PubMed] [Google Scholar]
- 32.Mohammadnejad Z, Ghanbari M, Ganjali R, et al. Association between vitamin D receptor gene polymorphisms and type 1 diabetes mellitus in Iranian population. Mol Biol Rep. 2012;39:831–837. doi: 10.1007/s11033-011-0805-3. [DOI] [PubMed] [Google Scholar]
- 33.Huang CM, Wu MC, Wu JY, Tsai FJ. No association of vitamin D receptor gene start codon fok 1 polymorphisms in Chinese patients with systemic lupus erythematosus. J Rheumatol. 2002;29:1211–1213. [PubMed] [Google Scholar]
- 34.Tajouri L, Ovcaric M, Curtain R, et al. Variation in the vitamin D receptor gene is associated with multiple sclerosis in an Australian population. J Neurogenet. 2005;19:25–38. doi: 10.1080/01677060590949692. [DOI] [PubMed] [Google Scholar]
- 35.Simon KC, Munger KL, Xing Y, Ascherio A. Polymorphisms in vitamin D metabolism related genes and risk of multiple sclerosis. Mult Scler. 2010;16:133–138. doi: 10.1177/1352458509355069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fukazawa T, Yabe I, Kikuchi S, et al. Association of vitamin D receptor gene polymorphism with multiple sclerosis in Japanese. J Neurol Sci. 1999;166:47–52. doi: 10.1016/s0022-510x(99)00112-4. [DOI] [PubMed] [Google Scholar]
- 37.Chang TJ, Lei HH, Yeh JI, et al. Vitamin D receptor gene polymorphisms influence susceptibility to type 1 diabetes mellitus in the Taiwanese population. Clin Endocrinol (Oxf) 2000;52:575–580. doi: 10.1046/j.1365-2265.2000.00985.x. [DOI] [PubMed] [Google Scholar]
- 38.Ozaki Y, Nomura S, Nagahama M, Yoshimura C, Kagawa H, Fukuhara S. Vitamin-D receptor genotype and renal disorder in Japanese patients with systemic lupus erythematosus. Nephron. 2000;85:86–91. doi: 10.1159/000045635. [DOI] [PubMed] [Google Scholar]
- 39.Niino M, Fukazawa T, Yabe I, Kikuchi S, Sasaki H, Tashiro K. Vitamin D receptor gene polymorphism in multiple sclerosis and the association with HLA class II alleles. J Neurol Sci. 2000;177:65–71. doi: 10.1016/s0022-510x(00)00336-1. [DOI] [PubMed] [Google Scholar]
- 40.Huang CM, Wu MC, Wu JY, Tsai FJ. Association of vitamin D receptor gene BsmI polymorphisms in Chinese patients with systemic lupus erythematosus. Lupus. 2002;11:31–34. doi: 10.1191/0961203302lu143oa. [DOI] [PubMed] [Google Scholar]
- 41.Taverna MJ, Sola A, Guyot-Argenton C, et al. Taq I polymorphism of the vitamin D receptor and risk of severe diabetic retinopathy. Diabetologia. 2002;45:436–442. doi: 10.1007/s00125-001-0769-2. [DOI] [PubMed] [Google Scholar]
- 42.Shimada A, Kanazawa Y, Motohashi Y, et al. Evidence for association between vitamin D receptor BsmI polymorphism and type 1 diabetes in Japanese. J Autoimmun. 2008;30:207–211. doi: 10.1016/j.jaut.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 43.Luo XY, Yang MH, Wu FX, et al. Vitamin D receptor gene BsmI polymorphism B allele, but not BB genotype, is associated with systemic lupus erythematosus in a Han Chinese population. Lupus. 2012;21:53–59. doi: 10.1177/0961203311422709. [DOI] [PubMed] [Google Scholar]
- 44.Sakulpipatsin W, Verasertniyom O, Nantiruj K, Totemchokchyakarn K, Lertsrisatit P, Janwityanujit S. Vitamin D receptor gene BsmI polymorphisms in Thai patients with systemic lupus erythematosus. Arthritis Res Ther. 2006;8:R48. doi: 10.1186/ar1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smolders J, Damoiseaux J, Menheere P, Tervaert JW, Hupperts R. Association study on two vitamin D receptor gene polymorphisms and vitamin D metabolites in multiple sclerosis. Ann N Y Acad Sci. 2009;1173:515–520. doi: 10.1111/j.1749-6632.2009.04656.x. [DOI] [PubMed] [Google Scholar]
- 46.Abbasi M, Rezaieyazdi Z, Afshari JT, Hatef M, Sahebari M, Saadati N. Lack of association of vitamin D receptor gene BsmI polymorphisms in patients with systemic lupus erythematosus. Rheumatol Int. 2010;30:1537–1539. doi: 10.1007/s00296-010-1504-4. [DOI] [PubMed] [Google Scholar]
- 47.Sioka C, Papakonstantinou S, Markoula S, et al. Vitamin D receptor gene polymorphisms in multiple sclerosis patients in northwest Greece. J Negat Results Biomed. 2011;10:3. doi: 10.1186/1477-5751-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cox MB, Ban M, Bowden NA, Baker A, Scott RJ, Lechner-Scott J. Potential association of vitamin D receptor polymorphism Taq1 with multiple sclerosis. Mult Scler. 2012;18:16–22. doi: 10.1177/1352458511415562. [DOI] [PubMed] [Google Scholar]
- 49.Adorini L, Penna G. Control of autoimmune diseases by the vitamin D endocrine system. Nat Clin Pract Rheumatol. 2008;4:404–412. doi: 10.1038/ncprheum0855. [DOI] [PubMed] [Google Scholar]
- 50.Szodoray P, Nakken B, Gaal J, et al. The complex role of vitamin D in autoimmune diseases. Scand J Immunol. 2008;68:261–269. doi: 10.1111/j.1365-3083.2008.02127.x. [DOI] [PubMed] [Google Scholar]
- 51.Cutolo M, Pizzorni C, Sulli A. Vitamin D endocrine system involvement in autoimmune rheumatic diseases. Autoimmun Rev. 2011;11:84–87. doi: 10.1016/j.autrev.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 52.Peelen E, Knippenberg S, Muris AH, et al. Effects of vitamin D on the peripheral adaptive immune system: a review. Autoimmun Rev. 2011;10:733–743. doi: 10.1016/j.autrev.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 53.Bhalla AK, Amento EP, Clemens TL, Holick MF, Krane SM. Specific high-affinity receptors for 1,25-dihydroxyvitamin D3 in human peripheral blood mononuclear cells: presence in monocytes and induction in T lymphocytes following activation. J Clin Endocrinol Metab. 1983;57:1308–1310. doi: 10.1210/jcem-57-6-1308. [DOI] [PubMed] [Google Scholar]
- 54.Provvedini DM, Tsoukas CD, Deftos LJ, Manolagas SC. 1,25-dihydroxyvitamin D3 receptors in human leukocytes. Science. 1983;221:1181–1183. doi: 10.1126/science.6310748. [DOI] [PubMed] [Google Scholar]
- 55.Veldman CM, Cantorna MT, DeLuca HF. Expression of 1,25-dihydroxyvitamin D(3) receptor in the immune system. Arch Biochem Biophys. 2000;374:334–338. doi: 10.1006/abbi.1999.1605. [DOI] [PubMed] [Google Scholar]
- 56.Deluca HF, Cantorna MT. Vitamin D: its role and uses in immunology. FASEB J. 2001;15:2579–2585. doi: 10.1096/fj.01-0433rev. [DOI] [PubMed] [Google Scholar]
- 57.Alroy I, Towers TL, Freedman LP. Transcriptional repression of the interleukin-2 gene by vitamin D3: direct inhibition of NFATp/AP-1 complex formation by a nuclear hormone receptor. Mol Cell Biol. 1995;15:5789–5799. doi: 10.1128/mcb.15.10.5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lemire JM, Archer DC, Beck L, Spiegelberg HL. Immunosuppressive actions of 1,25-dihydroxyvitamin D3: preferential inhibition of Th1 functions. J Nutr. 1995;125:1704S–1708S. doi: 10.1093/jn/125.suppl_6.1704S. [DOI] [PubMed] [Google Scholar]
- 59.Cippitelli M, Santoni A. Vitamin D3: a transcriptional modulator of the interferon-gamma gene. Eur J Immunol. 1998;28:3017–3030. doi: 10.1002/(SICI)1521-4141(199810)28:10<3017::AID-IMMU3017>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 60.Rigby WF, Stacy T, Fanger MW. Inhibition of T lymphocyte mitogenesis by 1,25-dihydroxyvitamin D3 (calcitriol) J Clin Invest. 1984;74:1451–1455. doi: 10.1172/JCI111557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lemire JM, Adams JS, Kermani-Arab V, Bakke AC, Sakai R, Jordan SC. 1,25-Dihydroxyvitamin D3 suppresses human T helper/inducer lymphocyte activity in vitro. J Immunol. 1985;134:3032–3035. [PubMed] [Google Scholar]
- 62.Reichel H, Koeffler HP, Tobler A, Norman AW. 1 alpha,25-Dihydroxyvitamin D3 inhibits gamma-interferon synthesis by normal human peripheral blood lymphocytes. Proc Natl Acad Sci U S A. 1987;84:3385–3389. doi: 10.1073/pnas.84.10.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Boonstra A, Barrat FJ, Crain C, Heath VL, Savelkoul HF, O’Garra A. 1Alpha,25-dihydroxyvitamin d3 has a direct effect on naive CD4(+) T cells to enhance the development of Th2 cells. J Immunol. 2001;167:4974–4980. doi: 10.4049/jimmunol.167.9.4974. [DOI] [PubMed] [Google Scholar]
- 64.Gorman S, Kuritzky LA, Judge MA, et al. Topically applied 1,25-dihydroxyvitamin D3 enhances the suppressive activity of CD4+ CD25+ cells in the draining lymph nodes. J Immunol. 2007;179:6273–6283. doi: 10.4049/jimmunol.179.9.6273. [DOI] [PubMed] [Google Scholar]
- 65.Daniel C, Sartory NA, Zahn N, Radeke HH, Stein JM. Immune modulatory treatment of trinitrobenzene sulfonic acid colitis with calcitriol is associated with a change of a T helper (Th) 1/Th17 to a Th2 and regulatory T cell profile. J Pharmacol Exp Ther. 2008;324:23–33. doi: 10.1124/jpet.107.127209. [DOI] [PubMed] [Google Scholar]
- 66.Yu S, Cantorna MT. The vitamin D receptor is required for iNKT cell development. Proc Natl Acad Sci U S A. 2008;105:5207–5212. doi: 10.1073/pnas.0711558105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Broux B, Pannemans K, Zhang X, et al. CX(3)CR1 drives cytotoxic CD4(+)CD28(−) T cells into the brain of multiple sclerosis patients. J Autoimmun. 2012;38:10–19. doi: 10.1016/j.jaut.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 68.Luckey D, Bastakoty D, Mangalam AK. Role of HLA class II genes in susceptibility and resistance to multiple sclerosis: studies using HLA transgenic mice. J Autoimmun. 2011;37:122–128. doi: 10.1016/j.jaut.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Moroni L, Bianchi I, Lleo A. Geoepidemiology, gender and autoimmune disease. Autoimmun Rev. 2012;11:A386–A392. doi: 10.1016/j.autrev.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 70.Sellner J, Kraus J, Awad A, Milo R, Hemmer B, Stuve O. The increasing incidence and prevalence of female multiple sclerosis–a critical analysis of potential environmental factors. Autoimmun Rev. 2011;10:495–502. doi: 10.1016/j.autrev.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 71.Soilu-Hanninen M, Airas L, Mononen I, Heikkila A, Viljanen M, Hanninen A. 25-Hydroxyvitamin D levels in serum at the onset of multiple sclerosis. Mult Scler. 2005;11:266–271. doi: 10.1191/1352458505ms1157oa. [DOI] [PubMed] [Google Scholar]
- 72.Correale J, Ysrraelit MC, Gaitan MI. Immunomodulatory effects of Vitamin D in multiple sclerosis. Brain. 2009;132:1146–1160. doi: 10.1093/brain/awp033. [DOI] [PubMed] [Google Scholar]
- 73.Soilu-Hanninen M, Laaksonen M, Laitinen I, Eralinna JP, Lilius EM, Mononen I. A longitudinal study of serum 25-hydroxyvitamin D and intact parathyroid hormone levels indicate the importance of vitamin D and calcium homeostasis regulation in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2008;79:152–157. doi: 10.1136/jnnp.2006.105320. [DOI] [PubMed] [Google Scholar]
- 74.van der Mei IA, Ponsonby AL, Dwyer T, et al. Vitamin D levels in people with multiple sclerosis and community controls in Tasmania, Australia. J Neurol. 2007;254:581–590. doi: 10.1007/s00415-006-0315-8. [DOI] [PubMed] [Google Scholar]
- 75.Smolders J, Menheere P, Kessels A, Damoiseaux J, Hupperts R. Association of vitamin D metabolite levels with relapse rate and disability in multiple sclerosis. Mult Scler. 2008;14:1220–1224. doi: 10.1177/1352458508094399. [DOI] [PubMed] [Google Scholar]
- 76.Hiremath GS, Cettomai D, Baynes M, et al. Vitamin D status and effect of low-dose cholecalciferol and high-dose ergocalciferol supplementation in multiple sclerosis. Mult Scler. 2009;15:735–740. doi: 10.1177/1352458509102844. [DOI] [PubMed] [Google Scholar]
- 77.Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA. 2006;296:2832–2838. doi: 10.1001/jama.296.23.2832. [DOI] [PubMed] [Google Scholar]
- 78.Kragt J, van Amerongen B, Killestein J, et al. Higher levels of 25-hydroxyvitamin D are associated with a lower incidence of multiple sclerosis only in women. Mult Scler. 2009;15:9–15. doi: 10.1177/1352458508095920. [DOI] [PubMed] [Google Scholar]
- 79.de Boer IH, Sachs MC, Cleary PA, et al. Circulating vitamin D metabolites and kidney disease in type 1 diabetes. J Clin Endocrinol Metab. 2012;97(12):4780–4788. doi: 10.1210/jc.2012-2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Borkar VV, Devidayal, Verma S, Bhalla AK. Low levels of vitamin D in North Indian children with newly diagnosed type 1 diabetes. Pediatr Diabetes. 2010;11:345–350. doi: 10.1111/j.1399-5448.2009.00589.x. [DOI] [PubMed] [Google Scholar]
- 81.Singh DK, Winocour P, Summerhayes B, Viljoen A, Sivakumar G, Farrington K. Are low erythropoietin and 1,25-dihydroxyvitamin D levels indicative of tubulo-interstitial dysfunction in diabetes without persistent microalbuminuria? Diabetes Res Clin Pract. 2009;85:258–264. doi: 10.1016/j.diabres.2009.06.022. [DOI] [PubMed] [Google Scholar]
- 82.Bener A, Alsaied A, Al-Ali M, et al. High prevalence of vitamin D deficiency in type 1 diabetes mellitus and healthy children. Acta Diabetol. 2009;46:183–189. doi: 10.1007/s00592-008-0071-6. [DOI] [PubMed] [Google Scholar]
- 83.Littorin B, Blom P, Scholin A, et al. Lower levels of plasma 25-hydroxyvitamin D among young adults at diagnosis of autoimmune type 1 diabetes compared with control subjects: results from the nationwide Diabetes Incidence Study in Sweden (DISS) Diabetologia. 2006;49:2847–2852. doi: 10.1007/s00125-006-0426-x. [DOI] [PubMed] [Google Scholar]
- 84.Bogaczewicz J, Sysa-Jedrzejowska A, Arkuszewska C, et al. Vitamin D status in systemic lupus erythematosus patients and its association with selected clinical and laboratory parameters. Lupus. 2012;21:477–484. doi: 10.1177/0961203311427549. [DOI] [PubMed] [Google Scholar]
- 85.Kim HA, Sung JM, Jeon JY, Yoon JM, Suh CH. Vitamin D may not be a good marker of disease activity in Korean patients with systemic lupus erythematosus. Rheumatol Int. 2011;31:1189–1194. doi: 10.1007/s00296-010-1442-1. [DOI] [PubMed] [Google Scholar]
- 86.Amital H, Szekanecz Z, Szucs G, et al. Serum concentrations of 25-OH vitamin D in patients with systemic lupus erythematosus (SLE) are inversely related to disease activity: is it time to routinely supplement patients with SLE with vitamin D? Ann Rheum Dis. 2010;69:1155–1157. doi: 10.1136/ard.2009.120329. [DOI] [PubMed] [Google Scholar]
- 87.Wright TB, Shults J, Leonard MB, Zemel BS, Burnham JM. Hypovitaminosis D is associated with greater body mass index and disease activity in pediatric systemic lupus erythematosus. J Pediatr. 2009;155:260–265. doi: 10.1016/j.jpeds.2009.02.033. [DOI] [PubMed] [Google Scholar]
- 88.Borba VZ, Vieira JG, Kasamatsu T, Radominski SC, Sato EI, Lazaretti-Castro M. Vitamin D deficiency in patients with active systemic lupus erythematosus. Osteoporos Int. 2009;20:427–433. doi: 10.1007/s00198-008-0676-1. [DOI] [PubMed] [Google Scholar]
- 89.Ruiz-Irastorza G, Egurbide MV, Olivares N, Martinez-Berriotxoa A, Aguirre C. Vitamin D deficiency in systemic lupus erythematosus: prevalence, predictors and clinical consequences. Rheumatology (Oxford) 2008;47:920–923. doi: 10.1093/rheumatology/ken121. [DOI] [PubMed] [Google Scholar]
- 90.Cantorna MT. Vitamin D and its role in immunology: multiple sclerosis, and inflammatory bowel disease. Prog Biophys Mol Biol. 2006;92:60–64. doi: 10.1016/j.pbiomolbio.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 91.Nordvik I, Myhr KM, Nyland H, Bjerve KS. Effect of dietary advice and n-3 supplementation in newly diagnosed MS patients. Acta Neurol Scand. 2000;102:143–149. doi: 10.1034/j.1600-0404.2000.102003143.x. [DOI] [PubMed] [Google Scholar]
- 92.Achiron A, Barak Y, Miron S, Izhak Y, Faibel M, Edelstein S. Alfacalcidol treatment in multiple sclerosis. Clin Neuro-pharmacol. 2003;26:53. doi: 10.1097/00002826-200303000-00001. [DOI] [PubMed] [Google Scholar]
- 93.Wingerchuk DM, Lesaux J, Rice GP, Kremenchutzky M, Ebers GC. A pilot study of oral calcitriol (1,25-dihydroxyvitamin D3) for relapsing-remitting multiple sclerosis. J Neurol Neurosurg Psychiatry. 2005;76:1294–1296. doi: 10.1136/jnnp.2004.056499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li X, Liao L, Yan X, et al. Protective effects of 1-alpha-hydroxyvitamin D3 on residual beta-cell function in patients with adult-onset latent autoimmune diabetes (LADA) Diabetes Metab Res Rev. 2009;25:411–416. doi: 10.1002/dmrr.977. [DOI] [PubMed] [Google Scholar]
- 95.Pitocco D, Crino A, Di Stasio E, et al. The effects of calcitriol and nicotinamide on residual pancreatic beta-cell function in patients with recent-onset type 1 diabetes (IMDIAB XI) Diabet Med. 2006;23:920–923. doi: 10.1111/j.1464-5491.2006.01921.x. [DOI] [PubMed] [Google Scholar]
- 96.Ruiz-Irastorza G, Gordo S, Olivares N, Egurbide MV, Aguirre C. Changes in vitamin D levels in patients with systemic lupus erythematosus: effects on fatigue, disease activity, and damage. Arthritis Care Res (Hoboken) 2010;62:1160–1165. doi: 10.1002/acr.20186. [DOI] [PubMed] [Google Scholar]
- 97.Antico A, Tampoia M, Tozzoli R, Bizzaro N. Can supplementation with vitamin D reduce the risk or modify the course of autoimmune diseases? A systematic review of the literature. Autoimmun Rev. 2012;12:127–136. doi: 10.1016/j.autrev.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 98.Roy E, Leduc M, Guegan S, et al. Specific maternal micro-chimeric T cells targeting fetal antigens in beta cells predispose to autoimmune diabetes in the child. J Autoimmun. 2011;36:253–262. doi: 10.1016/j.jaut.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 99.Bogdanos DP, Smyk DS, Rigopoulou EI, et al. Twin studies in autoimmune disease: genetics, gender and environment. J Autoimmun. 2012;38:J156–J169. doi: 10.1016/j.jaut.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 100.Staples JA, Ponsonby AL, Lim LL, McMichael AJ. Ecologic analysis of some immune-related disorders, including type 1 diabetes, in Australia: latitude, regional ultraviolet radiation, and disease prevalence. Environ Health Perspect. 2003;111:518–523. doi: 10.1289/ehp.5941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hypponen E, Laara E, Reunanen A, Jarvelin MR, Virtanen SM. Intake of vitamin D and risk of type 1 diabetes: a birth-cohort study. Lancet. 2001;358:1500–1503. doi: 10.1016/S0140-6736(01)06580-1. [DOI] [PubMed] [Google Scholar]
- 102.Agmon-Levin N, Mosca M, Petri M, Shoenfeld Y. Systemic lupus erythematosus one disease or many? Autoimmun Rev. 2012;11:593–595. doi: 10.1016/j.autrev.2011.10.020. [DOI] [PubMed] [Google Scholar]
- 103.Borchers AT, Naguwa SM, Shoenfeld Y, Gershwin ME. The geoepidemiology of systemic lupus erythematosus. Autoimmun Rev. 2010;9:A277–A287. doi: 10.1016/j.autrev.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 104.Fu SM, Deshmukh US, Gaskin F. Pathogenesis of systemic lupus erythematosus revisited 2011: end organ resistance to damage, autoantibody initiation and diversification, and HLA-DR. J Autoimmun. 2011;37:104–112. doi: 10.1016/j.jaut.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lehmann P, Homey B. Clinic and pathophysiology of photosensitivity in lupus erythematosus. Autoimmun Rev. 2009;8:456–461. doi: 10.1016/j.autrev.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 106.Breslin LC, Magee PJ, Wallace JM, McSorley EM. An evaluation of vitamin D status in individuals with systemic lupus erythematosus. Proc Nutr Soc. 2011;70:399–407. doi: 10.1017/S0029665111001613. [DOI] [PubMed] [Google Scholar]