Abstract
Background and objective
Molecular biomarkers are needed to refine prognostication and phenotyping of pulmonary hypertension (PH) patients. S100A12 is an emerging biomarker of various inflammatory diseases. This study aims to determine the prognostic value of S100A12 in PH.
Methods
Exploratory microarray analysis performed on peripheral blood mononuclear cells (PBMC) collected from idiopathic pulmonary fibrosis (IPF) patients suggested an association between S100A12 and both PH and mortality. So the current study was designed to evaluate for an association between S100A12 in peripheral blood collected from two well-phenotyped PH cohorts in two other centres to derive and validate an association between S100A12 protein serum concentrations and mortality.
Results
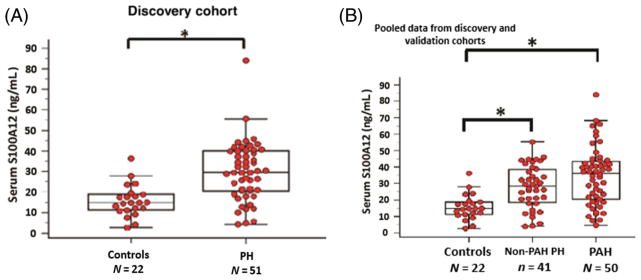
The majority of the patients in the discovery and validation cohorts were either World Health Organization (WHO) group 1 (pulmonary arterial hypertension (PAH)) or 3 (lung disease-associated) PH. In the discovery PH cohort, S100A12 was significantly increased in patients with PH (n = 51) compared to controls (n = 22) (29.8 vs 15.7 ng/mL, P < 0.001) and negatively correlated with cardiac output (r = −0.58, P < 0.001) in PH patients. When S100A12 data were pooled from both cohorts, PAH and non-PAH PH patients had higher S100A12 compared to healthy external controls (32.6, 30.9, 15.7 ng/mL; P < 0.001). S100A12 was associated with an increased risk in overall mortality in PH patients in both the discovery (n = 51; P = 0.008) and validation (n = 40; P < 0.001) cohorts.
Conclusion
S100A12 levels are increased in PH patients and are associated with increased mortality.
Keywords: biomarkers, inflammatory heart disease, prognosis, pulmonary hypertension, translational studies
INTRODUCTION
Pulmonary hypertension (PH) is a pathophysiological disorder of the pulmonary vasculature that has been divided into five major groups by the World Health Organization (WHO) classification.1 PH is known to complicate the majority of cardiovascular and respiratory diseases and it has been associated with significantly increased mortality regardless of the PH WHO group.2–5
Predicting prognosis in PH has been limited, as classifying and phenotyping patients with PH are largely ‘based on a relatively simple combination of patient characteristics and hemodynamics’.6 The National Heart, Lung, and Blood Institute Pulmonary Vascular Disease Phenomics (PVDomics; RFA-HL-14-027)7 and other groups8 are evaluating better ways to phenotype PH patients. The use of molecular biomarkers is one way to better phenotype these patients.6,9
S100A12 (Calgranulin C, EN-RAGE) is a member of the S100 family of calcium-binding proteins and exerts pleiotropic properties including chemotactic activity and activation of intracellular signalling cascades leading to inflammatory cytokine production and induction of oxidative stress,10 all of which have been implicated in the pathogenesis of pulmonary vascular disease. Recent studies have demonstrated that S100A12 can be of clinical value in the evaluation and assessment of several inflammatory disorders.11,12 Accordingly, blood concentration of S100A12 is an emerging biomarker of coronary artery disease as well as of acute and chronic lung disorders.13–16 Our study group has previously shown that S100A12 plasma concentrations were increased in patients with idiopathic pulmonary fibrosis (IPF) and correlated with poor outcomes.14 While others have shown that the expression of S100A12 in peripheral blood mononuclear cells (PBMC) is upregulated in PH patients compared to controls,17 the usefulness of blood S100A12 protein concentration as a prognostic biomarker in PH patients has not been established yet.
Following the exploratory observation that S100A12 gene expression was increased in IPF patients with PH compared to IPF patients without PH or healthy controls, we aimed to investigate the prognostic value of serum S100A12 protein concentrations in two independent prospective cohorts of patients with PH irrespective of PH aetiology. Some of the results of these studies have been previously reported in the form of an abstract.18
METHODS
Patients and samples
Source data included 113 individual patients with serum blood samples derived from two independent cohorts. Figure S1 in Supplementary Information summarizes the role of each of these cohorts.
Discovery cohort: The discovery cohort included 51 patients with PH (25 of which have pulmonary arterial hypertension (PAH)) and 22 age- and gender-matched healthy controls enrolled at the Division of Pulmonary and Critical Care Medicine, University of North Carolina.
Validation cohort: The validation cohort included 40 patients with PH enrolled at the section of Pulmonary, Critical Care, and Sleep Medicine at Yale University.
Diagnosis of PH was based on the revised guidelines of European Respiratory Society (ERS)/European Society of Cardiology (ESC) (2015)19 and fifth World Symposium on Pulmonary Hypertension.1,20 All patients and healthy donors provided written informed consent and a protocol incorporating biomarker studies that were approved by the respective Institutional Review Boards (IRB) of the University of Pittsburgh (approval number: IRB0610029), University of North Carolina (approval number: 09-0003) and Yale University (approval number: 1502015305).
Although all patients with PH had a right heart catheterization at some point, many of the patients’ right heart catheterizations were performed many years earlier. To avoid spurious associations, we included only right heart catheterization central haemodynamics performed within 1 year of blood draw; all others were considered missing.
S100A12 quantification
Patient samples were obtained at routine visits from consecutive patients during clinic visits or right heart catheterizations as appropriate. For the determination of S100A12 in serum in the two cohorts, we used enzyme-linked immunosorbent assay (ELISA) using recombinant S100A12 as standard according to manufacturer’s protocol (MBL International, Woburn, MA, USA).14 Serum samples were diluted 1:10. All assays were performed in duplicate, and the mean values were reported.
Statistical analysis
Sample descriptive data are expressed as mean ± SD. Categorical variables are expressed as counts/percentages. We used an unpaired (two-sample) Student’s t-test to compare the means of continuous variables between two groups, including, for example, comparing the S100A12 concentrations between PAH cohort and healthy controls, or between those who survived versus those who died. Bivariable relationships between continuous variables were evaluated by the Pearson’s correlation coefficient (r), including, for example, comparing S100A12 gene expression with PH severity. The significance of differences between patients and controls was determined using the Mann–Whitney U test.
To identify the optimal prognostic cut-off threshold of serum S100A12 concentrations, we applied receiver operator characteristic (ROC) curve analysis where the true positive rate (sensitivity) was plotted in function of the false positive rate (100 – specificity) for different concentrations of serum S100A12 in the discovery cohort. The ROC curve analysis revealed the cut-off value of serum S100A12 that was associated with mortality with the optimal sensitivity and specificity. ROC curve analysis as well as simple and multiple linear regression, Kaplan–Meier survival curves, and Cox proportional hazard model analyses were conducted using MedCalc version 14.0 and STATA software version 11 IC for Windows (2010; STATACorp, College Station, TX, USA). A two-sided alpha of 0.05 was considered statistically significant for all tests for effect modification.
RESULTS
Baseline characteristics of the discovery and validation PH cohorts
Healthy controls were recruited at the University of North Carolina and were age- and gender-matched to the discovery cohort patients. The validation cohort patients exhibited comparable haemodynamic profile (except for a lower cardiac output: 4.6 vs 5.5 L/min; P = 0.046) (Table 1). There were no significant differences between patients of both cohorts in the different clinical WHO PH groups’ distribution (Table 1), with a significant proportion being WHO group 1 PH (i.e. PAH) in both cohorts.
Table 1.
Baseline characteristics of patients in the discovery (UNC) and validation (Yale) cohorts
| External healthy controls (UNC) (n = 22) | Discovery PH cohort (UNC)* (n = 51) | Validation PH cohort (Yale) (n = 40) | P-value (discovery cohort vs controls) | P-value (discovery vs validation cohorts) | |
|---|---|---|---|---|---|
| Age (years) | 54 ± 8 | 55 ± 15 | 60 ± 12 | 0.58 | 0.07 |
| Female, n (%) | 16 (73%) | 30 (59%) | 30 (75%) | 0.16 | 0.11 |
| Mean PAP (mm Hg) | NA | 40 ± 13 | 45 ± 11 | NA | 0.055 |
| PVR (Wood units) | NA | 5.9 ± 5.4 | 7.0 ± 3.8 | NA | 0.32 |
| TD cardiac output (L/min) | NA | 5.5 ± 2.1 | 4.6 ± 1.5 | NA | 0.046 |
| TRV (m/s) | NA | 3.4 ± 1.0 | NA | NA | NA |
| S100A12 (ng/mL) | 15.7 ± 7.7 | 29.8 ± 14.4 | 34.4 ± 17.7 | <0.001 | 0.18 |
| WHO PH groups†: | NA | NS | |||
| PH—Group I (n %) | NA | 25 (49%) | 25 (63%) | ||
| PH—Group II (n %) | NA | 1 (2%) | 0 (0%) | ||
| PH—Group III (n %) | NA | 17 (33%) | 12 (30%) | ||
| PH—Group IV (n %) | NA | 5 (10%) | 1 (3%) | ||
| PH—Group V (n %) | NA | 3 (6%) | 2 (5%) |
Results are reported as mean ± SD, unless otherwise stated.
Discovery PH cohort (UNC) included 25 PAH patients and 26 non-PAH PH patients.
P-values were calculated using the Fisher’s exact test except for age, mean PAP, TD cardiac output where one-way ANOVA with Bonferroni correction or unpaired Student’s t-test was used.
ANOVA, analysis of variance; NA, Not applicable; NS: not significant; PAH, pulmonary arterial hypertension; PAP, pulmonary artery pressure; PH, pulmonary hypertension; PVR, pulmonary vascular resistance; TD, thermodilution; TRV, tricuspid regurgitation jet velocity; UNC, University of North Carolina; WHO, World Health Organization.
None of the patients had heritable PAH. The patient with WHO group 2 PH had severe mitral regurgitation; those with WHO group 3 PH were equally divided between COPD and interstitial lung disease as the aetiology of their lung disease (with a mean PAP of 37 ± 11 mm Hg; PVR 3.3 ± 1.8 Wood units). WHO group 5 PH patients were either renal failure patients or patients with pulmonary sarcoidosis.
Increased serum S100A12 concentration in PH patients correlating with reduced cardiac output
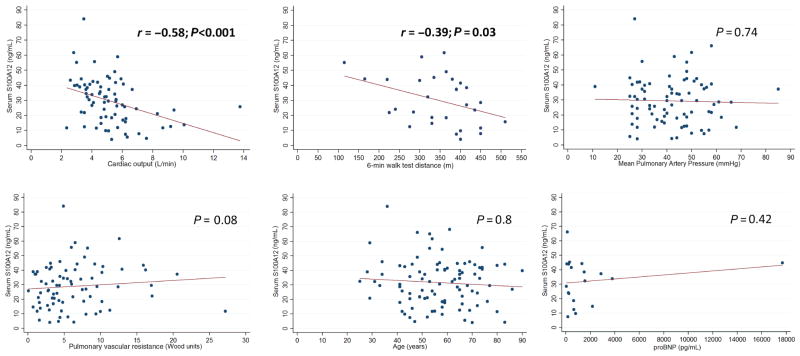
In the discovery cohort, serum S100A12 protein concentrations were significantly increased in PH patients (n = 51) compared to the healthy controls (n = 22) (29.8 ± 14.4 vs 15.7 ± 7.7 ng/mL; P < 0.001) (Fig. 1A, Table 1). Furthermore, serum S100A12 protein concentrations were negatively correlated with cardiac output measured by thermodilution (TD) on right heart catheterization (r = −0.58; P < 0.001), a marker of cardiovascular risk mortality (the difference in cardiac output is attributed to differential right heart strain, rather than left ventricular cardiomyopathy as this patient population was a PAH population and did not include patients with left heart disease). The serum S100A12 protein concentrations were also negatively but weakly correlated with 6-min walk distance (r = −0.39; P = 0.03) (Fig. 2). There was no correlation between serum S100A12 protein concentrations and other echocardiographic or invasive haemodynamic parameters of disease severity including mean pulmonary artery pressure (PAP; P = 0.74), tricuspid regurgitation jet velocity (TRV; P = 0.62) or pulmonary vascular resistance (PVR; P = 0.08). There was also no correlation between S100A12 concentrations and age (P = 0.8), or N-terminal pro B-type natriuretic peptide (proBNP; P = 0.42) (Fig. 2). There was no correlation either between proBNP (mean level 1880 ± 3966; normal vale is <300 pg/mL) and cardiac output (P = 0.29).
Figure 1.
(A) Serum A100A12 protein concentrations in healthy normal controls versus PH patients in the discovery cohort. (B) Serum S100A12 protein concentrations in healthy normal controls versus non-PAH PH patients versus PAH patients in the combined data from both the discovery and validation cohorts. Data are presented as box-and-whisker plots, with horizontal bars representing medians, top whisker representing maximal expression, and bottom whisker representing the 5th percentile, Mann–Whitney U test, *P < 0.001. PAH, pulmonary arterial hypertension; PH, pulmonary hypertension.
Figure 2.
Correlation scatterplot with best-fit line between serum levels of S100A12 levels and each of the following: cardiac output, 6-min walk test, mean pulmonary artery pressure, pulmonary vascular resistance, age, and proBNP levels. r, correlation coefficient;
 , fitted values;
, fitted values;
 , S100A12 level.
, S100A12 level.
Combined analysis of both cohorts revealed no significant differences in baseline characteristics, echocardiographic or haemodynamic parameters in patients with high serum S100A12 concentrations (≥21.2 ng/mL; n = 66) compared to those with lower serum S100A12 concentrations (<21.2 ng/mL; n = 25) except for TD cardiac output (4.8 vs 5.9 L/min, P = 0.02) and survival (Table 2). These data were similar between the two cohorts when analysed separately.
Table 2.
Baseline clinical and haemodynamic characteristics between high and low serum S100A12 combined patients in both (UNC & Yale) cohorts
| Variable | High S100A12 (≥21.2 ng/mL) (n = 66) | Low S100A12 (<21.2 ng/mL) (n = 25) | P-value |
|---|---|---|---|
| Age (years) | 58 ± 15 | 56 ± 13 | 0.67 |
| Female, n (%) | 45 (68%) | 15 (60%) | 0.46 |
| TRV | 3.4 ± 0.9 | 3.6 ± 1.1 | 0.66 |
| Mean PAP (mm Hg) | 41.4 ± 12.9 | 42.4 ± 11.8 | 0.75 |
| PVR (Wood units) | 6.5 ± 4.6 | 5.8 ± 5.5 | 0.54 |
| TD cardiac output (L/min) | 4.8 ± 1.9 | 5.9 ± 1.9 | 0.02 |
| S100A12 (ng/mL) | 38.9 ± 12.6 | 13.1 ± 5.4 | <0.0001 |
| Survival (years) | 2.1 ± 1.1 | 2.9 ± 0.7 | <0.001 |
| WHO PH groups: | NS | ||
| PH—Group I (n %) | 35 (53%) | 14 (53.8%) | |
| PH—Group II (n %) | 1 (1.5%) | 0 (0%) | |
| PH—Group III (n %) | 23 (34.8%) | 5 (19.2%) | |
| PH—Group IV (n %) | 4 (6.2%) | 4 (15.4%) | |
| PH—Group V (n %) | 3 (4.5%) | 3 (11.6%) |
Results are reported as mean ± SD, unless otherwise stated.
P-values were calculated using the Fisher’s exact test except for age, TRV, mean PAP, TD cardiac output, where unpaired Student’s t-test was used.
NS, not significant; PAP, pulmonary artery pressure; PH, pulmonary hypertension; PVR, pulmonary vascular resistance; TD, thermo-dilution; TRV, tricuspid regurgitation jet velocity; UNC, University of North Carolina; WHO, World Health Organization.
Combined analysis revealed that patients with PAH (n = 50; 32.6 ng/mL; P < 0.001) and patients with non-PAH PH (n = 41; 30.9 ng/mL; P < 0.001) exhibited significantly higher serum S100A12 concentrations compared to healthy normal controls (n = 22; 15.7 ng/mL) (Fig. 1B). There was no significant difference in S100A12 concentrations between the PAH and the non-PAH PH groups (P = 0.62), despite the more severe clinical pulmonary vascular disease pattern in PAH patients compared to PH patients as reflected by haemodynamic measurements (Table 3).
Table 3.
Baseline clinical and haemodynamic characteristics between patients with PAH and other groups of PH, from both discovery and validation cohorts
| Variable | PAH (n = 50) | PH (other than PAH) (n = 41) | P-value |
|---|---|---|---|
| Age (years) | 57 ± 14 | 57 ± 14 | 0.87 |
| Female, n (%) | 34 (68%) | 26 (63%) | 0.65 |
| Mean PAP (mm Hg) | 46 ± 13 | 37 ± 11 | 0.001 |
| PVR (Wood units) | 8.3 ± 5.3 | 3.5 ± 2.3 | <0.0001 |
| TD cardiac output (L/min) | 4.7 ± 1.5 | 5.7 ± 2.4 | 0.03 |
| S100A12 (ng/mL) | 32.6 ± 15.8 | 30.9 ± 16.3 | 0.62 |
| Survival (years) | 2.4 ± 1.0 | 2.3 ± 1.3 | 0.97 |
Results are reported as mean ± SD, unless otherwise stated.
P-values were calculated using the Fisher’s exact test except for age, mean PAP, TD cardiac output, where unpaired Student’s t-test was used.
PAH, pulmonary arterial hypertension; PAP, pulmonary artery pressure; PH, pulmonary hypertension; PVR, pulmonary vascular resistance; TD, thermodilution.
Increased serum S100A12 concentrations associate with increased mortality in both cohorts
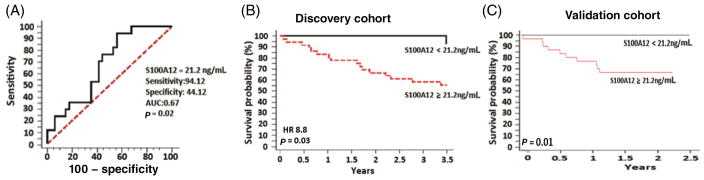
Serum S100A12 (as a continuous variable) was associated with an increased risk in overall mortality in PH patients in both the discovery (n = 51) (P = 0.008) and validation (n = 40) (P < 0.001) cohorts. Cardiac output (mean = 5.2 ± 1.9 L/min; within the normal range) on the other hand was not associated with mortality (P = 0.27). ROC curve analysis in the discovery cohort identified a serum S100A12 cut-off threshold of 21.2 ng/mL as the best fit for a significant predictive performance (as assessed by area under the curve (AUC) of 0.67; P = 0.02; Fig. 3A) that maximized specificity and sensitivity. Using this cut-off threshold to dichotomize the S100A12 variable, a significant association with mortality was observed in the discovery cohort (hazard ratio (HR) 8.8; P = 0.03) (Fig. 3B). Applying the same serum S100A12 cut-off threshold of 21.2 ng/mL to the second independent cohort validated the significant association with all-cause mortality (P = 0.01; Fig. 3C).
Figure 3.
(A) Receiver operator characteristic (ROC) curve for best-fit value of serum S100A12 protein level to predict mortality. (B) Kaplan–Meier survival curve using an ROC-derived value of serum S100A12 protein level of 21.2 ng/mL to dichotomize the discovery cohort. (C) Kaplan–Meier survival curve in the validation cohort using the serum S100A12 protein dichotomizing value of 21.2 ng/mL. AUC, area under the curve; HR, hazard ratio.
Serum S100A12 concentration, used as a continuous variable, remained a strong predictor of all-cause mortality in both the discovery (HR 1.08; P = 0.004) and validation (HR 1.10; P = 0.001) cohorts (Tables S1, S2 in Supplementary Information) after adjustment for age, gender, and when available haemodynamic parameters of disease severity, as was the dichotomized threshold (HR 8.8; P = 0.01). (We did not include 6-min walk distance or proBNP because they were only available for 16 patients in the validation cohort, and thus would make the model unstable).
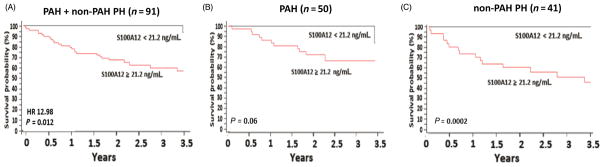
In a secondary analysis, serum S100A12 concentrations were associated with increased mortality in the combined discovery and validation cohorts; when survival data from both cohorts were pooled together, the serum S100A12 cut-off threshold of 21.2 ng/mL still predicted mortality (n = 91, HR 12.98; P = 0.012; Fig. 4A). High S100A12 expressers exhibited significantly shorter survival than low S100A12 expressers from both cohorts (median survival 2.1 vs 2.9 years, P < 0.001) (Table 2). There were no significant differences in the distribution of WHO PH clinical classification groups between high and low S100A12 expressers (Table 2).
Figure 4.
Combined discovery and validation cohorts. (A) Kaplan–Meier survival curve of all PH (PAH and non-PAH) patients. (B) Kaplan–Meier survival curve of PAH patients. (C) Kaplan–Meier survival curve of non-PAH PH patients. HR, hazard ratio; PAH, pulmonary arterial hypertension; PH, pulmonary hypertension.
The association between S100A12 concentration and survival did not reach statistical significance in the subgroup of patients with WHO group 1 PH (PAH; combined discovery and validation cohorts; n = 50; P = 0.06; Fig. 4B). There was a wide variability in the PAH-specific medications used in the PAH cohort, and consequently it had no impact on the above results. In the non-PAH PH subgroup, however, there was significant association between S100A12 level and mortality (n = 41; P = 0.0002; Fig. 4C).
DISCUSSION
This is the first study in the literature that shows a significant association between a novel serum protein bio-marker, S100A12, and mortality in multiple independent PH cohorts of varying aetiologies, including PAH. Serum S100A12 concentrations exhibited an increase from healthy controls in both the non-PAH PH and PAH patients, evidence that may reflect the increasing pulmonary vasculature pathology between these groups.
In an a priori subgroup analysis of only the PAH patients (n = 50), the association with mortality did not reach statistical significance (P = 0.06). However, in the overall group of all of the patients combined (main study results) and the non-PAH PH subgroup (a priori-decided subgroup), there was clear correlation between S100A12 levels and mortality despite having a relatively small sample size. The lack of association with mortality in the PAH subgroup is intriguing due to the limited sample size, rather than due an intrinsic difference in the pathophysiology between these two PH subgroups.
S100A12 is a member of the S100 family of calcium-binding proteins and acts as a danger-associated molecular pattern (DAMP) molecule.21,22 It increases with inflammation and its expression in humans is primarily in neutrophilic granulocytes,23 as well as structural cells, including endothelial, epithelial and smooth muscle cells.24 S100A12 binding to its major target proteins, receptor for advanced glycation end products (RAGE) or Toll-like receptor 4, activates the mitogen-activated protein (MAP) kinase (MAPK) and nuclear factor (NF)-kappa-B signalling pathways leading to production of pro-inflammatory cytokines10,25 as well as cell adhesion molecules ICAM1 and VCAM1,26 all of which known to be implicated in the pathogenesis of systemic or pulmonary vascular disease.27 To this end, S100A12 serum concentrations have been studied in the pathogenesis and prognosis of coronary artery disease12,28,29 as well as other chronic inflammatory disorders.10,24,25,30,31
Although S100A12 has intracellular activities, its predominant role is upon excretion. As mentioned above, ligation of S100A12 with its receptor RAGE induces activation of several adaptor molecules involved in NF-kappa-B and MAPK pathway, key mediators of cell survival and proliferation. Stress-activated MAPK including p38 have been implicated in the pathogenesis of PH. In particular, increased levels of activated (phosphorylated) p38 have been observed in the pulmonary vasculature from patients with idiopathic PAH, and inhibition of p38 signalling pathway through a selective antagonist led to reversal of PH in two experimental models of PH through mechanisms that involved restoration of hypoxia-induced endothelial dysfunction and IL-6 signalling pathway.32 However, it is currently unknown whether S100A12 is directly involved in enhanced proliferation and survival of vascular smooth muscle cells or endothelial dysfunction through interference with the MAPK or NF-kappa-B signalling pathway in the context of PH. This is an interesting premise and it is worth being addressed in the context of another study, as our study was not designed to delineate mechanisms of S100A12 actions.
The role of S100A12 in the pathogenesis of pulmonary vascular disease is currently unknown. Bull et al. reported differentially expressed genes that could reliably differentiate patients with PAH from controls, and S100A12 was among them.17 We, using a targeted proteomic approach, discovered that increased plasma S100A12 concentrations were included in a protein signature that could reliably predict mortality in two independent cohorts of patients with IPF.14 Considering that the clinical course of patients with IPF is often complicated and deteriorated by PH,33 we explored the relationship of S100A12 and PH in a publicly available data set generated by our group.34 Indeed, S100A12 PBMC gene expression was increased in IPF with PH compared to IPF patients without PH or healthy controls, and was positively correlated with indices of PH. These findings (summarized in Appendix S1, including Fig. S2 and Table S3 in Supplementary Information) were the basis of the current hereby-reported study.
Following this observation and considering the current unmet need for molecular biomarkers in patients with PH, our next step was to assess whether increased blood concentrations of S100A12 were associated with increased mortality in patients with PH. After showing that S100A12 serum concentrations were significantly increased in patients with PH compared to controls, we discovered an optimal cut-off threshold of serum S100A12 and tested its prognostic value in both the discovery cohort and then the validation cohort. The serum S100A12 cut-off threshold concentration of 21.2 ng/mL was significantly associated with mortality in those two independent cohorts, indicating a strong predictive signal in patients with PH, irrespective of disease aetiology.
S100A12 serum concentrations showed an association with survival and cardiac output, but not with other haemodynamic variables. It appears possible that elevated S100A12 concentrations represent a distinct molecular phenotype in which patients decompensate beyond the extent of pulmonary pressures. One could even speculate that increases in S100A12 concentrations identify patients with right ventricle dysfunction that is disproportionate of their pulmonary pressures and congestion as reflected by proBNP, but future prospective studies will need to assess this possibility.
Although the association between S100A12 has not been previously studied in PH, He et al. reported on a positive correlation between proBNP and plasma S100A12 levels in left heart failure patient population,29 which is different from the results reported above in PH patients. It is difficult to draw any concrete conclusions about such differential correlations of S100A12 and ventricular distension in the right versus left ventricle based on a single study. More studies in both right and left heart failure evaluating such a correlation need to be performed to better understand such potential physiologic interactions, or lack thereof.
The dissociation between S100A12 as a replicated predictor of mortality and haemodynamic parameters is novel, but has some precedent in the literature. Although PH is defined and its severity is quantified by haemodynamics,20 other studies have suggested that determinants of mortality are not solely related to haemodynamic impairment. The REVEAL registry risk score, for example, included (among many other non-haemodynamic variables) only two haemodynamic variables.35 Furthermore, these haemodynamic variables had to be dichotomized at significantly higher values that are uncommon in clinical practice (e.g. PVR at 32 Wood units and right atrial pressure at 20 mm Hg), limiting their clinical predictive value. Indeed, the currently available prognostic models in PH are limited,35 and need further refinement.36 There is no established threshold for a favourable or unfavourable prognosis in individual patients even for highly established bio-markers of clinically established significance such as BNP or proBNP leading to a recommendation that any comprehensive phenotyping algorithm should include biomarkers.6
Our study exhibited several limitations that should be considered cautiously. First, the IPF exploratory analysis data set was dichotomized as having PH or not based on echocardiographic studies, which have limited accuracy in estimating pulmonary artery pressures.37 However, using echocardiogram-derived variables is a practical and non-invasive way of estimating pulmonary artery pressures, and these pressures were measured directly by right heart catheterization in the discovery and validation cohorts. In addition, our study was not designed to determine whether S100A12 increases are specific for PH or pulmonary vascular disease. Increased blood S100A12 concentrations are predictive of worse outcomes in atherosclerosis and other vascular and inflammatory disorders.12,24,29,31 S100A12 has also significant roles in inflammatory signalling.12 However, our results are significantly novel and highlight the potential role of S100A12 as a molecular biomarker as well as an indicator of an inflammatory pathway previously unrecognized in the pathogenesis of PH. Last, our design was not powered to provide subgroup analysis and the relatively small sample size limited complete analysis and adjusting for other known prognostic variables.
In summary, this study shows that S100A12 is a strong predictor of mortality irrespective of central haemodynamics or ventricular congestion, and it should be considered as a biomarker for phenotype characterization and predicting disease progression in patients with PH. Further clinical studies enrolling highly characterized patients with PH are required to fully elucidate the role of S100A12 as a biomarker of disease progression and treatment responsiveness.
Supplementary Material
Appendix S1 Exploratory analysis.
Figure S1 Overall summary of the exploratory analysis and the two cohorts that comprise this study.
Figure S2 Pittsburgh GAP exploratory analysis.
Table S1 Multivariable Cox regression hazard models for all-cause mortality in the discovery cohort of PH patients.
Table S2 Multivariable Cox regression hazard models for all-cause mortality in the validation cohort of PH patients.
Table S3 Baseline characteristics of patients with IPF, IPF-PH and healthy controls of the GAP (gene expression) exploratory analysis.
SUMMARY AT A GLANCE.
S100A12 serum levels are elevated in patients with pulmonary hypertension and they are associated with increased mortality rates. These findings show that the S100A12 biomarker has a major role to play in determining the prognosis of pulmonary hypertension patients, and they would enrich deep phenotyping efforts in this patient population.
Acknowledgments
The authors gratefully acknowledge the IPF and PH patients who consented to participation in this study and the numerous clinical researchers involved in the collection of samples and clinical data. This work was funded by the National Institutes of Health (U01 HL108642 and R01 HL127349 to N.K. and F.A., UL1 RR 024153 and R03 HL095401, Pittsburgh Foundation M2010-0055, Pulmonary Hypertension Association Grant to F.A., R01 HL109033 to E.L.H., and R00HL114651 to J.H.C.).
Abbreviations
- HR
hazard ratio
- IPF
idiopathic pulmonary fibrosis
- MAPK
mitogen-activated protein kinase
- NF
nuclear factor
- PAH
pulmonary arterial hypertension
- PAP
pulmonary artery pressure
- PBMC
peripheral blood mononuclear cell
- PH
pulmonary hypertension
- proBNP
pro B-type natriuretic peptide
- PVR
pulmonary vascular resistance
- RAGE
receptor for advanced glycation end products
- ROC
receiver operatic characteristic
- TD
thermodilution
- TRV
tricuspid regurgitation jet velocity
- WHO
World Health Organization
- UNC
University of North Carolina
Footnotes
Disclosure statement
N.K. is currently a consultant to Pliant, Biogen-Idec, Boehringer-Ingelheim, Moerae Matrix; he is a recipient of investigator-initiated grants from Biogen Idec, and in the past Celgene and Gilead; N.K. and J.D.H.M. are inventors of a patent on the use of peripheral blood proteins in prediction of IPF outcomes. W.H.F. is on the Advisory Board & Speakers Bureau for Actelion, Gilead, United Therapeutics, & Bayer. H.J.F. is on the Advisory Board for Gilead, United Therapeutics, & Bayer. F.A. is the recipient of grants from MyoKardia and Gilead, and is a consultant for Janssen and Novartis.
Additional supplementary information can be accessed via the html version of this article at the publisher’s website.
References
- 1.Simonneau G, Gatzoulis MA, Adatia I, Celermajer D, Denton C, Ghofrani A, Gomez Sanchez MA, Krishna Kumar R, Landzberg M, Machado RF, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2013;62:D34–41. doi: 10.1016/j.jacc.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 2.Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the joint task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT) Eur Heart J. 2016;37:67–119. [Google Scholar]
- 3.Lajoie AC, Lauziere G, Lega JC, Lacasse Y, Martin S, Simard S, Bonnet S, Provencher S. Combination therapy versus monotherapy for pulmonary arterial hypertension: a meta-analysis. Lancet Respir Med. 2016;4:291–305. doi: 10.1016/S2213-2600(16)00027-8. [DOI] [PubMed] [Google Scholar]
- 4.McLaughlin VV, Shah SJ, Souza R, Humbert M. Management of pulmonary arterial hypertension. J Am Coll Cardiol. 2015;65:1976–97. doi: 10.1016/j.jacc.2015.03.540. [DOI] [PubMed] [Google Scholar]
- 5.Reque J, Quiroga B, Ruiz C, Villaverde MT, Vega A, Abad S, Panizo N, Lopez-Gomez JM. Pulmonary hypertension is an independent predictor of cardiovascular events and mortality in haemodialysis patients. Nephrol Ther. 2016;21:321–6. doi: 10.1111/nep.12595. [DOI] [PubMed] [Google Scholar]
- 6.Dweik RA, Rounds S, Erzurum SC, Archer S, Fagan K, Hassoun PM, Hill NS, Humbert M, Kawut SM, Krowka M, et al. ATS Committee on Pulmonary Hypertension Phenotypes. An official American Thoracic Society statement: pulmonary hypertension phenotypes. Am J Respir Crit Care Med. 2014;189:345–55. doi: 10.1164/rccm.201311-1954ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frantz RP. Pulmonary arterial hypertension or left heart disease with pulmonary hypertension? Toward noninvasive clarity, but time for a new paradigm. Eur Respir J. 2015;46:299–302. doi: 10.1183/13993003.00456-2015. [DOI] [PubMed] [Google Scholar]
- 8.Lutz K, Pauciulo M, Winslow C, Walsworth A, Gygi A, Reponen A, Barve M, Harley J, Marsolo K, Martin L, et al. National biological sample and data repository for pulmonary arterial hypertension. PH Professional Network Symposium. 2015:A34. [Google Scholar]
- 9.Gladwin MT. Translational advances in the field of pulmonary hypertension bench to bedside: how fundamental discoveries in science are advancing our understanding and therapy of pulmonary arterial hypertension. Am J Respir Crit Care Med. 2017;195:1–3. doi: 10.1164/rccm.201608-1637ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meijer B, Gearry RB, Day AS. The role of S100A12 as a systemic marker of inflammation. Int J Inflam. 2012;2012:907078. doi: 10.1155/2012/907078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao P, Wu M, Yu H, Huang Y, Wang Y, Wang W, Yin W. Serum S100A12 levels are correlated with the presence and severity of coronary artery disease in patients with type 2 diabetes mellitus. J Investig Med. 2013;61:861–6. doi: 10.2310/JIM.0b013e318292fb1e. [DOI] [PubMed] [Google Scholar]
- 12.Oesterle A, Bowman MA. S100A12 and the S100/Calgranulins: emerging biomarkers for atherosclerosis and possibly therapeutic targets. Arterioscler Thromb Vasc Biol. 2015;35:2496–507. doi: 10.1161/ATVBAHA.115.302072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen H, Fang X, Zhu H, Li S, He J, Gu P, Fan D, Han F, Zeng Y, Yu X, et al. Gene expression profile analysis for different idiopathic interstitial pneumonias subtypes. Exp Lung Res. 2014;40:367–79. doi: 10.3109/01902148.2014.933985. [DOI] [PubMed] [Google Scholar]
- 14.Richards TJ, Kaminski N, Baribaud F, Flavin S, Brodmerkel C, Horowitz D, Li K, Choi J, Vuga LJ, Lindell KO, et al. Peripheral blood proteins predict mortality in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2012;185:67–76. doi: 10.1164/rccm.201101-0058OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cockayne DA, Cheng DT, Waschki B, Sridhar S, Ravindran P, Hilton H, Kourteva G, Bitter H, Pillai SG, Visvanathan S, et al. Systemic biomarkers of neutrophilic inflammation, tissue injury and repair in COPD patients with differing levels of disease severity. PLoS One. 2012;7:e38629. doi: 10.1371/journal.pone.0038629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lorenz E, Muhlebach MS, Tessier PA, Alexis NE, Duncan Hite R, Seeds MC, Peden DB, Meredith W. Different expression ratio of S100A8/A9 and S100A12 in acute and chronic lung diseases. Respir Med. 2008;102:567–73. doi: 10.1016/j.rmed.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bull TM, Coldren CD, Moore M, Sotto-Santiago SM, Pham DV, Nana-Sinkam SP, Voelkel NF, Geraci MW. Gene microarray analysis of peripheral blood cells in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2004;170:911–9. doi: 10.1164/rccm.200312-1686OC. [DOI] [PubMed] [Google Scholar]
- 18.Ryu C, Tzouvelekis AE, Herazo-Maya J, Pan H, Adonteng-Boateng P, Li Q, Kaminski N, Ford HJ, Ahmad F, et al. S100A12 is elevated in patients with pulmonary hypertension and predicts mortality. American Thoracic Society Conference. 2016 193.1_MeetingAbstracts.A7832 [Abstract] [Google Scholar]
- 19.Galie N, Humbert M, Vachiery J-L, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Noordegraaf AV, Beghetti M, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. The joint task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS) Eur Respir J. 2015;46:903–75. doi: 10.1183/13993003.01032-2015. [DOI] [PubMed] [Google Scholar]
- 20.Hoeper MM, Bogaard HJ, Condliffe R, Frantz R, Khanna D, Kurzyna M, Langleben D, Manes A, Satoh T, Torres F, et al. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol. 2013;62:D42–50. doi: 10.1016/j.jacc.2013.10.032. [DOI] [PubMed] [Google Scholar]
- 21.Foell D, Wittkowski H, Roth J. Mechanisms of disease: a ‘DAMP’ view of inflammatory arthritis. Nat Clin Pract Rheumatol. 2007;3:382–90. doi: 10.1038/ncprheum0531. [DOI] [PubMed] [Google Scholar]
- 22.Roth J, Vogl T, Sorg C, Sunderkotter C. Phagocyte-specific S100 proteins: a novel group of proinflammatory molecules. Trends Immunol. 2003;24:155–8. doi: 10.1016/s1471-4906(03)00062-0. [DOI] [PubMed] [Google Scholar]
- 23.Guignard F, Mauel J, Markert M. Identification and characterization of a novel human neutrophil protein related to the S100 family. Biochem J. 1995;309(Pt 2):395–401. doi: 10.1042/bj3090395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Camoretti-Mercado B, Karrar E, Nunez L, Bowman MA. S100A12 and the airway smooth muscle: beyond inflammation and constriction. J Allergy Ther. 2012:3. doi: 10.4172/2155-6121.S1-007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang Z, Yan WX, Cai H, Tedla N, Armishaw C, Di Girolamo N, Wang HW, Hampartzoumian T, Simpson JL, Gibson PG, et al. S100A12 provokes mast cell activation: a potential amplification pathway in asthma and innate immunity. J Allergy Clin Immunol. 2007;119:106–14. doi: 10.1016/j.jaci.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 26.Vince RV, Chrismas B, Midgley AW, McNaughton LR, Madden LA. Hypoxia mediated release of endothelial microparticles and increased association of S100A12 with circulating neutrophils. Oxid Med Cell Longev. 2009;2:2–6. doi: 10.4161/oxim.2.1.7611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fares WH, Ford HJ, Ghio AJ, Aris RM. Safety and feasibility of obtaining wedged pulmonary artery samples and differential distribution of biomarkers in pulmonary hypertension. Pulm Circ. 2012;2:477–82. doi: 10.4103/2045-8932.105036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goyette J, Yan WX, Yamen E, Chung YM, Lim SY, Hsu K, Rahimi F, Di Girolamo N, Song C, Jessup W, et al. Pleiotropic roles of S100A12 in coronary atherosclerotic plaque formation and rupture. J Immunol. 2009;183:593–603. doi: 10.4049/jimmunol.0900373. [DOI] [PubMed] [Google Scholar]
- 29.He YY, Yan W, Liu CL, Li X, Li RJ, Mu Y, Jia Q, Wu FF, Wang LL, He KL. Usefulness of S100A12 as a prognostic biomarker for adverse events in patients with heart failure. Clin Biochem. 2015;48:329–33. doi: 10.1016/j.clinbiochem.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 30.van de Logt F, Day AS. S100A12: a noninvasive marker of inflammation in inflammatory bowel disease. J Dig Dis. 2013;14:62–7. doi: 10.1111/1751-2980.12012. [DOI] [PubMed] [Google Scholar]
- 31.Kang JH, Hwang SM, Chung IY. S100A8, S100A9 and S100A12 activate airway epithelial cells to produce MUC5AC via extracellular signal-regulated kinase and nuclear factor-kappaB pathways. Immunology. 2015;144:79–90. doi: 10.1111/imm.12352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Church AC, Martin DH, Wadsworth R, Bryson G, Fisher AJ, Welsh DJ, Peacock AJ. The reversal of pulmonary vascular remodeling through inhibition of p38 MAPK-alpha: a potential novel anti-inflammatory strategy in pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2015;309:L333–47. doi: 10.1152/ajplung.00038.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seeger W, Adir Y, Barbera JA, Champion H, Coghlan JG, Cottin V, De Marco T, Galie N, Ghio S, Gibbs S, et al. Pulmonary hypertension in chronic lung diseases. J Am Coll Cardiol. 2013;62:D109–16. doi: 10.1016/j.jacc.2013.10.036. [DOI] [PubMed] [Google Scholar]
- 34.Chien JW, Richards TJ, Gibson KF, Zhang Y, Lindell KO, Shao L, Lyman SK, Adamkewicz JI, Smith V, Kaminski N, et al. Serum lysyl oxidase-like 2 levels and idiopathic pulmonary fibrosis disease progression. Eur Respir J. 2014;43:1430–8. doi: 10.1183/09031936.00141013. [DOI] [PubMed] [Google Scholar]
- 35.Benza RL, Gomberg-Maitland M, Miller DP, Frost A, Frantz RP, Foreman AJ, Badesch DB, McGoon MD. The REVEAL registry risk score calculator in patients newly diagnosed with pulmonary arterial hypertension. Chest. 2012;141:354–62. doi: 10.1378/chest.11-0676. [DOI] [PubMed] [Google Scholar]
- 36.Fares WH, Bellumkonda L, Tonelli AR, Carson SS, Hassoun PM, Trow TK, Herzog EL, Kaminski N, Kholdani CA, Zhang L, et al. Right atrial pressure/pulmonary artery wedge pressure ratio: a more specific predictor of survival in pulmonary arterial hypertension. J Heart Lung Transplant. 2016;35:760–7. doi: 10.1016/j.healun.2015.12.028. [DOI] [PubMed] [Google Scholar]
- 37.Fisher MR, Forfia PR, Chamera E, Housten-Harris T, Champion HC, Girgis RE, Corretti MC, Hassoun PM. Accuracy of Doppler echocardiography in the hemodynamic assessment of pulmonary hypertension. Am J Respir Crit Care Med. 2009;179:615–21. doi: 10.1164/rccm.200811-1691OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Exploratory analysis.
Figure S1 Overall summary of the exploratory analysis and the two cohorts that comprise this study.
Figure S2 Pittsburgh GAP exploratory analysis.
Table S1 Multivariable Cox regression hazard models for all-cause mortality in the discovery cohort of PH patients.
Table S2 Multivariable Cox regression hazard models for all-cause mortality in the validation cohort of PH patients.
Table S3 Baseline characteristics of patients with IPF, IPF-PH and healthy controls of the GAP (gene expression) exploratory analysis.