Abstract
Bone remodeling is a lifelong process in which mature bone tissue is removed from the skeleton by bone resorption and is replenished by new during ossification or bone formation. The remodeling cycle requires both the differentiation and activation of two cell types with opposing functions. The osteoclast, which orchestrates bone resorption, and the osteoblast, which orchestrates bone formation. The differentiation of these cells from their respective precursors is a process which has been overshadowed by enigma, particularly because the precise osteoclast precursor has not been identified and because the identification of skeletal stem cells (SSC), which give rise to osteoblasts, is very recent. Latest advances in the area of stem cell biology have enabled us to gain a better understanding of how these differentiation processes occur in physiological and pathological conditions. In this review we postulate that modulation of stem cells during inflammatory conditions is a necessary prerequisite of bone remodeling and therefore an essential new component to the field of osteoimmunology. In this context, we highlight the role of transcription factor nuclear factor of activated T cells cytoplasmic 1 (NFATc1), because it directly links inflammation with differentiation of osteoclasts and osteoblasts.
Keywords: Hematopoietic Stem Cells, Skeletal Stem Cells, Mesenchymal Stem Cells, Bone, Bone Marrow, Osteoblasts, Osteoclasts, Osteoimmunology
Introduction
During the last decade an interdisciplinary research field has been developed focused on the crosstalk between the immune and bone systems. Although the interplay in between these systems has been recognized since the early 1970s, osteoimmunology started with studies on rheumatoid arthritis (RA), where a strong connection between activation of T lymphocytes and osteoclastogenesis was established [1, 2]. Since then, it has expanded to study the interplay of cells and crosstalk of signaling proteins between bone cells and the immune system [1].
Bone is a dynamic tissue in the body that regenerates itself throughout adult life. This remodeling cycle requires the activity of two cell types with opposing functions; osteoclasts, which orchestrate bone resorption, and osteoblasts, which orchestrate bone formation. Both of these cell types need to be constantly replenished, as terminally differentiated osteoclasts are short-lived and osteoblasts mature into post-mitotic osteocytes, which are embedded in bone [3]. The number of osteoclasts and osteoblasts on the bone surface does not get exhausted throughout life as they are constantly replenished by tissue specific stem cells. Albeit significant differences in the dynamics of osteoclast and osteoblast replenishment by stem cells, recent studies in both mice and human cells, have allowed a better understanding of the basic principles that govern these processes.
Stem Cells
In general, stem cells are a class of undifferentiated cells that have the remarkable potential to develop into specialized cell types in the body during development and maintenance of tissue homeostasis. Commonly, stem cells are derived from two main sources: from the inner cell mass of the blastocyst during embryological development (embryonic stem cells) and from adult tissues (adult stem cells). Adult stem cells are found among differentiated cells in various tissues and organs, and thus are also called somatic stem cells, where somatic refers to cells of the body that differ from germ cells (sperm or eggs). Adult stem cells are of particular interest in many pathological conditions as they have the capacity to self-renew and differentiate to all of the major specialized cell types of the tissue in which they are found, and thereby maintain the homeostasis and repair of that tissue.
Stem cells are therefore very important in bone homeostasis, as bone is continuously being remodeled throughout adulthood and undergoes repairs during fracture healing. The bone remodeling process requires both the differentiation and activation of two cell types with opposing functions: the osteoclast, a cell of hematopoietic origin that orchestrates bone resorption, and the osteoblast, a cell claimed to be of mesenchymal origin, which orchestrates bone formation. Two stem cell populations control the differentiation of these cells from their respective precursors in the bone marrow: the hematopoietic stem cells (HSC), which generate all the types of blood cells in the body and the skeletal stem cells (SSC) (which seem to correspond with a sub-population of mesenchymal stem cells; MSC), which generate bone, stroma and cartilage and are discussed below. Hence, in the coming years it will be important to establish the role of HSC and SSC on osteoimmunology. In the next sections we describe HSC and SSC and how they differentiate into osteoclasts and osteoblasts, respectively. We then describe our current understanding of the relationship between HSC, SSC and inflammation and address possible implications for bone homeostasis.
Hematopoietic stem cells (HSC) and osteoclastogenesis
The human body requires the replenishment of over fifty billion blood cells (leukocytes, erythrocytes and platelets) daily. This rapid turnover is possible due to HSC harbored in the bone marrow, capable of differentiating into all cells of the hematopoietic lineage, while maintaining a rather constant pool of HSC through self-renewal, thus avoiding cell exhaustion. In mice, different sets of markers have been identified to identify HSC including the original Lin− Sca-1+ c-kit+ (LSK) signature [4]. However, the LSK HSC compartment is heterogeneous and is generally divided into at least two multipotent subpopulations, namely Long-Term (LT)-HSC and Short-Term (ST)-HSC [5]. LT-HSCs have extensive (life-long) self-renewal potential and on commitment give rise to ST-HSCs with more restricted self-renewal capacity. Recently, the developmental gene homeobox B5 (Hoxb5) was shown to distinguish long-term HSC (LT-HSC; cells capable of sustained self-renewal for an organism’s lifetime) from short-term HSC (ST-HSC; cells with limited self-renewal capacity that do not repopulate secondary recipients in serial transplantation assays in mice) [6]. Other studies have shown that the ST-HSC population also exhibits heterogeneity comprising CD34−flt3−, CD34+flt3− and CD34+flt3+ cells [7].
HSC are also identified by the signaling lymphocyte activation molecule (SLAM) markers CD150+ CD244− CD48− [8]. HSC can commit into Multi-Potent Progenitor (MPP) identified as CD150− CD244+ CD48−, and a late MMP, CD150− CD244+ CD48+ [8], which are capable of giving rise to both lymphoid and myeloid lineages [9], but show very limited or no self-renewal potential [5]. These cells can then further commit into a common myeloid progenitor (CMP), characterized by the expression of FCγRlow and CD34+ [10]. In the presence of M-CSF, c-fms+ (CD115+), CMP can differentiate in the bone marrow into monoblasts, which then enter circulation and differentiate into immature promonocytes [11] CD13+/CD11b+, and mature monocytes CD11b+/CD14+ RANK+ osteoclast precursors which can further be differentiated to osteoclasts in response to RANKL signaling (Figure 1). Tissue resident CD14+ macrophages also can differentiate to osteoclasts. Terminally differentiated osteoclasts express tartrate-resistant acid phosphatase, cathepsin K, calcitonin receptor, and the ανβ3 integrin which facilitate the process of bone resorption. Therefore, during the commitment and differentiation of HSC into osteoclasts [12, 13], some intermediate, immature cell types can be identified based on specific expression of markers, as shown in Figure 2. However, this standard hierarchical model, although widely accepted, has been recently challenged with experiments demonstrating a certain degree of plasticity in the system. For example, different precursors rather than HSC can maintain hematopoiesis [14] and differentiation can occur without going through a specific intermediate cell type [9]. Similarly, various CD11b− and/or CD14− and/or CD11c+ populations have also been shown to differentiate to osteoclasts [15, 16]. Hence, it is prudent to consider the hierarchy shown in Figure 1 as a working, model in progress.
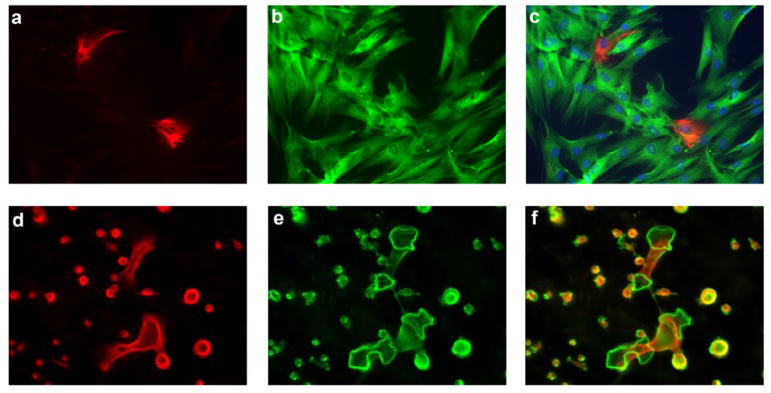
Figure 1.
A heterogeneous population of MSC and HSC gives rise to terminally differentiated cells with opposing functions in bone remodeling. Immunofluorescence microscopy of human bone marrow derived mesenchymal stem cells (MSC) (a–c). MSC after 14 days in culture showing homogenous expression of β-III-tubulin (green; a), and differential expression of nestin (red, b), highlighting the heterogeneous population of MSC (merged image, c). Immunofluorescence microscopy of mouse bone marrow derived HSC (d–f) cultured in the presence of MCSF and RANKL showing the homogeneous expression of α-tubulin (red, d), and the presence of multinucleated giant cells forming filamentous-actin ring structures (Phalloidin labeled actin in green, e), highlighting the heterogeneous population of osteoclast precursor cells as not all cells become terminally differentiated osteoclasts merged image (f). Original magnification for (a–c) 200x, and for (d–f) 60x.
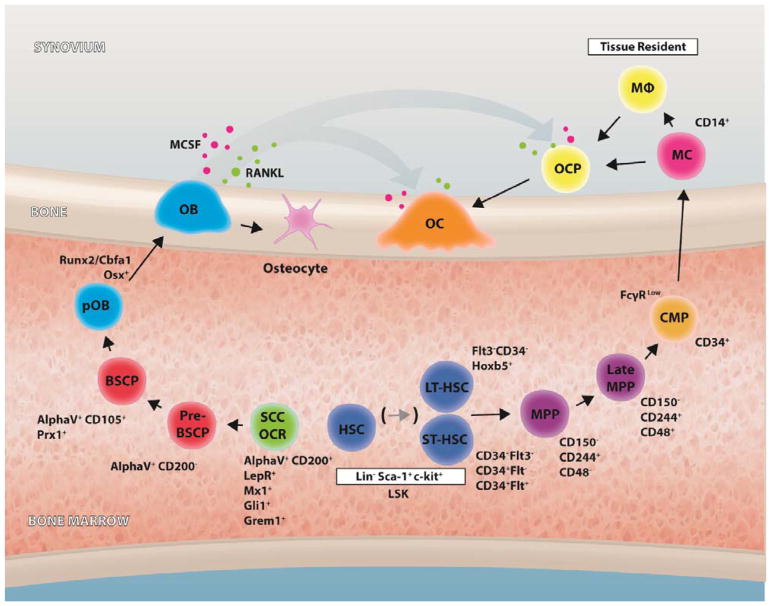
Figure 2.
Stem cell progenitors in bone homeostasis. Schematic graphical representation of the bone marrow microenvironment, one of the primary lymphoid organs, harbors immune cells including hematopoietic stem cells (HSCs), lymphocytes, monocytes/macrophages, and neutrophils, all of which interplay with bone cells such as osteoblasts, osteoclasts, and osteocytes. Schematic depicts the differentiation stages and early markers of HSC and SSC differentiation to osteoclasts and osteoblasts respectively. Long-term hematopoietic stem cells (LT-HSC) short-term hematopoietic stem cells (ST-HSC), Multipotent progenitor (MPP), Late multipotent progenitor (Late-MMP), common myeloid progenitor (CMP), monocyte (MC), macrophage (MΦ), osteoclast (OC). Skeletal stem cells (SSC), osteochondroreticular stem cell (OCR), pre-bone, stroma and cartilage progenitor (Pre-BSCP), bone, stroma and cartilage progenitor (BSCP), pre-osteoblast (pOB), osteoblast (OB) and osteocyte. Bone remodeling is regulated by macrophage colony stimulating factor (MCSF) and receptor activator for nuclear factor kappa B ligand (RANKL) at the bone surface.
Skeletal Stem Cells (SSC) and osteoblastogenesis
In addition to HSC, the bone marrow hosts a stem cell type known to differentiate into bone and other skeletal tissues [17, 18]. When bone marrow mononuclear cells are seeded and expanded in tissue culture flasks, a population of cells appears that acquires a fibroblastic morphology and maintains for several passages the potential to differentiate into bone, adipose cells and cartilage. These cells are commonly called Mesenchymal Stem Cells or Marrow Stromal Cells (MSC) [19] (Figure 1). However, it has been recently established that ex vivo expanded MSC differ from their in vivo counter-parts, which in the context of bone remodeling, are best identified as skeletal stem cells (SSC) [20]. The true nature of SSC in vivo has been elusive at least in part due to the lack of markers to identify this cell type in situ [21]. In addition, there is an apparent overlap in between the cells that gives rise to bone (SSC) and cells in the bone marrow capable of supporting hematopoiesis, serving as a key component of the so-called HSC niche [22]. Furthermore, there is strong evidence that MSC correspond with perivascular cells (pericytes) [23], serving as an explanation for why MSC can be isolated from virtually all vascularized tissues [24]. In consequence, at least three cell types in the bone marrow have been determined functionally, based on the expression of specific marker: SSC, HSC-supporting cells which are identified as CXCL12+ [25] Nestin+ [26], Prx1+ [27] or SCF+ [28], and pericytes expressing CD146+ [29]. To date, it remains unclear to what extent these three cell types overlap in terms of identity or differ from each other. For example, a population of CD146+ sub-endothelial cells in human bone marrow contains osteogenic progenitors that are also at the origin of the stromal cells that support hematopoiesis [22].
In mice, SSC have been recently identified as either Integrin alphaV+ CD200+ [30], Leptin-receptor (LepR)+ [31], Mx1+ [32], Gli-1+ [33] or Gremlin 1+ [34]. Gremlin 1+ have been also called osteochondroreticular (OCR) stem cells to highlight the ability of these cells to differentiate into osteoblasts, chondrocytes, and reticular marrow stromal cells, but not adipocytes. Since SSC/OCR have only recently been identified in vivo, it remains unknown if they correspond to the same or different cell types. Chan et al [30] have recently suggested a hierarchical commitment for SSC into osteoblasts, similar to the progressive stages of HSC into osteoclasts. In this model, integrin alphaV+ CD200+ cells commit into alphaV+ CD200− pre-bone, stroma and cartilage progenitor (BSCP) cells, which have no self-renewal properties. Pre-BSCP can then further commit into alphaV+ CD105+ BSCP. Experiments with transgenic mice have further demonstrated that Prx1+cells can give rise to osteoblasts and chondrocytes [35]. Hence, Prx1+ cells approach the definition of alphaV+ CD105+ BSCP [30, 36], although their contribution to stroma is not known. It is then likely that BSCP, with possibly more, undefined intermediate cell types, gives rise to proliferating pre-osteoblasts, which are characterized by the expression of the transcription factor Runx2/Cbfa1 and are completely committed cells into the osteogenic lineage [37]. Runx2/Cbfa1 is expressed restrictively in fetal development and Cbfa1 mutant mice have completely block of intramembranous and endochondral ossification owing to the maturational arrest of osteoblasts demonstrating the essential role of Cbfa1 in osteogenesis [38, 39]. Cbfa1 activates expression of Osterix [40] and pre-osteoblasts mature to give rise to calcium precipitating osteoblasts. During osteoblast differentiation, Runx2 and Osterix upregulate the expression of bone matrix protein genes including collagen type I alpha 1, integrin-binding sialoprotein, osteopontin and fibronectin 1 [41, 42]. Terminally differentiated osteoblasts finally mature into osteocytes (Figure 2).
Inflammation on HSC differentiation with implications on bone resorption
Inflammation is a protective response of the body to injury or infection. During inflammation, a high demand for myeloid and lymphoid cells causes HSC to divide and differentiate in order to supply the required cells. This expansion is normally not at the expense of the original HSC pool, because acute inflammation also increases self-renewal of the cells [43]. However, chronic inflammation can lead to HSC exhaustion and anemia [44]. HSC may sense inflammation directly in response to interferons, TNF, interleukin 1β, and other pro-inflammatory cytokines, reviewed in [45]. In addition, HSC may respond indirectly by changes triggered by inflammation in the stem cell niche, such as decreased SDF-1 secretion by osteoblasts induced by G-CSF, or the egress of bone marrow-resident macrophages [46, 47]. Interestingly, during inflammation, circulating HSC are increased, and primed to differentiate in situ into myeloid cells [48, 49]. Since HSCs have the capacity to differentiate into osteoclasts, it is not surprising that increased myelopoiesis is directly linked with increased osteoclastogenesis and bone loss in inflammatory conditions [50, 51]. In fact, numerous reports have shown that any disturbance in the number of myeloid precursors will significantly affect the rate of osteoclast formation [15] and inflammatory bone loss.
Although the exact osteoclast precursor(s) remains to be defined, a number of cell types (macrophages, monocytes, immature dendritic cells) and molecules have been described as potential osteoclastogenesis agents both in the presence and/or in the absence of exogenous RANK ligand (RANKL) in vivo and in vitro [52]. RANKL is produced by osteoblasts under physiological conditions, but also activated immune cells, including B and T lymphocytes, have also been described to secrete RANKL [53].
Although the concept that alternative pathways of osteoclastogenesis independent of RANKL exist is still a matter of debate, it is clearly evident that a few pro-inflammatory cytokines including TNF [54, 55] and IL-23 [56] regulate the activation of calcium signaling and nuclear factor of activated T cells cytoplasmic 1 (NFATc1). NFATc1−/ − cells are unable to generate osteoclasts despite normal development into the monocyte/ macrophage lineage highlighting the specific needs of osteoclastogenesis [57]. NFATc1 is a transcription factor activated by calcium signaling, as Ca2+ activates calcineurin, which in turn dephosphorylates multiple phosphoserines on NFAT, leading to its nuclear translocation and activation. NFATc1 is responsible for the regulation of genes related to osteoclast function as well as numerous genes non-essential to osteoclast function [58, 59]; Therefore the significance of this pathway may extend beyond our current understanding.
Inflammation on SSC differentiation with implications on bone formation
SSC, which also give rise to chondrocytes, and reticular marrow stromal cells, differentiate into pre-osteoblasts and then become osteoblasts on the bone surface. The signals that regulate the decision of progenitor cells to form osteoblasts are complex and partially understood [60]. Osteoblast regulation can be achieved from several signals. Transforming growth factor-β (TGF-β) signalling through activation of receptor type I (R-I) and receptor type II (R-II), transduces signals to Smads, which form a complex with Smad4 and then translocate into the nucleus where they interact with Runx2 also known as Cbfa1 transcription factor to trigger activation of osteoblast specific genes [42, 61]. Commonly Runx2 is considered the major transcription factor to trigger activation of osteoblast specific genes. However, NFATc1 has also been implicated in osteoblast differentiation, since it forms a complex with Osterix, and together cooperatively regulate osteogenesis [62]. Other groups independently of these studies have also confirmed these observations using the opposite approach where mice expressing a constitutively nuclear NFATc1 variant (NFATc1nuc) in osteoblasts develop high bone mass due to massive osteoblast overgrowth and enhanced osteoblast proliferation [63]. In keeping with these observations it was recently shown that NFATc1 acts downstream of BMP-2 signaling inducing alkaline phosphatase activity and nodule formation in an osteoblastic cell line [64]. Of note, other groups have shown that constitutively active NFATc1 in osteoblast cell lines inhibits osteoblastogenesis in vitro [65]. These data suggest that modulation of NFATc1 may differ in early versus late differentiation and possibly in precursor versus terminally differentiated cells. These findings may serve as explanation for why patients following organ transplantation and treated with inhibitors of the Calcineurin/NFATc1 pathway, such as cyclosporin A and FK506 often develop osteoporosis [66]. Of note, IL-23 which is known to activate NFATc1 also promotes osteogenic differentiation of MSC, by activation of the β-catenin pathway [67]. Similarly, pro-inflammatory cytokines IL-6 and IL-17 promote osteogenesis of MSC in vitro [68, 69]. Altogether, our current understanding suggests that acute inflammation promotes osteoblastogenesis. In fact, it is well documented that the acute inflammation stage following bone fracture is necessary to promote bone repair [70] and there is impaired fracture healing in the absence of TNF signaling [71] or chronic inflammation [72]. Nonetheless, recent evidence suggests that the anti-inflammatory cytokine IL-27 may promote bone formation, but inhibiting osteoclastogenesis and inhibiting apoptosis of osteoblasts [73]. Future work is required to elucidate if inflammation activates NFATc1 in SSC or the osteoblastic progeny, similarly as observed in osteoclast precursors.
Periosteal and endosteal bone-lining tissues contain resident macrophages (termed osteomacs [74]), which play an important role supporting osteoblastogenesis during bone repair. Macrophages secrete factors including TGF-β, osteopontin, 1,25-dihydroxy-vitamin D3 and BMP-2 [75]. However, the specific role of osteomacs, although likely related to anabolism, on bone repair are poorly understood [76].
Since SSC have only been recently identified, the effect of inflammation on these cells is largely unknown. On the other hand, ex vivo expanded MSC (Figure 2) are widely known to be effective immune suppressors and are promising therapeutic agents for steroid-refractory acute graft vs. host disease (GVHD) [77], Crohn’s disease [78] and allograft rejection after organ transplantation [79]. Also many other early stage clinical trials are on their way to test the efficacy of MSCs as immune suppressors [80]. MSC affect lymphocytes, monocytes and dendritic cells by either secretion of factors such as prostaglandin E 2 (PGE2), Indoleamine 2,3-dioxygenase (IDO) or cytokines, reviewed by [81]. Of note, a clinical dose of MSC is typically around 20 to 100 million cells per patient, while the frequency of SSC in the bone marrow is expected to be approximately one in 100,000 mononuclear cells [19]. Therefore, it remains unclear to what extent cells in the bone marrow (SSC, pericytes or others), that seem to correspond with MSC, also exert significant immune suppressive functions.
Concluding Remarks
The role of hematopoietic stem cells (HSC) has been largely acknowledged, since HSC give rise to both immune cells and osteoclasts. However, only recently have some of the mechanisms underlying the effect of inflammation on HSC differentiation been uncovered. In addition, the activity of a second type of stem cell in the bone marrow, identified as skeletal stem cells (SSC), has been demonstrated to be affected by the immune system. This is particularly important since, in chronic inflammatory diseases where excessive bone loss is commonly observed, changes at the bone surface may reflect changes within the bone marrow. Therefore it is evident that the cellular and molecular interplay is not only present on the bone surface where bone resorption occurs but rather it begins very early and well before bone cells are terminally differentiated.
Significance Statement.
During the last decade an interdisciplinary research field has been developed focused on the crosstalk in between the immune and bone systems. The role of hematopoietic stem cells (HSC) has been largely acknowledged, since HSC give rise to both, all immune cells and osteoclasts. However, only recently some of the mechanisms underlying the effect of inflammation on HSC differentiation have been uncovered. In addition, the activity skeletal stem cells (SSC) is also likely to be affected by the immune system.
Footnotes
Author Contributions
F.A. Fierro: Conception and design, Collection and/or assembly of data, Data analysis and interpretation, Manuscript writing and Final approval of manuscript; J.A. Nolta: Manuscript writing and Final approval of manuscript; I.E. Adamopoulos: Conception and design, Collection and/or assembly of data, Data analysis and interpretation, Manuscript writing and Final approval of manuscript.
References
- 1.Takayanagi H. Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nature reviews Immunology. 2007;7:292–304. doi: 10.1038/nri2062. [DOI] [PubMed] [Google Scholar]
- 2.Walsh MC, Kim N, Kadono Y, et al. Osteoimmunology: interplay between the immune system and bone metabolism. Annual review of immunology. 2006;24:33–63. doi: 10.1146/annurev.immunol.24.021605.090646. [DOI] [PubMed] [Google Scholar]
- 3.Aarden EM, Burger EH, Nijweide PJ. Function of osteocytes in bone. Journal of cellular biochemistry. 1994;55:287–299. doi: 10.1002/jcb.240550304. [DOI] [PubMed] [Google Scholar]
- 4.Spangrude GJ, Heimfeld S, Weissman IL. Purification and characterization of mouse hematopoietic stem cells. Science (New York, NY. 1988;241:58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- 5.Morrison SJ, Weissman IL. The long-term repopulating subset of hematopoietic stem cells is deterministic and isolatable by phenotype. Immunity. 1994;1:661–673. doi: 10.1016/1074-7613(94)90037-x. [DOI] [PubMed] [Google Scholar]
- 6.Chen JY, Miyanishi M, Wang SK, et al. Hoxb5 marks long-term haematopoietic stem cells and reveals a homogenous perivascular niche. Nature. 2016;530:223–227. doi: 10.1038/nature16943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang L, Bryder D, Adolfsson J, et al. Identification of Lin(-)Sca1(+)kit(+)CD34(+)Flt3-short-term hematopoietic stem cells capable of rapidly reconstituting and rescuing myeloablated transplant recipients. Blood. 2005;105:2717–2723. doi: 10.1182/blood-2004-06-2159. [DOI] [PubMed] [Google Scholar]
- 8.Kiel MJ, Yilmaz OH, Iwashita T, et al. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 9.Arinobu Y, Mizuno S, Chong Y, et al. Reciprocal activation of GATA-1 and PU.1 marks initial specification of hematopoietic stem cells into myeloerythroid and myelolymphoid lineages. Cell stem cell. 2007;1:416–427. doi: 10.1016/j.stem.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 10.Akashi K, Traver D, Miyamoto T, et al. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature. 2000;404:193–197. doi: 10.1038/35004599. [DOI] [PubMed] [Google Scholar]
- 11.Bain BJ. What is a promonocyte? American journal of hematology. 2013;88:919. doi: 10.1002/ajh.23548. [DOI] [PubMed] [Google Scholar]
- 12.Yavropoulou MP, Yovos JG. Osteoclastogenesis--current knowledge and future perspectives. Journal of musculoskeletal & neuronal interactions. 2008;8:204–216. [PubMed] [Google Scholar]
- 13.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 14.Sun J, Ramos A, Chapman B, et al. Clonal dynamics of native haematopoiesis. Nature. 2014;514:322–327. doi: 10.1038/nature13824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Charles JF, Hsu LY, Niemi EC, et al. Inflammatory arthritis increases mouse osteoclast precursors with myeloid suppressor function. The Journal of clinical investigation. 2012;122:4592–4605. doi: 10.1172/JCI60920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alnaeeli M, Penninger JM, Teng YT. Immune interactions with CD4+ T cells promote the development of functional osteoclasts from murine CD11c+ dendritic cells. J Immunol. 2006;177:3314–3326. doi: 10.4049/jimmunol.177.5.3314. [DOI] [PubMed] [Google Scholar]
- 17.Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell and tissue kinetics. 1970;3:393–403. doi: 10.1111/j.1365-2184.1970.tb00347.x. [DOI] [PubMed] [Google Scholar]
- 18.Owen M, Friedenstein AJ. Stromal stem cells: marrow-derived osteogenic precursors. Ciba Foundation symposium. 1988;136:42–60. doi: 10.1002/9780470513637.ch4. [DOI] [PubMed] [Google Scholar]
- 19.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science (New York, NY. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 20.Bianco P, Robey PG, Simmons PJ. Mesenchymal stem cells: revisiting history, concepts, and assays. Cell stem cell. 2008;2:313–319. doi: 10.1016/j.stem.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bianco P, Cao X, Frenette PS, et al. The meaning, the sense and the significance: translating the science of mesenchymal stem cells into medicine. Nature medicine. 2013;19:35–42. doi: 10.1038/nm.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sacchetti B, Funari A, Michienzi S, et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131:324–336. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 23.Crisan M, Yap S, Casteilla L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell stem cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 24.da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. Journal of cell science. 2006;119:2204–2213. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- 25.Sugiyama T, Kohara H, Noda M, et al. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25:977–988. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 26.Mendez-Ferrer S, Michurina TV, Ferraro F, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466:829–834. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greenbaum A, Hsu YM, Day RB, et al. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature. 2013;495:227–230. doi: 10.1038/nature11926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ding L, Saunders TL, Enikolopov G, et al. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012;481:457–462. doi: 10.1038/nature10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crisan M, Chen CW, Corselli M, et al. Perivascular multipotent progenitor cells in human organs. Ann N Y Acad Sci. 2009;1176:118–123. doi: 10.1111/j.1749-6632.2009.04967.x. [DOI] [PubMed] [Google Scholar]
- 30.Chan CK, Seo EY, Chen JY, et al. Identification and specification of the mouse skeletal stem cell. Cell. 2015;160:285–298. doi: 10.1016/j.cell.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou BO, Yue R, Murphy MM, et al. Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell stem cell. 2014;15:154–168. doi: 10.1016/j.stem.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park D, Spencer JA, Koh BI, et al. Endogenous bone marrow MSCs are dynamic, fate-restricted participants in bone maintenance and regeneration. Cell stem cell. 2012;10:259–272. doi: 10.1016/j.stem.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao H, Feng J, Ho TV, et al. The suture provides a niche for mesenchymal stem cells of craniofacial bones. Nature cell biology. 2015;17:386–396. doi: 10.1038/ncb3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Worthley DL, Churchill M, Compton JT, et al. Gremlin 1 identifies a skeletal stem cell with bone, cartilage, and reticular stromal potential. Cell. 2015;160:269–284. doi: 10.1016/j.cell.2014.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ouyang Z, Chen Z, Ishikawa M, et al. Prx1 and 3.2kb Col1a1 promoters target distinct bone cell populations in transgenic mice. Bone. 2013 doi: 10.1016/j.bone.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chan CK, Lindau P, Jiang W, et al. Clonal precursor of bone, cartilage, and hematopoietic niche stromal cells. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:12643–12648. doi: 10.1073/pnas.1310212110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Del Fattore A, Teti A, Rucci N. Bone cells and the mechanisms of bone remodelling. Frontiers in bioscience. 2012;4:2302–2321. doi: 10.2741/543. [DOI] [PubMed] [Google Scholar]
- 38.Komori T, Yagi H, Nomura S, et al. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89:755–764. doi: 10.1016/s0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
- 39.Otto F, Thornell AP, Crompton T, et al. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell. 1997;89:765–771. doi: 10.1016/s0092-8674(00)80259-7. [DOI] [PubMed] [Google Scholar]
- 40.Nakashima K, Zhou X, Kunkel G, et al. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108:17–29. doi: 10.1016/s0092-8674(01)00622-5. [DOI] [PubMed] [Google Scholar]
- 41.Modder UI, Roforth MM, Nicks KM, et al. Characterization of mesenchymal progenitor cells isolated from human bone marrow by negative selection. Bone. 2012;50:804–810. doi: 10.1016/j.bone.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ducy P, Zhang R, Geoffroy V, et al. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997;89:747–754. doi: 10.1016/s0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]
- 43.Takizawa H, Regoes RR, Boddupalli CS, et al. Dynamic variation in cycling of hematopoietic stem cells in steady state and inflammation. The Journal of experimental medicine. 2011;208:273–284. doi: 10.1084/jem.20101643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Bruin AM, Demirel O, Hooibrink B, et al. Interferon-gamma impairs proliferation of hematopoietic stem cells in mice. Blood. 2013;121:3578–3585. doi: 10.1182/blood-2012-05-432906. [DOI] [PubMed] [Google Scholar]
- 45.Baldridge MT, King KY, Goodell MA. Inflammatory signals regulate hematopoietic stem cells. Trends in immunology. 2011;32:57–65. doi: 10.1016/j.it.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.King KY, Goodell MA. Inflammatory modulation of HSCs: viewing the HSC as a foundation for the immune response. Nature reviews Immunology. 2011;11:685–692. doi: 10.1038/nri3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Winkler IG, Sims NA, Pettit AR, et al. Bone marrow macrophages maintain hematopoietic stem cell (HSC) niches and their depletion mobilizes HSCs. Blood. 2010;116:4815–4828. doi: 10.1182/blood-2009-11-253534. [DOI] [PubMed] [Google Scholar]
- 48.Massberg S, Schaerli P, Knezevic-Maramica I, et al. Immunosurveillance by hematopoietic progenitor cells trafficking through blood, lymph, and peripheral tissues. Cell. 2007;131:994–1008. doi: 10.1016/j.cell.2007.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mossadegh-Keller N, Sarrazin S, Kandalla PK, et al. M-CSF instructs myeloid lineage fate in single haematopoietic stem cells. Nature. 2013;497:239–243. doi: 10.1038/nature12026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Adamopoulos IE, Tessmer M, Chao CC, et al. IL-23 is critical for induction of arthritis, osteoclast formation, and maintenance of bone mass. J Immunol. 2011;187:951–959. doi: 10.4049/jimmunol.1003986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Adamopoulos IE, Suzuki E, Chao CC, et al. IL-17A gene transfer induces bone loss and epidermal hyperplasia associated with psoriatic arthritis. Annals of the rheumatic diseases. 2015;74:1284–1292. doi: 10.1136/annrheumdis-2013-204782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Adamopoulos IE, Mellins ED. Alternative pathways of osteoclastogenesis in inflammatory arthritis. Nature reviews Rheumatology. 2015;11:189–194. doi: 10.1038/nrrheum.2014.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Caetano-Lopes J, Canhao H, Fonseca JE. Osteoimmunology--the hidden immune regulation of bone. Autoimmunity reviews. 2009;8:250–255. doi: 10.1016/j.autrev.2008.07.038. [DOI] [PubMed] [Google Scholar]
- 54.Mukai T, Ishida S, Ishikawa R, et al. SH3BP2 cherubism mutation potentiates TNF-alpha-induced osteoclastogenesis via NFATc1 and TNF-alpha-mediated inflammatory bone loss. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2014;29:2618–2635. doi: 10.1002/jbmr.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yarilina A, Xu K, Chen J, et al. TNF activates calcium-nuclear factor of activated T cells (NFAT)c1 signaling pathways in human macrophages. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:1573–1578. doi: 10.1073/pnas.1010030108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shin HS, Sarin R, Dixit N, et al. Crosstalk among IL-23 and DNAX activating protein of 12 kDa-dependent pathways promotes osteoclastogenesis. J Immunol. 2015;194:316–324. doi: 10.4049/jimmunol.1401013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Asagiri M, Sato K, Usami T, et al. Autoamplification of NFATc1 expression determines its essential role in bone homeostasis. The Journal of experimental medicine. 2005;202:1261–1269. doi: 10.1084/jem.20051150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aliprantis AO, Ueki Y, Sulyanto R, et al. NFATc1 in mice represses osteoprotegerin during osteoclastogenesis and dissociates systemic osteopenia from inflammation in cherubism. The Journal of clinical investigation. 2008;118:3775–3789. doi: 10.1172/JCI35711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Charles JF, Coury F, Sulyanto R, et al. The collection of NFATc1-dependent transcripts in the osteoclast includes numerous genes non-essential to physiologic bone resorption. Bone. 2012;51:902–912. doi: 10.1016/j.bone.2012.08.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zuo C, Huang Y, Bajis R, et al. Osteoblastogenesis regulation signals in bone remodeling. Osteoporos Int. 2012 doi: 10.1007/s00198-012-1909-x. [DOI] [PubMed] [Google Scholar]
- 61.Chen G, Deng C, Li YP. TGF-beta and BMP Signaling in Osteoblast Differentiation and Bone Formation. Int J Biol Sci. 2012;8:272–288. doi: 10.7150/ijbs.2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Koga T, Matsui Y, Asagiri M, et al. NFAT and Osterix cooperatively regulate bone formation. Nature medicine. 2005;11:880–885. doi: 10.1038/nm1270. [DOI] [PubMed] [Google Scholar]
- 63.Winslow MM, Pan M, Starbuck M, et al. Calcineurin/NFAT Signaling in Osteoblasts Regulates Bone Mass. Developmental cell. 2006;10:771–782. doi: 10.1016/j.devcel.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 64.Mandal CC, Das F, Ganapathy S, et al. Bone Morphogenetic Protein-2 (BMP-2) Activates NFATc1 Transcription Factor via an Autoregulatory Loop Involving Smad/Akt/Ca2+ Signaling. The Journal of biological chemistry. 2016;291:1148–1161. doi: 10.1074/jbc.M115.668939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Choo MK, Yeo H, Zayzafoon M. NFATc1 mediates HDAC-dependent transcriptional repression of osteocalcin expression during osteoblast differentiation. Bone. 2009;45:579–589. doi: 10.1016/j.bone.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Leidig-Bruckner G, Hosch S, Dodidou P, et al. Frequency and predictors of osteoporotic fractures after cardiac or liver transplantation: a follow-up study. Lancet. 2001;357:342–347. doi: 10.1016/S0140-6736(00)03641-2. [DOI] [PubMed] [Google Scholar]
- 67.Tu B, Liu S, Liu G, et al. Macrophages derived from THP-1 promote the osteogenic differentiation of mesenchymal stem cells through the IL-23/IL-23R/beta-catenin pathway. Experimental cell research. 2015;339:81–89. doi: 10.1016/j.yexcr.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 68.Bastidas-Coral AP, Bakker AD, Zandieh-Doulabi B, et al. Cytokines TNF-alpha, IL-6, IL-17F, and IL-4 Differentially Affect Osteogenic Differentiation of Human Adipose Stem Cells. Stem cells international. 2016;2016:1318256. doi: 10.1155/2016/1318256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Croes M, Oner FC, van Neerven D, et al. Proinflammatory T cells and IL-17 stimulate osteoblast differentiation. Bone. 2016;84:262–270. doi: 10.1016/j.bone.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 70.Marsell R, Einhorn TA. The biology of fracture healing. Injury. 2011;42:551–555. doi: 10.1016/j.injury.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gerstenfeld LC, Cho TJ, Kon T, et al. Impaired fracture healing in the absence of TNF-alpha signaling: the role of TNF-alpha in endochondral cartilage resorption. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2003;18:1584–1592. doi: 10.1359/jbmr.2003.18.9.1584. [DOI] [PubMed] [Google Scholar]
- 72.Loi F, Cordova LA, Pajarinen J, et al. Inflammation, fracture and bone repair. Bone. 2016;86:119–130. doi: 10.1016/j.bone.2016.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shukla P, Mansoori MN, Kakaji M, et al. IL-27 Alleviates Bone Loss in Estrogen Deficient Conditions by Induction of Early Growth Response-2 Gene. The Journal of biological chemistry. 2017 doi: 10.1074/jbc.M116.764779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wu AC, Raggatt LJ, Alexander KA, et al. Unraveling macrophage contributions to bone repair. BoneKEy reports. 2013;2:373. doi: 10.1038/bonekey.2013.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pettit AR, Chang MK, Hume DA, et al. Osteal macrophages: a new twist on coupling during bone dynamics. Bone. 2008;43:976–982. doi: 10.1016/j.bone.2008.08.128. [DOI] [PubMed] [Google Scholar]
- 76.Cho SW. Role of osteal macrophages in bone metabolism. Journal of pathology and translational medicine. 2015;49:102–104. doi: 10.4132/jptm.2015.02.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Le Blanc K, Frassoni F, Ball L, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 78.Garcia-Olmo D, Herreros D, Pascual I, et al. Expanded adipose-derived stem cells for the treatment of complex perianal fistula: a phase II clinical trial. Dis Colon Rectum. 2009;52:79–86. doi: 10.1007/DCR.0b013e3181973487. [DOI] [PubMed] [Google Scholar]
- 79.Reinders ME, de Fijter JW, Roelofs H, et al. Autologous bone marrow-derived mesenchymal stromal cells for the treatment of allograft rejection after renal transplantation: results of a phase I study. Stem cells translational medicine. 2013;2:107–111. doi: 10.5966/sctm.2012-0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Trounson A, McDonald C. Stem Cell Therapies in Clinical Trials: Progress and Challenges. Cell stem cell. 2015;17:11–22. doi: 10.1016/j.stem.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 81.Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nature reviews Immunology. 2008;8:726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]