Abstract
Background:
The results of plasma amino acid patterns in samples from kidney transplant patients with good and impaired renal function using a targeted LC-MS/MS amino acid assay and a non-targeted metabolomics assay were compared.
Methods:
EDTA plasma samples were prospectively collected at baseline, 1, 2, 4 and 6 months post-transplant (n=116 patients, n=398 samples). Each sample was analyzed using both a commercial amino acid LC-MS/MS assay and a non-targeted metabolomics assay also based on MS/MS ion transitions. The results of both assays were independently statistically analyzed to identify amino acids associated with estimated glomerular filtration rates using correlation and partial least squares- discriminant analysis.
Results:
Although there was overlap between the results of the targeted and non-targeted metabolomics assays (tryptophan, 1-methyl histidine), there were also substantial inconsistencies, with the non-targeted assay resulting in more “hits” than the targeted assay. Without further verification of the hits detected by the non-targeted discovery assay, this would have led to different interpretation of the results. There were also false negative results when the non-targeted assay was used (hydroxy proline). Several of said discrepancies could be explained by loss of sensitivity during analytical runs for selected amino acids (serine and threonine), retention time shifts, signals above the range of linear detector response and integration of peaks not separated from background and interferences (aspartate) when the non-targeted metabolomics assay was used.
Conclusions:
Whenever assessment of a specific pathway such as amino acids is the focus of interest, a targeted seems preferable to a non-targeted metabolomics assay.
Keywords: amino acids, non-targeted metabolomics, targeted metabolomics, kidney transplantation, eGFR, multi-analyte LC-MS/MS, LC-MS/MS
1. Introduction
Metabolomics assays can be separated into two distinct approaches, targeted and non-targeted. Non-targeted metabolomics is also often described as unbiased, due to the fact that non-selective assay strategies are employed [1], whereas these strategies monitor a large group of unrelated metabolites. Targeted metabolomics, on the other hand, involves the quantification of a specific set of often related metabolites [2]. Although several analytical platforms can be used for both strategies, liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS), including ion traps and high-resolution mass spectrometers, are frequently the preferred analytical tools due to their high sensitivity and specificity [1–4]. While both targeted and non-targeted metabolomics utilize LC-MS/MS, there are often key differences in terms of sample preparation and separation techniques. While non-targeted strategies typically employ non-specific and rather non-selective sample preparation techniques to ensure that metabolites with a wide range of physicochemical properties can be monitored, targeted strategies use specific and optimized sample extraction techniques customized based on the physiochemical properties of the analytes in question to achieve the best possible specificity and sensitivity for the specific metabolites of interest [5]. Moreover, such physiochemical properties are also taken into account during analytical method development including mobile phase and column selection as well as design of the elution gradient. In contrast, as aforementioned, to achieve their purpose, non-targeted, non-biased strategies have to use a “one size fits all” analytical approach, which results in less specificity and sensitivity than more targeted approaches, negatively affecting the quality of data. Targeted metabolomics assays are often quantitative and can be validated to a much larger extent whereas non-targeted assays typically generate semi-quantitative data. Nevertheless, non-targeted assays are often used as a first screening assay in clinical biomarker discovery studies and only those metabolites that show significant differences or changes are further confirmed using targeted, quantitative assays [1].
The kidney plays a central role in the metabolism of amino acids and control of plasma concentrations [6]. As a result, samples with varying degrees of kidney function were analyzed. Here we present a direct comparison of circulating amino acid concentrations in 398 EDTA plasma samples prospectively, longitudinally collected from 116 patients with good and impaired kidney function before and until 6 months after de novo kidney transplantation as measured in parallel with a semi-quantitative, non-targeted metabolomics LC-MS/MS assay [7] and a targeted, commercial LC-MS/MS assay kit specifically developed to quantify amino acids in human plasma.
2. Materials and Methods
2.1. Study Population
Samples were collected during a prospective, phase 3b interventional, multi-center, parallel group, randomized, open-label clinical trial to compare the efficacy and safety of concentration-controlled everolimus with low-dose tacrolimus versus mycophenolate mofetil (MMF) with standard-dose tacrolimus to establish clinical non-inferiority (CRAD001AUS92, Novartis Pharmaceuticals, East Hanover, NJ). One-hundred and twenty of the subjects enrolled agreed to participate in the present biomarker sub-study. Four of these patients were excluded from the analysis due to incomplete sample sets. All clinical study protocols and their amendments were reviewed and approved by the study centers’ appropriate institutional review board. All patients gave their written informed consent. The study followed all applicable regulatory, institutional, national and international rules and regulations of good clinical practices (GCP) and was in compliance with the Declaration of Helsinki and its amendments. This clinical trial was monitored by a Drug Safety Monitoring Board and was registered at clinicaltrials.gov (NCT01025817).
After informed consent, samples were collected from each patient prior to transplantation (baseline), as well as 28 days, 2, 4 and 6 months post-transplant. Eight mL of K2-EDTA blood was collected and plasma was isolated by standard procedures. As stability of plasma samples is of concern, handling procedures to preserve integrity of the samples were employed [8–10] and all clinical personnel handling these samples was trained accordingly. Plasma was aliquoted and stored at −80°C within 4 hours after collection. In the meantime, samples were kept on ice or in a refrigerator at +4°C. Samples were shipped to the bioanalytical laboratory overnight on dry ice, where they were also kept at −80°C until analysis.
2.2. Targeted Analysis of Twenty-four Amino Acids in EDTA Plasma
Amino acid analysis in EDTA plasma samples was performed using the EZ: faast kit purchased from Phenomenex (Torrance, CA) following the manufacturer’s instructions. The EZ: faast amino acid analysis procedure involves a solid phase extraction step, derivatization and subsequent liquid/liquid extraction of the derivatized amino acids. The derivatized samples were then analyzed using LC-MS/MS.
2.2.1. Preparation of Standards.
The EZ: faast kit contained an internal standard mix consisting of homo-arginine, methionine-d3 and homo-phenylalanine at concentrations of 200 μmol/L and was added to each sample during extraction. In addition, two calibrator solutions were also received. Standard solution one contained: alpha-aminoadipic acid, cystine, 4-hydroxy proline, 1-methyl histidine, threonine, lysine, proline, aspartic acid, glycine, methionine, sarcosine, histidine, citrulline, isoleucine, ornithine, tyrosine, alanine, leucine, phenylalanine, valine, arginine, glutamic acid, and serine at concentrations of 200 μmol/L. Standard solution two contained: asparagine, glutamine and tryptophan, also at a concentration of 200 μmol/L, and was provided separately due to differences in stability. Using these standard mixes, calibrators were prepared at the following concentrations: 0.001, 0.01, 0.1, 0.5, 1, 5, 25, 100 and 200 μmol/L. Calibration curves were constructed by plotting the peak area ratios of the corresponding analyte and internal standard against nominal analyte concentrations of the aforementioned calibrators.
2.2.2. Extraction Procedure.
Samples were first diluted 1/5 (v/v) with HPLC-grade water (20 µL of plasma into 80 µL of water) and combined with 100 µL of internal standard solution. Solid phase extraction was carried out using sorbent packed tips that bind amino acids while interfering compounds are not retained. Following a wash step with 200 µL of N-propanol, amino acids were eluted with 200 µL of NaOH / N-propanol, 3/2, v/v) into a sample vial. Samples were then derivatized with 50 µL of propyl chloroformate at room temperature in aqueous solution for 1 min. The derivatization reaction is shown in Supplementary Figure 1. Hereafter, 100 µL isooctane was added, the sample was vortexed and allowed to react for an additional minute, during which the derivatized amino acids migrated into the organic layer for additional separation from interfering compounds. The organic layer was then removed, evaporated, and re-dissolved in aqueous mobile phase and analyzed on an LC/MS-MS system.
2.2.5. LC-MS/MS Analysis.
Samples were analyzed using an Agilent 1100 series HPLC system consisting of a G1312 binary pump, a G1322A vacuum degasser, and a G1316A thermostated column compartment (Agilent Technologies, Palo Alto, CA) in combination with a Leap CTC PAL auto sampler (Carrboro, NC). The HPLC system was interfaced with an ABSciex 4000 triple quadrupole mass spectrometer (Foster City, CA) operating with an electrospray ionization source (ESI) using nitrogen (purity: 99.99%). Five µL of the extracted sample were injected onto a 3.0 × 250 mm EZ: faast AAA-MS column 4 µm particle size, Phenomenex (Torrance, CA). The starting mobile phase concentrations were 68% 10 mM ammonium formate in methanol and 32% 10 mM ammonium formate buffer (aqueous) with a flow of 0.5 mL/min. The analytical column was kept at 25°C. A gradient from 32% to 83% organic mobile phase was run over 13.0 min. The column was then re-equilibrated for 4 min to starting conditions. The mass spectrometer was run in the multiple reaction monitoring (MRM) mode with the interface heated to 425°C. Nitrogen of >99.999% purity was used as Collision Activated Dissociation (CAD) and curtain gas. A list of the MRM transitions for each amino acid as well as a representative total ion chromatogram (TIC) are shown in Supplementary Table 1 and Supplementary Figure 2, respectively.
2.3. Non-targeted Metabolomics in EDTA Plasma
Plasma metabolomics profiles were assessed using a previously described semi-quantitative LC-MS/MS assay platform [7].
2.3.1. Extraction Procedure.
Fifty µL of plasma were combined with 30 µL of internal standard solution and 270 µL of methanol. Samples were vortexed for 10 minutes and then centrifuged for 10 minutes at 13 400·g at 4°C. Three hundred µL of supernatant was transferred to glass HPLC auto sampler vials.
2.3.2. LC-MS/MS Analysis.
Samples were analyzed using an Agilent 1100 series HPLC system consisting of a G1312 binary pump, a G1322A vacuum degasser, and a G1316A thermostated column compartment (Agilent Technologies, Palo Alto, CA). The HPLC system was interfaced with an ABSciex 5500 hybrid triple quadrupole/ linear ion trap mass spectrometer (Concord, ON, Canada) equipped with an ESI source operating in the positive/negative switch mode using nitrogen (purity: 99.99%). Eight microliters of the extracted sample were injected onto a 3.0 × 150 mm Luna hydrophilic interaction liquid chromatography (HILIC) column (3 µm particle size, Phenomenex, Torrance, CA). The mobile phases consisted of acetonitrile and 5 mM ammonium acetate in 95/5 (v/v) water/ acetonitrile supplemented with 20 mM ammonium hydroxide. The gradient began at 99% acetonitrile and was kept at starting conditions for 2 minutes. Hereafter, acetonitrile was decreased to 50% over the next 9.5 minutes and then decreased to 1% for the next 2.2 mins. These conditions were held for another 1.2 min and then the column was re-equilibrated for 3 minutes at starting conditions. The flow rate was 0.5 mL/ min. The analytical column was kept at 35°C. A list of the MRM transitions for the metabolites monitored and their ionization modes are shown in Supplementary Table 2.
2.3.3. Unsupervised Peak Integration.
As described in [4] no internal standards were used and metabolite were integrated and identified using AB Sciex MultiQuant (version 3.0.1). Reference [4] states that the largest peak of a specific ion transition was found to be correct for approximately 80–90% of the metabolites. Peaks were integrated and results were reported as peak areas in counts per seconds (cps). Results were used in the statistical analysis as automatically generated by the MultiQuant software without corrections, modifications and further verification (unsupervised) [4].
2.3.4. Supervised Peak Integration.
Other than in the unsupervised analysis, quantification was based on the internal standard methionine-d3, which was added in 0.1% aqueous formic acid containing a concentration of 50 µmol/L before extraction. Peaks were integrated using MultiQuant and the analyte/ internal standard peak areas [cps/cps] were calculated and used in the statistical analysis. Amino acid standard solutions as provided in the Phenomenex EZ-faast amino acid kit were injected to verify retention times. All ion chromatograms were reviewed to ensure that amino acid peaks were correctly identified and integrated.
2.4. Biostatistical Analysis
The goal of the biostatistical analysis was to assess potential differences of plasma amino acid patterns in samples from de novo kidney transplant patients with good and impaired renal function. The data was divided into two sets based on estimated glomerular filtration rates (eGFR) calculated using the Modification of Diet in Renal Disease (MDRD) equation [8]. Based on the RIFLE classification [9], an eGFR cut off of 60 mL/min/1.73m2 was used. Data sets based on samples from patients with eGFRs ≥ 60 mL/min/1.73m2 (n= 134) and < 60 mL/min/1.73m2 (n=264) were compared. The median eGFRs of the patients from which samples were assigned to the two data sets were 72.4 mL/min/1.73m2 (interquartile range: 65.7–84.0 mL/min/1.73m2) and 38.1 mL/min/1.73m2 (11.4– 49.6 mL/min/1.73m2). Individual amino acids between the two data sets were compared using two-sided independent t-tests. Since not normally distributed, data was log-transformed before statistical comparison. To adjust for multiple testing, alpha was Bonferroni corrected. Statistical analyses were carried out using the R software (version 3.1.2., The R Foundation for Statistical Computing, Vienna, Austria) and SPSS software (version 23.0, SPSS/IBM, Chicago, IL). Method comparison was carried out using MedCalc (version 16.2.0, MedCalc Software, Ostend, Belgium).
Moreover, the amino acid patterns generated by each of the three analysis strategies (targeted, non-targeted supervised, and non-targeted unsupervised) were independently analyzed using Markerview 1.2.1 (ABSciex, Foster City, CA) and MetaboAnalyst 3.0 [13,14]. This included, but was not limited to, partial least squares- discriminant analysis (PLS-DA [15]), PLS Variable Importance for Projection (VIP) statistics [16] and Pearson regression analysis.
3. Results
The results of the analytical strategies and their distribution statistics in the two data sets from patients with good and impaired kidney function are shown in Supplementary Table 3. The results of the amino acid concentrations measured using the targeted assay in samples from patients with eGFRs ≥ 60 mL/min/1.73m2 were comparable to those described for healthy adults in the literature [17–20] (please also see Supplementary Table 4), with the exception of arginine, the concentration of which appears to be lower. This is also consistent with the literature. It has been described before that transplant patients seem to be arginine-deficient and it has been hypothesized that, at least in part, this is associated with immunosuppressive drug treatment [21]. As these were pooled data sets from kidney transplant patients treated with two different immunosuppressant drug regimens (standard dose tacrolimus+ MMF or reduced dose tacrolimus+ everolimus), a potential treatment effect was assessed and no difference was found for any of the amino acids.
When the amino acid patterns measured using the targeted assay in samples from kidney transplant patients with good and impaired renal function were compared, the concentrations of 1-methyl histidine, 4-hydroxy proline, citrulline and cystine were significantly higher and that of tryptophan was significantly lower in samples from patients with impaired than in patients with good renal function (after Bonferroni correction of alpha, Table 1). 1-Methyl histidine, citrulline and cystine were also higher and tryptophan lower in samples from patients from impaired than in those from patients with good renal function when the non-targeted assay was used (Table 1). Nevertheless, there were several differences. In the supervised analysis of the amino acid MS/MS responses, in addition, the following amino acids were found different: aminoadipic acid was higher whereas alanine, lysine, methionine, proline, sarcosine, tyrosine and valine were lower in samples from patients with impaired than from patients with good renal function. Hydroxy proline, which was found higher in patients with eGFRs < 60 mL/min/1.73m2 than in samples from patients with eGFRs ≥ 60 mL/min/1.73m2 using the targeted assay was not different when the non-targeted assay was used. As shown in Table 1, there were further differences when the results of the supervised and unsupervised analyses of the results generated were compared with the non-targeted assay.
Table 1. Comparison of amino acid patterns in EDTA plasma samples from de novo kidney transplant patients with an eGFR ≥ 60 mL/min/1.73m2 (n=134) and eGFR < 60 mL/min/1.73m2 (n=264).
The distributions statistics is shown in detail in Supplementary Table 2. The geometric mean ratios are the geometric means of patients with eGFR < 60 mL/min/1.73m2 / eGFR ≥ 60 mL/min/1.73m2. Statistical comparison was carried out using two-sided independent t-test comparing patients in the groups with eGFRs ≥ 60 and < 60 mL/min/1.73m2 based after log-transformation. P-values in bold are significant after Bonferroni correction of alpha for multiple testing.
| Targeted | Non-targeted | |||||
|---|---|---|---|---|---|---|
| supervised | unsupervised | |||||
| Amino Acid | Geometric mean ratio [%] | p< | Geometric mean ratio [%] | p< | Geometric mean ratio [%] | p< |
| 1-Methyl histidine | 318.0 | 0.0001 | 219.8 | 0.0001 | 227.3 | 0.0001 |
| 4-Hydroxy proline | 219.7 | 0.0001 | 107.3 | 0.145 | 98.3 | 0.742 |
| Aminoadipic acid | 109.8 | 0.337 | 147.5 | 0.0001 | 78.4 | 0.0001 |
| Alanine | 91.2 | 0.118 | 84.9 | 0.306 | 207.5 | 0.0001 |
| Arginine | 79.8 | 0.006 | 86.2 | 0.003 | 199.5 | 0.0001 |
| Asparagine | 103.3 | 0.634 | 97.0 | 0.241 | 108.1 | 0.917 |
| Aspartate | 121.2 | 0.065 | NA | NA | 90.2 | 0.432 |
| Citrulline | 129.7 | 0.0001 | 122.6 | 0.0001 | 114.5 | 0.001 |
| Cystine | 131.3 | 0.0001 | 135.6 | 0.0001 | 126.5 | 0.002 |
| Glutamate | 99.6 | 0.953 | 92.6 | 0.096 | 85.8 | 0.077 |
| Glutamine | 97.5 | 0.336 | 96.0 | 0.027 | 89.5 | 0.001 |
| Histidine | 115.6 | 0.252 | 94.0 | 0.154 | 97.8 | 0.845 |
| Leucine | 100.7 | 0.914 | 98.4 | 0.341 | 90.5 | 0.001 |
| Lysine | 97.5 | 0.606 | 86.9 | 0.0001 | 115.9 | 0.716 |
| Methionine | 98.8 | 0.842 | 90.4 | 0.001 | 81.9 | 0.0001 |
| Ornithine | 93.9 | 0.443 | 91.5 | 0.014 | 82.4 | 0.025 |
| Phenylalanine | 103.3 | 0.585 | 94.8 | 0.012 | 81.9 | 0.017 |
| Proline | 98.3 | 0.713 | 94.5 | 0.0001 | 87.5 | 0.0001 |
| Sarcosine | 85.0 | 0.243 | 84.6 | 0.0001 | 81.2 | 0.459 |
| Serine | 96.9 | 0.561 | 91.5 | 0.005 | 87.5 | 0.775 |
| Threonine | 96.4 | 0.564 | 94.0 | 0.055 | 116.3 | 0.904 |
| Tryptophan | 73.7 | 0.0001 | 66.8 | 0.0001 | 61.7 | 0.0001 |
| Tyrosine | 84.9 | 0.029 | 78.0 | 0.0001 | 72.1 | 0.0001 |
| Valine | 99.6 | 0.956 | 84.4 | 0.001 | 139.3 | 0.611 |
Moreover, there were differences when the amino acid patterns were correlated with eGFR (Table 2). The five amino acids showing the best correlations (positive or inverse) after sample analysis using the targeted assay were tryptophan, 1-methyl histidine, hydroxy proline, citrulline and tyrosine. After analysis using the non-targeted assay (supervised) those were alanine, sarcosine, tryptophan, tyrosine and valine. Again, there were also differences when the correlation patterns after supervised and non-supervised analysis of data generated by the non-targeted assay were compared (Table 2). Only tryptophan and tyrosine were among the five amino acids with the best correlations in all three data sets.
Table 2. Comparison of the correlations of amino acid patterns in the different assays with eGFRs (n=398).
Data was analyzed using Pearson correlation as implemented in MetaboAnalyst 3.0 [10,11]. The amino acids are ranked based on correlation coefficients (r).
| Targeted | Non-targeted | Non-targeted | ||||||
|---|---|---|---|---|---|---|---|---|
| supervised | unsupervised | |||||||
| Amino acid | r | p< | Amino acid | r | p< | Amino acid | r | p< |
| Tryptophan | 0.448 | 0 | Alanine | 0.472 | 0 | Alanine | 0.456 | 0 |
| 1-Methyl histidine | −0.489 | 2.48E-25 | Sarcosine | 0.477 | 0 | Leucine | 0.465 | 0 |
| 4-Hydroxy proline | −0.355 | 2.83E-13 | Tryptophan | 0.727 | 0 | Proline | 0.416 | 0 |
| Citrulline | −0.334 | 8.33E-12 | Tyrosine | 0.609 | 0 | Tryptophan | 0.677 | 0 |
| Tyrosine | 0.307 | 1.00E-04 | Valine | 0.479 | 0 | Tyrosine | 0.566 | 0 |
| Serine | 0.183 | 0.0002 | 1-Methyl histidine | −0.741 | 1.29E-70 | Aminoadipic acid | −0.584 | 1.06E-37 |
| Alanine | 0.182 | 0.0003 | Citrulline | −0.504 | 5.69E-27 | Arginine | −0.469 | 3.28E-23 |
| Methionine | 0.160 | 0.0014 | Methionine | 0.352 | 4.74E-13 | Sarcosine | 0.384 | 1.78E-15 |
| Sarcosine | 0.158 | 0.0016 | Serine | 0.265 | 8.06E-08 | Methionine | 0.382 | 3.11E-15 |
| Threonine | 0.156 | 0.0018 | Ornithine | 0.249 | 4.70E-07 | Citrulline | −0.356 | 2.30E-13 |
| Cystine | −0.149 | 0.0029 | Threonine | 0.249 | 4.91E-07 | Phenylalanine | 0.348 | 8.69E-13 |
| Ornithine | 0.130 | 0.0094 | Lysine | 0.242 | 1.00E-06 | Valine | 0.329 | 1.69E-11 |
| Lysine | 0.119 | 0.0179 | Arginine | 0.235 | 2.13E-06 | 1-Methyl histidine | −0.296 | 1.78E-09 |
| Glutamine | 0.113 | 0.0243 | Phenylalanine | 0.235 | 2.17E-06 | Glutamine | 0.277 | 1.97E-08 |
| Valine | 0.104 | 0.0386 | Asparagine | 0.198 | 6.86E-05 | Ornithine | 0.267 | 6.55E-08 |
| Histidine | −0.080 | 0.1103 | Glutamine | 0.195 | 9.21E-05 | Serine | 0.165 | 0.00093 |
| Asparagine | 0.071 | 0.1587 | Proline | 0.174 | 0.00050 | Lysine | 0.156 | 0.00185 |
| Leucine | 0.067 | 0.1817 | Aminoadipic acid | −0.171 | 0.00060 | Glutamate | 0.143 | 0.00426 |
| Aminoadipic acid | −0.059 | 0.2374 | Cystine | −0.167 | 0.00083 | Cystine | −0.133 | 0.00771 |
| Phenylalanine | 0.053 | 0.2917 | Leucine-Isoleucine | 0.068 | 0.17583 | 4-Hydroxy proline | 0.108 | 0.03167 |
| Proline | 0.016 | 0.7522 | Glutamate | 0.051 | 0.30611 | Threonine | 0.107 | 0.03365 |
| Glutamate | −0.004 | 0.9396 | 4-Hydroxy proline | −0.045 | 0.37032 | Histidine | 0.091 | 0.06841 |
| Arginine | 0.004 | 0.9408 | Histidine | 0.043 | 0.38677 | Asparagine | 0.061 | 0.22416 |
Principal components analysis and partial least square models are frequently used for the analysis of complex metabolomics data sets [13], including the analysis of amino acid patterns [15]. VIP plot analyses, which are based upon PLS models, to assess the contribution of each predictor [16] are shown in Figure 1 and PLS-DA analysis in Supplementary Figure 2. A VIP score of 0.8 has been considered the cut-off between meaningful factors and those that can be eliminated from the model [16]. As shown in Figure 1, the VIP scores for amino acids based on the three analysis strategies differ. Analysis of the targeted data in the sample sets collected from patients with eGFRs ≥ 60 and < 60 mL/min/1.73m2 indicated that only three amino acids, hydroxy proline, 1-methyl histidine and alanine had VIP score >0.8, whereas hydroxy proline had the highest score (Figure 1). This was different for the non-targeted, supervised analysis, which showed eight amino acids with VIP scores > 0.8, but with hydroxy proline scoring low. The eight amino acids with >0.8 for the non-targeted, unsupervised analysis mostly matched those of the supervised analysis, albeit the scores were different. Aminoadipic acid scored >0.8 in the unsupervised, but not in the supervised analysis. On the other hand, citrulline had a VIP score of >0.8 in the supervised, but not in the unsupervised analysis.
Figure 1. Comparison of PLS Variable Importance for Projection (VIP) statistics.
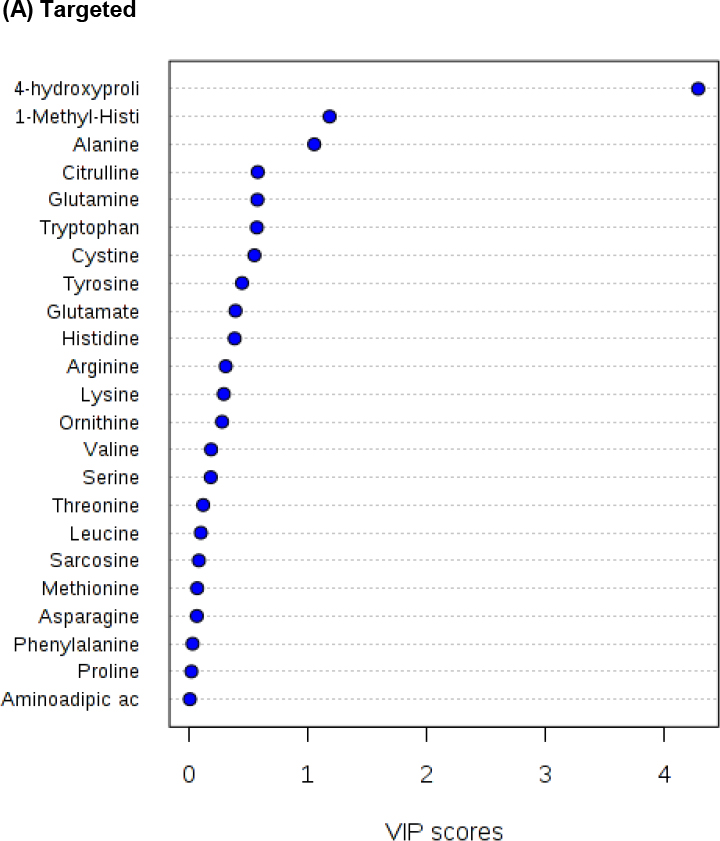
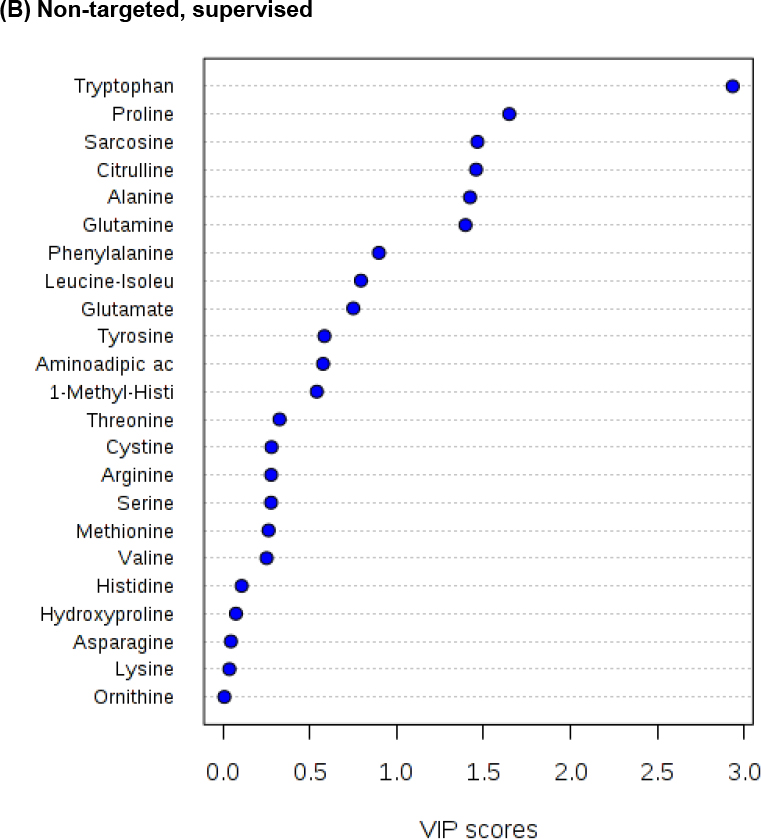

The VIP plot analyzes the contribution of each predictor by fitting the PLS model for both predictors and response, thus summarizing the contribution that each variable makes to the model [13]. A value of < 0.8 is considered small and makes the factor a candidate for deletion from the model [13]. The plots were generated using MetaboAnalyst 3.0 [10,11]. The PLS-DA analyses are compared in Supplementary Figure 2.
The results showed that there were substantial differences between the results of the three analysis strategies. While the analysis of the targeted data set consistently pointed towards 1-methyl histidine and hydroxy proline as being the amino acids most strongly associated with eGFR, overall tryptophan and proline showed the best association in the non-targeted data sets (please see Tables 1, 2 and Figure 1). As a next step, the data sets were further examined to explain said discrepancies. As shown in Supplementary Figure 3A, for selected amino acids, the non-targeted assay lost sensitivity with the number of injections. While this was the case for serine, this was not the case, for example, for tryptophan (Supplementary Figure 3A). Another amino acid that lost sensitivity during the analysis of a sample batch was threonine, while the response of internal standard methionine-d3 was stable. For the affected amino acids, this led to several results below the lower limit of quantification, which is physiologically unrealistic in plasma and introduced bias into the analysis depending on the position of the sample in the analytical sequence. Another problem were amino acids with shifting retention times, such as alanine, and amino acids that resulted in MS/MS responses above the linear range of the detector, such as leucine-isoleucine (Supplementary Figure 3B). Other amino acid signals, especially aspartate (Supplementary Figure 3C), did not result in peaks that could be distinguished from background and interferences. Nevertheless, the MultiQuant software, if unsupervised, returned a result. Therefore, in the supervised, non-targeted analysis, aspartate was not reported (please see Supplementary Table 2B). As aforementioned, one of the most notable discrepancies between the analyses of the data generated with the targeted and non-targeted assays is hydroxy proline. None of the factors as shown in Supplementary Figure 3 were found to be involved. In fact, in the non-targeted assay, hydroxy proline resulted in a well separated peak that could reliably be integrated (Supplementary Figure 4). Nevertheless, there was no correlation between the MS/MS response in the non-targeted assay and the hydroxy proline concentration measured using the targeted assay. As the scatterplot in Supplementary Figure 4 indicates, numerous samples with hydroxy proline concentrations at the lower end of the calibration curve in the targeted assay that had relatively high MS/MS detector responses in the non-targeted assay. The amino acid that had the highest correlation coefficient (r) between concentrations measured in the targeted and MS/MS detector responses in the non-targeted assay was tryptophan with r= 0.224 (p< 0.0001, Supplementary Figure 4). The results for tryptophan were consistent across the analyses of the three data sets (Tables 1, 2 and Figure 1).
4. Discussion
The differences between targeted and non-targeted assays, including their limitations, has frequently been discussed in reviews, however, there is surprisingly little experimental data directly and systematically comparing the results and conclusions drawn based on different bioanalytical metabolomics strategies [22].
Ideally, metabolomics comprehensively measures the entire metabolome, which usually constitutes a mostly undefined set of molecules [1,22–25]. Targeted metabolomics refers to an assay that focuses on one or several related compounds of interest [25], in the present case amino acids. On the other hand, there are several levels of non-targeted metabolomics assay and it is a matter of discussion when an assay can be referred to as a non-targeted metabolomics assay [22]. These can range from “finger printing” assays, which try to capture as many metabolite signals as possible, sometimes thousands, to multi-analyte assays such as the assay described by Yuan et al. [7], which is based on monitoring MS/MS ion transitions and focuses on a known set of 258 metabolites. The latter assay was chosen for several reasons. It is a frequently cited metabolomics assay that due to the use of MS/MS transitions allows for comparison with the targeted amino acid assay. Moreover, the limited number of metabolites facilitated the supervised peak identification and integration approach as well as the assessment of the explanations for the discrepancies between the different data sets. One of the major differences between the targeted and non-targeted assays in the present study were the extraction procedures. The Phenomenex EZ: faast extraction procedure achieves specificity of the extraction mainly through a combination of solid phase extraction using a specifically designed, proprietary sorbent cartridge [26], the derivatization reaction and the extraction of the derivatized amino acids into isooctane. In comparison, to retain as many different metabolites as possible, the non-targeted assay relied on a simple methanol protein precipitation and centrifugation step [7]. As discussed in [7], the non-targeted, unsupervised assay did not include internal standards, relied on the knowledge that for 80–90% of the metabolites the largest peak of a specific ion transition was found to be correct [7] and data analysis was automatically carried out by the MultiQuant software. In the supervised version of the assay, retention times after injection of a reference amino acid mixture were taken into account, all peak areas were normalized to the internal standard methionine-d3 and all extracted ion chromatograms were checked for correct integration as recommended by [22]. Even when the integration of the non-targeted assay was supervised, there were substantial differences in comparison to the targeted results. The major reasons for those were found to be the selective decrease in sensitivity of several amino acids during the analytical run, retention time shifts and MS/MS detector responses outside of the linear range. Yuan et al. [7] already mentioned that source contamination, likely due to the relatively unselective extraction procedure, is a problem with the assay and that routine source cleaning is recommended. To monitor sensitivity of the assay, we included an internal standard (methionine-d3). As internal standards are always added to each sample at the same concentrations, they are well suited to monitor decline of the mass spectrometry signal. Moreover, none of the runs measured more than 48 samples in one batch. The source was always cleaned between runs. However, the observations that sensitivity was selectively lost for a few specific amino acids while the internal standard signal remained stable and that this decline in sensitivity could occur as early as after 10 injections were unexpected. The targeted assay with its more selective, multiple step extraction procedure did not have this problem. An additional problem with the unsupervised approach was the incorrect integration and identification of peaks, such as aspartate. Although no obvious explanation for the discrepancy of the hydroxy proline results between the targeted and non-targeted assays was found, based on the scatter plot in Supplementary Figure 4 suggesting that the untargeted assay overestimated hydroxyl proline, matrix effects and/or interferences seem potential reasons. These are issues that a validation following commonly accepted recommendations such as [27,28] would detect [2].
The three data sets (targeted, non-targeted supervised, non-targeted unsupervised) were independently analyzed using a common biostatistical approach. False positive results of non-targeted assays are a well-known problem and thus further confirmation of “hits”, for example by injection of and comparison with authentic reference compounds and/or sample re-analyses using a targeted assay, is required [22,23,25]. Indeed, the non-targeted metabolomics assay in the present study yielded more hits than the targeted assay. These are likely false positives and this would have been detected in the established workflow of a metabolomics discovery study [22,23,25]. However, as our results indicate, false negatives are a problem that the standard metabolomics workflow cannot reconcile and that would have led to missing important associations between specific amino acids and kidney function. An example is hydroxy proline, which was the amino acid with the highest VIP score when targeted amino acid data was analyzed in the present study, but did not have a significant influence in the models based on untargeted data (Figure 1). Notwithstanding these differences, there were also consistencies between the bioanalytical approaches, such as tryptophan.
The present study can be compared to that published by Duranton et al. [29] assessing amino acid profiles in plasma and urine of patients in different stages of chronic kidney disease using LC-MS/MS in combination with the Biocrates AbsoluteIDQ p180 kit (Biocrates, Innsbruck, Austria). Like in the present study, in plasma, citrulline concentrations were found higher and tryptophan concentrations lower in patients with higher CKD stages (4–5) than in patients with better kidney function (CKD stages 2–3), and plasma concentrations of both citrulline (inversely) and tryptophan (positively) correlated with eGFR [29]. 1-Methyl histidine and hydroxyl proline concentrations were not reported in this study [29].
Comprehensive profiling of amino acid patterns in plasma from de novo transplant patients has not been described yet. The results of the present study indicate that 1-methyl histidine, 4-hydroxy proline, citrulline, cystine and tryptophan plasma concentrations are associated with eGFR in these patients and further follow up to assess potential clinical and diagnostic importance of these findings may be worthwhile.
5. Conclusions
In the present study, the effects of renal function in de novo kidney transplant patients on plasma amino acid patterns as assessed using three metabolomics bioanalytical strategies (targeted, non-targeted supervised, non-targeted unsupervised [7]) were compared. Although there was some overlap among the results, there were also substantial inconsistencies, with the non-targeted assay resulting in more “hits” than the targeted assay. Without further verification of the hits detected by the non-targeted discovery assay, this would have led to different interpretation of the study results and conclusions. Nevertheless, there were also false negative results, such as hydroxy proline, when the non-targeted assay was used. Using recommended metabolomics workflows, false positive results are detected during further verification of the hits of a non-targeted assay. False negative results, however, are more problematic as these lead to potentially important markers being overlooked. The results of the present study support the concept that, whenever assessment of a specific pathway is the focus of interest, a targeted is preferable to a non-targeted metabolomics assay [1], even if, as in the present study, this is an assay using MS/MS ion transitions and supervised peak identification and integration, which was more focused than many other non-targeted metabolomics assay strategies [22].
Supplementary Material
6. Acknowledgments
This study was supported by Novartis Pharmaceuticals (East Hanover, NJ) and the National Institutes of Health, grant NIH/NICHD R01 HD070511.
Non-standard Abbreviations
- CAD
collision-activated dissociation
- CKD
chronic kidney disease
- cps
counts per second
- EDTA
ethylenediaminetetraacetic acid
- eGFR
estimated glomerular filtration rate
- ESI
electrospray ionization
- GCP
good clinical practices
- HILIC
hydrophilic interaction liquid chromatography
- MDRD
modification of diet in renal disease
- MMF
mycophenolate mofetil
- MRM
multi-reaction monitoring
- PLS-DA
partial least squares- discriminant analysis
- TIC
total ion count
- VIP
variable importance in the projection partial least squares
- v/v
volume by volume
7. References
- 1.Christians U, Klawitter J, Hornberger A. How unbiased is non-targeted metabolomics and is targeted pathway screening the solution? Curr Pharm Biotechnol 2011; 12 (7): 1053–66. [DOI] [PubMed] [Google Scholar]
- 2.Naz S, Vallejo M, Garcia A, Barbas C. Method validation strategies involved in non-targeted metabolomics. J Chromatogr A 2014; 1353: 99–105. [DOI] [PubMed] [Google Scholar]
- 3.Bajad SU, Kimball EH, Yuan J. Separation and quantitation of water soluble cellular metabolites by hydrophilic interaction chromatography-tandem mass spectrometry. J Chromatogr A 2006; 1125: 76–88. [DOI] [PubMed] [Google Scholar]
- 4.Yoshida H, Mizukoshi T, Hirayama K. Comprehensive Analytical Method for the Determination of Hydrophilic Metabolites by High-Performance Liquid Chromatography and Mass Spectrometry. J Agric Food Chem. 2007. February 7;55(3):551–60 [DOI] [PubMed] [Google Scholar]
- 5.Christians U, Klepacki J, Shokati T, Klawitter J, Klawitter J. Mass spectrometry-based multiplexing for the analysis of biomarkers in drug development and clinical diagnostics-how much is too much? Microchem J 2012; 105: 32–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruza CC, Hernanz A, Madero R. Plasmatic amino acids in kidney transplantation in children. Clin Transplant 1998;12:445–53 [PubMed] [Google Scholar]
- 7.Yuan M, Breitkopf SB, Yang X, Asara JM. A positive/negative ion-switching, targeted mass spectrometry-based metabolomics platform for bodily fluids, cells, and fresh and fixed tissue. Nat Protoc 2012; 7(5): 872–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perry TL, Hansen S. Technical pitfalls leading to errors in the quantitation of plasma amino acids. Clin Chim Acta 1969; 25(1): 53–8. [DOI] [PubMed] [Google Scholar]
- 9.Davis JS, Darcy CJ, Piera K, McNeil YR, Woodberry T, Anstey NM. Ex-vivo changes in amino acid concentrations from blood stored at room temperature or on ice: implications for arginine and taurine measurements. BMC Clin Pathol 2009; 9: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takehana S, Yoshida H, Ozawa S, Yamazaki J, Shimbo K, Nakayama A, et al. The effects of pre-analysis sample handling on human plasma amino acid concentrations. Clin Chim Acta 2016; 455: 68–74. [DOI] [PubMed] [Google Scholar]
- 11.Coresh J, Astor BC, Greene T, Eknoyan G, Levey AS. Prevalence of chronic kidney disease and decreased kidney function in adult US population: Third rrnational health and nutrition examination survey. Am J Kidney Dis 2003; 41(1): 1–12. [DOI] [PubMed] [Google Scholar]
- 12. Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P , for the Workgroup ATNA: Acute renal failure— Definition, outcome measures, animal models, fluid therapy and information technology needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 2004; 8(4): R205–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xia J, Sinelnikov IV, Han B, Wishart DS. MetaboAnalyst 3.0--making metabolomics more meaningful. Nucleic Acids Res 2015; 43(W1): W251–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MetaboAnalyst: http://www.metaboanalyst.ca (last accessed 03/01/2016)
- 15.Jellum E, Harboe M, Bjune G, Wold S. Interpreting complicated chromatographic patterns. J Pharm Biomed Anal 1991; 9(8): 663–9. [DOI] [PubMed] [Google Scholar]
- 16.Wold S PLS for multivariate linear modeling, In: van de Waterbeemd H. Chemometric methods in molecular design. Methods and principles in medicinal chemistry, Weinheim (Germany): Verlag-Chemie; 1994: p. 195–218. [Google Scholar]
- 17.Stegink LD, Filer LJ Jr, Brummel MC, Baker GL, Krause WL, Bell EF, Ziegler EE. Plasma amino acid concentrations and amino acid ratios in normal adults and adults heterozygous for phenylketonuria ingesting a hamburger and milk shake meal. Am J Clin Nutr 1991; 53(3): 670–5. [DOI] [PubMed] [Google Scholar]
- 18.Laidlaw SA, Berg RL, Kopple JD, Naito H, Walker WG, Walser M. Patterns of fasting plasma amino acid levels in chronic renal insufficiency: results from the feasibility phase of the Modification of Diet in Renal Disease Study. Am J Kidney Dis 1994; 23(4): 504–13. [DOI] [PubMed] [Google Scholar]
- 19.Zhou Y, Qiu L, Xiao Q, Wang Y, Meng X, Xu R, et al. Obesity and diabetes related plasma amino acid alterations. Clin Biochem 2013; 46(15): 1447–52. [DOI] [PubMed] [Google Scholar]
- 20.Hakuno D, Hamba Y, Toya T, Adachi T. Plasma amino acid profiling identifies specific amino acid associations with cardiovascular function in patients with systolic heart failure. PLoS One 2015; 10(2): e0117325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andrade F, Rodríguez-Soriano J, Prieto JA, Elorz J, Aguirre M, Ariceta G,Martin S, et al. The arginine-creatine pathway is disturbed in children and adolescents with renal transplants. Pediatr Res. 2008; 64: 218–22. [DOI] [PubMed] [Google Scholar]
- 22.Theodoridis GA, Gika HG, Want EJ, Wilson ID. Liquid chromatography-mass spectrometry based global metabolite profiling: a review. Anal Chim Acta. 2012; 711: 7–16. [DOI] [PubMed] [Google Scholar]
- 23.Zamboni N, Saghatelian A, Patti GJ. Defining the metabolome: size, flux, and regulation. Mol Cell 2015; 58(4): 699–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alonso A, Marsal S, Julià A. Analytical methods in untargeted metabolomics: state of the art in 2015. Front Bioeng Biotechnol. 2015; 3: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patti GJ, Yanes O, Siuzdak G. Innovation: Metabolomics: the apogee of the omics trilogy. Nat Rev Mol Cell Biol. 2012; 13(4): 263–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Husek P Sorbent cartridge for solid phase extraction. United States Patent US 6,770,246B1, 2004.
- 27.CLSI. Chromatography-mass spectrometry methods; approved guideline CLSI document C62-A. Wayne, PA: Clinical Laboratory Standards Institute, 2014. [Google Scholar]
- 28.Sargent M (ed.), Guide to achieving reliable quantitative LC-MS measurements, RSC Analytical Methods Committee, 2013. ISBN 978–0-948926–27-3. [Google Scholar]
- 29.Duranton F, Lundin U, Gayrard N, Mischak H, Aparicio M, Mourad G, Daurès JP, Weinberger KM, Argilés A. Plasma and urinary amino acid metabolomic profiling in patients with different levels of kidney function. Clin J Am Soc Nephrol 2014; 9(1): 37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


