Abstract
Background
Genetic testing (GT) for prostate cancer (PCA) is rising, with limited insights regarding genetic counseling (GC) needs of males. Genetic Evaluation of Men (GEM) is a prospective multigene testing study for inherited PCA. Men undergoing GC were surveyed on knowledge of cancer risk and genetics (CRG) and understanding of personal GT results to identify GC needs.
Methods
GEM participants with or high-risk for PCA were recruited. Pre-test GC was in-person, with video and handout, or via telehealth. Post-test disclosure was in-person, by phone, or via telehealth. Clinical and family history data were obtained from participant surveys and medical records. Participants completed measures of knowledge of CRG, literacy, and numeracy pre-test and post-test. Understanding of personal genetic results was assessed post-test. Factors associated with knowledge of CRG and understanding of personal genetic results were examined using multivariable linear regression or McNemar’s test.
Results
Among 109 men who completed pre- and post-GT surveys, multivariable analysis revealed family history meeting hereditary cancer syndrome (HCS) criteria was significantly predictive of higher baseline knowledge (p=0.040). Of 101 men who responded definitively regarding understanding of results, 13 incorrectly reported their result (McNemar’s p <0.001). Factors significantly associated with discordance between reported and actual results included having a variant of uncertain significance (VUS) (p<0.001) and undergoing GC via pre-test video and post-test phone disclosure (p=0.015).
Conclusions
While meeting criteria for HCS was associated with higher knowledge of CRG, understanding of personal GT results was lacking among males with VUS. A more exploratory finding was lack of understanding of results among men who underwent GC utilizing video and phone. Studies optimizing GC strategies for males undergoing multigene testing for inherited PCA are warranted.
Keywords: genetic testing, genetic counseling, prostate cancer, multigene testing, understanding of test results
Introduction
Approximately 5-20% of prostate cancer (PCA) is due to a strong-to-moderate inherited genetic predisposition.1 Multiple genes have been associated with inherited PCA including BRCA1, BRCA2, and HOXB13, with data emerging regarding DNA mismatch repair genes also predisposing to PCA.1,2 Furthermore, tumor sequencing studies in metastatic PCA are identifying inherited mutations in a broader range of DNA repair genes in up to 20% of patients.3–5 Multiple commercial genetic testing laboratories now offer multigene tests for PCA, including genes with strong evidence of PCA predisposition, genes with lesser degree of evidence for PCA risk, and genes with limited/no data in the context of PCA.6–7 Furthermore, NCCN guidelines have expanded genetic testing recommendations for PCA to include testing men with high risk to metastatic disease, or even lower risk/earlier stage disease based upon family history of cancers suggestive of hereditary breast and ovarian cancer or Lynch syndrome.8–10 Indeed, multigene testing is included as a consideration for testing by the NCCN Prostate Cancer treatment guideline (Version 1.2018) including BRCA1/2, ATM, PALB2, and FANCA, to inform options for precision treatment, clinical trials, and active surveillance discussions.9 Two consensus conferences also addressed genetic testing for PCA – one providing expert guidance on multigene testing based upon familial and personal cancer features and considerations for management and the other focused on genetic testing in the advanced stage setting.7,11 Thus, the rapid expansion in guidelines and expert opinion regarding genetic testing for men with PCA will lead to increased multigene testing, necessitating focused efforts to optimize genetic counseling (GC) among males.
Research regarding genetic testing and counseling for PCA among males is limited. Early studies showed that men were generally interested in genetic testing.12–14 Interest was primarily higher among men with a documented family history of PCA or those reporting increased levels of worry about a PCA diagnosis.12–14 But there is limited data regarding knowledge and understanding of actual genetic test results among males undergoing genetic evaluation for inherited PCA. Current advances in genetic testing availability present an opportunity to explore knowledge and understanding of PCA risk and genetics to optimize the GC experience.
GC for inherited cancer risk involves intake of medical history, family cancer history, and other risk factors followed by discussion of cancer inheritance, genetic test options, types of genetic test results, cancer risks based on genetic test results, screening recommendations, risk reduction options, reproductive implications, and financial considerations.15–17 Multigene testing can raise the complexity of these discussions, necessitating review of patients’ understanding of their results to ensure appropriate screening, medical management, and sharing of results with family members regarding positive, uncertain, and negative genetic test results. In particular, understanding of variants of uncertain significance (VUS) can be a challenge. As multigene testing has increased, rates of VUS have also risen.6,18 VUS are reported to patients in their genetic test results, but have no implications on management at the time of reporting. VUS are followed over time by genetic testing laboratories for evidence in support of pathogenicity. VUS can be reclassified over time to “benign” or “likely pathogenic/pathogenic” depending on accumulating evidence, which necessitates that patients with VUS keep in contact with their genetics program over time to learn of any reclassification of their findings. These considerations regarding VUS need to be clearly understood by patients in order to proceed with appropriate screening, management, and follow-up. Data regarding understanding of personal genetic test results in males undergoing multigene testing for PCA are currently lacking.
Cancer GC has traditionally been practiced in person, with patients travelling to a health care facility to meet with a trained genetics counselor.19 The increasing uptake of genetic testing is leading to a higher demand for genetic counselors, with a need to explore alternate GC delivery methods, such as through telephone and telehealth. Alternate service delivery models have been tested and evaluated primarily in the context of GC for breast and ovarian cancers,20–22 but little research has been devoted specifically to genetic testing for inherited PCA risk and GC delivery in men.
Genetic Evaluation of Men (GEM) is a prospective multigene testing study for PCA susceptibility in the context of GC. Initial analysis of the GEM study found that 5.5% of 200 males had a genetic mutation identified from multigene testing, and 35% of the cohort had a VUS identified.6 We surveyed men pre-genetic testing and post-genetic testing regarding knowledge of cancer risk and genetics and understanding of their personal genetic test results for insights into the needs of men undergoing germline genetic testing for inherited PCA.
Materials and Methods
Eligibility criteria
Eligibility criteria for the GEM study have been described in detail previously.6 Briefly, men with PCA were referred for genetic evaluation from treatment clinics at Site 1 (Fox Chase Cancer Center - FCCC) and Site 2 (Sidney Kimmel Cancer Center - SKCC) at Thomas Jefferson University (TJU). Unaffected males were referred primarily from screening efforts at both institutions.23 Affected men from Site 1 and Site 2 were recruited from medical oncology, urology, and radiation oncology clinics. At Site 2, referrals are also from the GU Multidisciplinary Clinic where men with PCA receive multidisciplinary evaluation, treatment planning, and an opportunity to engage in genetic evaluation.24 Family history eligibility criteria for GEM are modeled after other cancers and include a combination of young age of PCA diagnosis, African American race, family history of PCA, family history of hereditary cancer syndrome (HCS)-associated cancers, and family history meeting strict criteria for HCS.6 Furthermore, pathologic eligibility criteria include Gleason >7, T3 disease, or metastatic disease.6 GEM is approved by the IRB at SKCC and FCCC.
GC and multigene testing
Participants of GEM undergo pre-test and post-test GC employing various counseling delivery methods. Figure I displays the study flow, counseling, and timepoints of survey administration. Site 1 employs a pre-test counseling video that shows a genetic counselor delivering counseling information, along with a printed handout of the slide deck of information presented in the video, followed by a research assistant answering participant questions and proceeding with informed consent for the study. Site 2 performs pre-test counseling by a genetic counselor and physician (VNG) in-person or by telehealth. For telehealth visits, the appointment is arranged through the electronic medical system, where the patient and provider log in at the scheduled time for counseling to be delivered over the virtual interface. Patients may use their ipad, computer, or smartphone for telehealth visits.
Figure I.
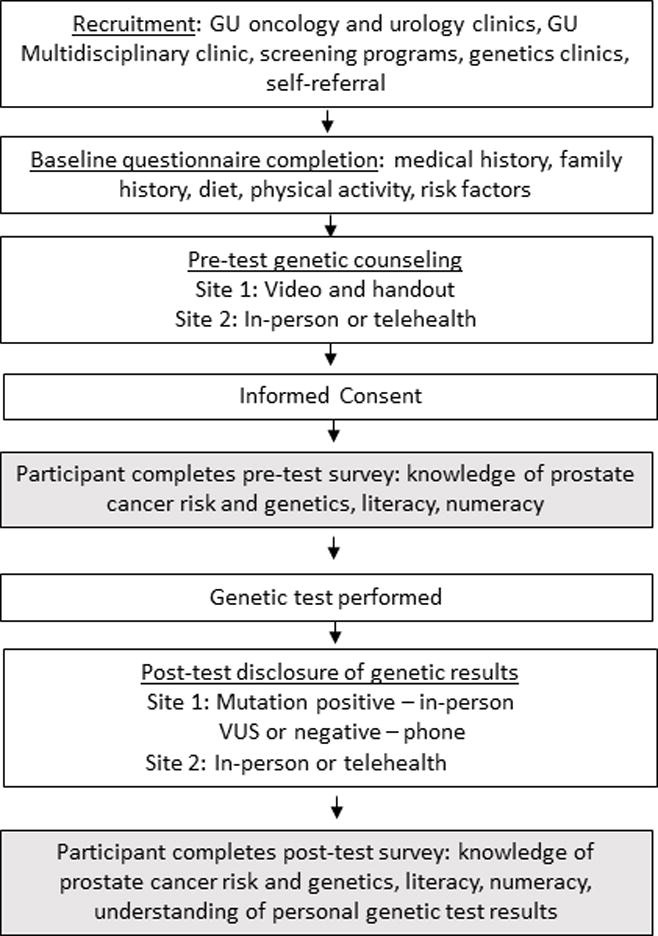
GEM Clinical Multigene Testing Study Flow
Informed consent was obtained after pre-test counseling. Blood was drawn for a 25-gene panel (later increased to 28-gene panel). Genetic testing was performed at a commercial genetic testing laboratory, with a clinical report generated. Approximately 4-8 weeks later, participants received their clinical genetic test results with a discussion of genetic findings, cancer risks, and screening recommendations. At Site 1, these results are delivered in-person if a participant has a mutation, by phone by a genetic counselor if a participant has a VUS, or by phone by the study coordinator if the test result was a negative result. Those with a VUS were encouraged by the genetic counselor to make an in-person appointment with a genetic counselor and medical provider (EO). At Site 2, all results are delivered in-person or by telehealth by the genetic counselor and medical provider (VNG).
Measures
Study measures were administered at two timepoints. A pre-test survey was administered after GC and prior to genetic testing, and a post-test survey was administered after genetic results were disclosed (Figure I)
Baseline questionnaires collected demographic and health history information including current age (at study entry), age at PCA diagnosis, personal medical history, family history of cancer, education level, marital status, race, and PCA stage/grade. The pre-test behavioral survey (administered after GC and informed consent) included measures of knowledge of PCA risk and genetics, health literacy, and numeracy. Knowledge of PCA risk and genetics was assessed using a 15-item scale adapted from prior studies of cancer genetics.14,25,26 Respondents answered each statement by marking “True” or “False.” Each correct answer was scored with a point, with higher scores reflecting greater knowledge. Scores could range from 0 to 15. Health literacy was measured using 3 items from the Short Test of Functional Health Literacy in Adults (STOHFLA) (Cronbach’s alpha 0.97).27 These items include: (1) “How often do you have someone help you read hospital materials?” (2) “How confident are you filling out medical forms by yourself?” and (3) “How often do you have problems learning about your medical condition because of difficulty understanding written information?” These items have been validated across studies and determined to be sensitive for identifying inadequate health literacy.27 Numeracy was assessed using a three-question survey used in prior studies and was scored as the total number of correct responses.28 Post-test survey re-tested on knowledge of PCA risk and genetics, health literacy, numeracy, and included an additional question addressing understanding of personal genetic test results: “I was found to carry a genetic mutation (a genetic change)”] (yes, no, don’t know).
Statistical methods
Participants completing both the pre- and post-test surveys were included in analyses. Participant demographic characteristics as well as family history of cancer were summarized with counts and percentages overall and within study site. Differences in subject characteristics between sites were assessed using Fisher’s exact tests and t-tests where appropriate. Associations between both pre-test knowledge and change in knowledge were assessed using linear regression. Univariable models were followed by multivariable models adjusting for all available covariates: age at consent, PCA diagnosis, family history of any cancer, status of any HCS, education level, literacy and numeracy.
In order to evaluate concordance of self-reported genetic test results with actual genetic test results, McNemar’s test of agreement was performed on the men who definitely reported their genetic test results; that is, excluding those who were unsure. Fisher’s exact tests and t-tests were used to explore univariable associations between discordance between self-reported genetic test results and genetic test results with demographic characteristics, family history, baseline knowledge and genetic test results (categorized as negative, VUS only, mutation). For all analyses, alpha was set at 0.05. All analyses were performed in SAS 9.4 (SAS Institute Inc., Cary, NC).
Results
Cohort characteristics
At the time of this analysis, 200 men were enrolled in the GEM clinical multigene testing study and all were offered survey completion at the pre-test and post-test timepoints. Of the 200 men, 109 completed both pre-test and post-test surveys, with a survey completion rate of 54.5%, and those were included in this analysis. There was a significant difference between responders and non-responders by HCS status, where 80% of those with HCS responded to the surveys vs. 44.8% without a HCS (p<0.001) (Supplementary Table I). The demographic characteristics of this cohort of 109 males is shown in Table I. The mean age of the cohort was 62.5 years (SD 8.1 years). White males comprised 80.7% of the cohort, and 86.2% of the cohort was married. Education status was at least some college or higher for 83.5% of the cohort. There was a significant difference in education level and PCA status by site (Supplementary Tables II-III). Greater percentage of participants at Site 1 had some college or less education while at Site 2 participants had college or higher education (p=0.025) (Supplementary Table II). PCA status was higher among participants at Site 2 compared to Site 1 (p<0.001) (Supplementary Table III).
Table I.
Demographic Characteristics of the Cohort Analyzed
| All | ||
|---|---|---|
| All | 109 | 100.0 |
| Site*, N (%) | ||
| Site 1 | 55 | 50.5 |
| Site 2 | 54 | 49.5 |
| Age, mean (SD) | 62.5 | 8.1 |
| Age, N (%) | ||
| 30–39 | 1 | 0.9 |
| 40–49 | 6 | 5.5 |
| 50–59 | 28 | 25.7 |
| 60–69 | 55 | 50.5 |
| 70–79 | 18 | 16.5 |
| 80–89 | 1 | 0.9 |
| Race, N (%) | ||
| No answer | 1 | 0.9 |
| White | 88 | 80.7 |
| Black or African American | 17 | 15.6 |
| Asian | 2 | 1.8 |
| Multiracial | 1 | 0.9 |
| Hispanic, N (%) | ||
| No answer | 7 | 6.4 |
| No | 101 | 92.7 |
| Yes | 1 | 0.9 |
| Ashkenazi Jewish, N (%) | ||
| No answer | 2 | 1.8 |
| No | 92 | 84.4 |
| Yes | 12 | 11.0 |
| Unsure | 3 | 2.8 |
| Marital Status, N (%) | ||
| No answer | 1 | 0.9 |
| Never Married | 3 | 2.8 |
| Married/Living with Partner | 94 | 86.2 |
| Separated/Divorced/Widowed | 11 | 10.1 |
| Education, N (%) | ||
| No answer | 1 | 0.9 |
| Less than high school | 1 | 0.9 |
| HS/GED or Vocational/Technical School | 16 | 14.7 |
| Some College/Associate’s Degree | 15 | 13.8 |
| Bachelor’s Degree or higher | 76 | 69.7 |
Site 1 provided pretest counseling with a video and handout. Post-test disclosure was in-person for men with a mutation and by phone for men with a variant of uncertain significance or negative results. Site 2 provided pretest counseling and post-test disclosure in-person or by telehealth.
Personal and family history characteristics are shown in Table II. Overall, 58.7% of males were diagnosed with PCA, and 40.4% of the cohort reported family history information meeting criteria for any of three HCS in which PCA has been implicated (Hereditary breast and ovarian cancer [HBOC], Lynch syndrome [LS], and hereditary PCA [HPC]). Family history of PCA was reported in 71.6% of the cohort, followed by family history of breast cancer in 47.7%.
Table II.
Personal and Family History Characteristics of Cohort Analyzed (n=109)
| All | ||
|---|---|---|
| Prostate Cancer Diagnosis, N (%) | ||
| No | 45 | 41.3 |
| Yes | 64 | 58.7 |
| Hereditary Cancer Syndrome, N (%) | ||
| No | 65 | 59.6 |
| Yes | 44 | 40.4 |
| HPC | 11 | 10.1 |
| LS | 3 | 2.8 |
| HBOC | 22 | 20.2 |
| HBOC&HPC | 4 | 3.7 |
| HBOC&LS | 4 | 3.7 |
| Family History of Cancer, N (%) | ||
| None | 7 | 6.4 |
| Any | 102 | 93.6 |
| Prostate | 78 | 71.6 |
| Breast | 52 | 47.7 |
| Ovarian | 14 | 12.8 |
| Colon | 26 | 23.9 |
| Pancreatic | 7 | 6.4 |
| Family History of Cancer (in FDRs only), N (%) | ||
| None | 12 | 11.0 |
| Any | 97 | 89.0 |
| Prostate | 62 | 56.9 |
| Breast | 30 | 27.5 |
| Ovarian | 3 | 2.8 |
| Colon | 15 | 13.8 |
| Pancreatic | 3 | 2.8 |
Abbreviations: HPC-Hereditary prostate cancer; LS-Lynch syndrome; HBOC-Hereditary breast and ovarian cancer; FDR-first-degree relative
Knowledge of cancer risk and genetics
Correct responses for the 15 knowledge items ranged from 12.8-86.2% for the pre-test survey and 22.9-95.4% for the post-test survey. The mean change in overall knowledge score from pre-test to post-test was 0.8 (SD 2.6, p=0.001). Table III displays results of univariable analysis of factors associated with knowledge of cancer risk and genetics from the pre-test survey. Higher pre-test knowledge of cancer risk and genetics was associated with meeting criteria for a HCS (p=0.006) and higher numeracy (p=0.025). On multivariable analysis, family history meeting criteria for a HCS remained significantly predictive of higher pre-test knowledge (p=0.040) (Table IV). No factors were associated with change in knowledge from pre-test to post-test timepoints.
Table III.
Univariable analysis of factors associated with pre-test cancer genetics knowledge
| Estimate | 95% CI | p-value | |
|---|---|---|---|
| Site* | |||
| Site 1 | REF | REF | — |
| Site 2 | 0.0 | (−1.20,1.27) | 0.959 |
| Age | |||
| + 10 years | −0.3 | (−1.02,0.50) | 0.504 |
| Prostate Cancer | |||
| Yes | −0.8 | (−2.07,0.41) | 0.187 |
| No | REF | REF | — |
| Family History | |||
| Yes | 2.3 | (−0.16,4.77) | 0.067 |
| No | REF | REF | — |
| HCS | |||
| Yes | 1.7 | (0.50,2.93) | 0.006 |
| No | REF | REF | — |
| Education | |||
| Less than high school | REF | REF | – |
| HS/GED or Vocational/Technical School | −0.8 | (−7.44,5.82) | 0.808 |
| Some College/Associate’s Degree | −0.7 | (−7.31,5.98) | 0.843 |
| Bachelor’s Degree or higher | 0.1 | (−6.35,6.59) | 0.971 |
| Literacy | |||
| + 1 point | 0.3 | (−0.55,1.18) | 0.471 |
| Numeracy | |||
| + 1 point | 0.8 | (0.11,1.55) | 0.025 |
Site 1 provided pretest counseling with a video + handout. Post-test disclosure was in-person for men with a mutation and by phone for men with a variant of uncertain significance or negative results. Site 2 provided pretest counseling and post-test disclosure in-person or by telehealth.
Table IV.
Multivariable analysis of factors associated with pre-test cancer genetics knowledge
| Estimate | 95% CI | p-value | |
|---|---|---|---|
| Intercept | 8.2 | (−2.22,18.64) | 0.121 |
| Site* | |||
| Site 1 | REF | REF | — |
| Site 2 | 0.5 | (−1.06,2.06) | 0.526 |
| Age | |||
| + 10 years | −0.0 | (−0.80,0.79) | 0.992 |
| Prostate Cancer | |||
| Yes | −1.5 | (−3.12,0.08) | 0.063 |
| No | REF | REF | — |
| Family History | |||
| Yes | 1.6 | (−1.01,4.20) | 0.227 |
| No | REF | REF | — |
| HCS | |||
| Yes | 1.4 | (0.07,2.70) | 0.040 |
| No | REF | REF | — |
| Education | |||
| Less than high school | REF | REF | — |
| HS/GED or Vocational/Technical School | −1.5 | (−8.21,5.12) | 0.647 |
| Some College/Associate’s Degree | −2.2 | (−8.77,4.45) | 0.518 |
| Bachelor’s Degree or higher | −1.8 | (−8.29,4.78) | 0.595 |
| Literacy | |||
| + 1 point | 0.0 | (−0.99,1.07) | 0.938 |
| Numeracy | |||
| + 1 point | 0.8 | (−0.05,1.65) | 0.063 |
Site 1 provided pretest counseling with a video + handout. Post-test disclosure was in-person for men with a mutation and by phone for men with a variant of uncertain significance or negative results. Site 2 provided pretest counseling and post-test disclosure in-person or by telehealth.
Understanding of personal genetic test results
Of 101 men who responded definitively regarding understanding of personal genetic test results (6 responded “don’t know” and 2 provided no answer), 88 men responded correctly regarding their genetic test result and 13 responded incorrectly, i.e. answering that they carry a genetic mutation when their result showed no mutation (McNemar’s p <0.001). Of these 13 men, 12 men reported they had a mutation when their test report revealed >= 1 VUS, and one male reported having a mutation when his result was negative. Overall, having a VUS genetic test result was significantly associated with incorrectly reporting genetic test results (p<0.001). Furthermore, undergoing GC utilizing pre-test video and post-test phone disclosure was also associated with lack of understanding genetic test results (p=0.015), which likely pertained to the participants receiving VUS results. Table V provides detailed personal and family history characteristics of participants, along with rates of discordance regarding self-report vs. actual test results. Other factors such as age, race, education status, literacy, or numeracy were not associated with discordance between reported vs. actual genetic test results. Interestingly, family history of a HCS (p=0.039) or family history of breast cancer (p=0.017) were associated with accurate understanding of personal genetic test results.
Table V.
Factors associated with understanding of personal genetic test results among males undergoing multigene testing for suspected inherited prostate cancer
| Discordance between self-reported vs. actual genetic test results | p-value | ||||
|---|---|---|---|---|---|
| No | Yes | ||||
| All | 88 | (100.0) | 13 | (100.0) | |
| Site* | 0.015 | ||||
| Site 1 | 41 | (46.6) | 11 | (84.6) | |
| Site 2 | 47 | (53.4) | 2 | (15.4) | |
| Age, Mean (SD) | 62.6 | (8.5) | 62.5 | (5.9) | 0.941 |
| Age, N (%) | >0.999 | ||||
| 30–49 | 6 | (6.8) | 0 | (0.0) | |
| 50–64 | 43 | (48.9) | 7 | (53.8) | |
| 65–89 | 39 | (44.3) | 6 | (46.2) | |
| Race, N (%) | >0.999 | ||||
| Not answered | 1 | (1.1) | 0 | (0.0) | |
| White | 73 | (83.0) | 11 | (84.6) | |
| Black or African American | 12 | (13.6) | 2 | (15.4) | |
| Asian | 1 | (1.1) | 0 | (0.0) | |
| Multiracial | 1 | (1.1) | 0 | (0.0) | |
| Education, N(%) | 0.617 | ||||
| Not answered | 1 | (1.1) | 0 | (0.0) | |
| Less than high school | 1 | (1.1) | 0 | (0.0) | |
| HS/GED or Vocational/Technical School | 11 | (12.5) | 2 | (15.4) | |
| Some College/Associate’s Degree | 12 | (13.6) | 3 | (23.1) | |
| Bachelor’s Degree or higher | 63 | (71.6) | 8 | (61.5) | |
| Knowledge Score, Mean (SD) | 10.1 | (2.9) | 9.0 | (3.5) | 0.283 |
| Literacy Score, Mean (SD) | 4.3 | (0.7) | 4.3 | (0.7) | 0.895 |
| Numeracy Score, Mean (SD) | 2.4 | (0.8) | 2.4 | (1.0) | 0.985 |
| Prostate Cancer Diagnosis, N (%) | 52 | (59.1) | 6 | (46.2) | 0.388 |
| Age of Diagnosis, Mean (SD) | 60.2 | (6.3) | 6 | (60.2) | 0.988 |
| Age of Diagnosis, N (%) | 0.354 | ||||
| 30–49 | 3 | (5.8) | 1 | (16.7) | |
| 50–64 | 34 | (65.4) | 3 | (50.0) | |
| 65–89 | 15 | (28.8) | 2 | (33.3) | |
| Family history of HCS, N (%) | 41 | (46.6) | 2 | (15.4) | 0.039 |
| Family History (FDRs), N (%) | |||||
| Cancer, any site | 81 | (92.0) | 10 | (76.9) | 0.118 |
| Prostate | 53 | (60.2) | 6 | (46.2) | 0.377 |
| Breast | 27 | (30.7) | 2 | (15.4) | 0.338 |
| Ovarian | 3 | (3.4) | 0 | (0.0) | >0.999 |
| Colon | 12 | (13.6) | 3 | (23.1) | 0.404 |
| Pancreatic | 3 | (3.4) | 0 | (0.0) | >0.999 |
| Family History (any relative), N (%) | |||||
| Cancer, any site | 84 | (95.5) | 11 | (84.6) | 0.171 |
| Prostate | 66 | (75.0) | 7 | (53.8) | 0.180 |
| Breast | 46 | (52.3) | 2 | (15.4) | 0.017 |
| Ovarian | 14 | (15.9) | 0 | (0.0) | 0.205 |
| Colon | 19 | (21.6) | 6 | (46.2) | 0.082 |
| Pancreatic | 7 | (8.0) | 0 | (0.0) | 0.590 |
| Genetic test result of VUS | 18 | (20.5) | 12 | (92.3) | <0.001 |
Site 1 provided pretest counseling with a video + handout. Post-test disclosure was in-person for men with a mutation and by phone for men with a variant of uncertain significance or negative results. Site 2 provided pretest counseling and post-test disclosure in-person or by telehealth.
Discussion
Genetic testing for inherited PCA is expected to increase with greater testing capability and expansion of guidelines.6–11 Recent updates to the NCCN guidelines include multigene testing for men with PCA factoring in family history and risk/stage of disease.9 With expected increased testing comes a need to provide men with meaningful GC to understand their test results. Furthermore, the rise in genetic testing is creating a greater need for access to GC, particularly with increasing awareness and acceptance of genetic testing among the general public.29,30 Since genetic testing for PCA is a relatively new field, focused efforts are needed to optimize the counseling experience for men undergoing genetic testing for inherited PCA.
Our results show that men with a personal/family history of HCS had higher knowledge of cancer risk and genetics, as well as greater understanding of personal genetic test results. When evaluating understanding by genetic results, having a VUS was associated with misconception of the interpretation of this finding, with 12 out of 13 men stating that they had a mutation when they actually had a VUS. Since VUS are reported in approximately a third of individuals who undergo multigene testing,6,18 it is important to address understanding of VUS since a substantial proportion of men undergoing multigene testing may have this finding. The potential risks of this lack of understanding include miscommunication of results to providers resulting possibly in unnecessary screening and/or risk reducing measures which may not be warranted. Furthermore, men and their families may have significant anxiety if they misunderstand a VUS finding as a mutation and subsequently pass on misinformation to relatives. Our data support the need to develop strategies to reinforce understanding of genetic findings, particularly VUS, among males undergoing multigene testing for PCA.
Our exploratory findings regarding understanding of personal genetic test results by mode of counseling delivery suggest that men who underwent GC utilizing a pre-test video and post-test phone disclosure had possible lack of an understanding of what their genetic test results meant than those who completed GC in person or via telehealth. This is particularly relevant for men who receive VUS findings, as these men had less understand their results. Since GC expertise is in demand, employing alternate delivery methods such as telephone, video, and telehealth will need to be thoughtfully utilized with attention to the needs and understanding of men undergoing genetic testing for inherited PCA. It has been suggested that patients may be most satisfied when they are allowed to choose the method of their GC,31 although this an area ripe for future research.
Personalized medicine and pre-symptomatic genetic testing has received an abundance of attention from the media and healthcare providers yet understanding the impact of these tests on patient understanding and behaviors has been slow to follow. This is particularly of concern for genetic testing and counseling for men, as studies in this patient population have not been as extensively performed. The public’s perception of the accuracy and utility of genetic testing is frequently overestimated,32 and was seen to some extent in the results of our study. Education delivered by a healthcare professional has been shown to increase a patient’s knowledge about their test results and their feelings of personal control, while decreasing levels of anxiety and cancer fatalism.33–35 Our results support developing and evaluating theoretically-driven, structured education protocols for PCA genetic testing that are also culturally appropriate and targeted to the specific psychological needs of the population.36
There are some limitations of this study that should be considered. The overall sample size is fairly small, and larger studies are needed to confirm our findings. Our cohort consisted of mostly White males with higher education level. Evaluation of genetic understanding is greatly needed in diverse populations to develop culturally-tailored strategies to reinforce understanding. Strategies for men with lower education levels are also needed. Completion of pre- and post-test surveys was 54.5%, with greater response rate among participants with a family history meeting criteria for a HCS, which may have impacted our findings. Additionally, misinterpretation of survey questions may have impacted responses. Confirmation of our findings is important. The exploratory results focused on understanding of test results by mode of counseling delivery were non-randomized by site and could have been confounded by site practice factors and patient populations. Our results point to the need for a formal randomized study testing various modes of counseling delivery focused on genetic testing for inherited PCA.
Conclusion
In summary, our results highlight the need for targeted strategies to reinforce understanding of genetic test results for greater impact on appropriate cancer management and enhanced patient experience with the genetic evaluation process. These efforts need to be focused in men receiving VUS results, as well as employing various models of counseling delivery for optimal GC and testing experience for men undergoing genetic evaluation for inherited PCA.
Supplementary Material
Acknowledgments
The GEM study is partially supported by Sidney Kimmel Cancer Center TIPS Pilot grant (108-27000-911621) and partially supported by PADOH (Grant #080-37270-AL0801). Efforts by FCCC investigators are funded in part by the FCCC CCSG (3P30CA006927). We are grateful to all participants of the study. Myriad Genetics provided genetic testing free of cost to study participants included in this analysis.
Footnotes
Conflict of Interest: All authors declare no conflicts of interest related to this manuscript.
References
- 1.Genetics of Prostate Cancer (PDQ®) National Cancer Institute; [Accessed February 1, 2018]. Available at: https://www.cancer.gov/types/prostate/hp/prostate-genetics-pdq. [Google Scholar]
- 2.Giri VN, Beebe-Dimmer J. Familial Prostate Cancer. Semin Oncol. 2016 Oct;43(5):560–565. doi: 10.1053/j.seminoncol.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schrader KA, Cheng DT, Joseph V, et al. Germline Variants in Targeted Tumor Sequencing Using Matched Normal DNA. JAMA Oncol. 2016;2(1):104–11. doi: 10.1001/jamaoncol.2015.5208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pritchard CC, Mateo J, Walsh MF, et al. Inherited DNA-Repair Gene Mutations in Men with Metastatic Prostate Cancer. N Engl J Med. 2016;375:443–453. doi: 10.1056/NEJMoa1603144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mandelker D, Zhang L, Kemel Y, Stadler ZK, Joseph V, Zehir A, et al. Mutation Detection in Patients With Advanced Cancer by Universal Sequencing of Cancer-Related Genes in Tumor and Normal DNA vs Guideline-Based Germline Testing. JAMA. 2017;318(9):825–835. doi: 10.1001/jama.2017.11137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giri VN, Obeid E, Gross L, et al. Inherited Mutations in Males Undergoing Multigene Panel Testing for PCA – Emerging Implications for Personalized Prostate Cancer Genetic Evaluation. Journal of Clinical Oncology – Precision Oncology. 2017 doi: 10.1200/PO.16.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giri VN, Knudsen KE, Kelly WK, Abida W, Andriole GL, Bangma C, et al. Role of Genetic Testing for Inherited Prostate Cancer Risk: Philadelphia Prostate Cancer Consensus Conference 2017. J Clin Oncol. 2017 Dec; doi: 10.1200/JCO.2017.74.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Genetic/Familial High-risk Assessment: Breast and Ovarian (Version 1.2018) [Accessed January 24, 2018]. Available at: https://www.nccn.org/professionals/physician_gls/pdf/genetics_screening.pdf.
- 9.NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Prostate Cancer (Version I.2018) [Accessed March 3, 2018]. Available at: https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf.
- 10.NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Prostate Cancer Early Detection (Version 2.2017) [Accessed February 16, 2018]. Available at: https://www.nccn.org/professionals/physician_gls/PDF/prostate_detection.pdf.
- 11.Gillessen S, Attard G, Beer TM, et al. Management of patients with advanced Prostate Cancer: The report of the advanced prostate cancer consensus conference APCCC 2017. Eur Urol. 2017 doi: 10.1016/j.eururo.2017.06.002. [DOI] [PubMed]
- 12.Diefenbach MA, Schnoll RA, Miller SM, Brower L. Genetic testing for Prostate Cancer: Willingness and predictors of interest. Cancer Practice. 2000;8(2):82–85. doi: 10.1046/j.1523-5394.2000.82006.x. [DOI] [PubMed] [Google Scholar]
- 13.Bratt O, Damber JE, Emanuelsson M, Kristoffersson U, Lundgren R, Olsson H, Gronberg H. Risk perception, screening practice and interest in genetic testing among unaffected men in families with hereditary prostate cancer. European Journal of Cancer. 2000;36(2):235–241. doi: 10.1016/s0959-8049(99)00272-5. [DOI] [PubMed] [Google Scholar]
- 14.Miesfeldt S, Jones SM, Cohn W, Lippert M, Haden K, Turner BL, Martin-Fries T, Clark SM. Men’s attitudes regarding genetic testing for hereditary prostate cancer risk. Urology. 2000;55(1):46–50. doi: 10.1016/s0090-4295(99)00400-8. [DOI] [PubMed] [Google Scholar]
- 15.Riley BD, Culver JO, Skrzynia C, et al. Essential elements of genetic cancer risk assessment, counseling, and testing: updated recommendations of the National Society of Genetic Counselors. J Genet Couns. 2012 Apr;21(2):151–61. doi: 10.1007/s10897-011-9462-x. [DOI] [PubMed] [Google Scholar]
- 16.Bradbury AR, Partick-Miller L, Long J, et al. Development of a tiered and binned GC model for informed consent in the era of multiplex testing for cancer susceptibility. Genet Med. 2015;17:485–492. doi: 10.1038/gim.2014.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Domchek SM, Bradbury AR, Garber JE, et al. Multiplex genetic testing for cancer susceptibility: out on the high wire without a net? J Clin Oncol. 2013;31(10):1267–70. doi: 10.1200/JCO.2012.46.9403. [DOI] [PubMed] [Google Scholar]
- 18.Balmaña J, Digiovanni L, Gaddam P, et al. Conflicting Interpretation of Genetic Variants and Cancer Risk by Commercial Laboratories as Assessed by the Prospective Registry of Multiplex Testing. J Clin Oncol. 2016 Sep 12; doi: 10.1200/JCO.2016.68.4316. pii: JCO684316. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen SA, Marvin ML, Riley BD, Vig HS, Rousseau JA, Gustafson SL. Identification of GC service delivery models in practice: A report from the NSGC Service Delivery Model Task Force. Journal of GC. 2013;22(4):411–421. doi: 10.1007/s10897-013-9588-0. [DOI] [PubMed] [Google Scholar]
- 20.Schwartz MD, Valdimarsdottir HB, Peshkin BN, et al. Randomized noninferiority trial of telephone vs. in-person GC for hereditary breast and ovarian cancer. J Clin Oncol. 2014;32:618–626. doi: 10.1200/JCO.2013.51.3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buchanan AH, Datta SK, Skinner CS, Hollowell GP, Beresford HF, Freeland T, Rogers B, Boling J, Marcom PK, Adams MB. Randomized Trial of Telegenetics vs. In-Person Cancer GC: Cost, Patient Satisfaction and Attendance. J Genet Couns. 2015 Dec;24(6):961–70. doi: 10.1007/s10897-015-9836-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buchanan AH, Rahm AK, Williams JL. Alternate Service Delivery Models in Cancer GC: A Mini-Review. Front Oncol. 2016 May 13;6:120. doi: 10.3389/fonc.2016.00120. eCollection 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giri VN, Beebe-Dimmer J, Buyyounouski M, Konski A, Feigenberg SJ, Uzzo RG, Hanks G, Godwin AK, Chen DYT, Gordon R, Cescon T, Raysor S, Watkins-Bruner D. Prostate Cancer Risk Assessment Program – A Ten-Year Update of Cancer Detection. J Urol. 2007;178(5):1920–1924. doi: 10.1016/j.juro.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 24.Giri VN, Gross L, Gomella LG, Hyatt C. How I Do It: GC and genetic testing for inherited prostate cancer. Can J Urol. 2016;23(2):8247–53. [PubMed] [Google Scholar]
- 25.Meiser B, Butow PN, Barratt AL, Schnieden V, Gattas M, Kirk J, Gaff C, Suthers G, Tucker K. Long-term outcomes of GC in women at increased risk of developing hereditary breast cancer. Patient Educ Couns. 2001;44(3):215–25. doi: 10.1016/s0738-3991(00)00191-9. [DOI] [PubMed] [Google Scholar]
- 26.Zilliacus EM, Meiser B, Lobb EA, Kelly PJ, Barlow-Stewart K, Kirk JA, Spigelman AD, Warwick LJ, Tucker KM. Are videoconferenced consultations as effective as face-to-face consultations for hereditary breast and ovarian cancer GC? Genet Med. 2011;13(11):933–41. doi: 10.1097/GIM.0b013e3182217a19. [DOI] [PubMed] [Google Scholar]
- 27.Chew LD, Bradley KA, Boyko EJ. Brief questions to identify patients with inadequate health literacy. Fam Med. 2004;36(8):588–94. [PubMed] [Google Scholar]
- 28.Schwartz LM, Woloshin S, Black WC, Welch HG. The role of numeracy in understanding the benefit of screening mammography. Ann Intern Med. 1997;127(11):966–72. doi: 10.7326/0003-4819-127-11-199712010-00003. [DOI] [PubMed] [Google Scholar]
- 29.Henneman L, Vermeulen E, van El CG, Claassen L, Timmermans DRM, Cornel MC. Public attitudes towards genetic testing revisited: comparing opinions between 2002 and 2010. European Journal of Human Genetics. 2013;21(8):793–799. doi: 10.1038/ejhg.2012.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vermeulen E, Henneman L, van El CG, Cornel MC. Public attitudes towards preventive genomics and personal interest in genetic testing to prevent disease: a survey study. European Journal of Public Health. 2014 Oct 1;24(5):768–775. doi: 10.1093/eurpub/ckt143. [DOI] [PubMed] [Google Scholar]
- 31.Baumanis L, Evans JP, Callanan N, Susswein LR. Telephoned BRCA 1/2 genetic test results: Prevalance, practice, and patient satisfaction. Journal of GC. 2009;18(5):447–463. doi: 10.1007/s10897-009-9238-8. [DOI] [PubMed] [Google Scholar]
- 32.Leighton JW, Valverde K, Bernhardt BA. The general public’s understanding and perception of direct-to-consumer genetic test results. Public Health Genomics. 2012;15(1):11–21. doi: 10.1159/000327159. [DOI] [PubMed] [Google Scholar]
- 33.Lerman C, Croyle RT, Tercyak KP, Hamann H. Genetic testing: Psychological aspects and implications. Journal of Consulting and Clinical Psychology. 2002;70(3):784–797. doi: 10.1037//0022-006x.70.3.784. [DOI] [PubMed] [Google Scholar]
- 34.Meiser B, Butow P, Friedlander M, Barratt A, Schnieden V, Watson M, Brown J, Tucker K. Psychological impact of genetic testing in women from high-risk breast cancer families. European Journal of Cancer. 2002;38(15):2025–2031. doi: 10.1016/s0959-8049(02)00264-2. [DOI] [PubMed] [Google Scholar]
- 35.Javitt GH, Hudson K. The right prescription for personalized genetic medicine. Personalized Medicine. 2007;4(2):115–118. doi: 10.2217/17410541.4.2.115. [DOI] [PubMed] [Google Scholar]
- 36.Bancroft EK, Castro E, Ardern-Jones A, Moynihan C, Page E, Taylor N, Eeles RA, Rowley E, Cox K. “It’s all very well reading and writing the letters in the genome, but it’s a long way to being able to write”: Men’s interpretations of undergoing genetic profiling to determine future risk of prostate cancer. Familial Cancer. 2014;13(4):625–635. doi: 10.1007/s10689-014-9734-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


